New Bisphosphomide Ligands, 1,3-Phenylenebis((diphenylphosphino)methanone) [1,3-
|
|
|
- Moses Bruce
- 5 years ago
- Views:
Transcription
1 New Bisphosphomide Ligands, 1,3-Phenylenebis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 ] and (2-Bromo-1,3-phenylene)bis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 Br]: Synthesis, Coordination behavior, DFT Calculations and Catalytic Studies Pawan Kumar a, Mujahuddin M. Siddiqui a, Yerrnaidu Reddi a, Joel T Mague b, Raghavan B. Sunoj a, Marvanji S. Balakrishna a*
2 [Ru 2 (η 6 -p-cymene) 2 Cl 4 {1,3 {Ph 2 PC(O)} 2 (C 6 H 4 )}] (5) [PdCl{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}](7) [NiBr{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}] (8) [PdBr{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}] (9) [Cu 2 (µ-cl) 2 {1,3-{Ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (10) [Cu 2 (µ-i) 2 {1,3-{Ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (12)
3 [Ag 2 (µ-clo 4 )(µ-cl){1,3-{ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (14) Figure S1. The optimized geometries of complexes 5, 7, 8, 9, 10, 12 and 14 at B3LYP/6-31G**, SDD level of theory. NBO Analysis Natural bonding orbital analysis provides details on the interaction between the occupied Lewis type (bond or lone pair) NBOs and unoccupied non-lewis type (anti bonding) NBOs in the molecule and which can be used as the measure of delocalization. This donor acceptor interaction can be described in terms of the second order perturbation interaction energy (E (2) ). These interaction energies are reported using the B3LYP/6-31G** and SDD for palladium metal as shown in Table S1. Table S1. The Second Order Perturbation Interaction Energies of Selected Donor and Acceptor NBOs of Palladium Pincer Complex 9 using the B3LYP Method Donor Acceptor E (2) (kcal/mol) C 1 C 51 Pd C 5 C 51 Pd P 13 Pd 61 P 14 Pd
4 P 13 Pd 61 C 51 Pd P 14 Pd 61 P 13 Pd P 14 Pd 61 C 9 P P 14 Pd 61 P 14 C P 14 Pd 61 P 14 C C 51 Pd 61 P 13 Pd C 51 Pd 61 P 14 Pd C 51 Pd 61 C 51 Pd Pd 61 C 51 Pd Pd 61 C 5 C Pd 61 P 13 Pd Pd 61 P 14 Pd The highest interaction energy is found to be kcal/mol between the donor NBO of BD (C 51 Pd 61) to acceptor NBOs of both BD* (P 13 Pd 61) and BD* (P 13 Pd 61). The over all interaction energy is found to be approximately kcal/mol. The slightly higher occupancy and good localized NBOs are obtained in P Pd bond ( electrons in both P Pd) than in C51 Pd bond ( electrons). Aim analysis Aim analysis is powerful method to explain the electron density between two atoms in the molecule. The eletron density is considered between the two atoms based on bond critical point (bcp) values
5 Table S2. Topological map showing bond paths and bond critical points for important weak interactions in palladium pincer complex 9 Bond path Interaction bcp *10-2 (a.u.) 1 Phenyl H Carbonyl O Phenyl H Carbonyl O Phosphorous and Palladium Phosphorous and Palladium Phenyl carbon and Palladium As shown in the table there are found to be two major interactions between palladium and phenyl carbon and palladium and phosphorous. The bcp of phenyl carbon and palladium is found to be higher than phosphorous and palladium atoms. Therefore the electron density is more between phenyl carbon and palladium reveals that the two atoms bind strongly. AIM analysis of Pd pincer complex 8 shows that hydrogen bonding interaction between C H O. Total electronic energies (in a.u) and Cartesian coordinates of geometries optimized at the B3LYP/6-31G*, SDD level of theory [Ru 2 (η 6 -p-cymene) 2 Cl 4 {1,3 {Ph 2 PC(O)} 2 (C 6 H 4 )}] (5) E ele = [PdCl{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}](7) E ele =
6
7 [NiBr{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}] (8) [PdBr{2,6-{Ph 2 PC(O)} 2 (C 6 H 3 )}] (9) E ele = E ele =
8 [Cu 2 (µ-cl) 2 {1,3-{Ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (10) [Cu 2 (µ-i) 2 {1,3-{Ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (12) E ele = E ele =
9
10
11 [Ag 2 (µ-clo 4 )(µ-cl){1,3-{ph 2 PC(O)} 2 (C 6 H 4 )} 2 ] (14) E ele =
12
13
How Partial Atomic Charges and Bonding. Orbitals Affect the Reactivity of Aluminum
 Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy. --- Influence of intramolecular interactions
 Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy --- Influence of intramolecular interactions Minfu Zhao, Xu Shan, Shanshan Niu, Yaguo Tang, Zhaohui Liu,
Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy --- Influence of intramolecular interactions Minfu Zhao, Xu Shan, Shanshan Niu, Yaguo Tang, Zhaohui Liu,
Hydrogen and Halogen Bonds are Ruled by the Same Mechanisms S.J.Grabowski
 1 Hydrogen and Halogen Bonds are Ruled by the Same Mechanisms S.J.Grabowski Electronic supplementary information All results presented were obtained at MP2/6-311++G(d,p) level, QTAIM was applied to calculate
1 Hydrogen and Halogen Bonds are Ruled by the Same Mechanisms S.J.Grabowski Electronic supplementary information All results presented were obtained at MP2/6-311++G(d,p) level, QTAIM was applied to calculate
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Valence electrons octet rule. Lewis structure Lewis structures
 Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds
 Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Coordinate Covalent Bond
 Coordinate Covalent Bond 1. The coordinate covalent bond is a special type of covalent bond in which shared pair of electrons between two atoms is contributed by one of the atoms only. The atom which contributes
Coordinate Covalent Bond 1. The coordinate covalent bond is a special type of covalent bond in which shared pair of electrons between two atoms is contributed by one of the atoms only. The atom which contributes
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
18-Electron Rule: Myth or Reality? An NBO Perspective
 18-Electron Rule: Myth or Reality? An NBO Perspective Eric CLOT UMR 5253 - CNRS, UM2, ENSCM, UM1 Interaction Experiment/Theory Experimental Chemists have Questions Where are the Nucleophilic and Electrophilic
18-Electron Rule: Myth or Reality? An NBO Perspective Eric CLOT UMR 5253 - CNRS, UM2, ENSCM, UM1 Interaction Experiment/Theory Experimental Chemists have Questions Where are the Nucleophilic and Electrophilic
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Photoinduced Water Oxidation at the Aqueous. GaN Interface: Deprotonation Kinetics of. the First Proton-Coupled Electron-Transfer Step
 Supporting Information Photoinduced Water Oxidation at the Aqueous Interface: Deprotonation Kinetics of the First Proton-Coupled Electron-Transfer Step Mehmed Z. Ertem,,,* eerav Kharche,,* Victor S. Batista,
Supporting Information Photoinduced Water Oxidation at the Aqueous Interface: Deprotonation Kinetics of the First Proton-Coupled Electron-Transfer Step Mehmed Z. Ertem,,,* eerav Kharche,,* Victor S. Batista,
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES
 N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
Lone Pairs: An Electrostatic Viewpoint
 S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
Acid Dissociation Constant
 CE 131 Lecture 37 Lewis Acids and Bases Chapter 16: pp. 800-802. Acid Dissociation Constant C 2 3 2 + 2 3 + + C 2 3-2 [ 3 + ][C 2 3-2 ] K = [ 2 ][C 2 3 2 ] [ 3 + ][C 2 3-2 ] K a = K [ 2 ] = [C 2 3 2 ]
CE 131 Lecture 37 Lewis Acids and Bases Chapter 16: pp. 800-802. Acid Dissociation Constant C 2 3 2 + 2 3 + + C 2 3-2 [ 3 + ][C 2 3-2 ] K = [ 2 ][C 2 3 2 ] [ 3 + ][C 2 3-2 ] K a = K [ 2 ] = [C 2 3 2 ]
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Investigation of hydrogen bonding between nitrosamine and sulfuric acid using Density Functional Theory
 doi: 10.2478/auoc-2014-0001 Ovidius University Annals of Chemistry Volume 25, Number 1, pp. 5-10, 2014 Investigation of hydrogen bonding between nitrosamine and sulfuric acid using Density Functional Theory
doi: 10.2478/auoc-2014-0001 Ovidius University Annals of Chemistry Volume 25, Number 1, pp. 5-10, 2014 Investigation of hydrogen bonding between nitrosamine and sulfuric acid using Density Functional Theory
Quick Tutorial on Natural Bond Order 3 Calculations Within Gaussian 09
 Quick Tutorial on Natural Bond Order 3 Calculations Within Gaussian 09 Benjamin Rudshteyn, and Victor S. Batista* victor.batista@yale.edu Yale University, Department of Chemistry 225 Prospect Street, New
Quick Tutorial on Natural Bond Order 3 Calculations Within Gaussian 09 Benjamin Rudshteyn, and Victor S. Batista* victor.batista@yale.edu Yale University, Department of Chemistry 225 Prospect Street, New
Electronic Supplementary Information. for
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information for Two Chiral Catalysts in Action: Insights on Cooperativity
Chapter 2: Alkanes MULTIPLE CHOICE
 Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
5.111 Principles of Chemical Science
 MIT penurseware http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 27.1 5.111 ecture 27
MIT penurseware http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 27.1 5.111 ecture 27
Acids and Bases. Acids and Bases
 BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
Ionic Versus Covalent Bonding
 Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
1. Determine the oxidation state of the metal centre and count the number of electrons.
 Exercise sheet : Organometallic chemistry Gunnar Bachem 1. Determine the oxidation state of the metal centre and count the number of electrons. 2. The metal fragment 1 reacts with the amine to give a carbene
Exercise sheet : Organometallic chemistry Gunnar Bachem 1. Determine the oxidation state of the metal centre and count the number of electrons. 2. The metal fragment 1 reacts with the amine to give a carbene
Periodicity HL (answers) IB CHEMISTRY HL
 Periodicity HL (answers) IB CHEMISTRY HL 13.1 First row d-block elements Understandings: Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and
Periodicity HL (answers) IB CHEMISTRY HL 13.1 First row d-block elements Understandings: Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and
Research Article. Studies on Derivatives Uracil stability in various solvents and different functional groups: A DFT Study
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(7):290-297 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Studies on Derivatives Uracil stability in various
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(7):290-297 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Studies on Derivatives Uracil stability in various
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Acid/Base stuff Beauchamp 1
 cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
B. Electron Deficient (less than an octet) H-Be-H. Be does not need an octet Total of 4 valence electrons
 B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
Molecular Orbitals of Ethene
 Molecular Orbitals of Ethene 1 Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" interestingly, this reaction is rare and very slow! Molecular Orbitals
Molecular Orbitals of Ethene 1 Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" interestingly, this reaction is rare and very slow! Molecular Orbitals
NH 3 inversion: Potential energy surfaces and transition states CH342L March 28, 2016
 N 3 inversion: Potential energy surfaces and transition states C342L March 28, 2016 Last week, we used the IR spectrum of ammonia to determine the splitting of energy levels due to inversion of the umbrella
N 3 inversion: Potential energy surfaces and transition states C342L March 28, 2016 Last week, we used the IR spectrum of ammonia to determine the splitting of energy levels due to inversion of the umbrella
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
NPA/NBO-Analysis. Examples POP =
 NPA/NBO-Analysis Examples POP = NBO Requests a full Natural Bond Orbital analysis, using NBO version 3 NPA Requests just the Natural Population Analysis phase of NBO. NBORead Requests a full NBO analysis,
NPA/NBO-Analysis Examples POP = NBO Requests a full Natural Bond Orbital analysis, using NBO version 3 NPA Requests just the Natural Population Analysis phase of NBO. NBORead Requests a full NBO analysis,
List of Figures Page Figure No. Figure Caption No. Figure 1.1.
 List of Figures Figure No. Figure Caption Page No. Figure 1.1. Cation- interactions and their modulations. 4 Figure 1.2. Three conformations of benzene dimer, S is not a minimum on the potential energy
List of Figures Figure No. Figure Caption Page No. Figure 1.1. Cation- interactions and their modulations. 4 Figure 1.2. Three conformations of benzene dimer, S is not a minimum on the potential energy
Inorganic chemistry 3-stage Lec. 2. Dr- leaqaa
 Inorganic chemistry 3-stage Lec. 2 Dr- leaqaa Chemical bonds types: Most chemical bonds fall into 2 categories depending on whether the valence e(s) are transferred or shared. Electron in ionic bond are
Inorganic chemistry 3-stage Lec. 2 Dr- leaqaa Chemical bonds types: Most chemical bonds fall into 2 categories depending on whether the valence e(s) are transferred or shared. Electron in ionic bond are
NAME: SECOND EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
Inorganic Pharmaceutical Chemistry. Coordination compounds
 Inorganic Pharmaceutical Chemistry Lecture No. 5 Date : 22/11 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Inorganic Pharmaceutical Chemistry Lecture No. 5 Date : 22/11 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia
 Research Journal of Chemical Sciences ISSN 2231-606X Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia Françoise Diendere *, Issaka Guiguemde and Abdouraman Bary Laboratoire
Research Journal of Chemical Sciences ISSN 2231-606X Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia Françoise Diendere *, Issaka Guiguemde and Abdouraman Bary Laboratoire
Jahn-Teller Distortions
 Selections from Chapters 9 & 16 The transition metals (IV) CHEM 62 Monday, November 22 T. Hughbanks Jahn-Teller Distortions Jahn-Teller Theorem: Nonlinear Molecules in orbitally degenerate states are inherently
Selections from Chapters 9 & 16 The transition metals (IV) CHEM 62 Monday, November 22 T. Hughbanks Jahn-Teller Distortions Jahn-Teller Theorem: Nonlinear Molecules in orbitally degenerate states are inherently
Chapter 2: Acids and Bases
 1. Which of the following statements is a correct definition for a Brønsted-Lowry acid? A) Proton acceptor C) Electron pair acceptor B) Electron pair donor D) Proton donor 2. Which of the following statements
1. Which of the following statements is a correct definition for a Brønsted-Lowry acid? A) Proton acceptor C) Electron pair acceptor B) Electron pair donor D) Proton donor 2. Which of the following statements
5.4. Electronic structure of water
 5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
Chemical Bonding 4.8. Valence Bond Theory Hybrid Orbital Theory Multiple Bonds High School Chem Solutions. All rights reserved.
 Chemical Bonding 4.8 Valence Bond Theory Hybrid Orbital Theory Multiple Bonds Valence Bond Theory Combines Lewis theory of filling octets by sharing pairs of electrons with the electron configuration of
Chemical Bonding 4.8 Valence Bond Theory Hybrid Orbital Theory Multiple Bonds Valence Bond Theory Combines Lewis theory of filling octets by sharing pairs of electrons with the electron configuration of
E4 Acids, Bases, and Salts
 E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Acids and Bases Q. Are acid-base properties of substances predictable
E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Acids and Bases Q. Are acid-base properties of substances predictable
Density Functional Theory Study of Exohedral Carbon Atoms Effect on Electrophilicity of Nicotine: Comparative Analysis
 Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Chapter 21: Transition Metals and Coordination Chemistry
 Chapter 21: Transition Metals and Coordination Chemistry Mg, Cr, V, Co Pt Fe complexes O2 Mo and Fe complexes: nitrogen fixation Zn: 150 Cu, Fe: Co: B12 21.1 Transition Metals show great similarities within
Chapter 21: Transition Metals and Coordination Chemistry Mg, Cr, V, Co Pt Fe complexes O2 Mo and Fe complexes: nitrogen fixation Zn: 150 Cu, Fe: Co: B12 21.1 Transition Metals show great similarities within
Chapter 5 - Homework solutions
 Chapter 5 - Homework solutions Q Ex 1,2,3,4,5,7,9,13,18,20,21,23, 27,29 and Prob. 3,4,6,7 1) Li Be B C N O F acidic Na Mg Al Si P S Cl amphoteric K Ca Ga Ge As Se Br basic Rb Sr In Sn Sb Te I Cs Ba Tl
Chapter 5 - Homework solutions Q Ex 1,2,3,4,5,7,9,13,18,20,21,23, 27,29 and Prob. 3,4,6,7 1) Li Be B C N O F acidic Na Mg Al Si P S Cl amphoteric K Ca Ga Ge As Se Br basic Rb Sr In Sn Sb Te I Cs Ba Tl
PAPER No.11 : Inorganic Chemistry-II MODULE No.1 : Π-acceptor ligand, metal carbonyls, bonding modes of CO, classification of metal carbonyls
 Subject Paper No and Title Module No and Title Module Tag 11: INORGANIC CHEMISTRY-III (METAL π- COMPLEXES AND METAL CLUSTERS) 1: π-acidity, Metal carbonyls, their classification and general features CHE_P11_M1
Subject Paper No and Title Module No and Title Module Tag 11: INORGANIC CHEMISTRY-III (METAL π- COMPLEXES AND METAL CLUSTERS) 1: π-acidity, Metal carbonyls, their classification and general features CHE_P11_M1
Supporting Information
 Supporting Information Page 2-4. The B3LYP optimized gas phase structures of [Bmim + Cl - ] Pd complex. Page 5-10. The B3LYP optimized gas phase structures of [Bmim + Cl - ] Pd 2 complex. Page 11-14. The
Supporting Information Page 2-4. The B3LYP optimized gas phase structures of [Bmim + Cl - ] Pd complex. Page 5-10. The B3LYP optimized gas phase structures of [Bmim + Cl - ] Pd 2 complex. Page 11-14. The
Acids and Bases. CHEM 102 T. Hughbanks. In following equilibrium, will reactants or products be favored? Strong acid (HCl) + Strong base (NaOH)
 Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
Coordination Compounds
 Coordination Compounds 1. What is a coordination compound composed of? a. Metal Ion b. Ligand c. Counter Ion 2. What is a complex ion? The metal ion and ligand combination. 3. What is a counter ion? An
Coordination Compounds 1. What is a coordination compound composed of? a. Metal Ion b. Ligand c. Counter Ion 2. What is a complex ion? The metal ion and ligand combination. 3. What is a counter ion? An
ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless
![ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless ph = - log [H3O+] Example: ph 7 = - log [ 1 x 10-7] [H3O+] = mole/liter units ph values are unitless](/thumbs/80/82250423.jpg) E4 Acids, Bases, and Salts Oct. 1517 and Oct. 2728* Session One of two session lab Complete Parts 1 and 2 in lab. Part 1. Structure and AcidBase Properties Q. Are properties of a compound predictable from
E4 Acids, Bases, and Salts Oct. 1517 and Oct. 2728* Session One of two session lab Complete Parts 1 and 2 in lab. Part 1. Structure and AcidBase Properties Q. Are properties of a compound predictable from
Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles,
 Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles, this process is endothermic. Step 2: Solvent particles
Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles, this process is endothermic. Step 2: Solvent particles
Oxidative Addition/Reductive Elimination 1. Oxidative Addition
 Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Predicting LA-LB reactions
 Predicting LA-LB reactions A chemical reaction is governed by G rxn
Predicting LA-LB reactions A chemical reaction is governed by G rxn
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Practice sheet #6: Molecular Shape, Hybridization, Molecular Orbitals.
 CH 101/103 - Practice sheet 3/17/2014 Practice sheet #6: Molecular Shape, Hybridization, Molecular Orbitals. 1. Draw the 3D structures for the following molecules. You can omit the lone pairs on peripheral
CH 101/103 - Practice sheet 3/17/2014 Practice sheet #6: Molecular Shape, Hybridization, Molecular Orbitals. 1. Draw the 3D structures for the following molecules. You can omit the lone pairs on peripheral
Chem 2100, Organic Chemistry I WS07, Dr. Rainer Glaser
 Chem 2100, rganic Chemistry I WS07, Dr. Rainer Glaser Exam #1 A, As, Ms, Intra- & Intermolecular Bonding. Sources, Properties, and Uses of Acyclic Alkanes. Friday, 2-23-2007, 9:00-9:55 am ame: Answer Key
Chem 2100, rganic Chemistry I WS07, Dr. Rainer Glaser Exam #1 A, As, Ms, Intra- & Intermolecular Bonding. Sources, Properties, and Uses of Acyclic Alkanes. Friday, 2-23-2007, 9:00-9:55 am ame: Answer Key
Stereoelectronic Interactions and Molecular Properties. An NBO-Based Study of Uracil
 5544 J. Phys. Chem. A 2003, 107, 5544-5554 Stereoelectronic Interactions and Molecular Properties. An NBO-Based Study of Uracil Eduardo M. Sproviero and Gerardo Burton* Departamento de Química Orgánica,
5544 J. Phys. Chem. A 2003, 107, 5544-5554 Stereoelectronic Interactions and Molecular Properties. An NBO-Based Study of Uracil Eduardo M. Sproviero and Gerardo Burton* Departamento de Química Orgánica,
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory
 AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Supporting Information
 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2004 Chem. Eur. J. 2004 Supporting Information for Chem. Eur. J. Supporting Information For Quantitative Evaluation of d-p Interaction in Copper(I)
Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2004 Chem. Eur. J. 2004 Supporting Information for Chem. Eur. J. Supporting Information For Quantitative Evaluation of d-p Interaction in Copper(I)
Lewis dot structures for molecules
 1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
Coordination Compounds. Compounds containing Transition Metals
 Coordination Compounds Compounds containing Transition Metals Coordination Compounds Transition Metals Sc 6 Cu 1st row Y 6 Ag 2nd row La 6 Au 3rd row Properties of metals Not as reactive as group 1 or
Coordination Compounds Compounds containing Transition Metals Coordination Compounds Transition Metals Sc 6 Cu 1st row Y 6 Ag 2nd row La 6 Au 3rd row Properties of metals Not as reactive as group 1 or
E4 Acids, Bases, and Salts
 E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Reminder: Pre-lab report, page 112, due at start of lab. Acids and Bases
E4 Acids, Bases, and Salts Session One of two session lab Complete Parts 1 and 2 in lab. If time allows, start or complete Part 3. Reminder: Pre-lab report, page 112, due at start of lab. Acids and Bases
Required Reading Material.
 JF Chemistry 1101 2010 Introduction to Physical Chemistry: Acid Base and Solution Equilibria. Dr Mike Lyons School of Chemistry melyons@tcd.ie Required Reading Material. Silberberg, Chemistry, 4th edition.
JF Chemistry 1101 2010 Introduction to Physical Chemistry: Acid Base and Solution Equilibria. Dr Mike Lyons School of Chemistry melyons@tcd.ie Required Reading Material. Silberberg, Chemistry, 4th edition.
Introduction to Organometallic Compounds. Metal. R MgX. Wilkinson s catalyst is used for hydrogenation of alkene and alkyne. Wilkinson's catalyst
 Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Solvent Scales. ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute
 Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
Solvent Scales ε α β α: solvent's ability to act as a hydrogen bond-donor to a solute Water 78 1.17 0.47 DMS 47 0.00 0.76 DM 37 0.00 0.76 Methanol 33 0.93 0.66 MPA 29 0.00 1.05 Acetone 21 0.08 0.43 Methylene
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Supporting Information for
 Supporting Information for Interplay of Halogen and - Charge-Transfer Bondings in Intermolecular Associates of Bromo- or Iododinitrobenzene with Tetramethyl-p-phenylenediamine. Sergiy V. Rosokha, * Eric
Supporting Information for Interplay of Halogen and - Charge-Transfer Bondings in Intermolecular Associates of Bromo- or Iododinitrobenzene with Tetramethyl-p-phenylenediamine. Sergiy V. Rosokha, * Eric
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
PCCP Accepted Manuscript
 PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published
PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published
Constructing a MO of NH 3. Nitrogen AO symmetries are
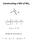 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
CHEMISTRY 121 AUTUMN 2009 CHAPTER 8 & 9 PROBLEMS
 Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Effects of Steric Interactions on the Relativistic Spin-Orbit. Tensors of Di-haloethenes
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 SUPPORTING INFORMATION: Effects of Steric Interactions on the Relativistic Spin-Orbit
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 SUPPORTING INFORMATION: Effects of Steric Interactions on the Relativistic Spin-Orbit
Supporting Information
 Supporting Information Computational details The first important issue we have encountered to accomplish our calculations has been the choice of the computational method. We decided to focus on the Density
Supporting Information Computational details The first important issue we have encountered to accomplish our calculations has been the choice of the computational method. We decided to focus on the Density
Supporting Information (DFT Calculations) Pd-Catalyzed C-H Functionalization of Acyldiazomethane. and Tandem Cross-Coupling Reactions
 Supporting Information (DFT Calculations) Pd-Catalyzed C-H Functionalization of Acyldiazomethane and Tandem Cross-Coupling Reactions Fei Ye,, Shuanglin Qu,, Lei Zhou,, Cheng Peng, Chengpeng Wang, Jiajia
Supporting Information (DFT Calculations) Pd-Catalyzed C-H Functionalization of Acyldiazomethane and Tandem Cross-Coupling Reactions Fei Ye,, Shuanglin Qu,, Lei Zhou,, Cheng Peng, Chengpeng Wang, Jiajia
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by Density Functional Theory Methods
 51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Chapter 2 Lecture Outline
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 2 Lecture Outline Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 2 Lecture Outline Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
- When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other.
 228 POLARITY - When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other. - When electrons are shared UNEVENLY,
228 POLARITY - When atoms share electrons, the electrons might not be EVENLY shared. Shared electrons may spend more time around one atomic nucleus than the other. - When electrons are shared UNEVENLY,
Ammonia and Amines. Four sp 3 hybridized orbitals. Three used for bonding and one for the lone pair of electrons. secondary 2. Et Me.
 Ammonia and Amines threefold axis of rotation 111 107 Four sp 3 hybridized orbitals. Three used for bonding and one for the lone pair of electrons. trigonal pyramidal primary 1 secondary 2 tertiary 3 quaternary
Ammonia and Amines threefold axis of rotation 111 107 Four sp 3 hybridized orbitals. Three used for bonding and one for the lone pair of electrons. trigonal pyramidal primary 1 secondary 2 tertiary 3 quaternary
Chemical Bonding. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chemical Bonding Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Valence Bond Theory VB theory predicts properties better than Lewis theory bonding schemes, bond strengths,
Chemical Bonding Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Valence Bond Theory VB theory predicts properties better than Lewis theory bonding schemes, bond strengths,
A series of organic conductors base upon partial oxidized chalcogene compounds like tetrachalcogenafulvalene.
 Abstract A series of organic conductors base upon partial oxidized chalcogene compounds like tetrachalcogenafulvalene. R R R a b R ( =, e) Fig.81: a) tetrachalkogenafulvalene b)chalkogenanthrene lectron-rich
Abstract A series of organic conductors base upon partial oxidized chalcogene compounds like tetrachalcogenafulvalene. R R R a b R ( =, e) Fig.81: a) tetrachalkogenafulvalene b)chalkogenanthrene lectron-rich
E5 Lewis Acids and Bases. Acids. Acids. Session one. Session two Lab: Parts 2B, 3 and 4
 E5 Lewis Acids and Bases Session one Pre-lab (p.141) due at start of lab. First hour: Discussion of E4 Lab: Parts 1and 2A Session two Lab: Parts 2B, 3 and 4 Acids Bronsted: Acids are proton donors and
E5 Lewis Acids and Bases Session one Pre-lab (p.141) due at start of lab. First hour: Discussion of E4 Lab: Parts 1and 2A Session two Lab: Parts 2B, 3 and 4 Acids Bronsted: Acids are proton donors and
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Nano theoretical studies of fmet-trna structure in protein synthesis of prokaryotes and its comparison with the structure of fala-trna
 African Journal of Microbiology Research Vol. 5(18), pp. 2667-2674, 16 September, 2011 Available online http://www.academicjournals.org/ajmr DOI: 10.5897/AJMR11.310 ISSN 1996-0808 2011 Academic Journals
African Journal of Microbiology Research Vol. 5(18), pp. 2667-2674, 16 September, 2011 Available online http://www.academicjournals.org/ajmr DOI: 10.5897/AJMR11.310 ISSN 1996-0808 2011 Academic Journals
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chemisorption VIII. NEVF 514 Surface Physics. Winter Term Troja, 16th December 2016
 Chemisorption František Máca VIII. NEVF 514 Surface Physics Winter Term 2016-2017 Troja, 16th December 2016 Chemisorption The knowledge of chemisorption phenomena requires the determination of the geometrical
Chemisorption František Máca VIII. NEVF 514 Surface Physics Winter Term 2016-2017 Troja, 16th December 2016 Chemisorption The knowledge of chemisorption phenomena requires the determination of the geometrical
Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals
 Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
PAPER No. 7: Inorganic chemistry II MODULE No. 5: Molecular Orbital Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II 5, Molecular Orbital Theory CHE_P7_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Ligand Field
NOT TO BE REMOVED FROM THE EXAMINATION HALL
 A copy of the Level III (FHEQ Level 6) Equation and Data Sheet booklet is provided. The use of hand-held, battery-operated, electronic calculators will be permitted subject to the regulations governing
A copy of the Level III (FHEQ Level 6) Equation and Data Sheet booklet is provided. The use of hand-held, battery-operated, electronic calculators will be permitted subject to the regulations governing
Ultraviolet-Visible Spectroscopy
 Ultraviolet-Visible Spectroscopy Introduction to UV-Visible Absorption spectroscopy from 160 nm to 780 nm Measurement of transmittance Conversion to absorbance * A=-logT=εbc Measurement of transmittance
Ultraviolet-Visible Spectroscopy Introduction to UV-Visible Absorption spectroscopy from 160 nm to 780 nm Measurement of transmittance Conversion to absorbance * A=-logT=εbc Measurement of transmittance
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals
 Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
