NWChem: Hartree-Fock, Density Functional Theory, Time-Dependent Density Functional Theory
|
|
|
- Berniece Fisher
- 5 years ago
- Views:
Transcription
1 NWChem: Hartree-Fock, Density Functional Theory, Time-Depent Density Functional Theory
2 Hartree-Fock! Functionality! Input! Wavefunctions! Initial MO vectors! Direct and semidirect algorithms! Convergence, files, and restarting 2
3 Hartree-Fock Functionality! Energies and gradients! Closed-shell (RHF)! Spin-restricted, high-spin open-shell (ROHF)! Spin-unrestricted open-shell (UHF)! Analytic second derivatives (RHF and UHF)! Finite point groups! Will be used as first step in all correlated methods (e.g. MP2, CC, etc )! Resolution of the identity (energy) 3
4 Input! SCF input block, e.g., scf triplet; uhf! Defaults! Restricted-spin wavefunction (ROHF)! Accuracy suitable for non-floppy molecule geometry optimization! Symmetry as defined in the geometry 4
5 Simple Example 3 B 1 CH 2 ROHF and UHF optimizations geometry units au C H symmetry c2v basis H library 3-21g; C library 3-21g scf; triplet; #default is ROHF task scf optimize scf; uhf; task scf optimize
6 Density-Functional Theory! Functionality! Input! XC functionals! Grid & Convergence options 6
7 DFT Functionality in a nutshell s Gaussian function-based DFT energies, gradients and second derivatives s finite symmetry s Exchange-Correlation functionals for Closed-Shell systems and Open-Shell systems
8 Exchange-Correlation Functionals! Pure Hartree-Fock Exchange! Traditional functionals: Density & density gradient! LDA, BP, BLYP, PBE, PW91,! Hybrid functionals: Inclusion of HF exchange! B3LYP, PBE0, BeckeHalfandHalf,! Meta functionals: Inclusion of kinetic energy! TPSS, PKZB, Minnesota functionals,! Range-separated functionals! CAM-B3LYP, LC-PBE0,! DFT + empirical dispersion (DFT+ D)! Based on Grimme s implementation! Double Hybrid functionals: DFT + MP2! Based on Grimme s implementation
9 Local Basis (Gaussian Basis Set) 9 Memory requirements! Largest quantities are the density, Fock, overlap, 1-electron matrices! Memory needed O(N 2 )! Replicated data O(N 2 ) per node! Distributed data O(N 2 ) for whole calculation Phys. Chem. Chem. Phys. 12, 6896 (2010) Computational Complexity! Main cost is the evaluation of the 2- electron integrals! Takes O(N 2 )-O(N 4 ) work! O(N 4 ) for small-medium systems! O(N 2 ) in the large N limit! Schwarz screening,! For large N the linear algebra becomes dominant at O(N 3 )! Matrix multiplication, diagonalization
10 NWChem: Gaussian Basis HF/DFT Gaussian based HF/DFT Finite systems (molecules, clusters, nanostructures)! Functionality! Exhaustive list of exchange-correlation functionals! Traditional xc functionals! Wide range of hybrid functionals (B3LYP, PBE0, BeckeH&H )! HF Exchange! Meta-GGA functionals! Minnesota functionals (M05, M06)! SIC and OEP! Range separated functionals (CAMB3LYP, LC-PBE0, BNL, )! DFT + D implementation (long-range empirical vdw)! Double hybrid functionals! Spin-orbit DFT! ECP, ZORA, DK! Constrained DFT! TDDFT for excited states à Optical spectroscopy! Various properties (NMR, Linear response, )! System sizes: ~150 atoms, basis functions are routine
11 NWChem: Gaussian DFT Scaling! Calculation on C 240! PBE0 functional, 6-31G*! Direct integral evaluation! Size 3600 basis functions! Timings for different components of the Kohn-Sham matrix construction! Fock 2e two electron integrals! Fock xc the DFT contribution! Diagonalization eigensolver
12 Input! DFT input block, e.g., dft mult 1! Defaults (similar to Hartree-Fock) Local density approximation (LDA) Accuracy suitable for non-floppy molecule geometry optimization Symmetry as defined in the geometry
13 Open Shell Input! DFT input block, e.g., dft mult 3! Unrestriced Open Shell Default (different from Hartree-Fock)! Recent RODFT implementation dft cgmin # quadratic conv. (required) mult 2 rodft
14 Minimal Input Example! Minimal input (all defaults) geometry; ne 0 0 0; basis; ne library cc-pvdz; task dft! Performs a closed-shell N 4 DFT calculation using the local density approximation on the neon atom (no fitting)
15 Simple DFT Input Example! Input with default DFT input (single point LDA calculation) echo # echoes the input in the output file start silane # name of files title silane # title of the calculation in output geometry si h h h h basis * library cc-pvdz task dft # specifies the task energy by default! EMSL Basis Set Exchange:
16 Changing the exchange-correlation echo start silane title silane geometry si h h h h basis * library cc-pvdz dft xc b3lyp # B3LYP task dft dft xc becke88 lyp #BLYP dft xc becke88 perdew86 Many other combinations possible
17 Important DFT keywords xc: controls the choice of the exchange-correlation convergence: controls the convergence (energy, density ) grid: specifies the grid mult: specifies the multiplicity odft: specify open shell calculation iterations: controls the number of iterations smear: useful for degenerate states SINGLET dft grid fine convergence energy 1e-08 xc b3lyp #B3LYP mult 1 TRIPLET dft odft grid fine convergence energy 1e-08 xc b3lyp #B3LYP mult 3
18 Putting it all together echo start silane title silane geometry si h h h h basis * library cc-pvdz dft grid fine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 task dft
19 Geometry Optimization echo start silane geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 task dft optimize
20 Frequencies echo start silane geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 task dft frequencies
21 Combining Calculations I echo start silane geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 task dft optimize task dft frequencies
22 Combining Calculations II geometry... basis * library cc-pvdz dft xc b3lyp #B3LYP mult 1 task dft optimize task dft frequencies dft odft xc becke88 lyp #BLYP mult 3 task dft optimize
23 Restarting Calculations echo restart silane geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 Restart files silane.db silane.movecs task dft
24 Using Old Vectors echo start silane geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp # B3LYP mult 1 vectors input old.movecs output b3lyp.movecs task dft
25 Organizing Your Files echo start silane permanent_dir /home/yourname/silane/b3lyp scratch_dir /scratch geometry si h h h h basis * library cc-pvdz dft grid xfine convergence energy 1e-08 xc b3lyp #B3LYP mult 1 task dft optimize
26 Customizing The Basis... geometry si h h h h basis si library 6-31G h1 library h sto-3g h2 library h 6-31g h3 library h 3-21g h4 library h 6-31g*...
27 Including empirical dispersion in DFT... geometry... basis... dft xc b3lyp disp vdw 2 s task dft optimize S. Grimme J. Comp. Chem (2004) S. Grimme J. Comp. Chem (2006)
28 Semi-empirical hybrid DFT + MP2 Double Hybrid Functionals... geometry... basis... dft xc HFexch 0.53 becke lyp 0.73 mp dftmp2 direct direct convergence energy 1e-8 iterations 100 S. Grimme, J. Chem. Phys., 124, (2006)
29 Other Capabilities! Charge density fitting (Dunlap scheme)! 4-center, 2-electron Coulomb integrals à 3-center integrals (N 3 )! Very fast for traditional DFT (pure density based functionals, no HF Exchange)! Cheaper and better parallel scaling! Direct or on-the-fly evaluation of integrals! All integrals evaluated as needed! Useful for large systems on large numbers of processors! Effective Core Potentials! Detailed documentation information available on
30 Charge-Density Fitting! Important difference between DFT and SCF Additional fitting basis set (reduces cost from N 4 --> N 3 ) geometry; ne 0 0 0; basis "ao basis" ne library "DZVP (DFT orbital) basis "cd basis" ne library "DGauss A1 DFT Coulomb Fitting task dft
31 Effective Core Potentials! Reduces the cost of calculation for heavy elements Additional input field required to define potential geometry; ne 0 0 0; ecp spherical * library Stuttgart_RSC_1997_ECP basis "ao basis" ni library "Stuttgart_RSC_1997_ECP" task dft
32 Grid Options! Numerical integration keywords and targets using Mura- Knowles radial and Lebedev angular quadratures: dft; grid xcoarse; (1d-4 au) dft; grid coarse; dft; grid medium; dft; grid fine; dft; grid xfine; (1d-5 au) (1d-6 au; default) (1d-7 au) (1d-8 au)! Addition quadrature choices, e.g., dft; grid eumac medium; dft; grid ssf lebedev 75 11; ( = G98 fine)
33 Modifying Accuracy! Controlling accuracy Density < tol_rho (10-10) are screened e.g., tolerances tol_rho 1.d-12 Schwarz screening is invoked for density*integral < 10-accCoul, acccoul default = 10 e.g., tolerances acccoul 12! When to change it? Diffuse basis/floppy molecules Changing from energy to optimizations, frequencies, etc. Don t forget to increase grid accuracy too!
34 Convergence! DIIS, level-shifting, and damping are available! Default is DIIS with no damping. Level-shifting is invoked when the HOMO-LUMO gap is less than hl_tol (default is 0.05 atomic units)! Control of DIIS, levelshifting, and damping: convergence lshift 0.1 damp 40 diis 5! When invoked can be by iteration count convergence ncydp 5! or by change in total energy convergence ncydp 0 dampon 1d6 \ dampoff 1d-2
35 Fractional occupation of MOs! The SMEAR keyword is useful in cases wit many degenerate states near the HOMO (e.g. metallic clusters). Molecular Orbitals near the gap will be occupied with a distribution a la Fermi-Dirac corresponding to a finite temperature.! SMEAR <real smear default 0.001>
36 Excited State Calculations with TDDFT
37 Time-Depent DFT Casida Formulation Perturbed density à first-order correction Linear response approach à frequency domain Cannot be used to describe excitations in intense fields! Working equations have M=N occ *N virt solutions! Dimension à tetradic (M*M)! Every root à cost of a HF or hybrid DFT calculation! Note that the vectors are normalized but differently so than your usual wavefunction! The orbital energy difference is a main term in the excitation energy! In the case of pure DFT with large molecules most of the integrals involving F xc vanish as this is a local kernel A B X 1 0 X ω * * = B A Y 0 1 Y ( X X) ( Y Y) 1 = ( ( r, r ) ( ( ) ) A = δδ ε ε + ia F + F jb ia, jb ij ab a i H xc B = ia F + F jb F ia, jb H xc xc f = ρ ) ( r) ρ( r ) Time-Depent Density Functional Theory, Marques et al, Springer 2006
38 Excited State Calculations with TDDFT geometry O H H basis O library 6-31G** H library 6-31G** dft xc b3lyp tddft nroots 10 notriplet task tddft energy
39 Excited State Sample Output Root 1 singlet b a.u. ( ev) Transition Moments X Y Z Transition Moments XX XY XZ Transition Moments YY YZ ZZ Transition Moments XXX XXY XXZ Transition Moments XYY XYZ XZZ Transition Moments YYY YYZ YZZ Transition Moments ZZZ Dipole Oscillator Strength Occ. 5 b2 --- Virt. 6 a X Root 2 singlet a a.u. ( ev) Transition Moments X Y Z Transition Moments XX XY XZ Transition Moments YY YZ ZZ Transition Moments XXX XXY XXZ Transition Moments XYY XYZ XZZ Transition Moments YYY YYZ YZZ Transition Moments ZZZ Dipole Oscillator Strength Occ. 5 b2 --- Virt. 7 b X
40 Excited State Spectrum Energy (ev)
41 Recent Applications (1) Formyl cation bound to a Bronsted acid site in a zeolite cavity Adsorption of aminotriazines on graphene using dispersion corrected DFT Dipole polarizabilities of water clusters Ground & Excited state properties of pure and N-doped TiO 2 rutile
42 Recent Applications (2) Charge transfer excitations in zinc porphyrin in aqueous solution Correct lowest excitation in the Adenine-Thymine base pair using range-separated functionals Excitations energies in the oligoporphyrin dimer Optical properties of silver clusters
43 Hands-On Exercises Tutorial exercises ================== hf-dft b3lyp: Shows how to perform a single point energy, geometry optimization and frequency calculation combined: Shows how to perform single point energy calculations with various exchange-correlation functionals restart: Shows how to restart a calculation files: Shows how to use the scratch and permanent directories multiplicity: Shows how to set the multiplicity in a calculation convergence: Shows how to specify other useful keywords in the dft block ecp: Shows how to use effective core potentials (ECP) direct: Shows how to perform direct calculations densityfitting: Shows how to use charge density fitting basis sets sodft: Shows how to perform calculation with a spin-orbit ecp explicitbasis: Shows how to specify the basis explicitly multiplestructures: Shows how to specify multiple structures multiplebasis: Shows how to specify multiple basis sets tddft h2o,2h2o,ethane,butane properties
44 44 Questions?
45 45 EXTRA MATERIAL
46 Hartree-Fock & Density Functional Theory I! The energy expression is derived from a single determinant wave function approximation! Replace the exchange with a exchange-correlation functional to go from Hartree-Fock à DFT! Implemented using various basis set approaches! Plane waves! Gaussian functions! Slater functions! Numerical atomic orbitals! Wavelets! Mixed basis sets! 46
47 Hartree-Fock & Density Functional Theory II Local Basis G D y DFT µν * E = Fµν Dµν + εi Cµ isµνcν j δij µν i j µν * µν µ i ν i i { occ} C C F = H + G + αg + βg + γg G G ϕ = φ () r = core J K X DFT C DFT µν µν µν µν µν µν = ( µν στ ) D J µν στ στ = ( µτ σν ) D K µν 1 2 στ στ = i µ C µ i µ { α, β, α, β, α β,...} ξ ρ ρ ρ ρ ρ ρ y f ξ ξ D µν r d! Minimize energy with respect to C µi and ε i! Gives! The total energy E! The molecular orbitals C µi! The orbital energies ε i Modern Quantum Chemistry, Ostlund & Szabo 47
Basic introduction of NWChem software
 Basic introduction of NWChem software Background NWChem is part of the Molecular Science Software Suite Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
Basic introduction of NWChem software Background NWChem is part of the Molecular Science Software Suite Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride. Dimer. Philip Straughn
 Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
DFT calculations of NMR indirect spin spin coupling constants
 DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
Multi-reference Density Functional Theory. COLUMBUS Workshop Argonne National Laboratory 15 August 2005
 Multi-reference Density Functional Theory COLUMBUS Workshop Argonne National Laboratory 15 August 2005 Capt Eric V. Beck Air Force Institute of Technology Department of Engineering Physics 2950 Hobson
Multi-reference Density Functional Theory COLUMBUS Workshop Argonne National Laboratory 15 August 2005 Capt Eric V. Beck Air Force Institute of Technology Department of Engineering Physics 2950 Hobson
Basic introduction of NWChem software
 Basic introduction of NWChem software Background! NWChem is part of the Molecular Science Software Suite! Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
Basic introduction of NWChem software Background! NWChem is part of the Molecular Science Software Suite! Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
QUANTUM CHEMISTRY FOR TRANSITION METALS
 QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
OVERVIEW OF QUANTUM CHEMISTRY METHODS
 OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
TDDFT in Chemistry and Biochemistry III
 TDDFT in Chemistry and Biochemistry III Dmitrij Rappoport Department of Chemistry and Chemical Biology Harvard University TDDFT Winter School Benasque, January 2010 Dmitrij Rappoport (Harvard U.) TDDFT
TDDFT in Chemistry and Biochemistry III Dmitrij Rappoport Department of Chemistry and Chemical Biology Harvard University TDDFT Winter School Benasque, January 2010 Dmitrij Rappoport (Harvard U.) TDDFT
Advanced Quantum Chemistry III: Part 3. Haruyuki Nakano. Kyushu University
 Advanced Quantum Chemistry III: Part 3 Haruyuki Nakano Kyushu University 2013 Winter Term 1. Hartree-Fock theory Density Functional Theory 2. Hohenberg-Kohn theorem 3. Kohn-Sham method 4. Exchange-correlation
Advanced Quantum Chemistry III: Part 3 Haruyuki Nakano Kyushu University 2013 Winter Term 1. Hartree-Fock theory Density Functional Theory 2. Hohenberg-Kohn theorem 3. Kohn-Sham method 4. Exchange-correlation
Density Func,onal Theory (Chapter 6, Jensen)
 Chem 580: DFT Density Func,onal Theory (Chapter 6, Jensen) Hohenberg- Kohn Theorem (Phys. Rev., 136,B864 (1964)): For molecules with a non degenerate ground state, the ground state molecular energy and
Chem 580: DFT Density Func,onal Theory (Chapter 6, Jensen) Hohenberg- Kohn Theorem (Phys. Rev., 136,B864 (1964)): For molecules with a non degenerate ground state, the ground state molecular energy and
Computational Chemistry I
 Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Oslo node. Highly accurate calculations benchmarking and extrapolations
 Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Tutorial I: IQ MOL and Basic DFT and MP2 Calculations 1 / 30
 Tutorial I: IQ MOL and Basic DFT and MP2 Calculations Q-Chem User Workshop, Denver March 21, 2015 1 / 30 2 / 30 Introduction to IQMOL DFT and MP2 Calculations 3 / 30 IQMOL and Q-CHEM IQMOL is an open-source
Tutorial I: IQ MOL and Basic DFT and MP2 Calculations Q-Chem User Workshop, Denver March 21, 2015 1 / 30 2 / 30 Introduction to IQMOL DFT and MP2 Calculations 3 / 30 IQMOL and Q-CHEM IQMOL is an open-source
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory
 Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
Institut Néel Institut Laue Langevin. Introduction to electronic structure calculations
 Institut Néel Institut Laue Langevin Introduction to electronic structure calculations 1 Institut Néel - 25 rue des Martyrs - Grenoble - France 2 Institut Laue Langevin - 71 avenue des Martyrs - Grenoble
Institut Néel Institut Laue Langevin Introduction to electronic structure calculations 1 Institut Néel - 25 rue des Martyrs - Grenoble - France 2 Institut Laue Langevin - 71 avenue des Martyrs - Grenoble
Module 6 1. Density functional theory
 Module 6 1. Density functional theory Updated May 12, 2016 B A DDFT C K A bird s-eye view of density-functional theory Authors: Klaus Capelle G http://arxiv.org/abs/cond-mat/0211443 R https://trac.cc.jyu.fi/projects/toolbox/wiki/dft
Module 6 1. Density functional theory Updated May 12, 2016 B A DDFT C K A bird s-eye view of density-functional theory Authors: Klaus Capelle G http://arxiv.org/abs/cond-mat/0211443 R https://trac.cc.jyu.fi/projects/toolbox/wiki/dft
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014
 Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Density Functional Theory - II part
 Density Functional Theory - II part antonino.polimeno@unipd.it Overview From theory to practice Implementation Functionals Local functionals Gradient Others From theory to practice From now on, if not
Density Functional Theory - II part antonino.polimeno@unipd.it Overview From theory to practice Implementation Functionals Local functionals Gradient Others From theory to practice From now on, if not
Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory.
 Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory. Walter Kohn receiving his Nobel Prize from His Majesty the King at the Stockholm
Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory. Walter Kohn receiving his Nobel Prize from His Majesty the King at the Stockholm
Post Hartree-Fock: MP2 and RPA in CP2K
 Post Hartree-Fock: MP2 and RPA in CP2K A tutorial Jan Wilhelm jan.wilhelm@chem.uzh.ch 4 September 2015 Further reading MP2 and RPA by Mauro Del Ben, Jürg Hutter, Joost VandeVondele Del Ben, M; Hutter,
Post Hartree-Fock: MP2 and RPA in CP2K A tutorial Jan Wilhelm jan.wilhelm@chem.uzh.ch 4 September 2015 Further reading MP2 and RPA by Mauro Del Ben, Jürg Hutter, Joost VandeVondele Del Ben, M; Hutter,
NWChem: Coupled Cluster Method (Tensor Contraction Engine)
 NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
Electron Correlation
 Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Orbital dependent correlation potentials in ab initio density functional theory
 Orbital dependent correlation potentials in ab initio density functional theory noniterative - one step - calculations Ireneusz Grabowski Institute of Physics Nicolaus Copernicus University Toruń, Poland
Orbital dependent correlation potentials in ab initio density functional theory noniterative - one step - calculations Ireneusz Grabowski Institute of Physics Nicolaus Copernicus University Toruń, Poland
计算物理作业二. Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit.
 计算物理作业二 Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit. In this exercise, basis indicates one of the following basis sets: STO-3G, cc-pvdz, cc-pvtz, cc-pvqz
计算物理作业二 Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit. In this exercise, basis indicates one of the following basis sets: STO-3G, cc-pvdz, cc-pvtz, cc-pvqz
LUMO + 1 LUMO. Tómas Arnar Guðmundsson Report 2 Reikniefnafræði G
 Q1: Display all the MOs for N2 in your report and classify each one of them as bonding, antibonding or non-bonding, and say whether the symmetry of the orbital is σ or π. Sketch a molecular orbital diagram
Q1: Display all the MOs for N2 in your report and classify each one of them as bonding, antibonding or non-bonding, and say whether the symmetry of the orbital is σ or π. Sketch a molecular orbital diagram
Exchange-Correlation Functional
 Exchange-Correlation Functional Aiichiro Nakano Collaboratory for Advanced Computing & Simulations Depts. of Computer Science, Physics & Astronomy, Chemical Engineering & Materials Science, and Biological
Exchange-Correlation Functional Aiichiro Nakano Collaboratory for Advanced Computing & Simulations Depts. of Computer Science, Physics & Astronomy, Chemical Engineering & Materials Science, and Biological
Dispersion Interactions from the Exchange-Hole Dipole Moment
 Dispersion Interactions from the Exchange-Hole Dipole Moment Erin R. Johnson and Alberto Otero-de-la-Roza Chemistry and Chemical Biology, University of California, Merced E. R. Johnson (UC Merced) Dispersion
Dispersion Interactions from the Exchange-Hole Dipole Moment Erin R. Johnson and Alberto Otero-de-la-Roza Chemistry and Chemical Biology, University of California, Merced E. R. Johnson (UC Merced) Dispersion
Molecular Simulation I
 Molecular Simulation I Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Jeffry D. Madura Department of Chemistry & Biochemistry Center for Computational Sciences
Molecular Simulation I Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Jeffry D. Madura Department of Chemistry & Biochemistry Center for Computational Sciences
Electric properties of molecules
 Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
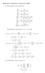 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Self-Consistent Implementation of Self-Interaction Corrected DFT and of the Exact Exchange Functionals in Plane-Wave DFT
 Self-Consistent Implementation of Self-Interaction Corrected DFT and of the Exact Exchange Functionals in Plane-Wave DFT Kiril Tsemekhman (a), Eric Bylaska (b), Hannes Jonsson (a,c) (a) Department of Chemistry,
Self-Consistent Implementation of Self-Interaction Corrected DFT and of the Exact Exchange Functionals in Plane-Wave DFT Kiril Tsemekhman (a), Eric Bylaska (b), Hannes Jonsson (a,c) (a) Department of Chemistry,
Exercise 1: Structure and dipole moment of a small molecule
 Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Lecture 5: More about one- Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory.
 Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lec20 Fri 3mar17
 564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
DFT with Hybrid Functionals
 DFT with Hybrid Functionals Sanliang Ling University College London 4th CP2K Tutorial, 31st August 4th September 2015, Zurich What are hybrid functionals? Hybrid functionals: mixing non-local Hartree-Fock
DFT with Hybrid Functionals Sanliang Ling University College London 4th CP2K Tutorial, 31st August 4th September 2015, Zurich What are hybrid functionals? Hybrid functionals: mixing non-local Hartree-Fock
Introduction to Computational Quantum Chemistry: Theory
 Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Combining density-functional theory and many-body methods
 Combining density-functional theory and many-body methods Julien Toulouse Université Pierre & Marie Curie and CNRS, Paris, France Vrije Universiteit Amsterdam, Netherlands November 2017 Outline 2/23 1
Combining density-functional theory and many-body methods Julien Toulouse Université Pierre & Marie Curie and CNRS, Paris, France Vrije Universiteit Amsterdam, Netherlands November 2017 Outline 2/23 1
MODELING MATTER AT NANOSCALES
 MODELING MATTER AT NANOSCALES 6. The theory of molecular orbitals for the description of nanosystems (part II) 6.0. Ab initio methods. Basis functions. Luis A. Monte ro Firmado digitalmente por Luis A.
MODELING MATTER AT NANOSCALES 6. The theory of molecular orbitals for the description of nanosystems (part II) 6.0. Ab initio methods. Basis functions. Luis A. Monte ro Firmado digitalmente por Luis A.
Lec20 Wed 1mar17 update 3mar 10am
 564-17 Lec20 Wed 1mar17 update 3mar 10am Figure 15.2 Shows that increasing the diversity of the basis set lowers The HF-SCF energy considerably, but comes nowhere near the exact experimental energy, regardless
564-17 Lec20 Wed 1mar17 update 3mar 10am Figure 15.2 Shows that increasing the diversity of the basis set lowers The HF-SCF energy considerably, but comes nowhere near the exact experimental energy, regardless
Chemistry 4560/5560 Molecular Modeling Fall 2014
 Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Supporting information for: First hyperpolarizability of collagen using the. point dipole approximation
 Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Pseudopotentials for hybrid density functionals and SCAN
 Pseudopotentials for hybrid density functionals and SCAN Jing Yang, Liang Z. Tan, Julian Gebhardt, and Andrew M. Rappe Department of Chemistry University of Pennsylvania Why do we need pseudopotentials?
Pseudopotentials for hybrid density functionals and SCAN Jing Yang, Liang Z. Tan, Julian Gebhardt, and Andrew M. Rappe Department of Chemistry University of Pennsylvania Why do we need pseudopotentials?
Computational chemistry with GAMESS: a very brief overview with examples
 Computational chemistry with GAMESS: a very brief overview with examples PHY-6120 Molecular Physics (Spring 2015), UConn Phys. Dept. Feb 17 th 2015 H = ħ2 2μ i Intro: V(R) for diatomic molecules + k Z
Computational chemistry with GAMESS: a very brief overview with examples PHY-6120 Molecular Physics (Spring 2015), UConn Phys. Dept. Feb 17 th 2015 H = ħ2 2μ i Intro: V(R) for diatomic molecules + k Z
IFM Chemistry Computational Chemistry 2010, 7.5 hp LAB2. Computer laboratory exercise 1 (LAB2): Quantum chemical calculations
 Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
ASSESSMENT OF DFT METHODS FOR SOLIDS
 MSSC2009 - Ab Initio Modeling in Solid State Chemistry ASSESSMENT OF DFT METHODS FOR SOLIDS Raffaella Demichelis Università di Torino Dipartimento di Chimica IFM 1 MSSC2009 - September, 10 th 2009 Table
MSSC2009 - Ab Initio Modeling in Solid State Chemistry ASSESSMENT OF DFT METHODS FOR SOLIDS Raffaella Demichelis Università di Torino Dipartimento di Chimica IFM 1 MSSC2009 - September, 10 th 2009 Table
Density Functional Theory
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard October 28, 2015 Density Functional Theory What is a Functional? A functional is a general mathematical quantity that represents a rule to convert a function
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard October 28, 2015 Density Functional Theory What is a Functional? A functional is a general mathematical quantity that represents a rule to convert a function
Dalton Quantum Chemistry Program
 1 Quotation from home page: Dalton Quantum Chemistry Program Dalton QCP represents a powerful quantum chemistry program for the calculation of molecular properties with SCF, MP2, MCSCF or CC wave functions.
1 Quotation from home page: Dalton Quantum Chemistry Program Dalton QCP represents a powerful quantum chemistry program for the calculation of molecular properties with SCF, MP2, MCSCF or CC wave functions.
Chemistry 3502/4502. Final Exam Part I. May 14, 2005
 Advocacy chit Chemistry 350/450 Final Exam Part I May 4, 005. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle
Advocacy chit Chemistry 350/450 Final Exam Part I May 4, 005. For which of the below systems is = where H is the Hamiltonian operator and T is the kinetic-energy operator? (a) The free particle
Advanced Electronic Structure Theory Density functional theory. Dr Fred Manby
 Advanced Electronic Structure Theory Density functional theory Dr Fred Manby fred.manby@bris.ac.uk http://www.chm.bris.ac.uk/pt/manby/ 6 Strengths of DFT DFT is one of many theories used by (computational)
Advanced Electronic Structure Theory Density functional theory Dr Fred Manby fred.manby@bris.ac.uk http://www.chm.bris.ac.uk/pt/manby/ 6 Strengths of DFT DFT is one of many theories used by (computational)
Time-Dependent Density-Functional Theory
 Summer School on First Principles Calculations for Condensed Matter and Nanoscience August 21 September 3, 2005 Santa Barbara, California Time-Dependent Density-Functional Theory X. Gonze, Université Catholique
Summer School on First Principles Calculations for Condensed Matter and Nanoscience August 21 September 3, 2005 Santa Barbara, California Time-Dependent Density-Functional Theory X. Gonze, Université Catholique
GEM4 Summer School OpenCourseWare
 GEM4 Summer School OpenCourseWare http://gem4.educommons.net/ http://www.gem4.org/ Lecture: Molecular Mechanics by Ju Li. Given August 9, 2006 during the GEM4 session at MIT in Cambridge, MA. Please use
GEM4 Summer School OpenCourseWare http://gem4.educommons.net/ http://www.gem4.org/ Lecture: Molecular Mechanics by Ju Li. Given August 9, 2006 during the GEM4 session at MIT in Cambridge, MA. Please use
CASSCF and NEVPT2 calculations: Ground and excited states of multireference systems. A case study of Ni(CO)4 and the magnetic system NArO
 CASSCF and NEVPT2 calculations: Ground and excited states of multireference systems. A case study of Ni(CO)4 and the magnetic system NArO The ground states of many molecules are often well described by
CASSCF and NEVPT2 calculations: Ground and excited states of multireference systems. A case study of Ni(CO)4 and the magnetic system NArO The ground states of many molecules are often well described by
Gaussian: Basic Tutorial
 Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
NWChem: Coupled Cluster Method (Tensor Contraction Engine)
 NWChem: Coupled Cluster Method (ensor Contraction Engine) What we want to solve H Ψ = E Ψ Many Particle Systems Molecular/Atomic Physics, Quantum Chemistry (electronic Schrödinger equations) Solid State
NWChem: Coupled Cluster Method (ensor Contraction Engine) What we want to solve H Ψ = E Ψ Many Particle Systems Molecular/Atomic Physics, Quantum Chemistry (electronic Schrödinger equations) Solid State
Molecular Mechanics: The Ab Initio Foundation
 Molecular Mechanics: The Ab Initio Foundation Ju Li GEM4 Summer School 2006 Cell and Molecular Mechanics in BioMedicine August 7 18, 2006, MIT, Cambridge, MA, USA 2 Outline Why are electrons quantum? Born-Oppenheimer
Molecular Mechanics: The Ab Initio Foundation Ju Li GEM4 Summer School 2006 Cell and Molecular Mechanics in BioMedicine August 7 18, 2006, MIT, Cambridge, MA, USA 2 Outline Why are electrons quantum? Born-Oppenheimer
Electronic structure calculations: fundamentals George C. Schatz Northwestern University
 Electronic structure calculations: fundamentals George C. Schatz Northwestern University Electronic Structure (often called Quantum Chemistry) calculations use quantum mechanics to determine the wavefunctions
Electronic structure calculations: fundamentals George C. Schatz Northwestern University Electronic Structure (often called Quantum Chemistry) calculations use quantum mechanics to determine the wavefunctions
HECToR CSE technical meeting, Oxford Parallel Algorithms for the Materials Modelling code CRYSTAL
 HECToR CSE technical meeting, Oxford 2009 Parallel Algorithms for the Materials Modelling code CRYSTAL Dr Stanko Tomi Computational Science & Engineering Department, STFC Daresbury Laboratory, UK Acknowledgements
HECToR CSE technical meeting, Oxford 2009 Parallel Algorithms for the Materials Modelling code CRYSTAL Dr Stanko Tomi Computational Science & Engineering Department, STFC Daresbury Laboratory, UK Acknowledgements
Generalized generalized gradient approximation: An improved density-functional theory for accurate orbital eigenvalues
 PHYSICAL REVIEW B VOLUME 55, NUMBER 24 15 JUNE 1997-II Generalized generalized gradient approximation: An improved density-functional theory for accurate orbital eigenvalues Xinlei Hua, Xiaojie Chen, and
PHYSICAL REVIEW B VOLUME 55, NUMBER 24 15 JUNE 1997-II Generalized generalized gradient approximation: An improved density-functional theory for accurate orbital eigenvalues Xinlei Hua, Xiaojie Chen, and
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory
 Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Hints on Using the Orca Program
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Hints on Using the Orca Program
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Hints on Using the Orca Program
Introduction to Computational Chemistry: Theory
 Introduction to Computational Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC andrew.gilbert@anu.edu.au 3023 Course Lectures Introduction Hartree Fock Theory Basis Sets Lecture 1 1 Introduction
Introduction to Computational Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC andrew.gilbert@anu.edu.au 3023 Course Lectures Introduction Hartree Fock Theory Basis Sets Lecture 1 1 Introduction
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
The successful wavefunction can be written as a determinant: # 1 (2) # 2 (2) Electrons. This can be generalized to our 2N-electron wavefunction:
 T2. CNDO to AM1: The Semiempirical Molecular Orbital Models The discussion in sections T2.1 T2.3 applies also to ab initio molecular orbital calculations. T2.1 Slater Determinants Consider the general
T2. CNDO to AM1: The Semiempirical Molecular Orbital Models The discussion in sections T2.1 T2.3 applies also to ab initio molecular orbital calculations. T2.1 Slater Determinants Consider the general
Basis Set for Molecular Orbital Theory
 Basis Set for Molecular Orbital Theory! Different Types of Basis Functions! Different Types of Atom Center Basis Functions! Classifications of Gaussian Basis Sets! Pseudopotentials! Molecular Properties
Basis Set for Molecular Orbital Theory! Different Types of Basis Functions! Different Types of Atom Center Basis Functions! Classifications of Gaussian Basis Sets! Pseudopotentials! Molecular Properties
ABC of DFT: Hands-on session 1 Introduction into calculations on molecules
 ABC of DFT: Hands-on session 1 Introduction into calculations on molecules Tutor: Alexej Bagrets Wann? 09.11.2012, 11:30-13:00 Wo? KIT Campus Nord, Flachbau Physik, Geb. 30.22, Computerpool, Raum FE-6
ABC of DFT: Hands-on session 1 Introduction into calculations on molecules Tutor: Alexej Bagrets Wann? 09.11.2012, 11:30-13:00 Wo? KIT Campus Nord, Flachbau Physik, Geb. 30.22, Computerpool, Raum FE-6
Introduction to numerical projects
 Introduction to numerical projects Here follows a brief recipe and recommendation on how to write a report for each project. Give a short description of the nature of the problem and the eventual numerical
Introduction to numerical projects Here follows a brief recipe and recommendation on how to write a report for each project. Give a short description of the nature of the problem and the eventual numerical
THE JOURNAL OF CHEMICAL PHYSICS 127,
 THE JOUNAL OF CHEMICAL PHYSICS 127, 214103 2007 Avoiding singularity problems associated with meta-gga generalized gradient approximation exchange and correlation functionals containing the kinetic energy
THE JOUNAL OF CHEMICAL PHYSICS 127, 214103 2007 Avoiding singularity problems associated with meta-gga generalized gradient approximation exchange and correlation functionals containing the kinetic energy
Using Web-Based Computations in Organic Chemistry
 10/30/2017 1 Using Web-Based Computations in Organic Chemistry John Keller UAF Department of Chemistry & Biochemistry The UAF WebMO site Practical aspects of computational chemistry theory and nomenclature
10/30/2017 1 Using Web-Based Computations in Organic Chemistry John Keller UAF Department of Chemistry & Biochemistry The UAF WebMO site Practical aspects of computational chemistry theory and nomenclature
DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY
 DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY A TUTORIAL FOR PHYSICAL SCIENTISTS WHO MAY OR MAY NOT HATE EQUATIONS AND PROOFS REFERENCES
DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY A TUTORIAL FOR PHYSICAL SCIENTISTS WHO MAY OR MAY NOT HATE EQUATIONS AND PROOFS REFERENCES
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Handbook of Computational Quantum Chemistry. DAVID B. COOK The Department of Chemistry, University of Sheffield
 Handbook of Computational Quantum Chemistry DAVID B. COOK The Department of Chemistry, University of Sheffield Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1998 CONTENTS 1 Mechanics and molecules 1 1.1
Handbook of Computational Quantum Chemistry DAVID B. COOK The Department of Chemistry, University of Sheffield Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1998 CONTENTS 1 Mechanics and molecules 1 1.1
Session 1. Introduction to Computational Chemistry. Computational (chemistry education) and/or (Computational chemistry) education
 Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Response Theory A (hopefully) gentle introduction
 Response Theory A (hopefully) gentle introduction Jeppe Olsen Department of Chemistry University of Aarhus September 14, 2017 Jeppe Olsen (Aarhus) Response Theory September 14, 2017 1 / 65 Contents Response
Response Theory A (hopefully) gentle introduction Jeppe Olsen Department of Chemistry University of Aarhus September 14, 2017 Jeppe Olsen (Aarhus) Response Theory September 14, 2017 1 / 65 Contents Response
Periodic Trends in Properties of Homonuclear
 Chapter 8 Periodic Trends in Properties of Homonuclear Diatomic Molecules Up to now, we have discussed various physical properties of nanostructures, namely, two-dimensional - graphene-like structures:
Chapter 8 Periodic Trends in Properties of Homonuclear Diatomic Molecules Up to now, we have discussed various physical properties of nanostructures, namely, two-dimensional - graphene-like structures:
Supplemental Material: Experimental and Theoretical Investigations of the Electronic Band Structure of Metal-Organic Framework of HKUST-1 Type
 Supplemental Material: Experimental and Theoretical Investigations of the Electronic Band Structure of Metal-Organic Framework of HKUST-1 Type Zhigang Gu, a Lars Heinke, a,* Christof Wöll a, Tobias Neumann,
Supplemental Material: Experimental and Theoretical Investigations of the Electronic Band Structure of Metal-Organic Framework of HKUST-1 Type Zhigang Gu, a Lars Heinke, a,* Christof Wöll a, Tobias Neumann,
Electronic correlation and Hubbard approaches
 Electronic correlation and Hubbard approaches Matteo Cococcioni Department of Chemical Engineering and Materials Science University of Minnesota Notable failures of LDA/GGA: transition-metal oxides Introduction
Electronic correlation and Hubbard approaches Matteo Cococcioni Department of Chemical Engineering and Materials Science University of Minnesota Notable failures of LDA/GGA: transition-metal oxides Introduction
This is called a singlet or spin singlet, because the so called multiplicity, or number of possible orientations of the total spin, which is
 9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
Modified Becke-Johnson (mbj) exchange potential
 Modified Becke-Johnson (mbj) exchange potential Hideyuki Jippo Fujitsu Laboratories LTD. 2015.12.21-22 OpenMX developer s meeting @ Kobe Overview: mbj potential The semilocal exchange potential adding
Modified Becke-Johnson (mbj) exchange potential Hideyuki Jippo Fujitsu Laboratories LTD. 2015.12.21-22 OpenMX developer s meeting @ Kobe Overview: mbj potential The semilocal exchange potential adding
Introduction to DFTB. Marcus Elstner. July 28, 2006
 Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
AN INTRODUCTION TO QUANTUM CHEMISTRY. Mark S. Gordon Iowa State University
 AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
7/29/2014. Electronic Structure. Electrons in Momentum Space. Electron Density Matrices FKF FKF. Ulrich Wedig
 Electron Density Matrices Density matrices Γ, an alternative to the wavefunction Ψ, for the description of a quantum system Electronic Structure The N-particle density matrix Electrons in Momentum Space
Electron Density Matrices Density matrices Γ, an alternative to the wavefunction Ψ, for the description of a quantum system Electronic Structure The N-particle density matrix Electrons in Momentum Space
Special 5: Wavefunction Analysis and Visualization
 Special 5: Wavefunction Analysis and Visualization Felix Plasser Institute for Theoretical Chemistry, University of Vienna COLUMBUS in China Tianjin, October 10 14, 2016 F. Plasser Wavefunction Analysis
Special 5: Wavefunction Analysis and Visualization Felix Plasser Institute for Theoretical Chemistry, University of Vienna COLUMBUS in China Tianjin, October 10 14, 2016 F. Plasser Wavefunction Analysis
One-Electron Hamiltonians
 One-Electron Hamiltonians Hartree-Fock and Density Func7onal Theory Christopher J. Cramer @ChemProfCramer 2017 MSSC, July 10, 2017 REVIEW A One-Slide Summary of Quantum Mechanics Fundamental Postulate:
One-Electron Hamiltonians Hartree-Fock and Density Func7onal Theory Christopher J. Cramer @ChemProfCramer 2017 MSSC, July 10, 2017 REVIEW A One-Slide Summary of Quantum Mechanics Fundamental Postulate:
DFT and beyond: Hands-on Tutorial Workshop Tutorial 1: Basics of Electronic Structure Theory
 DFT and beyond: Hands-on Tutorial Workshop 2011 Tutorial 1: Basics of Electronic Structure Theory V. Atalla, O. T. Hofmann, S. V. Levchenko Theory Department, Fritz-Haber-Institut der MPG Berlin July 13,
DFT and beyond: Hands-on Tutorial Workshop 2011 Tutorial 1: Basics of Electronic Structure Theory V. Atalla, O. T. Hofmann, S. V. Levchenko Theory Department, Fritz-Haber-Institut der MPG Berlin July 13,
XYZ of ground-state DFT
 XYZ of ground-state DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA January 5-9th, 2014 Kieron (UC Irvine) XYZ of ground-state
XYZ of ground-state DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA January 5-9th, 2014 Kieron (UC Irvine) XYZ of ground-state
Orbital Density Dependent Functionals
 Orbital Density Dependent Functionals S. Kluepfel1, P. Kluepfel1, Hildur Guðmundsdóttir1 and Hannes Jónsson1,2 1. Univ. of Iceland; 2. Aalto University Outline: Problems with GGA approximation (PBE, RPBE,...)
Orbital Density Dependent Functionals S. Kluepfel1, P. Kluepfel1, Hildur Guðmundsdóttir1 and Hannes Jónsson1,2 1. Univ. of Iceland; 2. Aalto University Outline: Problems with GGA approximation (PBE, RPBE,...)
Importing ab-initio theory into DFT: Some applications of the Lieb variation principle
 Importing ab-initio theory into DFT: Some applications of the Lieb variation principle Trygve Helgaker, Andy Teale, and Sonia Coriani Centre for Theoretical and Computational Chemistry (CTCC), Department
Importing ab-initio theory into DFT: Some applications of the Lieb variation principle Trygve Helgaker, Andy Teale, and Sonia Coriani Centre for Theoretical and Computational Chemistry (CTCC), Department
Electronic band structure, sx-lda, Hybrid DFT, LDA+U and all that. Keith Refson STFC Rutherford Appleton Laboratory
 Electronic band structure, sx-lda, Hybrid DFT, LDA+U and all that Keith Refson STFC Rutherford Appleton Laboratory LDA/GGA DFT is good but... Naive LDA/GGA calculation severely underestimates band-gaps.
Electronic band structure, sx-lda, Hybrid DFT, LDA+U and all that Keith Refson STFC Rutherford Appleton Laboratory LDA/GGA DFT is good but... Naive LDA/GGA calculation severely underestimates band-gaps.
Before we start: Important setup of your Computer
 Before we start: Important setup of your Computer change directory: cd /afs/ictp/public/shared/smr2475./setup-config.sh logout login again 1 st Tutorial: The Basics of DFT Lydia Nemec and Oliver T. Hofmann
Before we start: Important setup of your Computer change directory: cd /afs/ictp/public/shared/smr2475./setup-config.sh logout login again 1 st Tutorial: The Basics of DFT Lydia Nemec and Oliver T. Hofmann
DFT: Exchange-Correlation
 DFT: Local functionals, exact exchange and other post-dft methods Stewart Clark University of Outline Introduction What is exchange and correlation? Quick tour of XC functionals (Semi-)local: LDA, PBE,
DFT: Local functionals, exact exchange and other post-dft methods Stewart Clark University of Outline Introduction What is exchange and correlation? Quick tour of XC functionals (Semi-)local: LDA, PBE,
Same idea for polyatomics, keep track of identical atom e.g. NH 3 consider only valence electrons F(2s,2p) H(1s)
 XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
The LDA-1/2 method in exciting
 http://exciting-code.org The LDA-1/2 method in exciting Ronaldo Rodrigues Pelá Humboldt Universität zu Berlin Instituto Tecnológico de Aeronáutica Outline DFT-1/2 Exchange-correlation functionals Exact
http://exciting-code.org The LDA-1/2 method in exciting Ronaldo Rodrigues Pelá Humboldt Universität zu Berlin Instituto Tecnológico de Aeronáutica Outline DFT-1/2 Exchange-correlation functionals Exact
Computational Material Science Part II. Ito Chao ( ) Institute of Chemistry Academia Sinica
 Computational Material Science Part II Ito Chao ( ) Institute of Chemistry Academia Sinica Ab Initio Implementations of Hartree-Fock Molecular Orbital Theory Fundamental assumption of HF theory: each electron
Computational Material Science Part II Ito Chao ( ) Institute of Chemistry Academia Sinica Ab Initio Implementations of Hartree-Fock Molecular Orbital Theory Fundamental assumption of HF theory: each electron
Joint ICTP-IAEA Workshop on Fusion Plasma Modelling using Atomic and Molecular Data January 2012
 2327-3 Joint ICTP-IAEA Workshop on Fusion Plasma Modelling using Atomic and Molecular Data 23-27 January 2012 Qunatum Methods for Plasma-Facing Materials Alain ALLOUCHE Univ.de Provence, Lab.de la Phys.
2327-3 Joint ICTP-IAEA Workshop on Fusion Plasma Modelling using Atomic and Molecular Data 23-27 January 2012 Qunatum Methods for Plasma-Facing Materials Alain ALLOUCHE Univ.de Provence, Lab.de la Phys.
Beyond the Hartree-Fock Approximation: Configuration Interaction
 Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Lecture 4: Hartree-Fock Theory
 Lecture 4: Hartree-Fock Theory One determinant to rule them all, One determinant to find them, One determinant to bring them all and in the darkness bind them Second quantization rehearsal The formalism
Lecture 4: Hartree-Fock Theory One determinant to rule them all, One determinant to find them, One determinant to bring them all and in the darkness bind them Second quantization rehearsal The formalism
