MOLECULAR STRUCTURE, NBO AND HOMO-LUMO ANALYSIS OF QUERCETIN ON SINGLE LAYER GRAPHENE BY DENSITY FUNCTIONAL THEORY
|
|
|
- Edith O’Connor’
- 5 years ago
- Views:
Transcription
1 Digest Journal of Nanomaterials and Biostructures Vol. 13, No. 1, January - March 2018, p MOLECULAR STRUCTURE, NBO AND HOMO-LUMO ANALYSIS OF QUERCETIN ON SINGLE LAYER GRAPHENE BY DENSITY FUNCTIONAL THEORY M. SARANYA a, S. AYYAPPAN a*, R. NITHYA a, R. K. SANGEETHA b A. GOKILA b a Department of Physics, Government college of Technology, Coimbatore, , India b Department of Physics, Sri Eshwar College of Engineering, Coimbatore, , India Quercetin (3,5,7,3,4 - pentahydroxyflavone) is a member of flavonoids. Density functional theory has been employed to study the adsorption of quercetin on single layer graphene (QCT-SLG) was investigated with the basis set of 6-21G. The total density of states and partial density states of the titled molecules were performed at density functional theory method. The molecular electrostatic potential (MEP) mapping shows the binding interactions of quercetin with single graphene layer. Dipole moment, hyperpolarizability and quantum chemical parameters have been calculated by Hartree Fock (HF) approximation approach. The natural bonding orbital analyses (NBO) calculation confined that the occurrence of intramolecular charge transfer takes place within the molecules. From DFT, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of frontier orbital were obtained. (Received October 13, 2017; Accepted January 15, 2018) Keywords: QCT-SLG, Density Functional Theory, Hartree Fock, Molecular electrostatic potential, Natural Bonding orbital, HOMO-LUMO 1. Introduction Flavonoids are the most important class of polyphenolic compounds, which in addition to their important biological roles in plant pigmentation, nitrogen fixation, and chemical defense possess anti-cancer, anti-inflammatory, antibacterial, antiviral, and antiallergic properties that are a consequence of their antioxidant properties [1]. Flavonoids, found in leaves, barks, rinds, seeds, and flowers- frequently closely associated with Vitamin C and offering synergistic effects. Both flavonoids and vitamin C benefit plants by providing them with antioxidant protection and are also important for human health. Quercetin is a strong hydroxyantioxidant and major dietary flavonoid (flavonols) most common present in nature [2-4]. Hydroxy and oxy groups present in a quercetin structure have the ability to form complexes with various oxygen groups. It was commonly found in plants and fruits. Quercetin is the most abundant of the flavonoids which has 3 rings and 5 hydroxyl groups. Quercetin is found in many common foods including apple, tea, onion, nuts, berries, cauliflower, cabbage. Graphene, a single carbon layer with oxygen based functional groups bonded across the surface. The analysis of the interaction mechanism between flavonol molecules and the graphene surface has also led to relevant studies with regard to interaction energies and charge transfer between the molecules, structural changes and chemical properties of adsorbed molecules. Graphene is being focused for the scientific community due to it great potential in many applications such as biotechnology, nanobiomedicine or electronic devices [5-9]. The potential applications of graphene might open through chemical functionalization such as adsorption of molecules [10]. * Corresponding author: ayyappan@gct.ac.in
2 98 As a matter of fact, the functionalization and modification of graphene properties, with particular attention to the zero-gap band behavior, through covalent and non-covalent interactions with adsorbed moieties have a greater attention. Non-covalent interactions between organic molecules and the graphene surface lead to very subtle effects on the graphene π-electronic structure, rising from vander Waals forces, π π interactions and several other effects [11,12]. The charge transfer between electron donating or accepting adsorbed molecules and graphene induces changes in the carriers in graphene called molecular doping is a relevant approach to modify electronic properties of graphene [13-21]. The investigation of the interaction mechanism between flavonol molecules and the graphene surface has also led to relevant studies with regard to interaction energies and charge transfer as a function of the molecular structure and properties of adsorbed molecules [8-11, 17 36]. The influence of the adsorbed molecule on electronic properties of graphene is strongly depends upon their respective properties and their mutual interactions. However, it is difficult to predict which molecules could lead to new systems with the desired features. The theoretical calculations (such as density functional theory, DFT) are very useful tool to identification and quantification of the interactions between adsorbed molecules and graphene. Recently, density functional theory (DFT) method, have performed to evaluated chemical properties, Subsequently, the study of the electronic and molecular properties is one of the greater importance that helps to investigate the mechanism of the antioxidant activity of these compounds. Density functional theory (DFT) was used to study the adsorption of flavonols on the graphene surface, focusing our interest on flavonol features. Electronic properties such as electron affinity (EA), hardness (η), softness (S), electronegativity (χ) and electrophilic index (ω) are also evaluated. The frontier molecular orbitals (FMOs) and band gap energies are constructed and analyzed. The molecular electrostatic potential (MEP) showed that the reactive site for electrophilic and nucleophilic attacks and charge delocalization is characterized using natural bond orbital (NBO) analysis. Density of states (DOS), Projected Density of states (PDOS), Orbital Projected Density of states (OPDOS) for occupied and unoccupied molecular orbital is interpreted [37]. The present investigation gives a detailed DFT study on the interactions between a family of quercetin and graphene surface. 2. Computational details Density function theory method is used to calculate the HOMO-LUMO studies using B3LYP method with the basis set 6-21G (d,p) Gaussian 09 software programs [38-40]. The natural bonding orbital calculations were performed by NBO 5.0 program as executed in the Gaussian 09W program at B3LYP method. The total density of states and partial density of states of the QCT-SLG were calculated using multiwfn 3.3 program package [41]. XRD pattern of quercetin with graphene was recorded using an X-ray diffraction instrument (ULTIMA IV, Rigaku diffractometer) employing a cobalt (Co) tube at λ=1.78a, running at 40 kv and 30mA. 3. Results and discussion 3.1XRD Measurement The XRD pattern of quercetin with graphene is shown in Fig.1. The physical mixtures of quercetin with graphene exhibited a number of different peaks; However, these peaks were slightly shifted from their original positions, and also while new peaks were observed. The diffraction pattern for the single layered graphene with quercetin had a broad diffraction peak appearing at corresponds to (002) plane, which confirms the short range order in the stacked graphene sheets.
3 99 Fig. 1. Molecular structure and XRD pattern of QCT-SLG 3.2. Prediction of dipole moment and Hyperpolarizability The calculated dipole moment and Hyperpolarizability of single layered graphene with quercetin is done by HF method at the basis set of 6-21G(d,p) is shown in table 1. The third rank tensor of the hyperpolarizability can described by 3D matrix can be reduced to ten components due to Kleinmann symmetry [42]. The energy of the molecular systems is functioned with the presence of external electric field. The β components are described using coefficient in the Taylor series expansion of the energy in the external electric field. The external electric field depends on energy of unperturbed molecules, dipole moment, polarizability and first order hyperpolarizability of the molecules were calculated by finite field of approach and it is expressed as α tot = α xx + α yy + α zz / 3 β = (β 2 x + β 2 y + β 2 z) 1/2 β = [ (β xxx + β xyy + β xzz ) 2 + (β yyy + β xxy + β yzz ) 2 + (β zzz + β xxz + β yyz ) ] 1/2 The dipole moment of the titled of the compound is found to be Debye. The hyperpolarizability of the title of the molecules is x esu which is five times greater than urea. The NLO effect arises due to interaction of electromagnetic field and titled molecule. The high β value indicates the charge delocalization along the bonding axis and it is clearly show that charge transfer process involves in intermolecular orbital. Table 1 a) Calculated Dipole moment (µ), polarizability (α) and hyperpolarizability (β) of QCT-SLG by HF / 6-31G(d, p) method µ value HF/6-31G(d,p) µ x µ y µ z µ
4 100 Table 1 b) Calculated polarizability (α) and hyperpolarizability (β) of QCT-SLG by HF / 6-31G(d, p) method α value HF/6-31G(d, p) B3LYP α xx β value HF/6-31G(d,p) β xxx α yy β yyy α zz β zzz α xy β xyy α xz β xxy α yz β xxz Total a.u a.u β xzz β yzz β yyz β xyz Total 0.816x10-30 esu 3.3. HOMO-LUMO analysis The HOMO-LUMO energies are popular quantum mechanical descriptors which [43] play a major role in governing wide range of chemical interactions. The Frontier Molecular Orbital gives an insight about the reactivity of the molecule and the active site can be demonstrated by the distribution of frontier orbital. The HOMO-LUMO frontier orbital compositions for QCT-SLG calculated with the DFT/6-311G orbital energy level are shown in Fig.2 respectively. In order to evaluate the orbital energy level behavior of the title compounds, the third highest and highest occupied MO s (HOMO and HOMO-3), the lowest and the third values lowest unoccupied MO s (LUMO andlumo+3) are performed. The electronic absorption spectra corresponds to the transition from molecular orbital were the highest occupied molecular orbital and the lowest unoccupied molecular orbital of title of the compound are alpha molecular orbital level (242) and alpha molecular orbital level (243) respectively. The differences between the orbital energies were shown in table 2. The energy values of HOMO (242) orbital and LUMO (243) orbital were lying at an energy value of ev and ev respectively. The HOMO-LUMO energy gap was obtained at ev in the isolated gas molecular calculations. The HOMO-LUMO energy gap is higher implies the kinetic energy is higher and high chemical reactivity. Table 2 Theoretical transition levels between HOMO and LUMO and frontier orbital of QCT-SLG were calculated by B3LYP/6-31G (d,p) method Level MO Energy (ev) Level MO Energy (ev) E (ev) HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO HOMO LUMO
5 101 Fig. 2. Frontier molecular diagram of QCT-SLG 3.4. Natural Bonding Orbital analysis The strong intramolecular hyperconjugate interaction takes place between σc43-c44 and σ*c31-c32 and hence its electron density value is 1.84 which is present in SLG, whereas the intramolecular hyperconjugate interaction in the ring structure takes place in C88-O79. The natural population analysis of the QCT-SLG shows the Lewis structure 97.73% and non Lewis structure 2.266%. The result shows the charge transfer causing the stabilization occurs within the SLG with respect to QCT. Table 3. Second Order perturbation theory analysis of Fock matrix in NBO basis for single layered graphene by B3LYP/6-31G (d,p) method Donor NBO (i) ED/e Acceptor NBO (j) ED/e E(2) kcal/mol E(j)-E(i) a.u. C 1 - C C 11 - C C 7 - C C 19 - C C 9 - C C 19 - C C 11 - C C 1 - C C 11 - C C 21 - C C 19 - C C 9 - C C 19 - C C 29 - C C 21 - C C 11 - C C 21 - C C 31 - C C 29 - C C 19 - C C 29 - C C 39 - C C 31 - C C 21 - C C 31 - C C 43 - C C 33 - C C 21 - C C 39 - C C 29 - C C 43 - C C 31 - C C 49 - C C 39 - C F(i,j) a.u. C C 8 - C C C 10 - C
6 102 Donor NBO (i) ED/e Acceptor NBO (j) ED/e E(2) kcal/mol E(j)-E(i) a.u. C C 19 - C F(i,j) a.u. C C 32 - C C C 34 - C O 79 - C C 88 - C O 82 - C C 86 - C O 82 - C C 88 - C C O 79 - C C O 79 - C C C 88 - C C C 88 - C C O 80 - C C C 88 - C C O 81 - C C O 83 - C C O 84 - C C O 85 - C MEP Mapping Molecular electrostatic potential is highly informative concerning the nuclear and electronic charge distribution of the molecules besides it is a tool for interpretation and prediction of chemical reactivity. The MEP has proved to be extremely good for the description of noncovalent interactions particularly hydrogen bonds [44-46]. MEP is widely used as a reactivity map displaying most probable region for nucleophilic and electrophilic attacks. In the MEP surface, red colour refers to electron-rich (negative) region, blue colour refers to electron-poor (positive) region and green colour signifies zero electrostatic potential. In majority of the MEP surfaces, negative region is the preferred site for the electrophilic attack and positive region is preferred for nucleophilic attack. The electron concentrations at the MEP surface are indicated by different colours. The values of electron density increases in the following order Red > Orange > Yellow > Green > Blue In QCT-SLG, the electrophilic region occurs in the oxygen which is present in the quercetin molecule. The negative and positive electron density were occurs in the region of x10-2 a.u. to 2.304x10-2 a.u. The MESP at different points on the electron density isosurface is shown by the colour isosurface in the Fig.3. Red colour indicates the strong attraction which occurs in quercetin molecule due to oxygen atoms whereas blue colour which occurs in the overall region of single layered graphene. It is observed that nucleophilic attack is more predominant than electrophilic attack due to C-H molecules in SLG. Fig. 3. Molecular electrostatic potential mapping of SLG
7 3.6. Density of states The important application of DOS plot is to demonstrate the molecular orbital and their contribution of chemical bonding through the OPDOS plot [47]. The DOS plot results show that overlapping population in the molecular orbital. The OPDOS result shows that non bonding, bonding and antibonding interactions between the two orbital atom groups. The positive value of OPDOS indicating that the bonding interaction and negative value indicates the antibonding interactions and zero values indicates the non bonding interactions. The DOS plot gives the composition of group of orbital contributing to the molecular orbital. The PDOS and OPDOS plots are shown in the Fig.4. The plot for DOS is taken from the range of -1.50a.u. to +1.50a.u. The graph exhibits the orbital characteristics of different energy range. It is the major contribution from s orbital and p orbital basic function of carbon in the frontier molecular orbital. The partial density of states (PDOS) of the carbon atom of the title of the molecule exhibits the total density states of the molecules. The OPDOS of the carbon has just larger positive energy then the negative energy values. The carbon atoms which is present in SLG have more energy than the carbon atoms in QCT. 103 Fig. 4. Total and partial density of states diagram of QCT-SLG 3.7. Global chemical reactivity Density functional theory (DFT) [48] has been found to be successful in providing theoretical insights into the chemical reactivity and selectivity, in terms of qualitative chemical concepts like electronegativity (χ), hardness (η), softness(s), electrophilicity index(ω).the basic relationship of the density functional theory of chemical reactivity is precisely, the one established by Parr et al., [49], that links the chemical potential of DFT with the first derivative of the energy with respect to the n number of electrons, and therefore with the negative of the electronegativity χ. Ionization potential (I) is defined as the amount of energy required to remove an electron from a molecule [50]. Electron affinity (A) is defined as the energy released when a proton is added to a system [51]. It is related to the energy of the E HOMO and E LUMO through the equation: I = -E HOMO ; A = -E LUMO When the values of I and A are known, one can determine the electronegativity χ and the global hardness (η).the electronegativity is defined as the measure of the power of an atom or group of atoms to attract electrons towards itself [52], Hardness (η) can be defined within the DFT as the second derivative of the E with respect to N as v(r) property which measures both the stability and reactivity. It can be estimated by using the equation: I A/2 ; I A Chemical softness (S) is the measure of the capacity of an atom or group of atoms to receive electrons [53-54], it is estimated by using the equation: S=1/
8 104 Parr et al [55] have proposed electrophilicity index as a measure of energy lowering due to maximal electron flow between donor and acceptor. They defined electrophilicity index (ω) as follows. 2 /2 According to the definition, this index measures the propensity of chemical species to accept electrons. A good, reactive, nucleophile is characterized by lower value of μ, ω; and conversely a good electrophile is characterized by a high value of μ, ω. All the global quantities of SLG were calculated. Table 4 Calculated quantum chemical parameters of SLG-QCT by B3LYP/6-31G(d,p) method Quantum chemical parameters B3LYP/6-31G(d,p) Hardness(η) eV Chemical potential(µ) ev Ionization potential(i) kj/mol Electron affinity(a) kj/mol Energy gap(e g ) ev Electrophilicity index 0.5 ev Chemical softness (S) Conclusions The calculated hyperpolarizability of QCT-SLG is a five folders greater compared to the UREA, which shows high chemical reactivity between QCT-SLG. The HOMO-LUMO energy gap of higher order increases which shows the greater chemical reaction between QCT-SLG. The density of states of the QCT-SLG was analyzed and it exhibits the stabilization of the molecules which depends on carbon of SLG. The MESP provides the information on charge density distribution of the QCT-SLG. All the global parameters were calculated and analyzed. The study of theoretical insight can contribute significantly to the understanding of the stability of the QCT with SLG. References [1] E. Middelton, C. Kandaswami, in: J.B. Harborne 1994 [2] T. Leighton, C. Ginther,T. Huang, C.T. Ho, C.Y. Lee (Eds.), ACS, 1992 [3] P. Hollman, M. Hertog, M. Katan, Food Chem. 57, 43 (1996). [4] B. Havsteen, Biochem. Pharmacol. 32, 1141 (1983). [5] J. K. Wassei, RB Kaner (2013) Acc Chem Res 46:224 [6] H. Y. Mao, S. Laurent, W. Chen et al. Chem Rev 113, 3407(2013) [7] A. Bianco, Angew Chem Int Ed 52, 4986 (2013). [8] V. Palermo Chem Commun 2013(49), 2848(2013) [9] Y. H. Lu, W. Chen, Y.P. Feng, P.M. He, J Phys Chem B 113, 2 (2009). [10] V. Georgakilas, M. Otyepka, A.B. Bourlinos, V. Chandra, N. Kim, K.C. Kemp, P. Hobza, R. Zboril, K.S. Kim, Chem Rev 112, 6156 (2012). [11] J. Björk, F. Hanke, C.A. Palma, P. Samori, M. Cecchini, M. Persson, J Chem Phys Lett 1, 3407 (2010). [12] S. Gowtham, R.H. Scheicher, R. Ahuja, R. Pandey, S.P. Karna, Phys Rev B 76, (2007) [13] T.O. Wehling, K.S. Novoselov, S.V. Morozov, E. E. Vdovin, M. I. Katsnelson, A. K. Geim, I. L. Lichtenstein, Nano Lett 8, 173 (2008). [14] S. K. Saha, R. C. Chandrakanth, H. R. Krishnamurthy, Phys Rev B 80, (2009). [15] L. Tsetseris, S. T. Pantelides, Phys Rev B 85, (2012).
9 [16] A. J. Samuels, J. D. Carey ACS Nano 7, 279 (2013). [17] J.W. Li, Y.Y. Liu, L.H. Xie, J.Z. Shang, Y. Qian, M.D. Yi, W. Huang, Phys Chem 17, 491 (2015). [18] P.A. Denis, J Phys Chem, C117, 389 (2013). [19] I.S.S. Oliveira, R.H. Miwa, J Chem Phys 142, (2015). [20] T. Hu, I.C. Gerber, J Phys Chem C 117, 241 (2015). [21] M.A. Vincent, I.H. Hillier, J Chem Inf Model 54, 225 (2014). [22] D. Mollenhauer, C. Brieger, E. Voloshina, B. Paulus, J PhysChem C 119, 1898 (2015). [23] P.A. Denis, F. Iribarne, J Mol Struct THEOCHEM, 957, 114 (2010). [24] Q. Zhou, L. Yuan, X. Yang, Z. Fu, Y. Tang, C. Wang, H. Zhang, Chem Phys 440, 80 (2014). [25] S.D. Chakarova, E. Schröeder, B.I. Lundqvist, D.C. Langreth, Phys Rev Lett 96, (2006). [26] O. Leenaerts, B. Partoens, R.M. Peeters,Microelectron 40, 860 (2009). [27] I. Hamada, Phys Rev B, 86, (2012). [28] M. Galicia-Hernández, E. Chigo-Anota, M.T. Romero-de la Cruz, M. González-Melchor, G. Hernández-Cocoletzi, J Mol. Model 18, 3857 (2012). [29] P. Lazar, F. Karlicky, P. Jurecka, M.Kocman, E. Otyepkova, K. Safarova, J Am Chem Soc 135, 6372 (2013). [30] C. Lechner, A.F. Sax, J Phys Chem C 118, (2014). [31] L. Xu, X. Zhou, W.Q. Tian, T. Gao, Y.F. Zhang, S. Lei, Z.F. Liu, Angew Chem 126, 1 (2014). [32] C. Herrera, R. Alcalde, M. Atilhan, S. Aparicio, Phys Chem C 118, 9741 (2014). [33] Y. Wang, Z. Xu, Y.N. Moe, Chem Phys 406, 78 (2012). [34] Y. Zhao, Z. Hu, J Phys Chem B 117, (2013). [35] M. Vijayakumar, B. Schwenzer, V. Shutthanandan, J. Hu, J. Liu, I. A. Aksay, Nano Energy 3, 152 (2014). [36] N. Ramos-Berdullas, I. Perez-Juste, C. Van Alsenoy, M. Mandado, Phys Chem Chem Phys 17, 575 (2015). [37] W. Y. Huang, L. Fu, C. Y. Li, L. P. Xu, L. X. Zhang, W. M. Zhang, Journal of Food Science, 1239 (2017). [38] B. Miehlich, A. Savin, H. Stroll, H. Preuss, Chem. Phys. Lett. 157 (1987). [39] M. Frisch et al, Gaussian an 09 Rev. E. 01, (2009). [40] A. Frish, et al, A.B. Gassview, Gaussian Inc., Pittsburgh, PA, [41] Lu Tian, Feiwu Chen, Journal of Computational Chemistry 580 (2012). [42] D. A. Kleinman, Physical Review, [43] Z. Zhou, R.G. Parr, J. Am. Chem. Soc., 5720 (1990). [44] E. Scrocco, J. Tomasi, Adv. Quantum Chem. 115 (1979). [45] F. J. Luque, J.M. Lopez, M. Orozco, Theor. Chem. Acc. 343 (2000). [46] N. Okulik, A.H. Jubert, Int. Electron J. Mol. Des. 17 (2005). [47] M. Reinboth, S. Wolffram, G. Abraham, F.R. Ungemach, 198 (2010). 105
First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by Density Functional Theory Methods
 51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
EXPERIMENTAL AND THEORETICAL SPECTROSCOPIC STUDIES, CHARGE DISTRIBUTION ANALYSIS OF SINGLE LAYERED GRAPHENE
 Digest Journal of Nanomaterials and Biostructures Vol. 12, No. 1, January - March 2017, p. 127-134 EXPERIMENTAL AND THEORETICAL SPECTROSCOPIC STUDIES, CHARGE DISTRIBUTION ANALYSIS OF SINGLE LAYERED GRAPHENE
Digest Journal of Nanomaterials and Biostructures Vol. 12, No. 1, January - March 2017, p. 127-134 EXPERIMENTAL AND THEORETICAL SPECTROSCOPIC STUDIES, CHARGE DISTRIBUTION ANALYSIS OF SINGLE LAYERED GRAPHENE
DFT calculations of effective reactive sites of inositol
 Indian Journal of Chemistry Vol. 56A, July 2017, pp. 786-790 DFT calculations of effective reactive sites of inositol D Jeevitha a, K Sadasivam a, * & R Praveena b a Department of Physics, b Department
Indian Journal of Chemistry Vol. 56A, July 2017, pp. 786-790 DFT calculations of effective reactive sites of inositol D Jeevitha a, K Sadasivam a, * & R Praveena b a Department of Physics, b Department
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF M NITROACETANILIDE
 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
Vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N-phenylethanolamine by density functional method
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
Density Functional Theory Study of Exohedral Carbon Atoms Effect on Electrophilicity of Nicotine: Comparative Analysis
 Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone
 FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES
 Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Australian Journal of Basic and Applied Sciences
 AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes
 Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy. --- Influence of intramolecular interactions
 Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy --- Influence of intramolecular interactions Minfu Zhao, Xu Shan, Shanshan Niu, Yaguo Tang, Zhaohui Liu,
Valence electronic structure of isopropyl iodide investigated by electron momentum spectroscopy --- Influence of intramolecular interactions Minfu Zhao, Xu Shan, Shanshan Niu, Yaguo Tang, Zhaohui Liu,
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-fe 2
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
How Partial Atomic Charges and Bonding. Orbitals Affect the Reactivity of Aluminum
 Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
Structure, Electronic and Nonlinear Optical Properties of Furyloxazoles and Thienyloxazoles
 Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
International Journal of Materials Science ISSN Volume 12, Number 2 (2017) Research India Publications
 HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
Growth and Characterization of an Organic Crystal and DFT Studies of 2-amino 5-methyl Pyridinium Salicylate
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
Available online at WSN 61(2) (2017) EISSN
 Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Journal of Chemical and Pharmaceutical Research, 2015, 7(2): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Journal of Advanced Scientific Research DFT APPROACH ON CORROSION INHIBITION PERFORMANCE OF THIOSEMICARBAZONE DERIVATIVES ON METALLIC IRON
 Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):572-583 Ground state properties: Gross orbital charges,
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):572-583 Ground state properties: Gross orbital charges,
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8. Scholars Research Library (
 Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes
 Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum Chemical Designing of Novel Organic Non-Linear Optical Compounds
 Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
General Physical Chemistry II
 General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
Research & Development Centre, Bharathiar University, Coimbatore , India. 2
 EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes
 The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol. University of Science and Technology of China, Hefei, , China
 Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal.
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
Department of Physics, A.V.C. College, Mayiladuthurai, Tamilnadu, India.
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
Chapter 6. Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications
 Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Hong Zhou, Guang-Xiang Liu,* Xiao-Feng Wang and Yan Wang * Supporting Information
 Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene
 Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Computational Study of Arzanol and Comparison with the General Trends of Acylphloroglucinols
 Computational Study of Arzanol and Comparison with the General Trends of Acylphloroglucinols LILIANA MAMMIN Department of Chemistry University of Venda P/Bag X5050, Thohoyandou 0950 SUTH AFRICA email sasdestria@yahoo.com
Computational Study of Arzanol and Comparison with the General Trends of Acylphloroglucinols LILIANA MAMMIN Department of Chemistry University of Venda P/Bag X5050, Thohoyandou 0950 SUTH AFRICA email sasdestria@yahoo.com
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
First-principles Studies of Formaldehyde Molecule Adsorption on Graphene Modified with Vacancy, -OH, -CHO and -COOH Group
 2017 Asia-Pacific Engineering and Technology Conference (APETC 2017) ISBN: 978-1-60595-443-1 First-principles Studies of Formaldehyde Molecule Adsorption on Graphene Modified with Vacancy, -OH, -CHO and
2017 Asia-Pacific Engineering and Technology Conference (APETC 2017) ISBN: 978-1-60595-443-1 First-principles Studies of Formaldehyde Molecule Adsorption on Graphene Modified with Vacancy, -OH, -CHO and
1 Supporting information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 1 Supporting information 1.1 Separation of the chemical potentials of electrons and protons in
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 1 Supporting information 1.1 Separation of the chemical potentials of electrons and protons in
Quantum chemical origin of high ionization potential and low electron affinity of Tungsten Hexafluoride
 Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
arxiv: v1 [cond-mat.mtrl-sci] 9 Oct 2007
![arxiv: v1 [cond-mat.mtrl-sci] 9 Oct 2007 arxiv: v1 [cond-mat.mtrl-sci] 9 Oct 2007](/thumbs/72/66928159.jpg) Adsorption of H 2 O, NH 3, CO, NO 2, and NO on graphene: A first-principles study O. Leenaerts, B. Partoens, and F. M. Peeters Universiteit Antwerpen, Departement Fysica, Groenenborgerlaan 171, B-2020
Adsorption of H 2 O, NH 3, CO, NO 2, and NO on graphene: A first-principles study O. Leenaerts, B. Partoens, and F. M. Peeters Universiteit Antwerpen, Departement Fysica, Groenenborgerlaan 171, B-2020
Andrew Zahrt Denmark Group Meeting
 Andrew Zahrt Denmark Group Meeting 4.28.15 Introduction Early Publications/Hypotheses: Thermodynamic Product Stability Ground State Destabilization Transition State Stabilization Solvent Effects Gas Phase
Andrew Zahrt Denmark Group Meeting 4.28.15 Introduction Early Publications/Hypotheses: Thermodynamic Product Stability Ground State Destabilization Transition State Stabilization Solvent Effects Gas Phase
STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY
 Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Supporting Information for. Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table MA 02139
 Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds
 Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Structural and Electronic Properties of Folic Acid Adsorption on the Carbon Nanotubes: A Density Functional Theory Study
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2015, Vol. 31, No. (1): Pg. 345-351 Structural and Electronic
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2015, Vol. 31, No. (1): Pg. 345-351 Structural and Electronic
Supporting information for: First hyperpolarizability of collagen using the. point dipole approximation
 Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Quantum Chemical Simulations and Descriptors. Dr. Antonio Chana, Dr. Mosè Casalegno
 Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter Numbering of Chapters and Assigned Problems
 CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
Hydrogen Peroxide Adsorption on Graphene with Stone-Wales Defect
 JNS 4 (2014) 1-8 Hydrogen Peroxide Adsorption on Graphene with Stone-Wales Defect R. Majidi a, *, A. R. Karami b a Department of Physics, Shahid Rajaee Teacher Training University, Lavizan, 16788-15811
JNS 4 (2014) 1-8 Hydrogen Peroxide Adsorption on Graphene with Stone-Wales Defect R. Majidi a, *, A. R. Karami b a Department of Physics, Shahid Rajaee Teacher Training University, Lavizan, 16788-15811
J.Thi-Qar Sci. Vol.3 (1) July/2011
 ISSN 1991-8690 الترقيم الدولي - 0968 1661 Theoretical study of the Structural and Electronic Properties of the Phenol, Phenoxy Phenol-water complex and Phenoxy-water complex Kawkab A. H. Al-ali Sadiq M.
ISSN 1991-8690 الترقيم الدولي - 0968 1661 Theoretical study of the Structural and Electronic Properties of the Phenol, Phenoxy Phenol-water complex and Phenoxy-water complex Kawkab A. H. Al-ali Sadiq M.
Outline. Introduction: graphene. Adsorption on graphene: - Chemisorption - Physisorption. Summary
 Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
Supporting Information
 Supporting Information Uniformly Sized (112) Facet Co 2 P on Graphene for Highly Effective Photocatalytic Hydrogen Evolution Bin Tian, a, b Zhen Li, a, b Wenlong Zhen c and Gongxuan Lu *a a State Key Laboratory
Supporting Information Uniformly Sized (112) Facet Co 2 P on Graphene for Highly Effective Photocatalytic Hydrogen Evolution Bin Tian, a, b Zhen Li, a, b Wenlong Zhen c and Gongxuan Lu *a a State Key Laboratory
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
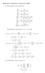 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Inclusion of X 3 N (X = Ti, Zr, Hf) in Fullerene C80
 Journal of Computational Methods in Molecular Design, 2015, 5 (1):33-38 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) Inclusion of X 3 N (X = Ti, Zr, Hf) in Fullerene C80
Journal of Computational Methods in Molecular Design, 2015, 5 (1):33-38 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) Inclusion of X 3 N (X = Ti, Zr, Hf) in Fullerene C80
Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table.
 1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
Electronic properties of aluminium and silicon doped (2, 2) graphyne nanotube
 Journal of Physics: Conference Series PAPER OPEN ACCESS Electronic properties of aluminium and silicon doped (2, 2) graphyne nanotube To cite this article: Jyotirmoy Deb et al 2016 J. Phys.: Conf. Ser.
Journal of Physics: Conference Series PAPER OPEN ACCESS Electronic properties of aluminium and silicon doped (2, 2) graphyne nanotube To cite this article: Jyotirmoy Deb et al 2016 J. Phys.: Conf. Ser.
International Journal of Pure and Applied Researches; Volume 1(1)/2015
 Computational tudies and Multivariate Analysis of Global and Local Reactivity Descriptors of Five Membered Heterocycles Molecules by Density Functional Theory (DFT). urendra Babu Department of Chemistry
Computational tudies and Multivariate Analysis of Global and Local Reactivity Descriptors of Five Membered Heterocycles Molecules by Density Functional Theory (DFT). urendra Babu Department of Chemistry
QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL AND PROPRANOLOLDERIVATIVES
 Vol. 9 No. 2 189 202 April June 2016 ISSN: 09741496 eissn: 09760083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL
Vol. 9 No. 2 189 202 April June 2016 ISSN: 09741496 eissn: 09760083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL
Journal of Physical and Theoretical Chemistry
 Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 11 (1) 1-13: Spring 214 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Investigation of relationship with electron configuration
Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 11 (1) 1-13: Spring 214 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Investigation of relationship with electron configuration
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 31, April 12, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters
 Appl Nanosci (2012) 2:359 364 DOI 10.1007/s13204-012-0092-x ORIGINAL ARTICLE A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters Ajanta
Appl Nanosci (2012) 2:359 364 DOI 10.1007/s13204-012-0092-x ORIGINAL ARTICLE A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters Ajanta
Bonding and Physical Properties The Molecular Orbital Theory
 Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Quantum Chemical Studies on the Inhibiting Effect of Bipyrazoles on Steel Corrosion in HCl
 ISS: 0973-4945; CODE ECJHAO E- Chemistry http://www.e-journals.net 2010, 7(2), 419-424 Quantum Chemical Studies on the Inhibiting Effect of Bipyrazoles on Steel Corrosion in HCl K. LAAREJ, M. BOUACHRIE
ISS: 0973-4945; CODE ECJHAO E- Chemistry http://www.e-journals.net 2010, 7(2), 419-424 Quantum Chemical Studies on the Inhibiting Effect of Bipyrazoles on Steel Corrosion in HCl K. LAAREJ, M. BOUACHRIE
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal.
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2017, Vol. 33, No. (3): Pg. 1071-1082 Structural and Theoretical
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2017, Vol. 33, No. (3): Pg. 1071-1082 Structural and Theoretical
Theoretical Study of the Mechanism of Corrosion Inhibition of Carbon Steel in Acidic Solution by 2-aminobenzothaizole and 2- Mercatobenzothiazole
 Int. J. Electrochem. Sci., 13 (2018) 3535 3554, doi: 10.20964/2018.04.50 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Theoretical Study of the Mechanism of Corrosion Inhibition
Int. J. Electrochem. Sci., 13 (2018) 3535 3554, doi: 10.20964/2018.04.50 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Theoretical Study of the Mechanism of Corrosion Inhibition
X-Shaped Donor Molecules Based on Benzo[2,1-b:3,4-b ]dithiophene for Organic Solar Cells Devices with PDIs as Acceptors.
![X-Shaped Donor Molecules Based on Benzo[2,1-b:3,4-b ]dithiophene for Organic Solar Cells Devices with PDIs as Acceptors. X-Shaped Donor Molecules Based on Benzo[2,1-b:3,4-b ]dithiophene for Organic Solar Cells Devices with PDIs as Acceptors.](/thumbs/75/72361771.jpg) Supporting Information X-Shaped Donor Molecules Based on Benzo[2,1-b:3,4-b ]dithiophene for Organic Solar Cells Devices with PDIs as Acceptors. Shamsa Bibi, Ping Li, Jingping Zhang * Faculty of Chemistry,
Supporting Information X-Shaped Donor Molecules Based on Benzo[2,1-b:3,4-b ]dithiophene for Organic Solar Cells Devices with PDIs as Acceptors. Shamsa Bibi, Ping Li, Jingping Zhang * Faculty of Chemistry,
Chapter 2. Methodology
 Chapter 2 Methodology Chapter 2 Methodology NORMA AUREA RANGEL-VÁZQUEZ 1 FRANCISCO RODRÍGUEZ FÉLIX 2 1 División de Estudios de Posgrado e Investigación del Instituto Tecnológico de Aguascalientes, Ave.
Chapter 2 Methodology Chapter 2 Methodology NORMA AUREA RANGEL-VÁZQUEZ 1 FRANCISCO RODRÍGUEZ FÉLIX 2 1 División de Estudios de Posgrado e Investigación del Instituto Tecnológico de Aguascalientes, Ave.
NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE
 Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Adsorption of H 2 O, CO 2, O 2, Ti and Cu on Graphene: A molecular modeling approach
 International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:12 No:06 234 Adsorption of H 2 O, CO 2, O 2, Ti and Cu on Graphene: A molecular modeling approach Hilal S Wahab a,*, Salam H. Ali b, Adi
International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:12 No:06 234 Adsorption of H 2 O, CO 2, O 2, Ti and Cu on Graphene: A molecular modeling approach Hilal S Wahab a,*, Salam H. Ali b, Adi
Effect of Lithium Doping on Hydrogen Adsorption of Defected. Graphene: A First-Principles Sudy
 Journal of Applied Chemistry Vol. 10, No37, 2016 Journal of Applied Chemistry Effect of Lithium Doping on Hydrogen Adsorption of Defected Article history: Received:23/Aug/2015 Graphene: A First-Principles
Journal of Applied Chemistry Vol. 10, No37, 2016 Journal of Applied Chemistry Effect of Lithium Doping on Hydrogen Adsorption of Defected Article history: Received:23/Aug/2015 Graphene: A First-Principles
Lone Pairs: An Electrostatic Viewpoint
 S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
S1 Supporting Information Lone Pairs: An Electrostatic Viewpoint Anmol Kumar, Shridhar R. Gadre, * Neetha Mohan and Cherumuttathu H. Suresh * Department of Chemistry, Indian Institute of Technology Kanpur,
Surface Transfer Doping of Diamond by Organic Molecules
 Surface Transfer Doping of Diamond by Organic Molecules Qi Dongchen Department of Physics National University of Singapore Supervisor: Prof. Andrew T. S. Wee Dr. Gao Xingyu Scope of presentation Overview
Surface Transfer Doping of Diamond by Organic Molecules Qi Dongchen Department of Physics National University of Singapore Supervisor: Prof. Andrew T. S. Wee Dr. Gao Xingyu Scope of presentation Overview
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Lecture 4: Band theory
 Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Electronic structure Crystal-field theory Ligand-field theory. Electronic-spectra electronic spectra of atoms
 Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
New Perspective on structure and bonding in water using XAS and XRS
 New Perspective on structure and bonding in water using XAS and XRS Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University, Sweden R. Ludwig Angew. Chem. 40, 1808 (2001)
New Perspective on structure and bonding in water using XAS and XRS Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University, Sweden R. Ludwig Angew. Chem. 40, 1808 (2001)
The Electronic Properties of SiC Graphene-Like: Doped and No-Doped Case
 Copyright 2011 American Scientific Publishers All rights reserved Printed in the United States of America Journal of Computational and Theoretical Nanoscience Vol. 8, 1 5, 2011 The Electronic Properties
Copyright 2011 American Scientific Publishers All rights reserved Printed in the United States of America Journal of Computational and Theoretical Nanoscience Vol. 8, 1 5, 2011 The Electronic Properties
Journal of Physical and Theoretical Chemistry
 Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 9 (4) 255-262: Winter 2013 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Theoretical thermodynamic study of CO and O
Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 9 (4) 255-262: Winter 2013 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Theoretical thermodynamic study of CO and O
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Homework 08 - Bonding Theories & IMF
 HW08 - Bonding Theories & IMF This is a preview of the published version of the quiz Started: Jun 4 at 11:4am Quiz Instructions Homework 08 - Bonding Theories & IMF Question 1 A sigma bond... stems from
HW08 - Bonding Theories & IMF This is a preview of the published version of the quiz Started: Jun 4 at 11:4am Quiz Instructions Homework 08 - Bonding Theories & IMF Question 1 A sigma bond... stems from
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Density Functional Theory Calculation of the Electronic Structure for C 20 Cage Fullerene حسابات نظرية دالة الكثافة للتركيب االلكتروني للفوليرين C 20
 Density Functional Theory Calculation of the Electronic Structure for C 20 Cage Fullerene حسابات نظرية دالة الكثافة للتركيب االلكتروني للفوليرين C 20 محمد اف ارح هادي هاشم نضال هادي طالب ليث عبيس اب ارهيم
Density Functional Theory Calculation of the Electronic Structure for C 20 Cage Fullerene حسابات نظرية دالة الكثافة للتركيب االلكتروني للفوليرين C 20 محمد اف ارح هادي هاشم نضال هادي طالب ليث عبيس اب ارهيم
one ν im: transition state saddle point
 Hypothetical Potential Energy Surface Ethane conformations Hartree-Fock theory, basis set stationary points all ν s >0: minimum eclipsed one ν im: transition state saddle point multiple ν im: hilltop 1
Hypothetical Potential Energy Surface Ethane conformations Hartree-Fock theory, basis set stationary points all ν s >0: minimum eclipsed one ν im: transition state saddle point multiple ν im: hilltop 1
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Uptake of OH radical to aqueous aerosol: a computational study
 Uptake of OH radical to aqueous aerosol: a computational study Grigory Andreev Karpov Institute of Physical Chemistry 10 Vorontsovo pole, Moscow, 105064, Russia Institute of Physical Chemistry and Electrochemistry
Uptake of OH radical to aqueous aerosol: a computational study Grigory Andreev Karpov Institute of Physical Chemistry 10 Vorontsovo pole, Moscow, 105064, Russia Institute of Physical Chemistry and Electrochemistry
1 Introduction. ISSN , England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11
 ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
NH 3 H 2 O N 2. Why do they make chemical bonds? Molecular Orbitals
 N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
First-Principle Studies on Adsorption of Cu + and Hydrated Cu + Cations on Clean Si(111) Surface
 CHEM. RES. CHINESE UNIVERSITIES 2010, 26(3), 472 478 First-Principle Studies on Adsorption of Cu + and Hydrated Cu + Cations on Clean Si(111) Surface CHENG Feng-ming 1,2, SHENG Yong-li 1,3, LI Meng-hua
CHEM. RES. CHINESE UNIVERSITIES 2010, 26(3), 472 478 First-Principle Studies on Adsorption of Cu + and Hydrated Cu + Cations on Clean Si(111) Surface CHENG Feng-ming 1,2, SHENG Yong-li 1,3, LI Meng-hua
Molecular Orbitals of Ethene
 Molecular Orbitals of Ethene 1 Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" interestingly, this reaction is rare and very slow! Molecular Orbitals
Molecular Orbitals of Ethene 1 Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" interestingly, this reaction is rare and very slow! Molecular Orbitals
Nanosheet-Constructed Porous BiOCl with Dominant {001} Facets for Superior Photosensitized Degradation
 Electronic Supplementary Information Nanosheet-Constructed Porous BiOCl with Dominant {001} Facets for Superior Photosensitized Degradation Dong-Hong Wang, ab Gui-Qi Gao, b Yue-Wei Zhang, a Li-Sha Zhou,
Electronic Supplementary Information Nanosheet-Constructed Porous BiOCl with Dominant {001} Facets for Superior Photosensitized Degradation Dong-Hong Wang, ab Gui-Qi Gao, b Yue-Wei Zhang, a Li-Sha Zhou,
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
Theoretical Investigation of Structural, Thermodynamical and HOMO, LUMO Analysis of 6-Aminopenicillanic Acid
 DOI:10.7598/cst2017.1329 Chemical Science Transactions ISSN:2278-3458 2017, 6(1), 173-178 RESEARCH ARTICLE Theoretical Investigation of Structural, Thermodynamical and HOMO, LUMO Analysis of 6-Aminopenicillanic
DOI:10.7598/cst2017.1329 Chemical Science Transactions ISSN:2278-3458 2017, 6(1), 173-178 RESEARCH ARTICLE Theoretical Investigation of Structural, Thermodynamical and HOMO, LUMO Analysis of 6-Aminopenicillanic
