Boron Oxides, Hydroxides, and Oxyanions *
|
|
|
- Angelina Simmons
- 6 years ago
- Views:
Transcription
1 OpenStax-CNX module: m Boron Oxides, Hydroxides, and Oxyanions * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License Oxides Boron oxide, B 2 O 3, is made by the dehydration of boric acid, (1). It is a glassy solid with no regular structure, but can be crystallized with extreme diculty. The structure consists of innite chains of triangular BO 3 unit (Figure 1). Boron oxide is acidic and reacts with water to reform boric acid, (1). (1) Figure 1: Glassy structure of B 2O 3. * Version 1.3: May 16, :55 pm
2 OpenStax-CNX module: m The reaction of B 2 O 3 with hydroxide yields the metaborate ion, (2), whose planar structure (Figure 2) is related to metaboric acid. Boron oxide fuses with a wide range of metal and non-metal oxides to give borate glasses. (2) Figure 2: Structure of the metaborate anion, [B 3O 6] Boric acid Boric acid, B(OH) 3, usually obtained from the dissolution of borax, Na 2 [B 4 O 5 (OH) 4 ], is a planar solid with intermolecular hydrogen bonding forming a near hexagonal layered structure, broadly similar to graphite (Figure 2). The inter layer distance is 3.18 Å. Figure 2: The hydrogen bonded structure of boric acid.
3 OpenStax-CNX module: m A summary of reactions of the reactivity of boric acid is shown in Figure 2. Figure 2: Selected reactions of boric acid. Adapted from F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 4 th Ed. Wiley Interscience (1980). Upon dissolution of boric acid in water, boric acid does not act as a proton acid, but instead reacts as a Lewis acid, (3). The reaction may be followed by 11 B NMR spectroscopy from the change in the chemical shift (Figure 3). (3)
4 OpenStax-CNX module: m Figure 3: The 11 B NMR chemical shift (ppm) of boric acid as a function of ph. Adapted from M. Bishop, N. Shahid, J. Yang, and A. R. Barron, Dalton Trans., 2004, Since the 11 B NMR shift is directly proportional to the mole fraction of the total species present as the borate anion (e.g., [B(OH) 4 ] - ) the 11 B NMR chemical shift at a given temperature, δ (obs), may be used to calculate both the mole fraction of boric acid and the borate anion, i.e., (4) and (5), respectively. Using these equations the relative speciation as a function of ph may be calculated for both boric acid (Figure 5). The ph at which a 50:50 mixture of acid and anion for boric acid is ca (4) (5)
5 OpenStax-CNX module: m Figure 5: The mole fraction of acid (black squares) and anion (white squares) forms of boric acid as a function of ph. Adapted from M. Bishop, N. Shahid, J. Yang, and A. R. Barron, Dalton Trans., 2004, In concentrated solutions, the borate ion reacts further to form polyborate ions. The identity of the polyborate is dependent on the ph. With increasing ph, B 5 O 6 (OH) 4-, (6), B 3 O 3 (OH) 4-, (7), and B 4 O 5 (OH) 4 2-, (8), are formed. The structure of each borate is shown in Figure 8. Once the ratio of B(OH) 3 to B(OH) 4 - is greater than 50%, only the mono-borate is observed. (6) (7) (8)
6 OpenStax-CNX module: m Figure 8: The structures of the borate anions (a) B 5O 6(OH) 4-, (b) B 3O 3(OH) 4-, and (c) B 4O 5(OH) Borax, the usual mineral form of boric acid, is the sodium salt, Na 2 [B 4 O 5 (OH) 4 ], which upon dissolution in water re-equilibrates to B(OH) Enough to make your hair curl In 1906, a German hairdresser, Charles Nessler who was living in London, decided to help his sister who was fed-up with having to put her straight hair in curlers. While looking for a solution, Nessler noticed that a clothesline contracted in a wavy shape when it was wet. Nessler wound his sister's hair on cardboard tubes; then he covered the hair with borax paste. After wrapping the tubes with paper (to exclude air) he heated the entire mass for several hours. Removing the paper and tubes resulted in curly hair. After much trial and error (presumably at his sisters discomfort) Nessler perfected the method by 1911, and called the process a permanent wave (or perm). The process involved the alkaline borax softening the hair suciently to be remodeled, while the heating stiened the borax to hold the hair into shape. The low cost of borax meant that Nessler's methods was an immediate success.
7 OpenStax-CNX module: m Metaboric acid Heating boric acid results in the partial dehydration to yield metaboric acid, HBO 2, (9). Metaboric acid is also formed from the partial hydrolysis of B 2 O 3. If the heating is carried out below 130 C, HBO 2 -III is formed in which B 3 O 3 rings are joined by hydrogen bonding to the hydroxide on each boron atom (Figure 9). Continued heating to 150 C results in HBO 2 -II, whose structure consists of BO 4 tetrahedra and B 2 O 5 groups chain linked by hydrogen bonding. Finally, heating above 150 C yields cubic HBO 2 -I with all the boron atoms tetrahedral. (9) Figure 9: Structure of the layered structure of HBO 2-III. 3 Borate esters Boric acid reacts with alcohols in the presence of sulfuric acid to form B(OR) 3 (Figure 2). This is the basis for a simple ame test for boron. Treatment of a compounds with methanol/sulfuric acid, followed by placing the reaction product in a ame results in a green ame due to B(OMe) 3. In the presence of diols, polyols, or polysaccharides, boric acid reacts to form alkoxide complexes. In the case of diols, the mono-diol [B(OH) 2 L] - (Figure 9a) and the bis-diol [BL 2 ] - (Figure 9b) complexes.
8 OpenStax-CNX module: m Figure 9: Structure of (a) the [B(OH) 2L] - and (b) the [BL 2] - anions formed by the reaction of boric acid (or borate) with a diol. Originally it was proposed that diols react with the anion rather than boric acid, (10). In contrast, it was suggested that the optimum ph for the formation of carboxylic acid complexes is under the condition where pk a (carboxylic acid) < ph < pk a (boric acid). Under these conditions it is the boric acid, B(OH) 3, not the borate anion, [B(OH) 4 ] -, that reacts to form the complex. The conversion of boric acid to borate ((3)) must occur through attack of hydroxide or the deprotonation of a coordinated water ligand, either of which is related to the pka of water. The formation of [B(OH) 2 L] - from B(OH) 3 would be expected therefore to occur via a similar initial reaction (attack by RO - or deprotonation of coordinated ROH) followed by a subsequent elimination of H 2 O and the formation of a chelate coordination, and would therefore be related to the pka of the alcohol. Thus the ph at which [B(OH) 2 L] - is formed relative to [B(OH) 4 ] - will depend on the relative acidity of the alcohol. The pk a of a simple alcohol (e.g., MeOH = 15.5, EtOH = 15.9) are close to the value for water (15.7) and the lowest ph at which [B(OH) 2 L] - is formed should be comparable to that at which [B(OH) 4 ] - forms. Thus, the formation of [B(OH) 2 L] - as compared to [B(OH) 4 ] - is a competition between the reaction of B(OH) 3 with RO - and OH - (Figure 11), and as with carboxylic acids it is boric acid not borate that reacts with the alcohol, (11). (10) (11)
9 OpenStax-CNX module: m Figure 11: Competition between hydroxide and 1,2-diol for complexation to boron in the aqueous boric acid/diol system. A key issue in the structural characterization and understanding of the diol/boric acid system is the assignment of the 11 B NMR spectral shifts associated with various complexes. A graphical representation of the observed shift ranges for boric acid chelate systems is shown in Figure 11. Figure 11: 11 B NMR spectroscopic shifts of chelate alkoxide compounds in comparison with boric acid and borate anion. The acronyms [B5] and [B6] are used to denote a borate complex of a 1,2-diol and 1,3- diol forming a chelate 5-membered and 6-membered ring cycle, respectively. Similarly, [B5 2] and [B6 2] denotes a borate complex of two 1,2-diols or 1,3-diols both forming [BL 2] -. Adapted from M. Bishop, N. Shahid, J. Yang, and A. R. Barron, Dalton Trans., 2004, Boric acid cross-linking of guar gum for hydraulic fracturing uids Thick gels of guar gum cross-linked with borax or a transition metal complex are used in the oil well drilling industry as hydraulic fracturing uids. The polysaccharide guaran (M w 10 6 Da) is the major (>85 wt%) component of guar gum, and consists of a (1 4)-β-D-mannopyranosyl backbone with α-d-galactopyranosyl side chain units attached via (1 6) linkages. Although the exact ratio varies between dierent crops of guar gum the general structure is consistent with about one galactose to every other mannose (Figure 11).
10 OpenStax-CNX module: m Figure 11: The repeat unit of guaran, a naturally occurring polysaccharide, consisting of (1 4)-β-Dmannopyranosyl units with α-d-galactopyranosyl side chains attached to about every other mannose via (1 6) linkages. The synthesis of a typical boric acid cross-linked guar gel, with viscosity properties suitable for use as a fracturing uid, involves mixing guar gum, water and boric acid, B(OH) 3, in a 10:2000:1 wt/wt ratio. Adjusting the ph to between 8.5 and 9 results in a viscous gel. The boron:guaran ratio corresponds to almost 2 boron centers per 3 monosaccharide repeat units. Such a large excess of boric acid clearly indicates that the system is not optimized for cross-linking, i.e., a signicant fraction of the boric acid is ineective as a cross-linking agent. The reasons for the ineciency of boric acid to cross-link guaran (almost 2 borate ions per 3 monosaccharide repeat unit are required for a viscous gel suitable as a fracturing uid): the most reactive sites on the component saccharides (mannose and galactose) are precluded from reaction by the nature of the guar structure; the comparable acidity (pk a ) of the remaining guaran alcohol substituents and the water solvent, results in a competition between cross-linking and borate formation; a signicant fraction of the boric acid is ineective in cross-linking guar due to the modest equilibrium (K eq ). 4 Bibliography F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 4 th Ed. Wiley Interscience (1980). M. Bishop, N. Shahid, J. Yang, and A. R. Barron, Dalton Trans., 2004, M. Van Duin, J. A. Peters, A. P. G. Kieboom, and H. Van Bekkum, Tetrahedron, 1984, 40, Zonal Isolation, Borate Crosslinked Fluids, by R. J. Powell and J. M Terracina, Halliburton, Duncan, OK (1998). R. Schechter, Oil Well Stimulation, Prentice-Hall Inc., Englewood Clis, NJ (1992). S. Kesavan and R. K. Prud'homme, Macromolecules, 1992, 25, 2026.
Organolithium Compounds *
 OpenStax-CNX module: m32444 1 Organolithium Compounds * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 One of the major uses of lithium
OpenStax-CNX module: m32444 1 Organolithium Compounds * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 One of the major uses of lithium
Ammonia * Andrew R. Barron. 1 Synthesis. 2 Structure
 OpenStax-CNX module: m32956 1 Ammonia * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Ammonia (NH 3 ) is a colorless, pungent gas
OpenStax-CNX module: m32956 1 Ammonia * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Ammonia (NH 3 ) is a colorless, pungent gas
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Ion Chromatography *
 OpenStax-CNX module: m50231 1 Ion Chromatography * Brett Virgin-Downey Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Introduction
OpenStax-CNX module: m50231 1 Ion Chromatography * Brett Virgin-Downey Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Introduction
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Organometallic Compounds of Magnesium *
 OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
BIOLOGY 101. CHAPTER 4: Carbon and the Molecular Diversity of Life: Carbon: the Backbone of Life
 BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
Lecture 21 Cations, Anions and Hydrolysis in Water:
 2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Name Date Class STATES OF MATTER
 13 STATES OF MATTER Each clue describes a vocabulary term. Read the clues and write the letters of each term on the lines. 1. Clue: the energy an object has because of its motion. 2. Clue: results from
13 STATES OF MATTER Each clue describes a vocabulary term. Read the clues and write the letters of each term on the lines. 1. Clue: the energy an object has because of its motion. 2. Clue: results from
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Section A. 1 at a given temperature. The rate was found to be first order with respect to the ester and first order with respect to hydroxide ions.
 2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Chapter 16: Ethers, Epoxides, and Sulfides
 Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Alcohols. Alcohol any organic compound containing a hydroxyl (R-OH) group. Alcohols are an extremely important organic source
 Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
UV-VISIBLE SPECTRAL ANALYSIS OF BORIC ACID IN DIFFERENT SOLVENTS: A CASE STUDY
 IJPSR (2015), Vol. 6, Issue 2 (Research Article) Received on 18 June, 2014; received in revised form, 28 August, 2014; accepted, 15 November, 2014; published 01 February, 2015 UV-VISIBLE SPECTRAL ANALYSIS
IJPSR (2015), Vol. 6, Issue 2 (Research Article) Received on 18 June, 2014; received in revised form, 28 August, 2014; accepted, 15 November, 2014; published 01 February, 2015 UV-VISIBLE SPECTRAL ANALYSIS
RHEOLOGICAL CHARACTERIZATION AND MODELING OF AQUEOUS GUAR GUM SOLUTIONS
 3 rd International Symposium on Food Rheology and Structure RHEOLOGICAL CHARACTERIZATION AND MODELING OF AQUEOUS GUAR GUM SOLUTIONS Marco Dressler, Peter Fischer, Erich J. Windhab Swiss Federal Institute
3 rd International Symposium on Food Rheology and Structure RHEOLOGICAL CHARACTERIZATION AND MODELING OF AQUEOUS GUAR GUM SOLUTIONS Marco Dressler, Peter Fischer, Erich J. Windhab Swiss Federal Institute
Biology 30 The Chemistry of Living Things
 Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Ethers. Synthesis of Ethers. Chemical Properties of Ethers
 Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Permanent Waves. ChemMatters April 1993 Page 8
 CLICK HERE FOR MAGAZINE PAGES ChemMatters April 1993 Page 8 Copyright 1993, American Chemical Society Permanent Waves By Roberta Baxter Since ancient times, people have experimented with many ways to change
CLICK HERE FOR MAGAZINE PAGES ChemMatters April 1993 Page 8 Copyright 1993, American Chemical Society Permanent Waves By Roberta Baxter Since ancient times, people have experimented with many ways to change
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY
 WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY enthalpy change of reaction, enthalpy change of combustion and standard molar enthalpy change of formation, Δ fh ϴ Hess s law and energy cycles concept
WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY enthalpy change of reaction, enthalpy change of combustion and standard molar enthalpy change of formation, Δ fh ϴ Hess s law and energy cycles concept
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
(1) Recall the different types of intermolecular interactions. (2) Look at the structure and determine the correct answer.
 MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
CHEM4. General Certificate of Education Advanced Level Examination January Unit 4 Kinetics, Equilibria and Organic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2011 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2011 Question 1 2 Mark
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Q.1 Draw structures for all amines of molecular formula C 4 H 11 N. Classify them as primary, secondary or tertiary amines.
 1 AMIES Structure ontain the 2 group. lassification primary (1 ) amines secondary (2 ) amines tertiary (3 ) amines quarternary (4 ) ammonium salts + 1 2 3 4 Aliphatic Aromatic methylamine, ethylamine,
1 AMIES Structure ontain the 2 group. lassification primary (1 ) amines secondary (2 ) amines tertiary (3 ) amines quarternary (4 ) ammonium salts + 1 2 3 4 Aliphatic Aromatic methylamine, ethylamine,
CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
 CHEM1902/4 2014-N-8 November 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 Name compounds E and G. E: G: Propose structures for compounds F, H and J. F H J Propose
CHEM1902/4 2014-N-8 November 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 Name compounds E and G. E: G: Propose structures for compounds F, H and J. F H J Propose
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Carboxylic Acids & Their Derivatives. Organic Chemistry, 2nd Edition David R. Klein
 Carboxylic Acids & Their Derivatives Organic Chemistry, 2nd Edition David R. Klein Introduction to Carboxylic Acids Carboxylic acids are compounds with a -COOH group. These compounds are abundant in nature,
Carboxylic Acids & Their Derivatives Organic Chemistry, 2nd Edition David R. Klein Introduction to Carboxylic Acids Carboxylic acids are compounds with a -COOH group. These compounds are abundant in nature,
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Chapter 13: Alcohols and Phenols
 Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
CHEM4. (JAN13CHEM401) WMP/Jan13/CHEM4. General Certificate of Education Advanced Level Examination January 2013
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2013 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2013 Question 1 2 Mark
Chemistry 1000 Lecture 22: Group 14 and Boron. Marc R. Roussel
 Chemistry 1000 Lecture 22: Group 14 and Boron Marc R. Roussel Group 14 In this group again, we see a full range of nonmetallic to metallic behavior: C is a nonmetal. Si and Ge are metalloids. Sn and Pb
Chemistry 1000 Lecture 22: Group 14 and Boron Marc R. Roussel Group 14 In this group again, we see a full range of nonmetallic to metallic behavior: C is a nonmetal. Si and Ge are metalloids. Sn and Pb
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
THE STABILITY CONSTANTS AND THERMODYNAMIC PARAMETERS OF BORATE - CARBOHYDRATE COMPLEXES BY ph MEASUREMENTS
 Journal of Bangladesh Chemical Society, Vol. 25(1), 15-20, 2012 THE STABILITY CONSTANTS AND THERMODYNAMIC PARAMETERS OF BORATE - CARBYDRATE COMPLEXES BY ph MEASUREMENTS FARIDA AKHTAR*, MD. ANISUR RAHMAN,
Journal of Bangladesh Chemical Society, Vol. 25(1), 15-20, 2012 THE STABILITY CONSTANTS AND THERMODYNAMIC PARAMETERS OF BORATE - CARBYDRATE COMPLEXES BY ph MEASUREMENTS FARIDA AKHTAR*, MD. ANISUR RAHMAN,
Thermodynamics of Borax Dissolution
 Thermodynamics of Borax Dissolution Introduction In this experiment, you will determine the values of H, G and S for the reaction which occurs when borax (sodium tetraborate octahydrate) dissolves in water.
Thermodynamics of Borax Dissolution Introduction In this experiment, you will determine the values of H, G and S for the reaction which occurs when borax (sodium tetraborate octahydrate) dissolves in water.
Carvone Reduction * Mary McHale. 1 Reduction of Carvone
 OpenStax-CNX module: m15745 1 Carvone Reduction * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Reduction of Carvone 1.1 Objective
OpenStax-CNX module: m15745 1 Carvone Reduction * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Reduction of Carvone 1.1 Objective
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
(2) After dissolving a solid in a solvent at high temperature, the solution is not filtered.
 Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Chapter 2 The Chemistry of Life
 Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Chapter Two: The Chemistry of Biology. The molecules of life make up the structure of cells Chemistry of biological molecule
 Chapter Two: The Chemistry of Biology The molecules of life make up the structure of cells Chemistry of biological molecule Atoms and Elements: Atoms: The basic units of all matter, containing three major
Chapter Two: The Chemistry of Biology The molecules of life make up the structure of cells Chemistry of biological molecule Atoms and Elements: Atoms: The basic units of all matter, containing three major
Basic Chemistry. Chapter 2 BIOL1000 Dr. Mohamad H. Termos
 Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø
 `1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
Carbonyl groups react via nucleophilic addition, with the mechanism being represented as follows:
 Aldehydes and Ketones Introduction Aldehydes and ketones are two similar homologous groups both having the carbonyl group: The Carbon on the carbonyl group is slightly positive wince the Oxygen is pulling
Aldehydes and Ketones Introduction Aldehydes and ketones are two similar homologous groups both having the carbonyl group: The Carbon on the carbonyl group is slightly positive wince the Oxygen is pulling
CHAPTER-9 NCERT SOLUTIONS
 CHAPTER-9 NCERT SOLUTIONS Question 9.1: Justify the position of hydrogen in the periodic table on the basis of its electronic configuration. Hydrogen is the first element of the periodic table. Its electronic
CHAPTER-9 NCERT SOLUTIONS Question 9.1: Justify the position of hydrogen in the periodic table on the basis of its electronic configuration. Hydrogen is the first element of the periodic table. Its electronic
9/8/17. K h D Base d c m m = 5 km 2 km = 2000 m
 9/6/17 Scientific Method Process to test hypothesis to answer a question Parts of the Scientific Method: Observation Question Research Hypothesis Experiment/ Procedure Analysis Results Control Group no
9/6/17 Scientific Method Process to test hypothesis to answer a question Parts of the Scientific Method: Observation Question Research Hypothesis Experiment/ Procedure Analysis Results Control Group no
Chem 263 Nov 3, 2016
 hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
Chapter 8: Ethers and Epoxides. Diethyl ether in starting fluid
 Chapter 8: Ethers and Epoxides Diethyl ether in starting fluid 8.1 Nomenclature of Ethers Ethers are usually named by giving the name of each alkyl or aryl group, in alphabetical order, followed by the
Chapter 8: Ethers and Epoxides Diethyl ether in starting fluid 8.1 Nomenclature of Ethers Ethers are usually named by giving the name of each alkyl or aryl group, in alphabetical order, followed by the
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
EXPERIMENTS 5-8: BACKGROUND OXIDATION REDUCTION REACTIONS IN ORGANIC CHEMISTRY: THE INTERCONVERSION OF
 EXPERIMENTS 5-8: BACKGRUND XIDATIN REDUCTIN REACTINS IN RGANIC CEMISTRY: TE INTERCNVERSIN F 4-tert-BUTYLCYCLEXANL AND 4-tert-BUTYLCYCLEXANNE 1 UTLINE AND GALS F TE PRJECT: This is a 4 week project that
EXPERIMENTS 5-8: BACKGRUND XIDATIN REDUCTIN REACTINS IN RGANIC CEMISTRY: TE INTERCNVERSIN F 4-tert-BUTYLCYCLEXANL AND 4-tert-BUTYLCYCLEXANNE 1 UTLINE AND GALS F TE PRJECT: This is a 4 week project that
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
18.1 Arenes benzene compounds Answers to Exam practice questions
 Pages 230 232 1 a) Benzene has a planar molecule ; with six carbon atoms in a regular hexagon. Each carbon atom forms a normal covalent ( ) bond with its two adjacent carbons atoms and a hydrogen atom.
Pages 230 232 1 a) Benzene has a planar molecule ; with six carbon atoms in a regular hexagon. Each carbon atom forms a normal covalent ( ) bond with its two adjacent carbons atoms and a hydrogen atom.
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
Topic 1: Quantitative chemistry
 covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
Important Note: We will NOT accept papers written in pencil back for re-marking after they have been returned to you. Please do not ask!
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Chemistry 1000 Lecture 24: Group 14 and Boron
 Chemistry 1000 Lecture 24: Group 14 and Boron Marc R. Roussel November 2, 2018 Marc R. Roussel Group 14 and Boron November 2, 2018 1 / 17 Group 14 In this group again, we see a full range of nonmetallic
Chemistry 1000 Lecture 24: Group 14 and Boron Marc R. Roussel November 2, 2018 Marc R. Roussel Group 14 and Boron November 2, 2018 1 / 17 Group 14 In this group again, we see a full range of nonmetallic
2 Answer all the questions. OH(g) ΔH = 91 kj mol 1 equation 1.1. (a) A pressure of between 50 and 100 atmospheres is used for this reaction.
 2 Answer all the questions. 1 Methanol is added to ethanol to make the ethanol unfit to drink. Methanol can be made by the following reaction. CO(g) + 2H 2 (g) CH 3 OH(g) ΔH = 91 kj mol 1 equation 1.1
2 Answer all the questions. 1 Methanol is added to ethanol to make the ethanol unfit to drink. Methanol can be made by the following reaction. CO(g) + 2H 2 (g) CH 3 OH(g) ΔH = 91 kj mol 1 equation 1.1
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water
 Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
(07) 2 (c) 2 (c) (i) Calculate the ph of this buffer solution at 25 oc (3 marks) (Extra space)
 7 The value of Ka for methanoic acid is 1.78 10 4 mol dm 3 at 25 oc. A buffer solution is prepared containing 2.35 10 2 mol of methanoic acid and Question 1: N/A 1.84 10 2 mol of sodium methanoate in 1.00
7 The value of Ka for methanoic acid is 1.78 10 4 mol dm 3 at 25 oc. A buffer solution is prepared containing 2.35 10 2 mol of methanoic acid and Question 1: N/A 1.84 10 2 mol of sodium methanoate in 1.00
Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes *
 OpenStax-CNX module: m34660 1 Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes * Jiebo Li Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m34660 1 Fourier Transform Infrared Spectroscopy of Metal Ligand Complexes * Jiebo Li Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Bonding and Dynamics. Outline Bonding and Dynamics Water Interactions Self Ionization of Water Homework
 Liquid Water Structure In liquid water, most of the water molecules have the same local environment as in ice but the long range structure of ice disappears due to motion of the molecules. Bonds between
Liquid Water Structure In liquid water, most of the water molecules have the same local environment as in ice but the long range structure of ice disappears due to motion of the molecules. Bonds between
Chapter 19 Carboxylic Acids
 Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Chem 3719 Klein Chapter Practice Problems
 Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS
 CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
Chemistry Honors Curriculum Pacing Guide
 Chemistry Honors Curriculum Guide 2013-2014 Areas Unit 1 - Scientific Inquiry Unit 2 - Measurement C-1.2 C-1.4 C-1.5 C-1.6 C-1.8 Daily - 4 days A/B - 2 days Use appropriate laboratory apparatuses, technology,
Chemistry Honors Curriculum Guide 2013-2014 Areas Unit 1 - Scientific Inquiry Unit 2 - Measurement C-1.2 C-1.4 C-1.5 C-1.6 C-1.8 Daily - 4 days A/B - 2 days Use appropriate laboratory apparatuses, technology,
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water
 Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Page 2. Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? alcohols C nh 2n+2O. aldehydes C nh 2n+1O
 Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
21.1 Introduction Carboxylic Acids
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. Klein, Organic Chemistry 1e 21-1 The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. Klein, Organic Chemistry 1e 21-1 The US produces over 2.5 million tons of acetic acid per year, which
Name Biology Chapter 2 Note-taking worksheet
 Name Biology Chapter 2 Note-taking worksheet The Nature of Matter 1. Life depends on Atoms 1. The study of chemistry starts with the basic unit of matter, the. 2. The atom was first used by the Greek philosopher
Name Biology Chapter 2 Note-taking worksheet The Nature of Matter 1. Life depends on Atoms 1. The study of chemistry starts with the basic unit of matter, the. 2. The atom was first used by the Greek philosopher
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
CHAPTER 23 HW: ENOLS + ENOLATES
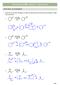 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Types of reactions: addition, elimination and substitution reactions
 OpenStax-NX module: m39090 1 Types of reactions: addition, elimination and substitution reactions Free igh School Science Texts Project This work is produced by OpenStax-NX and licensed under the reative
OpenStax-NX module: m39090 1 Types of reactions: addition, elimination and substitution reactions Free igh School Science Texts Project This work is produced by OpenStax-NX and licensed under the reative
Loudon Chapter 24 Review: Carbohydrates CHEM 3331, Jacquie Richardson, Fall Page 1
 CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
2014 Assessment Report. Chemistry Level 3
 National Certificate of Educational Achievement 2014 Assessment Report Chemistry Level 3 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 91391
National Certificate of Educational Achievement 2014 Assessment Report Chemistry Level 3 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 91391
