ENGINEERING SUMMARY SHEET AIR RESOURCES DIVISION
|
|
|
- Antony Greene
- 6 years ago
- Views:
Transcription
1 AFS #: Application #: FY DATE: 9/2/05 Page 1 of 4 DATE APPLICATION RECEIVED: November 25, 2003 PROJECT DESCRIPTION (A-Plus) submitted an application for the renewal of their State Permit to Operate, FP-S-0067 which expired on December 31, The existing permit covers RTAP and HAP emissions that are controlled by a wet scrubber. PROCESS DESCRIPTION A-Plus operates a job-shop for plating, etching and polishing various metal components. The chemical products used vary based on the workload of the facility. Chemical products used in the process consist of acidic and basic surface treatments, plating baths, ammonia and solvents. Attachment A lists the baths that were evaluated for this application. EMISSION CALCULATIONS A-Plus submitted data on chemical usage and estimated emissions for calendar year 2003 (actual purchases through 10/31/03 prorated to 12 months). The facility was also inspected in November 2003 by Alan Moulton who requested chemical purchasing records for the previous five years. All of this data was evaluated to determine the source s compliance status and applicability requirements. As an initial, conservative review, it was assumed that all of the chemicals purchased in a year are emitted. The details of this are in the spreadsheet h:\permitting\source Files\\ \A-Plus_ _Eng Sum(excel)_ Only the data for 2003 is printed and attached to this summary. The total VOC and HAP usage rates were added and are summarized below: Chemical Usage Rates (tpy) VOCs HAPs Estimating the potential emissions from the facility is difficult due to the nature of the operations (job-shop). The facility operates for 8 hr/day and 250 day/yr (2000 hr/yr). If one takes the highest annual usage rate and scales the number to 8760 hr/yr, the potential usage rate for VOCs would be 7.98 tpy and for HAPs it would be 9.86 tpy. These numbers are well below the major source thresholds for these compounds, and therefore it is concluded that this facility is a true minor source. The 2003 chemical usage rates were also compared to the de minimus emission rates listed in Env-A In most cases the daily usage rate was determined by taking the annual rate and dividing by 250 day/yr. For daily rates followed by an *, the rates listed in the table are based on expected maximum emission rates. There are eleven chemicals that had usage rates or emissions greater than the de minimus emission rates. The following discusses the assumptions and calculations made for these 11 chemicals to determine their emission rates. Two of the chemicals are solid metal compounds, barium and nickel sulfate. The usage rates are for the solid compound which is dissolved in a chemical bath and then plated onto the products. A-Plus utilizes electroless plating and the baths are not agitated in any way. It is assumed that the metals will not be emitted from the process; therefore these compounds are in compliance with Env-A Sodium hydroxide is used in relatively large quantities. This chemical is used in the wet scrubber as a means of neutralizing acid emissions. It is also used as part of a waste treatment system to neutralize liquid acid wastes. It is not expected that sodium hydroxide, as it is used by A-Plus, will be emitted into the air, therefore this compound is in compliance with Env-A Potassium Hydroxide is an alkali chemical used in one of the baths (Metex 662SE). Due to the low vapor pressure of this compound it is not expected to be emitted into the air, therefore this compound is in compliance with Env-A Hydrochloric and acetic acids are both volatile acids. For conservatism, it is assumed that 100% of these chemicals are emitted. All of the acetic acid is exhausted to a wet scrubber. The exhaust flow rate is 7500 acfm (7500/2119 = 3.54 m 3 /sec). The uncontrolled, adjusted in-stack concentrations of this acid is: 0.27 lb/hr * 10 6 /7.94 = μg/sec
2 AFS #: Application #: FY DATE: 9/2/05 Page 2 of / 3.54 / 400 = μg/ m 3 This is less than 50% of the 24-hr and annual AALs of 126 μg/ m 3 and 84 μg/ m 3, respectively. Therefore, this chemical is in compliance without the use of controls. Hydrochloric acid is used in five baths at the facility. Three of the baths are exhausted to the wet scrubber (removal efficiency 90%) and the remaining two have no exhaust. Because some of the emissions are fugitive, an in-stack calculation could not done for this pollutant. See the discussion below on modeling. The emissions of nitric acid were calculated by using a mass balance. Nitric acid is shipped off-site as a hazardous waste. Emissions are assumed to be equal to the usage rate minus the amount sent out as waste, 280 lbs/yr 106 lbs/yr = 174 lbs/yr. The estimated maximum daily emission rate is 8.4 lb/day. These numbers are greater than the de minimus emission rates. Nitric acid is used in a number of the baths at A-Plus. Some of these baths are exhausted to the wet scrubber (removal efficiency 70%) and some are not vented (fugitive emissions). Because of the fugitive emissions, an in-stack calculation for compliance determination is not possible. See the discussion below on modeling. The emission rates for sulfuric and phosphoric acids were determined by the source with an experiment to determine evaporation rates. It was determined that sulfuric acid evaporates at a rate of 0.19 g/ft 2 /hr (0.04 lbs/hr) and phosphoric acid evaporates at a rate of 0.23 g/ft 2 /hr (0.05 lbs/hr). (Note: these emission rates were compared to emission rates calculated from mass transfer equation for evaporation rates based on chemical properties see Attachment B for calculations. The emission rates used by A-Plus were higher and thus more conservative). If the baths were used for the maximum operating time of 8 hr/day, the emissions would exceed the de minimus emission rate. Like hydrochloric and nitric acids, some of the emissions of these acids are fugitive making an in-stack calculation invalid. See the discussion below on modeling. Methyl ethyl ketone (MEK) is a Volatile Organic Compound (VOC). It is assumed that all of the MEK used in the process is emitted into the air. MEK is emitted as a fugitive emission source; therefore an in-stack calculation is not possible See the discussion below on modeling. MODELING/DETAILED EMISSION ESTIMATES Various iterations of modeling was conducted for this source. Modeling was conducted during the previous permit renewal, in This modeling had all the acid emissions controlled and exhausted out of the scrubber stack. Since this is not how the facility actually operates, an updated modeling analysis was required. As an initial check, modeling was requested to be conducted on uncontrolled emissions. If the source was able to meet the AALs without controls, then no permit would be required. The results of this modeling showed that the impacts using uncontrolled emission rates were well above the AALs (the results of this preliminary modeling are not included in the modeling memo). During site visits to the facility, the amount of acid emitted through the exhaust vents was questioned. Due to the configuration of the ventilation systems for the process baths, a capture efficiency of less than 100% would be expected. A qualitative test was conducted using a smoke tube to estimate the capture efficiency of the baths. This test showed that the ventilation had a very good capture efficiency, but because of the qualitative nature of the test, an exact number was not determined. To be conservative, it is assumed that the capture efficiency of the exhaust system is 80%. During the summer months (when the weather is hot), the facility opens its overhead door to increase cross-ventilation through the process area. The smoke test for capture efficiency was conducted with the door open and with the door closed. No significant difference was noticed when the door was opened. As a conservative assumption, modeling was conducted for two scenarios: 1 the overhead door is open and all fugitive emissions are exhausted out of the door; and 2 the door is closed and there are no fugitive emissions out of the building (only emissions captured by the exhaust system were evaluated). Subsequent modeling was also done assuming all the fugitive emissions are eventually captured by the exhaust system and sent out the vent stack. In order to determine the emission rates of the various acids through the different exhausts, a more detailed picture of the facility was needed. The attached table, Detailed Acid Emission Calculations, lists the bath # s (corresponding with the updated facility plan submitted on April 5, 2005), the acids used in each bath and the proportional usage (see sample calculation at the bottom of the table). The proportional usage number was used to divide up the total emissions between the exhaust systems and fugitive emissions, as applicable. For baths that have ventilation an 80% capture efficiency was used, and for emissions through the scrubber, the
3 AFS #: Application #: FY DATE: 9/2/05 Page 3 of 4 appropriate removal efficiency was used. The following tables show the emission rates used as inputs to the dispersion modeling. Emission Rates for Existing Conditions Scrubber Stack Vent Stack Fugitive Annual (lb/hr) Daily (lb/hr) Annual (lb/hr) Daily (lb/hr) Annual (lb/hr) Daily (lb/hr) Nitric Acid Hydrochloric Acid Phosphoric Acid Sulfuric Acid Ammonia Methyl Ethyl Ketone A third, proposed scenario was also evaluated based on fugitive emissions exhausting out of the door and all captured acid emissions controlled and exhausted through the scrubber. Also, for this third scenario the emission rate for hydrochloric acid was decreased using an assumption that only 10% of the total usage is emitted (this is more realistic based on the volatility of acids. Using the mass transfer equation would give an approximate emission rate equal to 3% of the total usage). Emission Rates for Proposed Conditions All bath exhausts directed to scrubber only 10% HCl emitted Scrubber Stack Vent Stack Fugitive Annual (lb/hr) Daily (lb/hr) Annual (lb/hr) Daily (lb/hr) Annual (lb/hr) Daily (lb/hr) Nitric Acid Hydrochloric Acid Sulfuric Acid Results of the modeling analysis are detailed in a memo dated April 13, Basically, the results show that the source can meet the AALs as long as no fugitive emissions are allowed to exhaust through the overhead door. Additional modeling was completed in May In this analysis it was assumed that all the fugitive emissions are exhausted out the vent stack. This scenario showed predicted exceedances of the 24-hr AAL for sulfuric acid. If all the exhausted emissions are controlled and vented through the scrubber with the fugitives exhausting out of the vent stack, then the AALs are met. The source is planning on changing the exhaust configuration of its baths so that all baths with sulfuric acid are exhausted to the scrubber. REVIEW OF REGULATIONS State Regulations Env-A 600 Permitting (effective 7/28/04) (n) NO The facility is a true minor source (v) YES A permit is required for RTAPs because controls are required for compliance with AALs. Env-A 1200 Prevention, Abatement and Control of Stationary Source Air Pollution (effective 10/31/02) NO Potential VOC emissions are less than 50 tpy. Env-A 1400 Regulated Toxic Air Pollutants (effective 6/11/04) 1402 YES Facility able to show compliance with actual, controlled emissions using either the de minimus emission rate or modeling (see discussion above). At the time of permit issuance, the RTAPs in Attachment C were reviewed and determined to be in compliance. Env-A 2100 Particulate Matter and Visible Emissions Standards (effective 11/24/04) YES TSP emissions shall be limited according to the formula stated in ((b) YES visible emissions shall not exceed 20% Federal Regulations Not applicable
4 AFS #: Application #: FY DATE: 9/2/05 Page 4 of 4 CHANGES FROM PREVIOUS PERMIT The previous permit detailed calculations that the source could perform to show compliance with Env-A These calculations are not applicable to the current conditions at the facility and will not be included in the updated permit. The previous permit had specific emission limitations for various acids. These limits are no longer applicable to the source. The permit will require the source to maintain compliance with Env-A It has been shown that the emission rates listed in Attachment A are in compliance (note that these emission rates are different than the limits in the previous permit). The previous permit state the following operating conditions for the wet fume scrubber: gas throughput of 7500 acfm at 75 F; minimum pressure drop of 1 inch of water; minimum recycle rate of 4 gal/min of a 1% sodium hydroxide solution; and input solution ph range of 8.5 to 9.0. According to the source, there is currently no continuous monitoring done on the scrubber. The ph is measured with a meter and ph adjustment is performed by the manual addition of sodium hydroxide solution. The new permit will contain conditions to monitor and record ph readings and maintain the ph between 8.5 and 9.5. SUMMARY AND CONCLUSIONS In summary, the operations as applied for (based on 2003 chemical usage) will be capable of meeting all regulations and standards for air quality with the use of pollution control. A State Permit to Operate has been drafted.
5 ATTACHMENT A List of Process Baths Bath # Process/Chemistry Exhaust 1 Multikleen 1602 sodium hydroxide Vent 2 Rinse Vent 3 Rinse None 4 Rinse None 5 ElectroKleen SP sodium hydroxide Vent 6 Hydrochloric acid 20% Scrubber 7 Hydrochloric acid 20% Scrubber 9 Rinse None 10 Hydrochloric acid 10% Scrubber 12 Electrogleam-55 sulfuric and phosphoric acids 50% each Scrubber 14 Rinse None 16 Nitric acid 5% None 17 Rinse Vent 18 Nickel Strike nickel chloride in solution None 19 Rinse None 20 Rinse None 21 Rinse None 22 Nitric acid 20% and sodium dichromate 5% Scrubber 23 Nitric acid 20% Scrubber 24 Sulfuric acid 5% Scrubber 25 Rinse None 26 Hydrochloric acid 10% None 27 Nitric acid 10% None 29 Black Oxide sodium hydroxide Vent 30 Rinse None 31 Rinse None 32 Ultra-Black 407 sodium hydroxide Vent 35 Isoprep 44 proprietary soap solution Vent 36 Isoprep 35 sodium hydoxide Vent 37 Rinse None 38 Di-Oxide NC-9 nitric, sulfuric and hydrofluoric acids None 39 Rinse None 40 Nitric acid 10% and ammonium fluoride 5% Scrubber 41 Rinse None 42 Rinse None 43 ZA-3-L sodium hydroxide None 44 Rinse None 45 Zincate Strip nitric acid 20% None 46 Electroless Nickel nickel sulfate solution Scrubber 47 Rinse None 48 Electroless Nickel nickel sulfate solution Scrubber 49 Rinse None 50 Rinse None 54 Nickel Strip nitric acid 35% Scrubber 55 NiStrip 900P ammonium hydroxide solution Vent 56 Rinse Vent 57 Iridite 57A chromic acid solution None The following baths were not in use at the time of this application: 8, 11, 13, 15, 33, 34, and 51. Numbers 52 and 53 are not associated with process baths and are therefore not listed above.
6 ATTACHMENT C List of RTAPs and Emission Rates Evaluated RTAP CAS # Emission Rate (lb/day) Emission Rate (lb/yr) Compliance Determination Method Acetic Acid In-Stack Acetone De minimus Ammonia Modeling Dipropylene Glycol Monomethyl Ether De minimus Ethylenediamine De minimus Hydrochloric Acid Modeling Methyl Ethyl Ketone Modeling Nitric Acid Modeling Phosphoric Acid Modeling Propylene Glycol Monomethyl Ether De minimus Sulfuric Acid Modeling Notes: 1. The RTAPs listed in bold print are required to be controlled. The emission rates listed above show the controlled emission rates where controls are required in order to show compliance. 2. Annual emissions were evaluated based on actual operation of the Facility. 3. CAS = Chemical Abstract Service.
PA DEP SOUTHWEST REGIONAL OFFICE
 PA DEP SOUTHWEST REGIONAL OFFICE MEMO TO FROM Air Quality Permit File: OP-04-00043 / Thomas J. Joseph, P.E. Facilities Engineering Manager Air Quality Program THROUGH Mark R. Gorog, P.E. Environmental
PA DEP SOUTHWEST REGIONAL OFFICE MEMO TO FROM Air Quality Permit File: OP-04-00043 / Thomas J. Joseph, P.E. Facilities Engineering Manager Air Quality Program THROUGH Mark R. Gorog, P.E. Environmental
Technical Contact. Randy Moore. (910) Highway 421 North Wilmington, NC 28401
 NORTH CAROLINA DIVISION OF AIR QUALITY Air Permit Review Permit Issue Date: Facility Data Applicant (Facility s Name): Wilbara, LLC Facility Address: Wilbara, LLC SIC: 2819 / Industrial Inorganic Chemicals
NORTH CAROLINA DIVISION OF AIR QUALITY Air Permit Review Permit Issue Date: Facility Data Applicant (Facility s Name): Wilbara, LLC Facility Address: Wilbara, LLC SIC: 2819 / Industrial Inorganic Chemicals
Form S Chemical Reporting Challenges. Rich Bizzozero, Director Office of Technical Assistance May 2018
 Form S Chemical Reporting Challenges Rich Bizzozero, Director Office of Technical Assistance May 2018 Before You Get Started Review and know what chemicals are on the list Know what chemicals are used
Form S Chemical Reporting Challenges Rich Bizzozero, Director Office of Technical Assistance May 2018 Before You Get Started Review and know what chemicals are on the list Know what chemicals are used
Safety Manual > Incompatible Chemicals Partial Listing
 Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
FUNDERMAX GMBH TEST REPORT
 FUNDERMAX GMBH TEST REPORT SCOPE OF WORK SEFA 3 2010, 2.1 Analysis of Max Compact Interior Plus White and Black Plaques REPORT NUMBER 103600635GRR 001a ISSUE DATE 25 September 2018 PAGES 22 DOCUMENT CONTROL
FUNDERMAX GMBH TEST REPORT SCOPE OF WORK SEFA 3 2010, 2.1 Analysis of Max Compact Interior Plus White and Black Plaques REPORT NUMBER 103600635GRR 001a ISSUE DATE 25 September 2018 PAGES 22 DOCUMENT CONTROL
IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS
 IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS When a chemical or solution has not been labeled, was improperly labeled, or its label has deteriorated, become obscured or illegible, it becomes an unknown.
IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS When a chemical or solution has not been labeled, was improperly labeled, or its label has deteriorated, become obscured or illegible, it becomes an unknown.
Test Report For: FunderMax GmbH. MAX Resistance 2. SEFA , 2.1 Chemical/Stain Resistances
 4700 Broadmoor SE, Suite 200 Kentwood, MI 49512 Telephone: 616-656-7401 Facsimile: 616-656-2022 www.intertek-etlsemko.com P.O. No.: MP Page 1 of 9 Test Report For: FunderMax GmbH MAX Resistance 2 SEFA
4700 Broadmoor SE, Suite 200 Kentwood, MI 49512 Telephone: 616-656-7401 Facsimile: 616-656-2022 www.intertek-etlsemko.com P.O. No.: MP Page 1 of 9 Test Report For: FunderMax GmbH MAX Resistance 2 SEFA
Materials of Construction and Chemical Compatibility for Sensing and Control Products
 ADVANCED MATERIALS HANDLING TECHNICAL NOTE Materials of Construction and Compatibility for Sensing and Control Products INTRODUCTION Entegris Sensing and Control products are designed for use in highly
ADVANCED MATERIALS HANDLING TECHNICAL NOTE Materials of Construction and Compatibility for Sensing and Control Products INTRODUCTION Entegris Sensing and Control products are designed for use in highly
INSTRUCTIONS ON EVERY AP EXAM:
 Most Common Reaction Types: 1. Acid-base neutralization (both weak & strong) 2. Nonmetal and metal oxides with water 3. Active metals with water 4. Single replacement redox 5. Double replacement precipitation
Most Common Reaction Types: 1. Acid-base neutralization (both weak & strong) 2. Nonmetal and metal oxides with water 3. Active metals with water 4. Single replacement redox 5. Double replacement precipitation
Naming salts. Metal Acid Salt. Sodium hydroxide reacts with Hydrochloric acid to make Sodium chloride
 Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
Safe Installation and Use of the NanoRam Immersion Probe
 Safe Installation and Use of the NanoRam Immersion Probe Description: NanoRam Immersion Probe - 12 inch length; Immersion Probe Disposable Protective Sleeve Model Number: NR2-IMP; NR2-IMPS Part Number:
Safe Installation and Use of the NanoRam Immersion Probe Description: NanoRam Immersion Probe - 12 inch length; Immersion Probe Disposable Protective Sleeve Model Number: NR2-IMP; NR2-IMPS Part Number:
Name Honors Chemistry / /
 Name Honors Chemistry / / Redox Reactions Rules for Assigning Oxidation Numbers Oxidation state of: Charge Examples Neutral monoatomic or molecular elements 0 Na(s), Cl 2 (g), S 8 (s), O 2 (g), Hg(l) Fluorine
Name Honors Chemistry / / Redox Reactions Rules for Assigning Oxidation Numbers Oxidation state of: Charge Examples Neutral monoatomic or molecular elements 0 Na(s), Cl 2 (g), S 8 (s), O 2 (g), Hg(l) Fluorine
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118
 WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
GL Chromatodisc. Usage Type Description. GL Chromatodisc Type A GL Chromatodisc Type P GL Chromatodisc Type N 13AI
 syringe filters promises reproducible results from the filtration of organic and aqueous solutions in HPLC. Trace amount of samples containing impurities or contaminants can be easily removed, resulting
syringe filters promises reproducible results from the filtration of organic and aqueous solutions in HPLC. Trace amount of samples containing impurities or contaminants can be easily removed, resulting
2017 Public Annual Report Toxic Substance Reduction Act Downsview Woodworking Ltd.
 2017 Public Annual Report Toxic Reduction Act 1.0 Basic Facility Information (Downsview Kitchens) is specialized in the manufacture and delivery of custom kitchen cabinets that meet the most stringent
2017 Public Annual Report Toxic Reduction Act 1.0 Basic Facility Information (Downsview Kitchens) is specialized in the manufacture and delivery of custom kitchen cabinets that meet the most stringent
Chemical Storage According to Compatibility
 Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Chemical Storage Guidelines
 Storage Guidelines Do not store excess chemical containers on the benchtops, designate a storage location and return containers to that location after each use. Do not store chemicals in the fume hood.
Storage Guidelines Do not store excess chemical containers on the benchtops, designate a storage location and return containers to that location after each use. Do not store chemicals in the fume hood.
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
ChemTag. Visual Leak Detection Products. Nowtek introduces: Water Alkali. ChemTag Tape on Floor. After Chemical Exposure
 introduces: ChemTag Tape on Floor After Chemical Exposure ChemTag Acid Water Alkali Visual Leak Detection Products After Chemical Exposure to ChemTag Rings on Pipe Purpose If there is a chemical leak at
introduces: ChemTag Tape on Floor After Chemical Exposure ChemTag Acid Water Alkali Visual Leak Detection Products After Chemical Exposure to ChemTag Rings on Pipe Purpose If there is a chemical leak at
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes
 w- LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes by Daniel J. Vaughan This paper is focused on how not to make waste or how to prevent pollution at the source. I know
w- LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes by Daniel J. Vaughan This paper is focused on how not to make waste or how to prevent pollution at the source. I know
Unit 15 Solutions and Molarity
 Unit 15 s and Molarity INTRODUCTION In addition to chemical equations chemists and chemistry students encounter homogeneous mixtures or solutions quite frequently. s are the practical means to deliver
Unit 15 s and Molarity INTRODUCTION In addition to chemical equations chemists and chemistry students encounter homogeneous mixtures or solutions quite frequently. s are the practical means to deliver
We will not cover solution stoichiometry on Exam 1.
 Chemistry 1A Fall 2017 Exam 1 Review I. Chapter 1 Terms: macroscale and nano scale atoms and molecules elements and compounds (nanoscale and macroscale definitions) mixtures structure of solid, liquid,
Chemistry 1A Fall 2017 Exam 1 Review I. Chapter 1 Terms: macroscale and nano scale atoms and molecules elements and compounds (nanoscale and macroscale definitions) mixtures structure of solid, liquid,
Chem 2115 Experiment #10. Acids, Bases, Salts, and Buffers
 Chem 2115 Experiment #10 Acids, Bases, Salts, and Buffers OBJECTIVE: The goal of this series of experiments is to investigate the characteristics of acidic and basic solutions. We will explore the neutralization
Chem 2115 Experiment #10 Acids, Bases, Salts, and Buffers OBJECTIVE: The goal of this series of experiments is to investigate the characteristics of acidic and basic solutions. We will explore the neutralization
Experiment 8 - Double Displacement Reactions
 Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
HOW TO MAKE STANDARD SOLUTIONS FOR CHEMISTRY
 HOW TO MAKE STANDARD SOLUTIONS FOR CHEMISTRY Phillip Bigelow Chemists make two common types of "standard solutions": Molar solutions Normal solutions Both of these solutions are concentrations (or strengths
HOW TO MAKE STANDARD SOLUTIONS FOR CHEMISTRY Phillip Bigelow Chemists make two common types of "standard solutions": Molar solutions Normal solutions Both of these solutions are concentrations (or strengths
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Safe Operating Procedure
 Safe Operating Procedure (Reviewed 1/09) USE AND STORAGE OF PEROXIDE-FORMING CHEMICALS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Some common laboratory
Safe Operating Procedure (Reviewed 1/09) USE AND STORAGE OF PEROXIDE-FORMING CHEMICALS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Some common laboratory
Chemistry 283g- Experiment 4
 EXPEIMENT 4: Alkenes: Preparations and eactions elevant sections in the text: Fox & Whitesell, 3 rd Ed. Elimination eactions of Alcohols: pg. 426-428, 431-432 Electrophilic Addition to Alkenes: pg. 484-488,
EXPEIMENT 4: Alkenes: Preparations and eactions elevant sections in the text: Fox & Whitesell, 3 rd Ed. Elimination eactions of Alcohols: pg. 426-428, 431-432 Electrophilic Addition to Alkenes: pg. 484-488,
AIR. Ambient, Indoor, Workplace Air and Stack Emissions Proficiency Testing Scheme. Sample Preparation Instructions Round 1
 General Instructions Ambient, Indoor, Workplace Air and Sample Instructions Round 1 Sample Storage All samples should be stored in accordance with the instructions provided on the sample labels from the
General Instructions Ambient, Indoor, Workplace Air and Sample Instructions Round 1 Sample Storage All samples should be stored in accordance with the instructions provided on the sample labels from the
NOTE: This method does not include all of the. specifications (e.g., equipment and supplies) and procedures
 1923 METHOD 111 ) DETERMINATION OF POLONIUM-210 EMISSIONS FROM STATIONARY SOURCES NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling
1923 METHOD 111 ) DETERMINATION OF POLONIUM-210 EMISSIONS FROM STATIONARY SOURCES NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling
Page No.: The Department of Environmental Health and Safety is responsible for;
 1 Introduction The have been developed in order to minimize the risk of accidental chemical reactions and exposure resulting from the improper storage of hazardous chemicals. The chemical storage procedures
1 Introduction The have been developed in order to minimize the risk of accidental chemical reactions and exposure resulting from the improper storage of hazardous chemicals. The chemical storage procedures
Acids, Bases, Salts, & Buffers
 Acids, Bases, Salts, & Buffers 1.55 ns CONDUCTIVITY METER Part 1A Conductivity Measurements of Acids & Bases 1.55 ns CONDUCTIVITY METER X X H2O HCl CH3COOH HNO3 CH3CH2CH2NH2 KOH NaOH NH3 CH3CH2OH X DI
Acids, Bases, Salts, & Buffers 1.55 ns CONDUCTIVITY METER Part 1A Conductivity Measurements of Acids & Bases 1.55 ns CONDUCTIVITY METER X X H2O HCl CH3COOH HNO3 CH3CH2CH2NH2 KOH NaOH NH3 CH3CH2OH X DI
Identification of ions and gases Assignment
 Name: ( ) ate: lass: Identification of ions and gases ssignment 1. n excess of sodium hydroxide is added to an aqueous solution of salt X and boiled. mmonia gas is only given off after aluminium foil is
Name: ( ) ate: lass: Identification of ions and gases ssignment 1. n excess of sodium hydroxide is added to an aqueous solution of salt X and boiled. mmonia gas is only given off after aluminium foil is
Which fertiliser would improve the quality of this soil most effectively?
 1 farmer s soil is very low in both nitrogen (N) and phosphorus (P). Which fertiliser would improve the quality of this soil most effectively? 2 Which compound is not used as a fertiliser? ammonium sulfate
1 farmer s soil is very low in both nitrogen (N) and phosphorus (P). Which fertiliser would improve the quality of this soil most effectively? 2 Which compound is not used as a fertiliser? ammonium sulfate
Kjeldahl Method. Quantiative analysis
 e-learning for Quantiative analysis Kjeldahl Method Introduction Nitrogen is one of the five major elements found in organic materials such as protein. This fact was recognized by a Danish chemist, Johan
e-learning for Quantiative analysis Kjeldahl Method Introduction Nitrogen is one of the five major elements found in organic materials such as protein. This fact was recognized by a Danish chemist, Johan
Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class
 Chemical Inventory Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class 1 SUGGESTED SHELF STORAGE PATTERN - ORGANIC Organic
Chemical Inventory Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class 1 SUGGESTED SHELF STORAGE PATTERN - ORGANIC Organic
CHEMICAL RESISTANCE LIST
 1,1,1 Trichloroethane C 2 H 3 CI 3 100 2-Butanoxim C 4 H 9 NO 100 COOH 1 60 COOH 5 COOH 5 60 COOH 10 COOH COOH 20 Acetone CH 3 COCH 3 100 Alaun KAl( ) 2 Aluminium Chloride AlCl 3 Aluminium Chloride AlCl
1,1,1 Trichloroethane C 2 H 3 CI 3 100 2-Butanoxim C 4 H 9 NO 100 COOH 1 60 COOH 5 COOH 5 60 COOH 10 COOH COOH 20 Acetone CH 3 COCH 3 100 Alaun KAl( ) 2 Aluminium Chloride AlCl 3 Aluminium Chloride AlCl
Unit 4: Chemical Changes (Higher Content)
 Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Hints for Strong Ion Exchange Resins
 Hints for Strong Ion Exchange Resins Chromatography Application Note AN98 Abstract Ion exchange columns are a powerful means of isolating and purifying compounds, but their use is limited due to lack of
Hints for Strong Ion Exchange Resins Chromatography Application Note AN98 Abstract Ion exchange columns are a powerful means of isolating and purifying compounds, but their use is limited due to lack of
Solid: mg, g, kg; oz, lb. Numeric; From client inventory
 Client Name INVENTORY INPUT SAMPLE JENSEN HUGHES CHI Team Building Name Sample Internal ID / Record # Chemical Name Mixture? (Multiple CAS) Components of Mixture Percent of Mixture Concentration (Single
Client Name INVENTORY INPUT SAMPLE JENSEN HUGHES CHI Team Building Name Sample Internal ID / Record # Chemical Name Mixture? (Multiple CAS) Components of Mixture Percent of Mixture Concentration (Single
Summer Assignment Part 2
 Summer Assignment Part 2 Name: 1. Metric Conversions. Remember 1 cm 3 = 1 ml 1 L = 1 dm 3 ITEM GIVEN METRIC UNIT DESIRED METRIC UNIT A 8.43 cm mm B 2.41 x 10 2 cm m C 294.5 nm cm D 1.445 x 10 4 m km E
Summer Assignment Part 2 Name: 1. Metric Conversions. Remember 1 cm 3 = 1 ml 1 L = 1 dm 3 ITEM GIVEN METRIC UNIT DESIRED METRIC UNIT A 8.43 cm mm B 2.41 x 10 2 cm m C 294.5 nm cm D 1.445 x 10 4 m km E
Extra Questions. Chemical Formula IUPAC Name Ionic, Molecular, or Acid. ethanol. sulfurous acid. titanium (IV) oxide. gallium sulfate.
 Chemistry 30 Recap Chemistry 20 Complete the following chart: Extra Questions Name: Chemical Formula IUPAC Name Ionic, Molecular, or Acid PbI2 (s) ethanol NaHS (aq) sulfurous acid H2O2 (l) titanium (IV)
Chemistry 30 Recap Chemistry 20 Complete the following chart: Extra Questions Name: Chemical Formula IUPAC Name Ionic, Molecular, or Acid PbI2 (s) ethanol NaHS (aq) sulfurous acid H2O2 (l) titanium (IV)
APC Spring Break Take-Home Exam Instructions
 APC Spring Break Take-Home Exam Instructions Complete all exam questions on separate paper. Show all work to receive credit. Partial credit will be awarded! Staple all papers together. Do NOT include the
APC Spring Break Take-Home Exam Instructions Complete all exam questions on separate paper. Show all work to receive credit. Partial credit will be awarded! Staple all papers together. Do NOT include the
SpillSolv. Chemical Spill Treatment Kits
 SpillSolv Chemical Spill Treatment Kits Features & Benefits Compe SpillSolv Chemical Spill Treatment Kits EMD Chemicals offers the next generation in spill cleanup technology. SpillSolv Chemical Treatment
SpillSolv Chemical Spill Treatment Kits Features & Benefits Compe SpillSolv Chemical Spill Treatment Kits EMD Chemicals offers the next generation in spill cleanup technology. SpillSolv Chemical Treatment
mohd faisol mansor/chemistry form 4/chapter 7 CHAPTER 7 ACIDS AND BASES HCl (g) H 2 O H + (aq) + Cl - (aq) NaOH(s) H 2 O Na + (aq) + OH - (aq)
 CHAPTER 7 ACIDS AND BASES Arrhenius Theory An acid is a chemical compound that produces hydrogen ions, H + or hydroxonium ions H3O + when dissolve in water. A base defined as a chemical substance that
CHAPTER 7 ACIDS AND BASES Arrhenius Theory An acid is a chemical compound that produces hydrogen ions, H + or hydroxonium ions H3O + when dissolve in water. A base defined as a chemical substance that
NJ MATERIALS ACCOUNTING & POLLUTION PREVENTION PLANNING
 NJ MATERIALS ACCOUNTING & POLLUTION PREVENTION PLANNING NJ DEPARTMENT OF ENVIRONMENTAL OFFICE OF POLLUTION PREVENTION & RIGHT TO KNOW www.state.nj.us/dep/opppc JUNE 15, 2010 Purpose of the NJ RPPR To collect
NJ MATERIALS ACCOUNTING & POLLUTION PREVENTION PLANNING NJ DEPARTMENT OF ENVIRONMENTAL OFFICE OF POLLUTION PREVENTION & RIGHT TO KNOW www.state.nj.us/dep/opppc JUNE 15, 2010 Purpose of the NJ RPPR To collect
User Initials. Date. User Initials. Date
 Name & ID (Print): PI (subject to change): Signature & : Research Focus (subject to change): Chemical Handling and Equipment Extreme Hazards Acetic Acid, Glacial Ammonium Fluoride Ammonium Hydroxide Aqua
Name & ID (Print): PI (subject to change): Signature & : Research Focus (subject to change): Chemical Handling and Equipment Extreme Hazards Acetic Acid, Glacial Ammonium Fluoride Ammonium Hydroxide Aqua
Unit V: Solutions. A. Properties of Solutions. B. Concentration Terms of Solutions. C. Mass Percent Calculation. D. Molarity of Solutions
 Unit V: Solutions A. Properties of Solutions B. Concentration Terms of Solutions C. Mass Percent Calculation D. Molarity of Solutions E. Solution Stoichiometry F. Dilution Problems 5-A Properties of Solutions
Unit V: Solutions A. Properties of Solutions B. Concentration Terms of Solutions C. Mass Percent Calculation D. Molarity of Solutions E. Solution Stoichiometry F. Dilution Problems 5-A Properties of Solutions
Sizes 48" x 96" (1.22m x 2.44m) Custom sheet sizes available upon request
 Applications Decorative paneling Partitions Water features Signage Furniture Displays and more... Attributes Excellent UV properties High impact resistance - 17x greater than glass and 4x greater than
Applications Decorative paneling Partitions Water features Signage Furniture Displays and more... Attributes Excellent UV properties High impact resistance - 17x greater than glass and 4x greater than
CSUS Department of Chemistry Experiment 3 Chem.1A
 Experiment 3: Reactions in Aqueous Solutions: Pre lab Name: 10 points Due at the beginning of lab. Section: 1. Precipitation Reactions a. On the reverse side of this page or on a separate piece of paper,
Experiment 3: Reactions in Aqueous Solutions: Pre lab Name: 10 points Due at the beginning of lab. Section: 1. Precipitation Reactions a. On the reverse side of this page or on a separate piece of paper,
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione
 Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
NAMING IONIC COMPOUNDS. ammonium sulfide. iron(ii) carbonate. barium phosphate. titanium(iv) sulfide. Spelling matters!
 67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
Part 01 - Notes: Reactions & Classification
 Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Concentration of Solutions
 CHAPTER 4 Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table compares these three ways
CHAPTER 4 Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table compares these three ways
Write the chemical formulas for these polyatomic ions:
 Write the chemical formulas for these polyatomic ions: 1. sulfate 2. phosphate 3. carbonate 4. hydroxide 5. nitrate 6. ammonium 7. acetate 1of 29 Answers: 1. SO 2 4 2. PO 3 4 3. CO 2 3 4. OH 5. NO 3 6.
Write the chemical formulas for these polyatomic ions: 1. sulfate 2. phosphate 3. carbonate 4. hydroxide 5. nitrate 6. ammonium 7. acetate 1of 29 Answers: 1. SO 2 4 2. PO 3 4 3. CO 2 3 4. OH 5. NO 3 6.
SOLUTIONS. Solutions - page
 SOLUTIONS For gases in a liquid, as the temperature goes up the solubility goes. For gases in a liquid, as the pressure goes up the solubility goes. Example: What is the molarity of a solution with 2.0
SOLUTIONS For gases in a liquid, as the temperature goes up the solubility goes. For gases in a liquid, as the pressure goes up the solubility goes. Example: What is the molarity of a solution with 2.0
Did you know that there are 4 ways of making salt? cgrahamphysics.com
 Did you know that there are 4 ways of making salt? Starter 1. Recap methods of making and naming salts. 2. Recap the term 'NeutralizaBon' and indicators. 3. Make your own salt through pracbcal method.
Did you know that there are 4 ways of making salt? Starter 1. Recap methods of making and naming salts. 2. Recap the term 'NeutralizaBon' and indicators. 3. Make your own salt through pracbcal method.
CHM 152 Lab 5: Qualitative Analysis updated May, 2011
 CHM 152 Lab 5: Qualitative Analysis updated May, 2011 Introduction In this lab you will see how it s possible to separate a mixture using many of the common reactions you ve learned in General Chemistry
CHM 152 Lab 5: Qualitative Analysis updated May, 2011 Introduction In this lab you will see how it s possible to separate a mixture using many of the common reactions you ve learned in General Chemistry
A cream precipitate formed
 Q1. (a) Some scientists thought that the waste water from a waste disposal factory contained two sodium halides. They tested a sample of the waste water. They added three reagents, one after the other,
Q1. (a) Some scientists thought that the waste water from a waste disposal factory contained two sodium halides. They tested a sample of the waste water. They added three reagents, one after the other,
Chemistry 20 Lesson 17 Solubility
 Chemistry 20 Lesson 17 Solubility The ability of one compound to dissolve in another compound is called solubility. The term solubility can be used in two senses, qualitatively and quantitatively. Qualitatively,
Chemistry 20 Lesson 17 Solubility The ability of one compound to dissolve in another compound is called solubility. The term solubility can be used in two senses, qualitatively and quantitatively. Qualitatively,
1. The elements on the periodic table are arranged by increasing A) atomic weight. B) atomic number. C) valence electrons. D) mass number.
 1. The elements on the periodic table are arranged by increasing A) atomic weight. B) atomic number. C) valence electrons. D) mass number. 2. Periodic law states which of the following? A) Physical and
1. The elements on the periodic table are arranged by increasing A) atomic weight. B) atomic number. C) valence electrons. D) mass number. 2. Periodic law states which of the following? A) Physical and
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
! "#$%&'$!()! *+&$(,)#$! *+&$(,)#$! *+&$(,)#$!
 Learning Checkpoint, p. 197 1. A solution with a ph of 11 is basic. 2. A solution with a ph of 5 is acidic. 3. The ph of pure water is 7 (neutral).! "#$%&'$!()! *+&$(,)#$! 4. No, litmus paper does not
Learning Checkpoint, p. 197 1. A solution with a ph of 11 is basic. 2. A solution with a ph of 5 is acidic. 3. The ph of pure water is 7 (neutral).! "#$%&'$!()! *+&$(,)#$! 4. No, litmus paper does not
AP Study Questions
 ID: A AP 16.4-16.7 Study Questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1 What is the ph of an aqueous solution at 25.0 C in which [H + ] is 0.0025
ID: A AP 16.4-16.7 Study Questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1 What is the ph of an aqueous solution at 25.0 C in which [H + ] is 0.0025
9.4 Naming and Writing Formulas for Acids and Bases. What s the name of the acid responsible for the crisp taste in this drink?
 CHEMISTRY & YOU What s the name of the acid responsible for the crisp taste in this drink? There s a certain acid that gives many soft drinks their crisp, enjoyable taste. 1 How do you determine the name
CHEMISTRY & YOU What s the name of the acid responsible for the crisp taste in this drink? There s a certain acid that gives many soft drinks their crisp, enjoyable taste. 1 How do you determine the name
Hach Method Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis
 Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
- Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS
 63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
Sodium, Na. Gallium, Ga CHEMISTRY Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 11.1 to 11.4.
 Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 11.1 to 11.4 Forms of Carbon The Halogens (Group 17 What is a halogen? Any element in group
Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 11.1 to 11.4 Forms of Carbon The Halogens (Group 17 What is a halogen? Any element in group
Class III Wastewater Laboratory Analyst Examination Study Guide 2008
 Class III Wastewater Laboratory Analyst Examination Study Guide 2008 Specific laboratory analyses included on Class III Exam: All Information and tests from Class I and Class II Plus the following - Biological
Class III Wastewater Laboratory Analyst Examination Study Guide 2008 Specific laboratory analyses included on Class III Exam: All Information and tests from Class I and Class II Plus the following - Biological
25. Qualitative Analysis 2
 25. Qualitative Analysis 2 This experiment uses a series of wet chemistry analytical tests to determine the functional group present in a series of known and an unknown compound. Each student receives
25. Qualitative Analysis 2 This experiment uses a series of wet chemistry analytical tests to determine the functional group present in a series of known and an unknown compound. Each student receives
NAMING IONIC COMPOUNDS
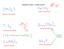 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS
 APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind.
 Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind. 1. calcium + oxygen 2. cupric carbonate 3. aluminum + hydrochloric
Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind. 1. calcium + oxygen 2. cupric carbonate 3. aluminum + hydrochloric
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions)
 Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
HSW NORM-JECT SYRINGES CHEMICAL RESISTANCE CHART
 A Beer + + Acetic acid (10-60%) + O Benzaldehyde + O Acetic acid (1-10%) + + Benzene O - Acetic acid (80-100%) + O Benzene sulfonic acid + + Acetic anhydride + + Benzoic acid + Acetone + + Bismuth carbonate
A Beer + + Acetic acid (10-60%) + O Benzaldehyde + O Acetic acid (1-10%) + + Benzene O - Acetic acid (80-100%) + O Benzene sulfonic acid + + Acetic anhydride + + Benzoic acid + Acetone + + Bismuth carbonate
Experiment 4 Stoichiometry: The Reaction of Iron with Copper(II) Sulfate
 CEAC 105 GENERAL CHEMISTRY Experiment 4 Stoichiometry: The Reaction of Iron with Copper(II) Sulfate Purpose: To enhance the understanding of stoichiometry, a reaction between iron and copper (II) sulfate
CEAC 105 GENERAL CHEMISTRY Experiment 4 Stoichiometry: The Reaction of Iron with Copper(II) Sulfate Purpose: To enhance the understanding of stoichiometry, a reaction between iron and copper (II) sulfate
Chemical Reactions. Chemical changes are occurring around us all the time
 Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
ICSE Board. Class X Chemistry. Board Paper Time: 1½ hrs Total Marks: 80
 ICSE Board Class X Chemistry Board Paper 2013 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
ICSE Board Class X Chemistry Board Paper 2013 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
PREVENTING HAZARDOUS WASTE FINES
 University of Medicine and Dentistry of New Jersey EOHSS FACTSHEET PREVENTING HAZARDOUS WASTE FINES In the last few years institutions such as MIT, University of New Hampshire, Yale, Stanford, and Boston
University of Medicine and Dentistry of New Jersey EOHSS FACTSHEET PREVENTING HAZARDOUS WASTE FINES In the last few years institutions such as MIT, University of New Hampshire, Yale, Stanford, and Boston
Magnetic Flowmeter Material Selection Guide
 Magnetic Flowmeter Material Selection Guide www.sparlinginstruments.com Introduction The purpose of this guide is to provide a general resource for the selection of materials for the magnetic flowmeter.
Magnetic Flowmeter Material Selection Guide www.sparlinginstruments.com Introduction The purpose of this guide is to provide a general resource for the selection of materials for the magnetic flowmeter.
Chemical Changes. Introduction
 Chemical Changes Introduction Chemical changes occur around us all the time. Perhaps one of the most common chemical changes that we have seen for a number of years is the destruction of marble and limestone
Chemical Changes Introduction Chemical changes occur around us all the time. Perhaps one of the most common chemical changes that we have seen for a number of years is the destruction of marble and limestone
Solubility Rules. Electrolytes, Weak and Strong. Examples. Another Example:
 Electrolytes, Weak and Strong Electrolytes are compounds that ionize in water to produce aqueous solutions that conduct an electric current. Nonelectrolytes are substances that do not ionize, remain as
Electrolytes, Weak and Strong Electrolytes are compounds that ionize in water to produce aqueous solutions that conduct an electric current. Nonelectrolytes are substances that do not ionize, remain as
Fluid Compatibility Table
 Acetaldehyde C A A A B D C A A Acetate Solvents A B A A D D A A Acetic Acid, crude C B A A D D A A Acetic Acid, pure D B A A D D A A Acetic Acid, 10% C B A A B D D A A Acetic Acid, 80% C B A A C D D A
Acetaldehyde C A A A B D C A A Acetate Solvents A B A A D D A A Acetic Acid, crude C B A A D D A A Acetic Acid, pure D B A A D D A A Acetic Acid, 10% C B A A B D D A A Acetic Acid, 80% C B A A C D D A
Figure 1. Pore size distribution
 Product Information '2:(;Ã237,325(Ã/ÃDQGÃ9 Polymeric Adsorbents Dow has developed a new polymeric adsorbent type for the concentration of organics from air and water. Key features of these adsorbents are:
Product Information '2:(;Ã237,325(Ã/ÃDQGÃ9 Polymeric Adsorbents Dow has developed a new polymeric adsorbent type for the concentration of organics from air and water. Key features of these adsorbents are:
CHEM 200/202. Professor Gregory P. Holland Office: GMCS-213C. All s are to be sent to:
 CHEM 200/202 Professor Gregory P. Holland Office: GMCS-213C All emails are to be sent to: chem200@mail.sdsu.edu My office hours will be held in GMCS-212 on Monday from 12:00 pm to 2:00 pm or by appointment.
CHEM 200/202 Professor Gregory P. Holland Office: GMCS-213C All emails are to be sent to: chem200@mail.sdsu.edu My office hours will be held in GMCS-212 on Monday from 12:00 pm to 2:00 pm or by appointment.
OSH in Medical Laboratory
 OSH in Medical Laboratory Mr. K K LEUNG M(OSH) HRD HAHO Induction Program for Newly Recruited AMT 28 March 2008 Contents 1. Ordinance and Regulations 2. HA Safety Manuals 3. Chemical Safety 4. Biological
OSH in Medical Laboratory Mr. K K LEUNG M(OSH) HRD HAHO Induction Program for Newly Recruited AMT 28 March 2008 Contents 1. Ordinance and Regulations 2. HA Safety Manuals 3. Chemical Safety 4. Biological
Exploring Equilibria
 Exploring Equilibria Name: Chem 112 This experiment explores a variety of equilibrium systems. A reference Table of Reactions is attached to aid in your explanations. In this qualitative lab, your observations,
Exploring Equilibria Name: Chem 112 This experiment explores a variety of equilibrium systems. A reference Table of Reactions is attached to aid in your explanations. In this qualitative lab, your observations,
DETERMINING IONIC FORMULAS strontium oxide
 69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
ACID-BASE EXTRACTION
 ACID-BASE EXTRACTION An acid-base extraction is a type of liquid-liquid extraction. It typically involves different solubility levels in water and an organic solvent. The organic solvent may be any carbon-based
ACID-BASE EXTRACTION An acid-base extraction is a type of liquid-liquid extraction. It typically involves different solubility levels in water and an organic solvent. The organic solvent may be any carbon-based
La Salle College High School AP Chemistry Summer Homework Assignment
 La Salle College High School AP Chemistry Summer Homework Assignment DUE on first day of class! Email me with questions as a last resort: stottm@lschs.org Name: 1. Metric Conversions. Remember 1 cm = 1
La Salle College High School AP Chemistry Summer Homework Assignment DUE on first day of class! Email me with questions as a last resort: stottm@lschs.org Name: 1. Metric Conversions. Remember 1 cm = 1
Lab #14: Qualitative Analysis of Cations and Anions
 Lab #14: Qualitative Analysis of Cations and Anions Objectives: 1. To understand the rationale and the procedure behind the separation for various cations and anions. 2. To perform qualitative analysis
Lab #14: Qualitative Analysis of Cations and Anions Objectives: 1. To understand the rationale and the procedure behind the separation for various cations and anions. 2. To perform qualitative analysis
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Identification of an Unknown Compound through Mass Correlations
 EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
EXPERIMENT Identification of an Unknown Compound through Mass Correlations PURPOSE To carry out a series of decomposition reactions for five different unknown, and use stoichiometry in order to identify
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION
 METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
AP CHEMISTRY CHAPTER 8 PROBLEM SET #3. 1. Determine if the following pairs would form a solution. Explain your answer. a.
 NAME: AP CHEMISTRY CHAPTER 8 PROBLEM SET #3 1. Determine if the following pairs would form a solution. Explain your answer. a. C 2 H 6 and water b. PbCl 2 and water c. I 2 and water d. F 2 and CH 4 2.
NAME: AP CHEMISTRY CHAPTER 8 PROBLEM SET #3 1. Determine if the following pairs would form a solution. Explain your answer. a. C 2 H 6 and water b. PbCl 2 and water c. I 2 and water d. F 2 and CH 4 2.
Name Hour. Acids, Bases, Salts and Neutralization. Practice Test A
 Name Hour Acids, Bases, Salts and Neutralization Practice Test A Objective 1: Solve problems involving the molarity of a solution 1. What is the molarity of a solution made by dissolving 2.5 moles of sodium
Name Hour Acids, Bases, Salts and Neutralization Practice Test A Objective 1: Solve problems involving the molarity of a solution 1. What is the molarity of a solution made by dissolving 2.5 moles of sodium
International Advanced Level Chemistry Advanced Subsidiary Unit 3: Chemistry Laboratory Skills I
 Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 3: Chemistry Laboratory Skills I Candidate Number Wednesday 7 May
Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 3: Chemistry Laboratory Skills I Candidate Number Wednesday 7 May

 155 Find the ph of the solution
155 Find the ph of the solution