466. The Electronic Orbitals, Shpes, and flpectra of Polyatomic Molecules. Part I. AH, Molecules.
|
|
|
- Wesley Gilmore
- 6 years ago
- Views:
Transcription
1 2.260 Walsh : The Electronic Orbitals, Shapes, and 466. The Electronic Orbitals, Shpes, and flpectra of Polyatomic Molecules. Part I. AH, Molecules. By A. D. WALSH. The electronic orbitals possible for an AH, molecule when linear are correlated with those possible for the molecule when non-linear. Qualitative curves of binding energy versus LHAH are given for the various orbitals. These curves are then used to explain and predict (i) the shapes and (ii) the electronic spectra and associated characteristics of AH, molecules. AH, molecules containing 4 valency electrons should be linear in their ground states. AH, molecules containing 5-8 valency electrons should be bent in their ground states. THE purpose of this paper is first to correlate the electronic orbitals of a bent with a linear AH, molecule, next to decide whether a given orbital becomes more or less tightly bound with increase of the angle HAH, and then to use the resulting graphs of binding energy versus apex angle to explain and predict the shapes and spectra of AH, molecules. Linear AH, MoZeczcZes.T-The lowest-energy intra-valency-shell orbitals of a linear AH, molecule, may, on the assumption that these are built solely from s and fi atomic orbitals, be described as follows: (i) Two orbitals binding the hydrogen atoms to the central atom. These may be thought of as each formed by the overlap of an sfi hybrid atomic orbital on A with the 1s orbital of hydrogen. If so regarded they will be orbitals of a-type predominantly localized one in each A-H distance. If, however, one were discussing a transition involving excitation of an electron from one of the bonding orbitals, one could not regard the electron as coming from one A-H link or the other-the two are indistinguishable. If one first conceives of the orbitals as completely localized one has to take combinations of them in order to express this indistinguishability. These combinations are either in-phase or out-of-phase and may be labelled og and 6, respectively. (ii) A z,~ orbital. This is simply a 9 orbital localized on the central atom and pointing in a direction at 90" to the HAH line. It is non-bonding. Since there are two such directions that are independent but equivalent (except for a rotation by go"), the orbital is two-fold
2 [1953] Spectra of Polyatomic Molecules. Part I degenerate. If the apex angle were changed from 180", the degeneracy must become split. The order of decreasing binding energy of these orbitals is agj cu, xu. Bent AH, MoZecuZes.-A bent AH, molecule belongs to the symmetry class C2v. The definitions of the symbols appropriate to the non-localized orbitals of such a molecule are given below. The z axis bisects the HAH angle and lies in the molecular plane. The y axis also lies in the molecular plane and is parallel with the H H line. C,(z) means a rotation by 180" about the z axis. Q@).means a reflection in the xz plane. + and - mean respectively that the wave function does not or does change sign when one of the symmetry operations C,(z) or c&) is carried out. c2 (4 4Y) L u b, * b, * * Certain authors reverse the definitions of b, and b,. The lowest-energy intra-valency-shell orbitals of an AH, molecule whose apex angle is 90" are then as follows : (i) Two orbitals binding the hydrogen atoms to the central atom. These may be thought of, in the first place, as each formed by the overlap of a pure p atomic orbital on A with the 1s orbital of H. This would be to think of the orbitals as predominantly localized, one in each A-H distance. As with the bonding orbitals of the linear molecule, however, for discussions of spectroscopic transitions involving these orbitals they must be regarded as non-localized. The equivalent non-localized orbitals are simply the in-phase or out-of-phase combinations of the two localized orbitals, and, being no longer localized, may, by the above table of definitions, be labelled a, and b, respectively. (ii) A fi orbital on atom A pointing in the x direction. The foregoing Table shows that the molecular symbol to be applied to this orbital is b,. (iii) An s orbital on atom A. It is non-bonding. The Table shows that the molecular symbol to be applied to this orbital is a,. Correlation of the Orbitals for Bent and Lifiear Mo1ecuZes.-Clearly, if the HAH angle is gradually increased, the a, and the b, bonding orbitals must eventually become the as and the Q, orbitals respectively of the linear molecule. At the same time the binding energy of the orbitals must increase. The reasons for this may be seen by thinking of the orbitals in either their localized or their non-localized forms. For the localized forms, the reasons are : (1) the orbitals are built solely from p atomic orbitals of A in the 90" molecule but partly from an s atomic orbital of A in the linear molecule; (2) an sfi hybrid valency gives rise to a stronger bond than does a pure fi valency (see Walsh, Discuss. Faraday SOC., 1947, 2, 18). When considering the non-localized forms, it is convenient to form first the two possible group orbitals of the H, group, viz., 1s + 1s and 1s - 1s. To do this automatically takes account of the symmetry of the molecule. The components from which the non-localized molecular orbitals are built may then be written as follows : Bent molecule Linear molecule, - > Is + Is, ug Is - Is, (3, papm TI4 PW 0, SAr 0, Components of the same species may then be "mixed" to form the actual molecular orbitals. This procedure leads to the same end as taking Combinations of the localized orbitals. We now introduce three principles : (i) in the 90" molecule the SA orbital does not mix (" hybridize ") with the other orbitals * ; (ii) whether or not an orbital becomes more tightly bound with change of angle is determined primarily by whether or not it changes from being built from a fi orbital of A to being built from an s orbital of A; and (iii) if no change of A valencies from which the orbital is built occurs when the angle is changed, the following subsidiary effect determines whether the orbital becomes more * Introduced to make the description of the non-localized bond orbitals consistent with that of the localized ones.
3 2262 IVnLsh : T?azc Electronic Orbitals, Shapes, uptd or less tightly bound: if the orbital is anti-bonding between the end atoms it is most tightly bound when the latter are as far apart as possible (k., in the linear molecule) ; if it is bonding between the end atoms it is most tightly bound when the latter are as near together as possible (Le., in the 90" molecule). The al-as bond orbital is thus built from a combination of 1s + 1s and p, in the 90" molecule, from a combination of 1s + 1s and SA in the linear molecule, and from a combination of 1s + Is, s ~ and, p, in the intermediate molecule. It follows that the orbital is more tightly bound in the linear thafi in the bent molecule. The b,-au orbital is built from a combination of the 1s - 1s and 6, components in both the bent and the linear molecule ; but from principle (iii), it is more tightly bound in the linear molecule since it is anti-bonding between the end atoms. Clearly, as the HAH angle is increased, the b, orbital of the bent molecule must become one of the two x,, orbitals of the linear molecule. To a first approximation, the orbital is the same when the apex angle is 90" as when it is 180". We may therefore suppose its binding energy to remain approximately constant as the angle changes. Increase of the apex angle from 90" implies mixing of the als~ orbital with the alpt orbital on A. The (zi bond orbital, instead of being built from a pure f~ valency of A, becomes more and more built also from the s orbital of A; while at the same time the non-bonding orbital, instead of being built solely from the pure s orbital of A, becomes more and more built from a p, orbital on A. In the linear molecule, the bonding orbital has become built from an orbital of A that is solely s, while the non-bonding orbital has become built solely from a 9, orbital of A. In other words, as the HAH angle increases from 90" to 180", the bond orbital increases in binding energy, while the non-bonding orbital originally labelled qs-4 decreases in binding energy until at 180" it becomes one o the degenerate xu orbitals. At 90" the non-bonding U ~SA. orbital must be much more tightly bound than the nonbonding b,j5 orbital. Where it lies in relation to the b, and a, bond orbitals is best decided by appeal to experimental facts. Shapes of Actual AH, MoZecuZes.-The Figure is a correlation diagram between the orbitals possible for bent and linear AH, molecules. It incorporates the conclusions reached above as to whether a particular orbital rises or falls in energy as the HAH angle is changed.* The Figure does not include nzz the possible intra-valency-shell orbitals, but only the lowest-lying ones. Two others are possible and are referred to below. There is no particular reason to make the Figure include all and only all the intra-valency-shell orbitals ; for an infinite number of extra-valency-shell orbitals is also possible, the lowest of which (see the discussion below of the spectrum of water vapour) will be comparable in energy with the highest intra-valency-shell orbitals. It is just as arbitrary to make the Figure show six orbitals as to make it show four; and, indeed, for the purpose of interpreting observed spectra it is less desirable since it is the highest intra-valency-shell orbitals that are most likely to lie above or mix with the lowest extra-valency-shell orbitals. There is no doubt about the four lowest-lying orbitals-they are intra-valency-shell in type; but the fifth and sixth orbitals in order of energy are probably not both simple intra-valency-shell orbitals. A similar point should be borne in mind in reading all the * Added in Proof.-The drop from left to right of the ul-u, orbital curve, due to principle (ii), is offset by a smaller rise due to principle (iii). The rise from left to right of the ul-ru orbital curve is not offset. The Figure has therefore been drawn with the u,-u, and b,-u,, curves falling from left to right by similar amounts; and both falling considerably less than the uiqu curve rises.
4 I Sfiectra of Polyatomic Molecules. Part I papers of this series, where the correlation diagrams show what are thought to be the lowest energy orbitals and do not necessarily include all the intra-valency shell orbitals. Each of the curves must be a maximum or a minimum on the 180" line, since from 180" to 270" the curves must repeat their behaviour from 180" to 90". The two most tightly bound orbitals have a minimum on the 180" line. One therefore expects that all *4H, molecules containing only four valency electrons will be linear in their ground states. As far as is known, this is true. As examples, the BeH, and HgH, molecules are expected to be linear in their ground states. On the other hand, the ground states of AH, molecules containing 5, 6, 7, or 8 valency electrons are expected to be bent because at least one electron has to be placed in the a, non-bonding orbital. Similarly, the first excited state of HgH, or BeH, should be bent. This a, orbital rises steeply from left to right in the Figure because, as already explained, it changes from a pure s orbital to a pure + orbital. The ground state of the H,O molecule, with eight valency electrons, has therefore a bent nuclear configuration. The CH, molecule, with six valency electrons, is similarly expected to be bent. The actual value of the apex angle cannot be predicted, while the Figure remains merely qualitative. If the angle is close to 180", the ground state of the molecule may be a triplet, the configuration lying lower in energy than (a 1) 2( b2) 2(a,) (b 1),3B, (1) (a1)2(b2)~(a1)2,1al (2) because the existence of electron repulsion offsets the small energy difference of the (a,) and (b,) orbitals. If, on the other hand, the apex angle is considerably less than NO", the ground state will be a singlet, having the configuration (2). By analogy with H,O and in view of the fact that the two most weakly bound electrons of the H,O molecule lie in the b, orbital and should therefore have comparatively little effect on the apex angle, the second possibility seems the more Likely. The small decrease that has been observed in the apex angle in the series H,O, H,S, H,Se, H,Te may be due either to the a,-x, curve rising even more steeply or to the bonding orbital curves falling less steeply as one passes from H,O to H,Te. However, the total range of the decrease is only 14". Spectra of AH, Molecules.-(i) Spectrum of H20. In the ground state of the H20 molecule all the orbitals represented in the Figure are fully occupied. These are the only low-lying orbitals of the molecule. No electronic spectrum of H,O is therefore expected until comparatively short wave-lengths are reached. In agreement, the first absorption of the molecule occurs as a continuum between 1830 and 1500 A (Amm. ca A ; Hopfield, Phys. Review, 1950, 71, 560). In the Figure the b, and the upper a, curve have been drawn to cross at an angle of ca. 110". The reason for this is as follows. Three ionization potentials of H,O are known (Price and Sugden, Trans. Faraday Soc., 1948, 44, 108, 116), viz., v, v, 16.2 & 0-3 V. A good Rydberg series is known leading to the first limit (Price, J. Chem. PJzys., 1936, 4, 147). A cruder Rydberg series is also known leading to the third limit (Henning, Ann. Physik, 1924, 13, 599). Rydberg series are only likely for the excitation of lone-pair electrons (or, sometimes, of weakly bonding or anti-bonding electrons) ; excitation of an electron from a strongly bonding orbital is hardly likely to give a series of discrete transitions. For this reason it seem best to interpret the first and third limits as due respectively to ionization from the (b,) and (a,) lone-pair orbitals; leaving the second limit to represent ionization of the (b,) bonding electrons. The ground state configuration of H,O is therefore written as (al)2(al)2(b2)2(b1)2 instead of (al)2(b2)2((al)2(b1)2. The main conclusions of this paper would not, however, be altered if the order of the (a,) and (b,) orbitals were reversed.* Two intra-valency-shell orbitals other than those shown in the Figure are possible. These are highly anti-bonding in nature, being obtained by considering out-of-phase overlap of an oxygen valency with the hydrogen 1s valency. The lower of the two is of * Price and Sugden (Zoc. cit.) assume that (b,) is more tightly bound than (al).
5 2264 Walsh : The Electro.lltic Orbitals, Shapes, and species il-og, where the bar indicates the anti-bonding nature. For spectroscopic purposes it must be considered as non-localized and therefore built by the in-phase overlap of the two hydrogen 1s atomic orbitals, these interacting out-of-phase with an oxygen valency that is 2s in the linear molecule and 2pz in the 90 molecule. Because of its anti-bonding nature, it may well lie so high that transition to it requires as much energy as transition to the lowest extra-valency-shell (Rydberg) orbital. In other words, the longest wavelength allowed transition of H20 may be formulated where the uppermost (al) orbital refers either to the anti-bonding intra-valency-shell orbital or to the 3s orbital of the oxygen atom. The A absorption has already been interpreted as due to transition of an electron from (b,) to (3sal) (Mulliken, J. Chem. Phys., 1935, 3, 506; Price, Teegan, and Walsh, Proc. Roy. SOC., 1950, A, 201, 600). Its continuous nature, however, makes it best considered as involving also transition to a repulsive upper state containing an electron in the anti-bonding (d,) orbital (cf. Walsh, unpublished work). According to Wilkinson and Johnston (J. Chem. Phys., 1950, 18, 190) the absorption actually consists of three diffuse bands superimposed on a continuum. Rathenau (2. Physik, 1934, 87, 32) had earlier found structure in the continuum, stating that a frequency difference crn.-l was present. The diffuse bands found by Wilkinson and Johnston are at 1608, 1648, and 1718 A. It is possible that they represent the Rydberg transition while the continuum represents the intra-valency-shell transition. The maximum molecular extinction coefficient is about 10oO.* It seems therefore that the transition(s) must be regarded as allowed, in agreement with the present assignments. Because of this, one expects either or both of the two totally symmetrical vibrational frequencies of the upper state to appear in the spectrum. It is possible that the separation of the 1608 and the 1648 band (1520 cm.-l) represents the bending vibration v2 (1595 cm. l in the ground state), and that the separation of the 1608 and the 1718 A band (3388 cm.-l) represents the stretching vibration v1 (3655 cm.-l in the ground state). The smallness of the reductions in these frequencies from the ground-state values would accord with only one quantum of each vibration appearing. It seems that comparatively little change of the molecular dimensions occurs on excitation, in agreement with the transition being of an electron from one non-bonding orbital (whose binding energy changes little with change of HOH angle) to another. (ii) Spectrum of NH,. In the ground state of the NH, radical only one electron lies in the (b,) orbital. A long-wave-length transition.... should therefore be possible. It represents an allowed transition. The further, longwave-length, transit ion. * * (b2)(b,)2,2b2 f+ * * (b2)2(b1),2b1 is forbidden. The allowed transition should be polarized in the x direction, Le., perpendicularly to the molecular plane. Since the transition involves transfer of an electron from the orbital represented in the Figure by the steep al-x, curve to the orbital represented by the approximately horizontal bl-x, line, it should result in an increase of the apex angle in the equilibrium form of the upper state. On the other hand, since the (a,) and the (b,) orbital are non-bonding, there should be little change of N-H length. The so-called ammonia a band, lying in the visible region, has long been attributed (though without proof) to the NH, radical. It was first observed by Eder (Denkschr. * Wilkinson and Johnston record intensities in terms of the atmospheric absorption coefficient at 30 C. Their values have been multiplied by (22.4 x 303)/(2-30 x 273) in order to obtain molecular extinction coefficients. The present discussion assumes that the bands observed by them in the continuum are not due to impurities. There is some doubt of this, however, since Rathenau s findings are not entirely in agreement with those of Wilkinson and Johnston. (3)
6 [ Spectra of Polyatomic Molecules. Part I Wien. Akad., 1893, 60, 1) in the ammonia-oxygen flame. It was later found to occur (more weakly) in the spectrum of an uncondensed discharge through ammonia (Rimmer, Proc. Roy. Soc., 1923, A, 103, 696). The latter method of obtaining it supports the identification of the emitter as a decomposition product of NH,. The band also occurs in the spectra of the hydrogen-nitrous oxide (Fowler and Badami, ibid., 1931, A, 133, 325; Gaydon, ibid., 1942, A, 181, 197) and the methane-nitrous oxide flames (Gaydon, Zoc. cit.). Infra-red bands observed in the spectrum of the former flame have been provisionally assigned to an extension of the band (Gaydon, LOG. cit.). Rimmer's highresolution photographs showed the great complexity of the band and the many lines (some 3000) present. The fine structure appears much too complex for the emitter to be the diatomic radical NH. Its attribution to NH, therefore seems very plausible. If the attribution is correct, then the interpretation of the band can hardly be other than as the emission transition (3). Analysis of the observed rotational structure should proceed in the light of the expectations formulated above.* That the structure should be so complex is not surprising (i) if the HNH angle is such that the molecule is not even an approximate symmetric top t and (ii) if, as expected here, the apex angle changes markedly in the transition. (iii) Spectrum of CH,. If the ground state of the molecule is a singlet, then the following is the longest-wave-length absorption transition : Mulliken (quoted by Venkateswarlu, Phys. Review, 1950, 77, 676) has earlier made the same assignment. The transition is allowed, with polarization perpendicular to the main symmetry axis. It should probably occur at quite long wave-lengths (cf. the supposed NH, transition above). It would result in an increase of apex angle in the equilibrium form of the upper state. On the other hand, there should be little change of C-H length. The Figure thus enables very specific statements to be made about the expected spectrum of CH,, which should be helpful in the search currently being undertaken in various laboratories for that spectrum. It is worth noting what the expectations would be if the ground state of the CH, molecule should turn out to be a triplet. This implies that the molecule is nearly linear in the ground state and we shall therefore use the a, x, nomenclature for its orbitals. Consider only transitions with no change of multiplicity : the lowest energy transition, ViZ., (09) (Qu) (xu) (xu) P3 g f- (Gg) (%) (xu) (xu),3 Cg- is forbidden. There will be no allowed transitions until comparatively short wavelengths (probably, by analogy with H,O, not until wave-lengths (2500 A). The longest wave-length allowed transition will be 311u +-- 3Zg- whose interpretation may be or where (Q;) stands for either the 3s carbon atom orbital or the anti-bonding orbital referred to above in the discussion of the H,O spectrum. If the CH, molecule is found to have a strong transition at wave-lengths considerably longer than 2500& it shodd be safe to conclude that (a) the apex angle in the ground state is considerably less than 180" and (b) the ground state is a singlet. * Since this paper was written, Dr. D. A. Ramsay has informed me that Professor Herzberg and he have now (a) proved that the ammonia a bands are due to NH,, (b) obtained the system in absorption between 4500 and 7400 A, and (c) shown, in agreement with the present expectations, that the upper and the lower state differ Considerably in the geometrical arrangement of the nuclei (see Herzberg and Ramsay, J. Chew Phys., 1952, 20, 347; Discuss. Favaday SOL, 1953, 14, 11). Dr. Ramsay has also pointed out to me that the same assignment of the bands as made here has been given earlier by Mulliken and quoted as a personal communication in a paper by Swings, McKellar, and Minkowski (Astrophys. J., 1943, 98,-142). f According to the Figure the apex angle in the ground state should be close to that of the H,O molecule.
7 2266 WaZsh : The Electronic Orbitals, Shapes, and Relation to Other Work.-In the following papers we shall show that, as with AH, molecules, the shape of a molecule in its ground state depends primarily on the number of valency electrons. In general terms, the recognition of this is of course not new (see, e.g., Cassie, Nature, 1933, 131, 438; Penney and Sutherland, Proc. Roy. SOC., 1936, A, 156, 654), though the newer data now available frequently enable the older generalizations to be extended and made more precise. There seems, however, to have been only one previous attempt to plot a correlation diagram between the orbitals possible for a polyatomic molecule in each of two nuclear configurations, and to use such a diagram to discuss both the shapes (in excited as well as ground states) and electronic spectra of molecules. This was by Mulliken (Rev. Mod. Phys., 1942,14,204) who plotted a correlation diagram for the limited case of AB, molecules. He did not specifically apply his diagram to AH, molecules. He also said that he was unable to give any simple explanation of why particular curves should rise or fall with increase of angle. His diagram was either empirical or based upon unpublished computations. UNIVERSITY OF LEEDS. [Received, May 15th, J
(2, CARBONYL GROUP. C=-C double bond, (xx)2 a pair forming the second bond. The xx electrons MECHANISM OF LONG WA VE-LENGTH ABSORPTION OF THE
 312 PHYSICS: MCMURR Y AND MULLIKEN PROC. N. A. S. ' In accordance with the rule that isobars nearly never differ by one in their charge numbers, table 1 contains only three nuclei with both symbols, p
312 PHYSICS: MCMURR Y AND MULLIKEN PROC. N. A. S. ' In accordance with the rule that isobars nearly never differ by one in their charge numbers, table 1 contains only three nuclei with both symbols, p
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Chemistry 543--Final Exam--Keiderling May 5, pm SES
 Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
Lecture 9 Electronic Spectroscopy
 Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
Lecture 9 Electronic Spectroscopy Molecular Orbital Theory: A Review - LCAO approximaton & AO overlap - Variation Principle & Secular Determinant - Homonuclear Diatomic MOs - Energy Levels, Bond Order
QUANTUM MECHANICS AND MOLECULAR STRUCTURE
 6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
In the fourth problem set, you derived the MO diagrams for two complexes containing Cr-Cr bonds:
 Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
wbt Λ = 0, 1, 2, 3, Eq. (7.63)
 7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Vibrational Autoionization in Polyatomic molecules
 Vibrational Autoionization in Polyatomic molecules S.T. Pratt Annu. Rev. Phys. Chem. 2005. 56:281-308 2006. 12. 4. Choi, Sunyoung 1 Schedule 12/4 (Mon) - Introduction - Theoretical background 12/6 (Wed)
Vibrational Autoionization in Polyatomic molecules S.T. Pratt Annu. Rev. Phys. Chem. 2005. 56:281-308 2006. 12. 4. Choi, Sunyoung 1 Schedule 12/4 (Mon) - Introduction - Theoretical background 12/6 (Wed)
Symmetry: Translation and Rotation
 Symmetry: Translation and Rotation The sixth column of the C 2v character table indicates the symmetry species for translation along (T) and rotation about (R) the Cartesian axes. y y y C 2 F v (x) T x
Symmetry: Translation and Rotation The sixth column of the C 2v character table indicates the symmetry species for translation along (T) and rotation about (R) the Cartesian axes. y y y C 2 F v (x) T x
Molecular Physics. Attraction between the ions causes the chemical bond.
 Molecular Physics A molecule is a stable configuration of electron(s) and more than one nucleus. Two types of bonds: covalent and ionic (two extremes of same process) Covalent Bond Electron is in a molecular
Molecular Physics A molecule is a stable configuration of electron(s) and more than one nucleus. Two types of bonds: covalent and ionic (two extremes of same process) Covalent Bond Electron is in a molecular
Assumed knowledge. Chemistry 2. Learning outcomes. Electronic spectroscopy of polyatomic molecules. Franck-Condon Principle (reprise)
 Chemistry 2 Lecture 11 Electronic spectroscopy of polyatomic molecules Assumed knowledge For bound excited states, transitions to the individual vibrational levels of the excited state are observed with
Chemistry 2 Lecture 11 Electronic spectroscopy of polyatomic molecules Assumed knowledge For bound excited states, transitions to the individual vibrational levels of the excited state are observed with
EXPLAINING THE GEOMETRY OF SIMPLE MOLECULES USING MOLECULAR ORBITAL ENERGY-LEVEL DIAGRAMS BUILT BY USING SYMMETRY PRINCIPLES
 Quim. Nova, Vol. XY, No. 00, 17, 200_ http://dx.doi.org/10.21577/01004042.20170198 EXPLAINING THE GEOMETRY OF SIMPLE MOLECULES USING MOLECULAR ORBITAL ENERGYLEVEL DIAGRAMS BUILT BY USING SYMMETRY PRINCIPLES
Quim. Nova, Vol. XY, No. 00, 17, 200_ http://dx.doi.org/10.21577/01004042.20170198 EXPLAINING THE GEOMETRY OF SIMPLE MOLECULES USING MOLECULAR ORBITAL ENERGYLEVEL DIAGRAMS BUILT BY USING SYMMETRY PRINCIPLES
VSEPR Model. Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds
 Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
4 Diatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
l ; Y l,l-1, Y l,-1+1; etc., plus a single non-degenerate function Y l,0, in axial symmetry.
 Chapter 6 Along "Reaction Paths", Orbitals Can be Connected One-to-One According to Their Symmetries and Energies. This is the Origin of the Woodward-offmann Rules I. Reduction in Symmetry As fragments
Chapter 6 Along "Reaction Paths", Orbitals Can be Connected One-to-One According to Their Symmetries and Energies. This is the Origin of the Woodward-offmann Rules I. Reduction in Symmetry As fragments
CHEM J-5 June 2014
 CHEM1101 2014-J-5 June 2014 The molecular orbital energy level diagrams for H 2, H 2 +, H 2 and O 2 are shown below. Fill in the valence electrons for each species in its ground state and label the types
CHEM1101 2014-J-5 June 2014 The molecular orbital energy level diagrams for H 2, H 2 +, H 2 and O 2 are shown below. Fill in the valence electrons for each species in its ground state and label the types
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter IV: Electronic Spectroscopy of diatomic molecules
 Chapter IV: Electronic Spectroscopy of diatomic molecules IV.2.1 Molecular orbitals IV.2.1.1. Homonuclear diatomic molecules The molecular orbital (MO) approach to the electronic structure of diatomic
Chapter IV: Electronic Spectroscopy of diatomic molecules IV.2.1 Molecular orbitals IV.2.1.1. Homonuclear diatomic molecules The molecular orbital (MO) approach to the electronic structure of diatomic
CHEM1901/ J-5 June 2013
 CHEM1901/3 2013-J-5 June 2013 Oxygen exists in the troposphere as a diatomic molecule. 4 (a) Using arrows to indicate relative electron spin, fill the left-most valence orbital energy diagram for O 2,
CHEM1901/3 2013-J-5 June 2013 Oxygen exists in the troposphere as a diatomic molecule. 4 (a) Using arrows to indicate relative electron spin, fill the left-most valence orbital energy diagram for O 2,
Electronic Spectroscopy of Polyatomics
 Electronic Spectroscopy of Polyatomics We shall discuss the electronic spectroscopy of the following types of polyatomic molecules: 1. general AH 2 molecules, A = first-row element 2. formaldehyde 3. benzene
Electronic Spectroscopy of Polyatomics We shall discuss the electronic spectroscopy of the following types of polyatomic molecules: 1. general AH 2 molecules, A = first-row element 2. formaldehyde 3. benzene
We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows:
 We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows: The beryllium sp orbitals overlap with hydrogen Is orbitals (the hydrogen's electrons are shown in the above orbital diagram
We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows: The beryllium sp orbitals overlap with hydrogen Is orbitals (the hydrogen's electrons are shown in the above orbital diagram
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
Inorganic Chemistry with Doc M. Day 19. Transition Metals Complexes IV: Spectroscopy Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields 2. Term symbols and the method of microstates
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
4 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
 CHEMISTRY & YOU Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates.
CHEMISTRY & YOU Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates.
8.3 Bonding Theories > Chapter 8 Covalent Bonding. 8.3 Bonding Theories. 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding
 Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights
Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Lesmahagow High School AHChemistry Inorganic and Physical Chemistry Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Shapes of Molecules and Polyatomic Ions 1 Shapes of molecules Bonding and Electronegativity Revision A Covalent
Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Shapes of Molecules and Polyatomic Ions 1 Shapes of molecules Bonding and Electronegativity Revision A Covalent
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Hybridization of Orbitals
 Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Valence Bond Model and Hybridization
 Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Lecture 4 Model Answers to Problems
 Self-Study Problems / Exam Preparation Construct and annotate a valence MO diagram for H 2 CN -. Use your diagram to explain why the neutral radical is more stable than the anion. (this is an old exam
Self-Study Problems / Exam Preparation Construct and annotate a valence MO diagram for H 2 CN -. Use your diagram to explain why the neutral radical is more stable than the anion. (this is an old exam
Chem 673, Problem Set 5 Due Thursday, November 29, 2007
 Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Molecular Term Symbols
 Molecular Term Symbols A molecular configuration is a specification of the occupied molecular orbitals in a molecule. For example, N : σ gσ uπ 4 uσ g A given configuration may have several different states
Molecular Term Symbols A molecular configuration is a specification of the occupied molecular orbitals in a molecule. For example, N : σ gσ uπ 4 uσ g A given configuration may have several different states
Chapter 8 Problem Solutions
 Chapter 8 Problem Solutions 1. The energy needed to detach the electron from a hydrogen atom is 13.6 ev, but the energy needed to detach an electron from a hydrogen molecule is 15.7 ev. Why do you think
Chapter 8 Problem Solutions 1. The energy needed to detach the electron from a hydrogen atom is 13.6 ev, but the energy needed to detach an electron from a hydrogen molecule is 15.7 ev. Why do you think
Literature values: ΔH f, gas = % error Source: ΔH f, solid = % error. For comparison, your experimental value was ΔH f = phase:
 1 Molecular Calculations Lab: Some guideline given at the bottom of page 3. 1. Use the semi-empirical AM1 method to calculate ΔH f for the compound you used in the heat of combustion experiment. Be sure
1 Molecular Calculations Lab: Some guideline given at the bottom of page 3. 1. Use the semi-empirical AM1 method to calculate ΔH f for the compound you used in the heat of combustion experiment. Be sure
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together.
 Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital
 Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES
 Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES LEARNING OBJECTIVES To build models of selected molecules using VSEPR theory. To illustrate patterns of molecular shapes. BACKGROUND The shapes exhibited
Chemistry 212 MOLECULAR STRUCTURES AND GEOMETRIES LEARNING OBJECTIVES To build models of selected molecules using VSEPR theory. To illustrate patterns of molecular shapes. BACKGROUND The shapes exhibited
Ionic Versus Covalent Bonding
 Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
Ionic Versus Covalent Bonding Ionic compounds are formed when electrons are transferred from one atom to another The transfer of electrons forms ions Each ion is isoelectronic with a noble gas Electrostatic
LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
Periodic Trends. Homework: Lewis Theory. Elements of his theory:
 Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
Quantum Chemistry. NC State University. Lecture 5. The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy
 Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Chemical Bonding & Structure
 Chemical Bonding & Structure Further aspects of covalent bonding and structure Hybridization Ms. Thompson - HL Chemistry Wooster High School Topic 14.2 Hybridization A hybrid orbital results from the mixing
Chemical Bonding & Structure Further aspects of covalent bonding and structure Hybridization Ms. Thompson - HL Chemistry Wooster High School Topic 14.2 Hybridization A hybrid orbital results from the mixing
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
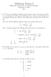 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Molecular orbitals, potential energy surfaces and symmetry
 Molecular orbitals, potential energy surfaces and symmetry mathematical presentation of molecular symmetry group theory spectroscopy valence theory molecular orbitals Wave functions Hamiltonian: electronic,
Molecular orbitals, potential energy surfaces and symmetry mathematical presentation of molecular symmetry group theory spectroscopy valence theory molecular orbitals Wave functions Hamiltonian: electronic,
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
CHEMISTRY 112 LECTURE EXAM II Material
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions
 CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
Chem 673, Problem Set 5 Due Thursday, December 1, 2005
 otton, Problem 9.3 (assume D 4h symmetry) Additional Problems: hem 673, Problem Set 5 Due Thursday, December 1, 2005 (1) Infrared and Raman spectra of Benzene (a) Determine the symmetries (irreducible
otton, Problem 9.3 (assume D 4h symmetry) Additional Problems: hem 673, Problem Set 5 Due Thursday, December 1, 2005 (1) Infrared and Raman spectra of Benzene (a) Determine the symmetries (irreducible
Chapter 6 Vibrational Spectroscopy
 Chapter 6 Vibrational Spectroscopy As with other applications of symmetry and group theory, these techniques reach their greatest utility when applied to the analysis of relatively small molecules in either
Chapter 6 Vibrational Spectroscopy As with other applications of symmetry and group theory, these techniques reach their greatest utility when applied to the analysis of relatively small molecules in either
Lesmahagow High School CfE Advanced Higher Chemistry. Unit 2 Organic Chemistry and Instrumental Analysis. Molecular Orbitals and Structure
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
NPTEL/IITM. Molecular Spectroscopy Lectures 1 & 2. Prof.K. Mangala Sunder Page 1 of 15. Topics. Part I : Introductory concepts Topics
 Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Inorganic Chemistry with Doc M. Fall Semester, 2011 Day 19. Transition Metals Complexes IV: Spectroscopy
 Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Inorganic Chemistry with Doc M. Fall Semester, 011 Day 19. Transition Metals Complexes IV: Spectroscopy Name(s): lement: Topics: 1. The visible spectrum and the d-orbitals 3. Octahedral fields. Term symbols
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
6.2. Introduction to Spectroscopic states and term symbols
 Chemistry 3820 Lecture Notes Dr. M. Gerken Page62 6.2. Introduction to Spectroscopic states and term symbols From the number of absorption bands we have already seen that usually more d-d transitions are
Chemistry 3820 Lecture Notes Dr. M. Gerken Page62 6.2. Introduction to Spectroscopic states and term symbols From the number of absorption bands we have already seen that usually more d-d transitions are
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
The problem of the motion of a particle attracted by two fixed centers. moving under the influence of two fixed centers of Coulomb attraction as a
 466 PHYSICS: E. U. CONDON electron interaction with the molecule by that between the electron and equivalent dipole, one has that vibration transitions associated with electronic excitation of the molecule
466 PHYSICS: E. U. CONDON electron interaction with the molecule by that between the electron and equivalent dipole, one has that vibration transitions associated with electronic excitation of the molecule
X-Ray transitions to low lying empty states
 X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
INTRODUCTION. Fig. 1.1
 1 INTRODUCTION 1.1 SYMMETRY: AN INTRODUCTION In nature, when we see the fascinating world of plants, flowers, birds, architectural buildings (Lotus Temple of Delhi, Taj Mahal, Ashoka Pillar, Rastrapati
1 INTRODUCTION 1.1 SYMMETRY: AN INTRODUCTION In nature, when we see the fascinating world of plants, flowers, birds, architectural buildings (Lotus Temple of Delhi, Taj Mahal, Ashoka Pillar, Rastrapati
Let the longest wavelength of Balmer series is
 1: If the radius of first Bohr orbit of H-atom is x, then de-broglie wavelength of electron in 3 rd orbit is nearly: (A) 2 x (B) 6 x (C) 9x x (D) 3 3h Solution: mvr 3 = 2 9 x 3h mv = mv = 1 2 h = = 6 x
1: If the radius of first Bohr orbit of H-atom is x, then de-broglie wavelength of electron in 3 rd orbit is nearly: (A) 2 x (B) 6 x (C) 9x x (D) 3 3h Solution: mvr 3 = 2 9 x 3h mv = mv = 1 2 h = = 6 x
Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts
 1 Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical species
1 Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical species
Rethinking Hybridization
 Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics
 Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
To visualize the three-dimensional structures of some common molecules. To obtain bond angle, bond length, and hybridization data for molecules.
 Molecular Geometry PURPOSE A B C To explore some simple molecular structures. To explore the relationship between bond order and bond length. To explore resonance structures. GOALS To compare Lewis structures
Molecular Geometry PURPOSE A B C To explore some simple molecular structures. To explore the relationship between bond order and bond length. To explore resonance structures. GOALS To compare Lewis structures
Chemical Bonding. The Octet Rule
 Chemical Bonding There are basically two types of chemical bonds: 1. Covalent bonds electrons are shared by more than one nucleus 2. Ionic bonds electrostatic attraction between ions creates chemical bond
Chemical Bonding There are basically two types of chemical bonds: 1. Covalent bonds electrons are shared by more than one nucleus 2. Ionic bonds electrostatic attraction between ions creates chemical bond
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Chapter 12. Molecular Structure
 Chapter 12 Molecular Structure Chapter Map Models Advantages and Disadvantages (1) They help us to visualize, explain, and predict chemical changes. Because a model is a simplified version of what we think
Chapter 12 Molecular Structure Chapter Map Models Advantages and Disadvantages (1) They help us to visualize, explain, and predict chemical changes. Because a model is a simplified version of what we think
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chm 363. Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra. Mr. Linck. Version 1.5 March 9, 2017
 Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Downloaded from
 Points to Remember Class: XI Chapter Name: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical
Points to Remember Class: XI Chapter Name: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry. Zachary Chin, Alex Li, Alex Liu
 A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
 CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester Christopher J. Cramer. Lecture 30, April 10, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 442 Review for Exam 2. Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative (3D) components.
 Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Molecular-Orbital Theory
 Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
Prof. Dr. I. Nasser atomic and molecular physics -551 (T-11) April 18, 01 Molecular-Orbital Theory You have to explain the following statements: 1- Helium is monatomic gas. - Oxygen molecule has a permanent
Electronic Spectra of Complexes
 Electronic Spectra of Complexes Interpret electronic spectra of coordination compounds Correlate with bonding Orbital filling and electronic transitions Electron-electron repulsion Application of MO theory
Electronic Spectra of Complexes Interpret electronic spectra of coordination compounds Correlate with bonding Orbital filling and electronic transitions Electron-electron repulsion Application of MO theory
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron pair repulsion (VSEPR)
Chemical Bonding II: and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron pair repulsion (VSEPR)
CHEMISTRY XL-14A CHEMICAL BONDS
 CHEMISTRY XL-14A CHEMICAL BONDS July 16, 2011 Robert Iafe Office Hours 2 July 18-July 22 Monday: 2:00pm in Room MS-B 3114 Tuesday-Thursday: 3:00pm in Room MS-B 3114 Chapter 2 Overview 3 Ionic Bonds Covalent
CHEMISTRY XL-14A CHEMICAL BONDS July 16, 2011 Robert Iafe Office Hours 2 July 18-July 22 Monday: 2:00pm in Room MS-B 3114 Tuesday-Thursday: 3:00pm in Room MS-B 3114 Chapter 2 Overview 3 Ionic Bonds Covalent
Physical Chemistry Lab II CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible.
 Physical Chemistry Lab II Name: KEY CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible. Constants: c = 3.00 10 8 m/s h = 6.63 10-34 J s 1 Hartree = 4.36 10-18
Physical Chemistry Lab II Name: KEY CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible. Constants: c = 3.00 10 8 m/s h = 6.63 10-34 J s 1 Hartree = 4.36 10-18
Lewis Structure and Electron Dot Models
 Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Ch. 7 The Quantum Mechanical Atom. Brady & Senese, 5th Ed.
 Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
