Historical Developments in Chemistry. SC.912.P Major Models of the Atom SC.912.N.3.3 Scientific Law vs. Scientific Theory
|
|
|
- Arleen Campbell
- 6 years ago
- Views:
Transcription
1 Historical Developments in Chemistry SC.912.P Major Models of the Atom SC.912.N.3.3 Scientific Law vs. Scientific Theory
2 Atoms vs. Elements Element: pure substance that cannot be separated into simpler substances Made up of only 1 kind of atom Combine to form compounds Atom: the smallest particle into which an element can be divided and still be the same substance Atoms make up elements Democratus (400 BC) -If you cut a substance in half forever you end up with an uncuttable particle = atom
3 John Dalton ( ) 1. All matter is made of atoms; atoms are indivisible and indestructible. 2. All atoms of a given element are identical in mass and properties. 3. Atoms join with other atoms to make new substances. Atoms hard; dense (like billiard balls) *Disclaimer* Modern Atomic Theory elaborates more, but Dalton s theory became the theoretical foundation in chemistry (e.g. atoms can be destroyed in nuclear reactions but not by chemical reactions, different kinds of atoms = isotopes)
4 J.J. Thomson ( )- -Discovered the electron -Realized the accepted model of an atom did not account for negatively or positively charged particles - Plum Pudding model; explained some of the atom s electrical properties, but failed to recognize the atom s positive charges as particles.
5 Thomson s Cathode Ray Tube Experiment YouTube URL: Or search YouTube for Cathode Ray Tube
6 Thomson s Cathode Ray Tube Experiment YouTube URL: Or search YouTube for Discovery of the Electron: Cathode Ray Tube Experiment
7 Ernest Rutherford ( )- - Gold Foil experiment showed the mass of an atom was concentrated in a nucleus - Nuclear Model of the Atom - Did not yet theorize about neutrons (not discovered until 1932 by James Chadwick) Fun-fact Ernest Rutherford was Thomson s student!...who disproved Thomson s Plum Pudding model.
8 Note: All you need to understand about alpha particles at this point is that they are positively charged particles
9 Rutherford s Gold Foil Experiment YouTube URL: Or search YouTube for Rutherford Gold Foil Experiment Backstage Science
10 Niels Bohr ( )- - Electron Shell Model (or the Quantum Model) - Suggested that: - electrons in an atom revolve around the nucleus only in certain discrete, separate orbits, or shells
11 n Erwin Schroedinger ( )- - Mathematical model (wave functions) for the distribution of electrons in an atom Electron Cloud Model - Probability of where electrons are - 3-dimensional model better describes 3-dimensional space - Bohr s model was only 1- dimensional Werner Heisenberg ( ) - Uncertainty principle the position and velocity cannot both be measured at the same time - Since you could never with great certainty measure more than one property of a particle, you could only work with probability & mathematical formulations
12 Law vs. Theory Scientific LAW A set of observed regularities expressed in a concise verbal or mathematical statement A statement that a particular phenomena always occurs if certain conditions are present Describes what nature does under certain conditions (predicts what will happen) Resists change over time Scientific THEORY An explanation for an observation (or series of observations) that is substantiated by a considerable body of evidence A body of knowledge and explanatory concepts that seek to increase our understanding (explain) a major phenomenon in nature Explains how nature works
13 Sooo what about Models? Constructed to help explain complex concepts
14 LAW or THEORY? Sir Isaac Newton s of Gravity LAW Gravity is described as-is, not explained in terms of how it probably came about Ms. O., what s gravity? Ms. O, why is there gravity?
15 Practice The law of gravity describes a relationship between two masses. The more massive an object is or the closer it is to another body, the more the gravitational attraction between the two objects. However, the law is limited because it cannot explain which of the following? A) what gives the larger object more mass B) why we experience gravity on earth and not in space C) why there is a gravitational attraction in the first place D) why does the smaller object also attract the larger object
16 Practice The law of gravity describes a relationship between two masses. The more massive an object is or the closer it is to another body, the more the gravitational attraction between the two objects. However, the law is limited because it cannot explain which of the following? A) what gives the larger object more mass B) why we experience gravity on earth and not in space C) why there is a gravitational attraction in the first place D) why does the smaller object also attract the larger object
17 What is this model called? The Plum Pudding model Who created this model? Thomson What is he most known for discovering? The electron Who disproved it? Rutherford Which experiment disproved it? Gold Foil Experiment
18 Whose theory does the other model represent? Rutherford s NUCLEAR Model What is the main difference Which model represents Bohr s atomic theory? between the two models? Bohr s model requires discrete electron orbits
19 Whose model laid the foundation for modern Atomic Theory? Dalton What did his theory say? (Hint: 3 things) 1. All matter is made up of atoms, which are indivisible 2. All atoms of a given element are the same mass and properties 3. Atoms join with other atoms to make new substances
20 Summary Dalton laid the theoretical foundation for Atomic Theory Thomson discovered the electron using Cathode Ray experiment; developed Plum Pudding Model Rutherford disproved Plum Pudding Model; replaced it with Nuclear Model after conducting Gold Foil Experiment which provided evidence for an dense, positively-charged atomic nucleus Bohr Replaced Rutherford s model with the electron shell model (or Quantum theory); key feature = discrete electron orbits with specific energy levels that prevent negatively-charged electrons from collapsing into the positively-charged nucleus Shroedinger electron cloud model; said that the position and location of electrons in an atom is better described with probabilities (a three-dimensional model rather than Bohr s one-dimensional model) Scientific Theory well-supported explanations of phenomena (WHY x happens) Scientific Law well-supported descriptions of phenomena (WHAT happens when x occurs) **Theories do not become laws, and laws do not become theories**
21 Anatomy of the Atom SC.912.P.8.4 Structure & Composition of Atoms
22 Word Bank Construct/Draw an atomic model Label using the word bank, and Please include the electrical charge PROTON - electrical charge? NEUTRON - electrical charge? ELECTRON - electrical charge? NUCLEUS - electrical charge? ELECTRON CLOUD electrical charge?
23 PROTONS CHARGE of proton? (+) Positive LOCATION of proton? Nucleus SUPPORT THESE ANSWERS USING WHAT YOU KNOW ABOUT THE SCIENTISTS WHO HELPED DEVELOP ATOMIC THEORY E.g. Particular experimental observation? An inference?
24 ELECTRONS CHARGE of electron? (-) negative LOCATION of electron? Electron cloud SUPPORT THESE ANSWERS USING WHAT YOU KNOW ABOUT THE SCIENTISTS WHO HELPED DEVELOP ATOMIC THEORY E.g. Particular experimental observation? An inference?
25 NEUTRONS CHARGE of neutron? 0 - Neutral (no charge) LOCATION of neutron? nucleus We did not discuss neutrons so this one is given to you
26 NUCLEUS Electrical charge of nucleus? (+) positive Location of nucleus? In the center of the atom SUPPORT THESE ANSWERS USING WHAT YOU KNOW ABOUT THE SCIENTISTS WHO HELPED DEVELOP ATOMIC THEORY. E.g. Particular experimental observation? An inference?
27 ELECTRON CLOUD Electrical charge of electron cloud? (-) negative Location of electron cloud? Everywhere that isn t the nucleus SUPPORT THESE ANSWERS USING WHAT YOU KNOW ABOUT THE SCIENTISTS WHO HELPED DEVELOP ATOMIC THEORY. E.g. Particular experimental observation? An inference?
28
29 Forces of Attraction/Repulsion > electrostatic forces: affects objects that have charge more mass in an atom = larger electron cloud = larger magnitude of distortions in the electron distribution => Dipoles! DIPOLE: a charge separation
30 Atomic Parts & What to Know Subatomic Particles Proton, Neutron, Electron Location Nucleus or Electron Cloud? Charge Positive, Negative, or Neutral? Forces of attraction and repulsion between subatomic particles E.g. electrical charge (opposite charges attract, like charges repel) RELATIVE SIZE of proton, neutron, and electron Which is the SMALLEST?
31 ACTIVITY/HOMEWORK (it s only homework if you do not finish in class) Another form of note-taking/organizing information into a GRAPHIC ORGANIZER. Your Task: Draw/Make a graphic organizer that summarizes the Historical Developments of Atomic Theory Please include the following information: - Name of scientists (in chronological order) -Their discovery or idea -Their experiment (if applicable) -The name of their model and a sketch of it -What makes each theory/idea different from the last? (This part does not apply to Democratus)
32 ACTIVITY/HOMEWORK (it s only homework if you do not finish in class) Another form of note-taking/organizing information into a GRAPHIC ORGANIZER. Your Task: Draw/Make a graphic organizer that summarizes the atomic structure Please include the following information: - the 3 subatomic particles (charge, location, relative size) - the 2 different atomic structures (charge, location) - label a picture of an atom that includes all of the above
What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms.
 Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
Atomic Models. A model uses familiar ideas to explain unfamiliar facts observed in nature. A model can be changed as new information is collected.
 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
7.1 Development of a Modern Atomic Theory
 7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
The idea of an atom began about 400 B.C. with many Greek philosophers, like Democritus, working to figure out what everything was made of.
 The idea of an atom began about 400 B.C. with many Greek philosophers, like Democritus, working to figure out what everything was made of. Always move & join together Atomas means indivisible I m Aristotle
The idea of an atom began about 400 B.C. with many Greek philosophers, like Democritus, working to figure out what everything was made of. Always move & join together Atomas means indivisible I m Aristotle
EARLY VIEWS: The Ancient Greeks
 Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C.
 Chapter 5 Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C. Democritus thought atoms were indivisible & indestructible Lacked experimental support
Chapter 5 Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C. Democritus thought atoms were indivisible & indestructible Lacked experimental support
SNC1D1 History of the Atom
 SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
Democritus & Leucippus (~400 BC) Greek philosophers: first to propose that matter is made up of particles called atomos, the Greek word for atoms
 AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester?
 Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Get out your diagram from your research paper. Get out a sheet of paper to take some notes on.
 Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
The History of the Atom. How did we learn about the atom?
 The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
PROGRESSION OF THE ATOMIC MODEL
 PROGRESSION OF THE ATOMIC MODEL By 1808, it was widely accepted that matter was made up of ELEMENTS, which consisted of tiny PARTICLES called ATOMS. After 2000 years - DEMOCRITUS was right all along John
PROGRESSION OF THE ATOMIC MODEL By 1808, it was widely accepted that matter was made up of ELEMENTS, which consisted of tiny PARTICLES called ATOMS. After 2000 years - DEMOCRITUS was right all along John
Dalton Thompson Rutherford Bohr Modern Model ("Wave. Models of the Atom
 Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Atomic Structure. A model uses familiar ideas to explain unfamiliar facts observed in nature.
 Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Early Ideas About Matter
 Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
The structure of Atom III
 The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
Bellwork: 2/6/2013. atom is the. atom below. in an atom is found in the. mostly. 2. The smallest part of an. 1. Label the parts of the
 Bellwork: 2/6/2013 1. Label the parts of the atom below. B 2. The smallest part of an atom is the. 3. The majority of the mass in an atom is found in the. A C 4. An atom is made up of mostly. Bellwork:
Bellwork: 2/6/2013 1. Label the parts of the atom below. B 2. The smallest part of an atom is the. 3. The majority of the mass in an atom is found in the. A C 4. An atom is made up of mostly. Bellwork:
Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester?
 Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
In many ways, Dalton's ideas are still useful today. For example, they help us to understand elements, compounds, and molecules.
 History of the Atom Name: Reading excerpt from Absorb Chemistry for GCSE by Lawrie Ryan http://www.absorblearning.com/chemistry/demo/units/lr301.html Introduction Our understanding of the physical world
History of the Atom Name: Reading excerpt from Absorb Chemistry for GCSE by Lawrie Ryan http://www.absorblearning.com/chemistry/demo/units/lr301.html Introduction Our understanding of the physical world
History of the OBJECTIVES. ESSENTIAL QUESTION What evidence is there for the existence of atoms and their sub-atomic particles?
 History of the 09/15/2016 OBJECTIVES Understand the law of definite proportions. Define a scientific law and identify how observations become a law. Explain that a scientific theory is not established
History of the 09/15/2016 OBJECTIVES Understand the law of definite proportions. Define a scientific law and identify how observations become a law. Explain that a scientific theory is not established
9/23/2012. Democritus 400 B.C. Greek philosopher Proposed that all materials are made from atoms. Coined Greek word atmos, meaning indivisible.
 Mr. Sudbury Atoms are too small to see with your eyes. Atoms are too small to see with the most powerful microscopes. Scientist use models to explain atoms. A scientific model is an representation containing
Mr. Sudbury Atoms are too small to see with your eyes. Atoms are too small to see with the most powerful microscopes. Scientist use models to explain atoms. A scientific model is an representation containing
Democritus 460 BC 370 BC. First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible.
 Democritus 460 BC 370 BC First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible. Democritus 460 BC 370 BC Problems with theory: 1. Did not explain chemical
Democritus 460 BC 370 BC First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible. Democritus 460 BC 370 BC Problems with theory: 1. Did not explain chemical
Chapter #1 - Atomic Structure
 Chapter #1 - Atomic Structure Atomic Theories Democritus (460-340 BC) Democritus believed that all matter consisted of extremely small particles that could not be divided. He called them atoms from the
Chapter #1 - Atomic Structure Atomic Theories Democritus (460-340 BC) Democritus believed that all matter consisted of extremely small particles that could not be divided. He called them atoms from the
Memorial to a Scientist
 Memorial to a Scientist 1. My Question of Inquiry: Use this sheet to outline how you will collect and present the information to the class. My Group s Scientist: 1 Part I: Memorial to a Scientist: John
Memorial to a Scientist 1. My Question of Inquiry: Use this sheet to outline how you will collect and present the information to the class. My Group s Scientist: 1 Part I: Memorial to a Scientist: John
Atomic Theory Development
 Atomic Theory Development Born as early as 400 BC, it took more than 2000 years before Science was ready to accept the idea of atomic structure of matter and another 150 years to develop a good model!
Atomic Theory Development Born as early as 400 BC, it took more than 2000 years before Science was ready to accept the idea of atomic structure of matter and another 150 years to develop a good model!
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
Chapter 11 Study Questions Name: Class:
 Chapter 11 Study Questions Name: Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. The discovery of which particle proved that the atom
Chapter 11 Study Questions Name: Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. The discovery of which particle proved that the atom
Nuclear Chemistry. Atomic Structure Notes Start on Slide 20 from the second class lecture
 Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
1 Development of the Atomic Theory
 CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
AP Atomic Structure Models
 AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
The History of Atomic Theory Chapter 3--Chemistry
 The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
Chap 4 Bell -Ringers
 Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Chapter 4. Atomic Structure
 Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
DEMOCRITUS - A philosopher in the year 400 B.C. - He didn t do experiments and he wondered if atoms kept on being divided, that there would only be
 DEMOCRITUS A philosopher in the year 400 B.C. He didn t do experiments and he wondered if atoms kept on being divided, that there would only be one undividable particle left. He discovered that this was
DEMOCRITUS A philosopher in the year 400 B.C. He didn t do experiments and he wondered if atoms kept on being divided, that there would only be one undividable particle left. He discovered that this was
Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON
 Unit 1: Atoms Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON The History of Discovering the Atom The Timeline of Discovery 1. Philosophical Era 2. Alchemical Era 3. Classical
Unit 1: Atoms Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON The History of Discovering the Atom The Timeline of Discovery 1. Philosophical Era 2. Alchemical Era 3. Classical
The Beginning of the Atom
 The Beginning of the Atom Democritus s Atomic Philosophy 460BC 370BC ~ Believed atoms were indivisible and indestructible Dalton took it and ran with it 2000 yrs later! Jan 31 3:33 PM Dalton and the Atomic
The Beginning of the Atom Democritus s Atomic Philosophy 460BC 370BC ~ Believed atoms were indivisible and indestructible Dalton took it and ran with it 2000 yrs later! Jan 31 3:33 PM Dalton and the Atomic
Structure of the Atom. Atomic Components
 Chapter 19 Atoms 0 Matter is anything that takes up space and has mass. All matter is made of atoms. 0 Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board,
Chapter 19 Atoms 0 Matter is anything that takes up space and has mass. All matter is made of atoms. 0 Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board,
Chapter 3. Atom. Table of Contents. 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology
 Atom Table of Contents 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology The History of The Atom Warm up Make a list of inferences about any properties of objects
Atom Table of Contents 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology The History of The Atom Warm up Make a list of inferences about any properties of objects
Atomic Theory. Developing the Nuclear Model of the Atom. Saturday, January 20, 18
 Atomic Theory Developing the Nuclear Model of the Atom Democritus Theory: Atom, the indivisible particle c. 300 BC Democritus Problem: No scientific evidence c. 300 BC Dalton Theory: The solid sphere model
Atomic Theory Developing the Nuclear Model of the Atom Democritus Theory: Atom, the indivisible particle c. 300 BC Democritus Problem: No scientific evidence c. 300 BC Dalton Theory: The solid sphere model
Atomic Theory. The History of Atomic Theory
 Atomic Theory The History of Atomic Theory This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels.
Atomic Theory The History of Atomic Theory This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels.
Investigating Atoms and Atomic Theory
 Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Early Atomic Models. Atoms: the smallest particle of an element that retains the properties of that element.
 Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
1 Development of the Atomic Theory
 CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
democritus (~440 bc) who was he? theorized: A Greek philosopher
 democritus (~440 bc) who was he? A Greek philosopher theorized: Everything in the world is made up small particles that we cannot see The shape of these particles determine the properties of a substance
democritus (~440 bc) who was he? A Greek philosopher theorized: Everything in the world is made up small particles that we cannot see The shape of these particles determine the properties of a substance
Where are we? Check-In
 Where are we? Check-In ü Building Blocks of Matter ü Moles, molecules, grams, gases, ü The Bohr Model solutions, and percent composition Coulomb s Law ü Empirical and Molecular formulas Photoelectron Spectroscopy
Where are we? Check-In ü Building Blocks of Matter ü Moles, molecules, grams, gases, ü The Bohr Model solutions, and percent composition Coulomb s Law ü Empirical and Molecular formulas Photoelectron Spectroscopy
The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal.
 Section 1 Development of the Atomic Theory Bellringer The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal. Color exists by convention,
Section 1 Development of the Atomic Theory Bellringer The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal. Color exists by convention,
Ancient Greek Models of Atoms
 Atomic Theory Ancient Greek Models of Atoms The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the
Atomic Theory Ancient Greek Models of Atoms The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.1 Introduction to Atoms
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
Atomic Theory. Democritus to the Planetary Model
 Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
Name Class Date. Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY
 Skills Worksheet Directed Reading A Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY Write the letter of the correct answer in the space provided. 1. Around 440 BCE, who thought
Skills Worksheet Directed Reading A Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY Write the letter of the correct answer in the space provided. 1. Around 440 BCE, who thought
SNC1D CHEMISTRY 2/8/2013. ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P ) Atomic Theory. Atomic Theory
 SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
Name Period Date Engage-Atoms 1. What does Bill cut in half?
 Name Period Date Engage-Atoms https://www.youtube.com/watch?v=fmuskig2exi 1. What does Bill cut in half? 2. By cutting this item in half he tries to prove that there are pieces that are uncut- table called
Name Period Date Engage-Atoms https://www.youtube.com/watch?v=fmuskig2exi 1. What does Bill cut in half? 2. By cutting this item in half he tries to prove that there are pieces that are uncut- table called
Atomic Structure. How do you discover and study something you can t see?
 Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video
 Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
What is matter? Matter is anything that has mass and takes up space. What is matter made of??
 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter Remember???? The Cell theory - 3
What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter Remember???? The Cell theory - 3
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
BEGINNING OF ATOMIC THEORY
 Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. What does the word atom mean? a. dividable b. invisible c. hard particles d. not able to
Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. What does the word atom mean? a. dividable b. invisible c. hard particles d. not able to
Updating the Atomic Theory
 Updating the Atomic Theory Three major differences between modern atomic theory and Dalton s atomic theory 1. Atoms are NOT indivisible. They are made up of smaller particles: electrons, protons and neutrons.
Updating the Atomic Theory Three major differences between modern atomic theory and Dalton s atomic theory 1. Atoms are NOT indivisible. They are made up of smaller particles: electrons, protons and neutrons.
The Development of Atomic Theory
 The Development of Atomic Theory Ideas & Theories in Science Change Our theory about the atom has changed over time as new studies are done. Even though no one has ever seen an atom up close we are still
The Development of Atomic Theory Ideas & Theories in Science Change Our theory about the atom has changed over time as new studies are done. Even though no one has ever seen an atom up close we are still
Directed Reading B. Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY. is a(n). DALTON S ATOMIC THEORY BASED ON EXPERIMENTS
 Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Atomic Theory. Past and Present: pieces of a puzzle
 Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Unit 3 Lesson 1 The Atom. Copyright Houghton Mifflin Harcourt Publishing Company
 As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
Physics 30 Modern Physics Unit: Atomic Basics
 Physics 30 Modern Physics Unit: Atomic Basics Models of the Atom The Greeks believed that if you kept dividing matter into smaller and smaller pieces, you would eventually come to a bit of matter that
Physics 30 Modern Physics Unit: Atomic Basics Models of the Atom The Greeks believed that if you kept dividing matter into smaller and smaller pieces, you would eventually come to a bit of matter that
CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS
 CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Dalton s Atomic theory ** 2. J J Thomson Experiments *** 3. Rutherford s Scattering Experiments
CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Dalton s Atomic theory ** 2. J J Thomson Experiments *** 3. Rutherford s Scattering Experiments
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Chapter 4: Atomic Structure Section 4.1 Defining the Atom
 Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
Major upsetting discoveries: Today s Objectives/Agenda. Notice: New Unit: with Ms. V. after school Before Friday 9/22.
 Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
Atomic Theory Timeline Project
 Atomic Theory Timeline Project MAKE AN ATOMIC THEORY TIMELINE! Directions: 1) Read the information about the scientists and theories that have developed over time about matter and the atom in the Atomic
Atomic Theory Timeline Project MAKE AN ATOMIC THEORY TIMELINE! Directions: 1) Read the information about the scientists and theories that have developed over time about matter and the atom in the Atomic
Atomic Structure. ppst.com
 Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
History and Structure of the Atom. From Democritus to...
 1 History and Structure of the Atom From Democritus to... History of Atomic Theory 2 Democritus (from about 440 BC) coined the term atom which means uncuttable He felt that if you kept cutting matter smaller
1 History and Structure of the Atom From Democritus to... History of Atomic Theory 2 Democritus (from about 440 BC) coined the term atom which means uncuttable He felt that if you kept cutting matter smaller
History of Atomic Theory
 Unit 2 The Atom History of Atomic Theory A. Democritus and Aristotle Democritus named the "atom" - means indivisible Dalton (with work of Lavoisier, Proust, and Gay-Lussac) 1. atomic theory - first based
Unit 2 The Atom History of Atomic Theory A. Democritus and Aristotle Democritus named the "atom" - means indivisible Dalton (with work of Lavoisier, Proust, and Gay-Lussac) 1. atomic theory - first based
Atom s Structure. Chemistry is the study of the structure, characteristics and behavior of matter. The basic
 AlMarzooqi 1 Hamad AlMarzooqi Professor Kafle ESL 015 26 February 2013 Atom s Structure Chemistry is the study of the structure, characteristics and behavior of matter. The basic unit of matter is an atom,
AlMarzooqi 1 Hamad AlMarzooqi Professor Kafle ESL 015 26 February 2013 Atom s Structure Chemistry is the study of the structure, characteristics and behavior of matter. The basic unit of matter is an atom,
Honors Ch3 and Ch4. Atomic History and the Atom
 Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Make sure this is handed in!
 Make sure this is handed in! Based on the 3 groups in early atomic history, pick one of the groups and explain how they progressed the current knowledge of atoms and elements at their time. OR Explain
Make sure this is handed in! Based on the 3 groups in early atomic history, pick one of the groups and explain how they progressed the current knowledge of atoms and elements at their time. OR Explain
Webquest: Atomic Theories and Models. Answer the following questions regarding the progression of the model of the atom.
 Chemistry Ms. Ye Name Date Block Webquest: Atomic Theories and Models Answer the following questions regarding the progression of the model of the atom. Early Ideas About Atoms: Go to http://galileo.phys.virginia.edu/classes/252/atoms.html
Chemistry Ms. Ye Name Date Block Webquest: Atomic Theories and Models Answer the following questions regarding the progression of the model of the atom. Early Ideas About Atoms: Go to http://galileo.phys.virginia.edu/classes/252/atoms.html
UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE
 S T U D E N T N O T E S P r e - A P C h e m i s t r y U N I T 4 Page 1 NAME PERIOD UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE STUDENT OBJECTIVES: Your fascinating teachers would like you amazing learners
S T U D E N T N O T E S P r e - A P C h e m i s t r y U N I T 4 Page 1 NAME PERIOD UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE STUDENT OBJECTIVES: Your fascinating teachers would like you amazing learners
Unit 2 Atomic Structure and Nuclear Chemistry
 Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
a. According to Dalton, what is inside the atom? Nothing, the atom it the smallest
 Unit 3: Review SCIENTIFIC THEORIES Dalton theorized that atoms were the smallest particle and could not be divided. Atoms can bond with one another in whole number ratios to form compounds but cannot be
Unit 3: Review SCIENTIFIC THEORIES Dalton theorized that atoms were the smallest particle and could not be divided. Atoms can bond with one another in whole number ratios to form compounds but cannot be
Atomic Theory The earliest recorded information about changes in matter come from the early Greeks so it is often referred to as the Greek Model. Anci
 Atomic Theory A theory - a thoroughly tested explanation. With more information the explanations change, hopefully the explanations get better, explain more behaviors. While the old theory still works,
Atomic Theory A theory - a thoroughly tested explanation. With more information the explanations change, hopefully the explanations get better, explain more behaviors. While the old theory still works,
Atomic Structure. Chemistry Mr. McKenzie
 Atomic Structure Chemistry Mr. McKenzie How was the understanding of the atom developed? John Dalton (1766-1844) - developed a model to explain observations made at the time 1. Elements are made of tiny
Atomic Structure Chemistry Mr. McKenzie How was the understanding of the atom developed? John Dalton (1766-1844) - developed a model to explain observations made at the time 1. Elements are made of tiny
Atomic Structure. History of Atomic Theory
 Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Early Models of the Atom
 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms,
Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms,
SCH3U1 This presentation and more can be found at
 SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
Origins of the Atom. Atoms: The Building Blocks of Matter. Let s Get Ready to Rumble. Aristotle s Theory of the Atom CHAPTER 3
 Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Understanding the Atom 1.3
 Understanding the Atom 1.3 Introduction to Atoms 1.What are some ways that scientists have described the atom? 2.What are the parts of the atom, and how are they arranged? 3.How are atoms of all elements
Understanding the Atom 1.3 Introduction to Atoms 1.What are some ways that scientists have described the atom? 2.What are the parts of the atom, and how are they arranged? 3.How are atoms of all elements
Rhonda Alexander IC Science Robert E. Lee
 Rhonda Alexander IC Science Robert E. Lee Atom The smallest particle of an element that retains all of the chemical properties of the element. The Theory & Evidence for John Dalton s Atomic Theory: Around
Rhonda Alexander IC Science Robert E. Lee Atom The smallest particle of an element that retains all of the chemical properties of the element. The Theory & Evidence for John Dalton s Atomic Theory: Around
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
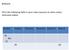 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
Binder. Notes: DO NOW
 CONTENT OBJECTIVE: SWBAT EXPLAIN HOW THE MODEL OF THE ATOM HAS EVOLVED. Binder HW for checking: 2.1 HW SKILL OBJECTIVES: SWBAT STOP AND THINK ALOUD TO MAKE SURE OF THEIR UNDERSTANDING SWBAT HIGHLIGHT FOR
CONTENT OBJECTIVE: SWBAT EXPLAIN HOW THE MODEL OF THE ATOM HAS EVOLVED. Binder HW for checking: 2.1 HW SKILL OBJECTIVES: SWBAT STOP AND THINK ALOUD TO MAKE SURE OF THEIR UNDERSTANDING SWBAT HIGHLIGHT FOR
Ch4 and Ch5. Atomic History and the Atom
 Ch4 and Ch5 Atomic History and the Atom Ch4.2 What are atoms? Atoms are the smallest part of an element that still has the element s properties. Ch. 4.3 The Atom is Defined 400 B.C. the Greek philosopher
Ch4 and Ch5 Atomic History and the Atom Ch4.2 What are atoms? Atoms are the smallest part of an element that still has the element s properties. Ch. 4.3 The Atom is Defined 400 B.C. the Greek philosopher
Atomic Theory. Why do we believe that all matter is made of atoms?
 Atomic Theory Why do we believe that all matter is made of atoms? 1. Law of definite composition: Compounds (like H 2 O) contain the same elements in the same proportions by mass regardless of the size
Atomic Theory Why do we believe that all matter is made of atoms? 1. Law of definite composition: Compounds (like H 2 O) contain the same elements in the same proportions by mass regardless of the size
Chemistry. Chapter 14 Section 1
 Chemistry Chapter 14 Section 1 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter There
Chemistry Chapter 14 Section 1 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter There
4-1 Notes. Defining the Atom
 4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
Warm Up 1: CH 4 RG Pass it up!
 WU: A number is not a measurement without a Thermal expansion of metals = draw a particle drawing of a solid under these 3 conditions 30 o C, room temp, 1000 o C What is air made of? What does the law
WU: A number is not a measurement without a Thermal expansion of metals = draw a particle drawing of a solid under these 3 conditions 30 o C, room temp, 1000 o C What is air made of? What does the law
Where it came from and what we know now
 Where it came from and what we know now History of the Atom The first mention of the atom came from Democritus in ancient Greece He suggested that the universe was made up of small, indivisible units called
Where it came from and what we know now History of the Atom The first mention of the atom came from Democritus in ancient Greece He suggested that the universe was made up of small, indivisible units called
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr
 Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
Name: Date: Blk: Dalton Thomson Rutherford Bohr THOMSON
 Name: Date: Blk: NOTES: ATOMIC STRUCTURE I. History of the Atom Dalton Thomson Rutherford Bohr 1803 1897 1909 1913 1. DALTON - everything is made of atoms - different elements combine to form compounds
Name: Date: Blk: NOTES: ATOMIC STRUCTURE I. History of the Atom Dalton Thomson Rutherford Bohr 1803 1897 1909 1913 1. DALTON - everything is made of atoms - different elements combine to form compounds
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
