Ch.10 Alkyl Halides. Organic halides are valuable as industrial solvents, inhaled anesthetics in medicine, refrigerants, and pesticides.
|
|
|
- Donald Owens
- 6 years ago
- Views:
Transcription
1 Ch.10 Alkyl alides Organic halides are valuable as industrial solvents, inhaled anesthetics in medicine, refrigerants, and pesticides. F F C C F Trichloroethylene (a solvent) alothane (an inhaled anesthetic) F C F C Dichlorodifluoromethane (arefrigerant) omoethane (a fumigant)
2 Ch.10 Alkyl alides a lot of pharmaceutical compounds are organic halides N N 200 times more potent than morphine at blocking pain in animals Epibatidine
3 10.1 Naming Alkyl alides Ch.10 Alkyl alides named in the same way as alkanes, treat the halogen as a substituent Step 1 Step 2 Name the parent hydrocarbon: Find the longest carbon chain. If a double bond or triple bond is present, parent chain must contain it. Numbering: Begin at the end nearer the first substituent, regardless of whether it is alkyl or halo omo-4,5-dimethylheptane
4 Ch.10 Alkyl alides (a) di-, tri-, tetra-...: same kind of halogens (b) list in alphabetical order for different halogens ,3-Dichloro-4-methylhexane omo-3-chloro-4-methylpentane
5 Ch.10 Alkyl alides Step 3 Begin at the end nearer the substituent (either alkyl or halo) that has alphabetical precedence omo-5-methylhexane common names C 3 I Iodomethane (or Methyl iodide) 2-Chloropropane (or Isopropyl chloride) omocyclohexane (or Cyclohexyl bromide)
6 Ch.10 Alkyl alides 10.2 Structure of Alkyl alides sp 3, tetrahedral Bond length Bond strength C F C C C I 139 pm kj/mol kcal/mol Electrophilic carbon in C-X δ - X δ + C electrophilic site
7 Ch.10 Alkyl alides 10.3 Preparation of Alkyl alides Additions of X, X 2 to alkenes: X 2 X X X X C 3 3 C X=, X=, or I
8 adical chlorination Ch.5 An Overview of Organic eactions 3 C + hv 3 C + initiation hv (UV) 2 propagation (a) 3 C + C 3 + (b) C C + (c) epeat steps (a) and (b) until termination
9 Ch.5 An Overview of Organic eactions C 3 C 3 3 C termination + 2 C C C 3 + C 3 3 C C 3
10 Ch.10 Alkyl alides 10.4 adical alogenation of Alkanes a poor method of alkyl halide synthesis: poly-halogenation 3 C + hv 3 C + hv C hv C 3 + hv C 4 +
11 Ch.10 Alkyl alides hv dichloro-, trichloro, : 70 hv + + dichloro-, trichloro, : 65
12 Ch.10 Alkyl alides eactivity toward halogenation relative reactivity + 1 o C: 6 2 o C: 4 30% / 6 = 5% 70% / 4 = 17.5% 30 : 70 2 o : 1 o = 17.5 : 5 = 3.5 : o C: 1 1 o C: 9 35% / 1 = 35% 65% / 9 = 7.2% 35 : 65 3 o : 1 o = 35 : 7.2 = 5 : 1 1 o : 2 o : 3 o = 1 : 3.5 : 5.0
13 Ch.10 Alkyl alides eactivity toward halogenation C C C primary < secondary < tertiary reactivity
14 Ch.10 Alkyl alides Bond dissociation energy: weaker 3 o C- bond is most reactive Table 5.3 Bond dissociation energy (D) 1 o C- bond 2 o C- bond 3 o C- bond 420 kj/mol (100 kcal/mol) 401 kj/mol (96 kcal/mol) 390 kj/mol (93 kcal/mol) bond strength Stability of radicals C C C primary < secondary < tertiary stability
15 Ch.10 Alkyl alides An explanation of the relationship between reactivity and bond strength in radical chlorination relies on the ammond postulate. - C- abstraction step is slightly exorgonic; developing radical character in the TS - alkyl substituents stabilize the transition state leading the radical intermediate Therefore, the relative rate of formation of radicals is the same as their stability order.
16 Ch.10 Alkyl alides eaction energy diagram for alkane chlorination C 3 '- + 2 C 2 3 C Energy ' + '- eaction progress (reaction coordinate)
17 Ch.10 Alkyl alides Alkane brominarion: highly selective hv + 2 >99 : 1 - C- abstraction by. is much less exergonic; the transition state for bromination resembles the alkyl radicals more closely than does the transition state for chlorination, and the stability of that radical is therefore more important for bromination than for chlorination. C 3 C 3 3 C C + X 3 C C + X C 3 C 3 o = -50 kj for X= o = +13 kj for X=
18 Ch.10 Alkyl alides TS 1 TS 2 (C 3 ) 3 C + (C 3 ) 3 C + TS 1 has much more radical character than TS 2 Selectivity v.s.. eactivity
19 Ch.10 Alkyl alides 10.5 Allylic omination of Alkenes 1942, Karl Ziegler; allylic bromination - 2 reacts with alkene - use NBS for selective allylic bromination O N (NBS) O O + N allylic position hv, C 4 85% O
20 Ch.10 Alkyl alides radical mechamism O O O N + N + 2 hv O
21 Ch.10 Alkyl alides Selective bromination: allylic radical is relatively stable alkyl: 400 kj/mol (96 kcal/mol) allylic: 360 kj/mol (87 kcal/mol) vinylic: 445 kj/mol (106 kcal/mol)
22 Ch.10 Alkyl alides adical stability C C C C C C C C C vinylic < methyl < primary < secondary < tertiary < allylic less stable stability more stable
23 Ch.10 Alkyl alides 10.6 Stability of the Allyl adical: esonance evisited esonance stabilization C C C The unpaired electron is delocalized over the extended π-orbital: the unpaired electron is equally shared between the two terminal carbons.
24 Ch.10 Alkyl alides Allylic bromination of unsymmetrical alkene NBS, C 4 unsymmetrical radicals + 83% 17% reaction of less hindered primary end is favored
25 Ch.10 Alkyl alides Synthesis of conjugated diene: NBS KO hv, C 4
26 Ch.10 Alkyl alides Example: 3 C 3 C a NBS a 3 C 3 C + 3 C 3 C hv, C 4 b b 3 C 3 C + 3 C 3 C
27 Ch.10 Alkyl alides 10.7 Preparing Alkyl alides from Alcohols Alcohol to alkyl halide:,, I ; limited to 3 o alcohols (1 o and 2 o alcohols: slow, high temp.) -O + X -X + 2 O (X =,, I) eactivity of alcohols under acidic condition C O C O C O C O methyl < primary < secondary < tertiary less reactive reactivity more reactive
28 Ch.10 Alkyl alides 3 C O (g) Et 2 O, 0 o C 3 C + 2 O 90%
29 Ch.10 Alkyl alides 1 o and 2 o alcohols: use SO 2, P 3 O SO 2 O Pyridine O + SO % 3 O P 3 Et 2 O, 0 o C 3 + P(O) 3 86% - less acidic condition: no acid sensitive side reactions
30 Ch.10 Alkyl alides 10.8 eactions of Alkyl alides: Grignard eagents Victor Grignard: Grignard reagent -X + Mg -Mg-X = 1 o, 2 o, 3 o alkyl, aryl, alkenyl X =,, I - activation of Mg required (I 2, C 2 C 2..) - reactivity: I > > (F is not reactive)
31 Ch.10 Alkyl alides Mg Et 2 O Mg Mg Et 2 O Mg Mg Et 2 O Mg
32 Ch.10 Alkyl alides Grignard reagent: strong base and strong nucleophile δ + δ - MgX C basic and nucleophilic Grignard reagents react with weak acids such as 2 O, O, COO, N 2 Convert -X to Mg -Mg- 3 O + -
33 Ch.10 Alkyl alides 10.9 Organometallic Coupling eactions alkyl lithium reagent + 2 Li pentane Li n-butyllithium copper reagent: Gilman reagent ( 2 CuLi) 2 C 3 Li + CuI Et 2 O (C 3 ) 2 Cu - Li + + LiI Lithium dimethylcuprate (a Gilman reagent)
34 Ch.10 Alkyl alides Organo copper reagents: undergo coupling reactions with alkyl halides (,, I, but not F) to form C-C bonds (C 3 ) 2 Cu - Li + + Lithium dimethylcuprate -I Et 2 O 0 o C -C 3 + LiI + C 3 Cu
35 Ch.10 Alkyl alides Coupling reactions with alkenyl halides: vinyl, aryl halides I n-bu 2 CuLi n-bu + n-buli + LiI I Me 2 CuLi Me + MeLi + LiI mechanism - X + ' Cu ' Li + ' Cu ' ' + ' Cu
36 Ch.10 Alkyl alides Oxidation and eduction in Organic Chemistry Oxidation: eduction: Decreases electron density (increase oxidation state) on carbon by: forming one of these: C-O, C-N, C-X or breaking this: C- (addition of C-O bonds) Increase electron density (decrease oxidation state) on carbon by: forming this : C- or breaking one of these : C-O, C-N, C-X (addition of C- bonds) 3 C 2 hv 3 C oxidation: C- bond broken and C- bond formed 3 C 1. Mg 2. 3 O + 3 C reduction: C- bond broken and C- bond formed
37 Ch.10 Alkyl alides oxidation: two new bonds 2 formed between carbon and a 2 C C 2 2 C C 2 more electronegative element reduction: two new bonds 2 formed between carbon and a 2 C C 2 2 C C 2 less electronegative element 2 C C 2 2 C C 2 neither oxidation nor reduction: one new C- bond and one new C- bond formed
38 Ch.10 Alkyl alides Oxidation levels C 3 C 3 C 2 =C 2 C C C 3 O C 2 =O COO CO 2 C 3 C 2 2 C 3 C 4 C 3 N 2 C 2 =N C N Low oxidation state igh oxidation state
39 Work Naturally Occuring Organohalides organohalogen compounds: drugs, solvents, toxins... N N N O O O N O Jasplakinolide O O disrupts formation of the actin microtubles that make up the skeleton of cellular organelles - many organisms use organohalogen compounds for self-defence - marine organisms produce diverse organohalogen compounds
Bond length (pm) Bond strength (KJ/mol)
 hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Chapter 10. BrCH 2 CH 2 CH 2 CCH 2 Br CH 3. CH 3 CCH 2 CH 2 Cl CH 3 CHCH 2 CH 2 CHCH Give IUPAC names for the following alkyl halides:
 hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
Alkyl halides. Substitutive nomenclature. Nomenclature of Alkyl halides. Primary, Secondary and Tertiary Alkyl halides 3/12/2012
 Alkyl halides A compound with a halogen atom bonded to one of the sp 3 hybrid carbon atoms of an alkyl group + - C Primary, Secondary and Tertiary Alkyl halides Alkyl: alogen,, is directly bonded to sp3
Alkyl halides A compound with a halogen atom bonded to one of the sp 3 hybrid carbon atoms of an alkyl group + - C Primary, Secondary and Tertiary Alkyl halides Alkyl: alogen,, is directly bonded to sp3
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
Chapter 10 Free Radicals
 hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
Organometallic Reagents
 Making - bonds rganometallic eagents [hapter 3 Section 3.4; http://ochem.jsd.claremont.edu/tutorials.htm#] alletrin I (aid ) creating - bonds allows for making larger organic molecules from smaller molecules
Making - bonds rganometallic eagents [hapter 3 Section 3.4; http://ochem.jsd.claremont.edu/tutorials.htm#] alletrin I (aid ) creating - bonds allows for making larger organic molecules from smaller molecules
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Chapter 10 - Organohalides
 Give the name for each of the following alkyl halides. Chapter 10 - Organohalides 1. H C C H H 1,1,2-trichloroethane 2. CHI 3 triiodomethane F 3. F C C H, Halothane F 2-bromo-2-chloro-1,1,1-trifluoroethane
Give the name for each of the following alkyl halides. Chapter 10 - Organohalides 1. H C C H H 1,1,2-trichloroethane 2. CHI 3 triiodomethane F 3. F C C H, Halothane F 2-bromo-2-chloro-1,1,1-trifluoroethane
William H. Brown & Christopher Foote
 William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
Chem 263 Nov 3, 2016
 hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Chapter 04 Alcohols and Alkyl Halides part 01
 hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
Organic Chemistry(I) Chapter 3
 Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Introduction to Alkanes
 Introduction to Alkanes Alkanes do not react with most reagents for two reasons. First, carbon-carbon and carbon-hydrogen single bonds are very strong due to good orbital overlap. Second, the carbon-hydrogen
Introduction to Alkanes Alkanes do not react with most reagents for two reasons. First, carbon-carbon and carbon-hydrogen single bonds are very strong due to good orbital overlap. Second, the carbon-hydrogen
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Krypton Argon. Bromine Chlorine. Fluorine Selenium Oxygen
 1 ydrogen 1.0079 3 Li Lithium 6.941 4 Be Beryllium 9.0122 79 Au Gold 196.9665 1A (1) 2A (2) 3B (3) 3A (13) 4A (14) 5A (15) 6A (16) 7A (17) 8A (18) 4B (4) 5B (5) 6B (6) 7B (7) 8B (8) 8B (9) 8B (10) 1B (11)
1 ydrogen 1.0079 3 Li Lithium 6.941 4 Be Beryllium 9.0122 79 Au Gold 196.9665 1A (1) 2A (2) 3B (3) 3A (13) 4A (14) 5A (15) 6A (16) 7A (17) 8A (18) 4B (4) 5B (5) 6B (6) 7B (7) 8B (8) 8B (9) 8B (10) 1B (11)
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chem 341 Jasperse Ch Handouts 1
 Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chem 263 Nov 7, elimination reaction. There are many reagents that can be used for this reaction. Only three are given in this course:
 hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
Aryl Halides. Structure
 Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Homework - Chapter 9 Chem 2310
 Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chemistry 2000 Lecture 18: Reactions of organic compounds
 hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
Reactions of Haloalkanes
 Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
What is the major product of the following reaction?
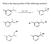 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Monday /3/2017. (Lecture No. 3-4 : Alkanes )
 Babylon University / Pharmacy Collage Department of Pharmaceutical Chemistry Monday.. 13-16/3/2017 (Lecture No. 3-4 : Alkanes ) Classification by structure: the family The basis of organic chemistry, we
Babylon University / Pharmacy Collage Department of Pharmaceutical Chemistry Monday.. 13-16/3/2017 (Lecture No. 3-4 : Alkanes ) Classification by structure: the family The basis of organic chemistry, we
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
08. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16
 08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
Organomagnesium (Grignard) and organolithium reagents
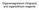 rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Chapter 15. Reactions of Aromatic Compounds. 1. Electrophilic Aromatic Substitution Reactions
 hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
CHEM 241 ALCOHOLS AND ALKYL HALIDES CHAP 5 ASSIGN
 CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
acetaldehyde (ethanal)
 hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Reaction of alkanes with bromine / chlorine in UV light. This is the overall reaction, but a more complex mixture of products is actually formed
 8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
12.5 Organometallic Compounds
 12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Organic Chemistry. for Students of Medicine and Biology 大学化学 III 和大学化学 III(2)
 Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
Straight. C C bonds are sp 3 hybridized. Butane, C 4 H 10 H 3 C
 Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Allylic and Benzylic Reactivity
 17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Model 1 Homolysis Reactions are Highly Endothermic
 Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
11/26/ Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds
 9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
Organic Chemistry SL IB CHEMISTRY SL
 Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #2 Alkyl alides: Their Synthesis by alogenation of Alkanes and Their Nucleophilic Substitution Reactions. Friday, ctober
hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #2 Alkyl alides: Their Synthesis by alogenation of Alkanes and Their Nucleophilic Substitution Reactions. Friday, ctober
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Lecture 14: October 4, 2018
 CM 223 Organic Chemistry I Prof. Chad Landrie C 3 Ea + O 3 C O 2 reactant intermediate Lecture 14: October 4, 2018 Ch. 6: Section 6.8: Bond Dissociation Energies and Reaction Enthalpy Ch. 17: Sections:
CM 223 Organic Chemistry I Prof. Chad Landrie C 3 Ea + O 3 C O 2 reactant intermediate Lecture 14: October 4, 2018 Ch. 6: Section 6.8: Bond Dissociation Energies and Reaction Enthalpy Ch. 17: Sections:
