What is the major product of the following reaction?
|
|
|
- Mae Goodman
- 6 years ago
- Views:
Transcription
1 What is the major product of the following reaction?
2 Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane D) 1-bromo-3-methylbutane H3CH2CCH3CH3Br
3 Which of the following alkanes will give more than one monochlorination product upon treatment with chlorine and light? A) 2,2-dimethylpropane B) cyclopropane C) ethane D) 2,3-dimethylbutane H3CCH3CH3CH3Cl2hvH3CCH3CH3CH3Cl+H3CCH2ClCH3CH
4 Which of the following is the major product of the chlorination of methane if a large excess of methane is used? A) CH 3 Cl B) CH 2 Cl 2 C) CH 3 CH 2 Cl D) CCl 4
5 Consider the reaction of 2-methylpropane with a halogen. With which halogen will the product be almost exclusively 2-halo-2- methylpropane? A) F 2 B) Cl 2 C) Br 2 D) I 2 H3CCH3CH3Br2hvH3CCH3CH3BrCl2hvH3CCH3CH3Cl+ClH2CCH
6 The alllyl carbon is the carbon adjacent to a double bond. The allylic radical is more stable than a 3 radical.
7 Because allylic C H bonds are weaker than other sp 3 hybridized C H bonds, the allylic carbon can be selectively halogenated using NBS in the presence of light or peroxides. NBS contains a weak N Br bond that is homolytically cleaved with light to generate a bromine radical, initiating an allylic halogenation reaction. Propagation then consists of the usual two steps of radical halogenation.
8
9 NBS also generates a low concentration of Br 2 needed in the second chain propagation step (Step [3] of the mechanism). The HBr formed in Step [2] reacts with NBS to form Br 2, which is then used for halogenation in Step [3] of the mechanism.
10 Thus, an alkene with allylic C H bonds undergoes two different reactions depending on the reaction conditions. How is the vicinal dibromide formed?
11 Halogens add to π bonds because halogens are polarizable. The electron rich double bond induces a dipole in an approaching halogen molecule, making one halogen atom electron deficient and the other electron rich (X δ+ X δ ). The electrophilic halogen atom is then attracted to the nucleophilic double bond, making addition possible.
12 Why does a low concentration of Br 2 (from NBS) favor allylic substitution (over ionic addition to form the dibromide)? The key to getting substitution is to have a low concentration of bromine (Br 2 ). The Br 2 produced from NBS is present in very low concentrations. A low concentration of Br 2 would first react with the double bond to form a low concentration of the bridged bromonium ion. The bridged bromonium ion must then react with more bromine (in the form of Br ) in a second step to form the dibromide. If concentrations of both intermediates the bromonium ion and Br are low (as is the case here), the overall rate of addition is very slow, and the products of the very fast and facile radical chain reaction predominate.
13 Predict the products. Br NBShv H2CCHCH3Br2 H2CHCCH3BrBr NBShv H2CCHCH2Br
14 Halogenation at an allylic carbon often results in a mixture of products. Consider the following example: A mixture results because the reaction proceeds by way of a resonance stabilized radical.
15 Predict the products. H3CCHCHCH3NBShv BrH2CCHCHCH3+ H2CCHHCCH3Br H2CCH2CCH3H2CCH3NBShv H2CCCHCH3H2CCH3B BrH2CCHCCH3H2CC
16 CH2NBShv CH2Br+ CH2Br
17 Radical Additions to Double Bonds HBr adds to alkenes to form alkyl bromides in the presence of heat, light, or peroxides. The regioselectivity of the addition to unsymmetrical alkenes is different from that in addition of HBr in the absence of heat, light or peroxides. The addition of HBr to alkenes in the presence of heat, light or peroxides proceeds via a radical mechanism.
18 Hydrohalogenation Electrophilic Addition of HX The mechanism of electrophilic addition consists of two successive Lewis acid-base reactions. In step 1, the alkene is the Lewis base that donates an electron pair to H Br, the Lewis acid, while in step 2, Br is the Lewis base that donates an electron pair to the carbocation, the Lewis acid.
19 With an unsymmetrical alkene, HX can add to the double bond to give two constitutional isomers, but only one is actually formed: This is a specific example of a general trend called Markovnikov s rule. Markovnikov s rule states that in the addition of HX to an unsymmetrical alkene, the H atom adds to the less substituted carbon atom that is, the carbon that has the greater number of H atoms to begin with.
20
21 Note that in the first propagation step, the addition of Br to the double bond, there are two possible paths: 1.Path [A] forms the less stable 1 radical. 2.Path [B] forms the more stable 2 radical. The more stable 2 radical forms faster, so Path [B] is preferred.
22 The radical mechanism illustrates why the regioselectivity of HBr addition is different depending on the reaction conditions.
23 HBr adds to alkenes under radical conditions, but HCl and HI do not. This can be explained by considering the energetics of the reactions using bond dissociation energies. Both propagation steps for HBr addition are exothermic, so propagation is exothermic (energetically favorable) overall. For addition of HCl or HI, one of the chain propagating steps is quite endothermic, and thus too difficult to be part of a repeating chain mechanism.
24 H3CCHCH2HBr H3CHCCH3Br H3CCHCHBrROORCH3CH3 H3CHCCHCH3CH3B HClhv Cl
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
ORGANIC - BROWN 8E CH.8 - HALOALKANES, HALOGENATION AND RADICALS
 !! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
!! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Organic Chemistry(I) Chapter 3
 Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical
 C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Organic Chemistry. Radical Reactions
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Chapter 5. 3-Chloro-2-methylpentane. Cl 2. 2-Chloro-2-methylpentane. 1-Chloro-2-methylpentane. Cl 2-Chloro-4-methylpentane. 1-Chloro-4-methylpentane
 hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chemistry 2000 Lecture 18: Reactions of organic compounds
 hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Organic Mechanisms 1
 Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. A) I B) II C) III D) IV E) V
 Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Chapter 15. Free Radical Reactions
 Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Radical Reac)ons of Alkanes
 Radical Reac)ons of Alkanes Types of Steps in Reac)on Mechanisms Bond forma)on or breakage can be symmetrical or unsymmetrical Symmetrical- homoly)c Unsymmetrical- heteroly)c Indica)ng Steps in Mechanisms
Radical Reac)ons of Alkanes Types of Steps in Reac)on Mechanisms Bond forma)on or breakage can be symmetrical or unsymmetrical Symmetrical- homoly)c Unsymmetrical- heteroly)c Indica)ng Steps in Mechanisms
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
An Overview of Organic Reactions
 An Overview of Organic Reactions Radical Reactions Reactions involving symmetrical bond breaking and bond forming omolytic bond breaking omogenic bond formation Radical Reaction with alkanes and uv light
An Overview of Organic Reactions Radical Reactions Reactions involving symmetrical bond breaking and bond forming omolytic bond breaking omogenic bond formation Radical Reaction with alkanes and uv light
Organic Chemistry. Why are these compounds called Organic. What is a Hydrocarbon? Questions: P167 Read
 Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
ORGANIC CHEMISTRY 307
 ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
CHEM Exam 2 October 25, 2007
 CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Chapter 10 Free Radicals
 hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Organic Chemistry. Alkenes
 Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane.
 Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
4. A sample of 0.200g of an organic compound was subjected to combustion analysis and
 1. A sample of 0.205g of an organic compound was subjected to combustion analysis and produced 0.660g of carbon dioxide, 0.225g of water and nothing else. Its RMM is 82. 2. A sample of 0.400g of an organic
1. A sample of 0.205g of an organic compound was subjected to combustion analysis and produced 0.660g of carbon dioxide, 0.225g of water and nothing else. Its RMM is 82. 2. A sample of 0.400g of an organic
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Classification of Reactions by:
 lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
ELECTROPHILIC ADDITIONS OF ALKENES AS THE COUNTERPART OF ELIMINATIONS
 ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
Straight. C C bonds are sp 3 hybridized. Butane, C 4 H 10 H 3 C
 Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
CHEM 241 ALCOHOLS AND ALKYL HALIDES CHAP 5 ASSIGN
 CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Name: Chapter 3: The Nature Of Organic Reactions: Alkenes
 Name: Chapter 3: The Nature Of Organic Reactions: Alkenes 1 Vocabulary cis-trans isomerism: E,Z designation: Addition: Elimination: Substitution: Rearrangement: Homolytic: Heterolytic Homogenic: Heterogenic:
Name: Chapter 3: The Nature Of Organic Reactions: Alkenes 1 Vocabulary cis-trans isomerism: E,Z designation: Addition: Elimination: Substitution: Rearrangement: Homolytic: Heterolytic Homogenic: Heterogenic:
An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A
 An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A How many nonequivalent protons does the following structure have? 4 Reading from left to right,
An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A How many nonequivalent protons does the following structure have? 4 Reading from left to right,
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Chapter 5. Reactions of Alkenes and Alkynes
 Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
William H. Brown & Christopher Foote
 William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
Rearrangement: a single reactant rearranges its
 Chapter 5: An overview of organic reactions 5.1 Kinds of organic reactions Even though there are hundreds of reactions to study, organic chemistry is governed by only a few key ideas that determine chemical
Chapter 5: An overview of organic reactions 5.1 Kinds of organic reactions Even though there are hundreds of reactions to study, organic chemistry is governed by only a few key ideas that determine chemical
Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide
 Q1. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3) 2C=CH 2 Name of mechanism... Mechanism (4)
Q1. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3) 2C=CH 2 Name of mechanism... Mechanism (4)
CH320/328 M Spring 2014
 CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more single bonds between them)
 1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
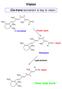 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
6.10 Acid-Catalyzed Hydration of Alkenes. Acid-Catalyzed Hydration of Alkenes
 Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
CHEM Lecture 6
 EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide.
 Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
