Structure at the isoelectric point: The predominant form of this tripeptide at ph 1 has a net charge of: (circle one)
|
|
|
- Sandra Harrington
- 6 years ago
- Views:
Transcription
1 ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by writing the correct isoelectric point value in the box beside each amino acid. Arg pi = Glu pi = 3.08 Val pi = II. (18 points) Draw the full structure (including stereochemistry) for the tripeptide Cys-Pro-Arg (all L amino acids) in the predominant form it would exist at its isoelectric point. Use the Fischer projections. tructure at the isoelectric point: The predominant form of this tripeptide at p 1 has a net charge of: (circle one) The predominant form of this tripeptide at p 11 has a net charge of: (circle one) III. (20 points) Draw in the boxes below the full structures (including stereochemistry) of the products from each of the following reactions. (1) 2 F 3 (C 2 C 3 ) 3 2. Cl, 2,! Cl (2) itro-containing product 6 Cl salt dimethylformamide (solvent) 2 C 2 C 1 10
2 ame Key 21 F07-Final exam Page 3 IV. (20 points) Consider the esterification reaction (A to ) shown below. Cl C(C 3 ) 2 C(C3 ) 2 A (1) (16 points) Which of the following would result in an increase in the amount of ester formed? 2 If the following is done: Yield of would increase (circle one) (2) Excess C(C 3 ) 2 is added. Yes o ac(c 3 ) 2 is used in place of C(C 3 ) 2 Yes o C(C 3 ) 2 is removed during the reaction Yes o Dilute aqueous Cl is used instead of concentrated Cl Yes o If isotopically labeled - 18 C(C 3 ) 2 (circle one) is used, the 18 label would end up in : V. (32 points) Treatment of L-phenylalanine with excess methanol in the presence of mol equiv of gaseous Cl at room temperature produces the Cl salt of L-phenylalanine methyl ester in 9% yield. Provide in the box below a step-by-step mechanism, using the curved arrow convention, for the formation of this methyl ester Cl salt from L-phenylalanine. You may use - and - as a general acid and its conjugate base, respectively. 2 3 Cl (gas, mol equiv) C 3 C 3 room teperature, hr 3 9% Cl 3 3 C 3 3 C 3 3 C 3 3 C 3 3 C 3 3 C
3 ame Key 21 F07-Final exam Page VI. (18 points) A levulinyl group[c 3 C(=)C 2 C 2 C(=)-] is an extremely versatile hydroxyl-protecting group. A team of scientists at The cripps Research Institute in La Jolla, CA, reported in 2003 that the levulinate group (indicated by a rectangular box) in differentially protected disaccharide C can be deprotected selectively to provide alcohol D in quantitative yield [Proc. atl. Acad. ci., UA 2003, 100, 797]. CCl 3 C D CCl 3 (1) The methods used to achieve this selective deprotection take advantage of the difference in electrophilicity between aldehyde/ketone and ester carbonyl groups. Among these methods known, the use of a is most convenient. In the reaction shown below, the levulinate ester of cyclohexanol, E, is treated with a to afford cyclohexanol (F). The by-product (G) from this reaction has the molecular formula of C 8 2. Provide in the box below the structure of this compound. E a C 3 0 C G (C 8 2 ) (2) When a levulinate such as E is treated with hydrazine ( 2-2 ) in acetic acid, the levulinate-protected alcohol undergoes facile deprotection (see below). Draw in the box provided the structure of the by-product. It might be easier if you solve # (3) before this question. E 2-2 C 3 C room temperature min F F (C 8 2 ) (3) The reaction of levulinate E with hydrazine first produces its hydrazone derivative, which further undergoes an intramolecular reaction to produce. Draw in the box below the structure of this hydrazone derivative of E
4 ame Key 21 F07-Final exam Page VII. (22 points) Provide in the box below a step-by-step mechanism for the following reaction using the curved arrow convention. Use - and - for the base and the conjugate acid, respectively. Make sure to show the mechanism for the catalytic cycle, i.e., regeneration of - [or C(C 3 ) 3 ]. You do not need to rationalize the stereochemistry of the new chiral centers created [Tetrahedron Lett. 2007, 8, 683]. C 2 C 3 K C(C 3 ) 3 (catalytic) tetrahydrofuran (solvent) 69% C 2 C 3 : C 2 C 3 C 2 C 3 C 2 C 3 : C 2 C 3 C 2 C 3 22 VIII. (22 points) Provide in the box below a step-by-step mechanism for the following acid-catalyzed reaction using the curved arrow convention. Use - and - for the acid and the conjugate base, respectively. p-ts (catalytic) acetone (solvent) 66% 22
5 ame Key 21 F07-Final exam Page 6 IX. (39 points) Complete the following transformations as indicated by the data provided. Clearly indicate the stereochemistry where applicable. (1) (2) 3 C 2 Which amino acid is this? Use its 3-letter abbreviation as your answer. er Cl answer C 3 to p ~1, (C 2 C 3 ) 3 C 2 2 C2 2, Pd/C 3 with added DCC r/ 3 CC(=) or r/f 3 CC(=) (3) [J. rg. Chem. 200, 69, 1038] C 2 n-uli TF (solvent) 2. Cl C 3 () [J. rg. Chem. 2007, 72, 91] C 3 3 LiCu(C 3 ) 2 TF (solvent) 2. aq Cl C (Z-isomer) C 3 Ac Ac Ac = 3C r (gas) C 3 Ac (acetyl) Ac C 2 Cl 2 (solvent) C 3 Ac Ac Ac r Cs 2!-anomer C C 3 Ac Ac Ac Csr
6 ame Key 21 F07-Final exam Page 7 X. (3 points) Complete the following transformations as indicated by the data provided. Clearly indicate the stereochemistry where applicable. equential experimental steps must be numbered. (1) Li TF (solvent) 2. aq Cl (2) [Tetrahedron 2007, 63, 8] C C 2 (2 mol equiv) K TF (solvent) 2. C 2 r (3) [J. rg. Chem. 2007, 72, 93] C Kr 3 C 3 C C 3 C 3 C 3 C 3 C 3 p-ts 3 C toluene,! 3 C C 3 2 C 3 C 8 18 () [Tetrahedron Lett. 2007, 8, 623] C 2 C 2 C 3 2 LDA TF (solvent) -30 C dianion diastereomers C 2 C 3 (1 mol equiv) 2. aq Cl C 2 C 3 diastereomeric mixture at a new chiral center (show that stereochemistry with ) 3 C C 3 2 C 3 C 2C 3 C 2C 3 3 C p-ts C 3
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point:
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point: O O
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points and B indicated below and (ii)
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points and B indicated below and (ii)
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
(4) (5) (6) a. b. H. explanation: explanation:
 ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
H H H OH OH H OH H O 1 CH 2 OH
 Name 215 F07-Exam No. 3 Page 2 I. (29 points) Streptomycetes are soil-dwelling, filamentous, Gram-positive saprophytic bacteria. They are responsible for over 50% of the known microbial metabolites, including
Name 215 F07-Exam No. 3 Page 2 I. (29 points) Streptomycetes are soil-dwelling, filamentous, Gram-positive saprophytic bacteria. They are responsible for over 50% of the known microbial metabolites, including
H CH 2 -OH (4) H b. (5) H H. (6) a. b.
 ame Key 15 F010-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
ame Key 15 F010-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
H CH 2 -OH (4) H b. H H (5) (6) a. b.
 ame 215 F010-Exam o. 2 Page 2 I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
ame 215 F010-Exam o. 2 Page 2 I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
R or S? oxidation #: hybridization:
 Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
C C H 2. O p-tsoh (catalytic) H 3 O +! O
 ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
R or S? 2) oxidation numbers of the designated carbon atoms: note: An oxidation number must have a sign. F 3 C OCH 3 oxidation #: OH.
 ame Key 5 0-Exam o. Page. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) esignate in each of the boxes below the stereochemistry
ame Key 5 0-Exam o. Page. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) esignate in each of the boxes below the stereochemistry
R or S? 2) oxidation numbers of the designated carbon atoms: note: An oxidation number must have a sign. F 3 C OCH 3 oxidation #: OH.
 ame 5 F0-Exam o. Page I. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) Designate in each of the boxes below the stereochemistry
ame 5 F0-Exam o. Page I. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) Designate in each of the boxes below the stereochemistry
EXAMINATION 2 Chemistry 3A
 1 EXAMINATIN 2 Chemistry 3A Name: KEY Print first name before second! Use capital letters! SID #: Peter Vollhardt April 11, 2017 GSI (if you are taking Chem 3AL): Please provide the following information
1 EXAMINATIN 2 Chemistry 3A Name: KEY Print first name before second! Use capital letters! SID #: Peter Vollhardt April 11, 2017 GSI (if you are taking Chem 3AL): Please provide the following information
Chem 332, Exam 4. Spring Provide reagents for the following transformations (2 pts each) NaOH , KOH. 4) H2/Pd NO 2. 1) AlCl 3 O 2) H 2 NNH 2
 AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
(2) After dissolving a solid in a solvent at high temperature, the solution is not filtered.
 Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Exam 4. Name CHEM 210. PBr 3. HBr. 1) NaH 2) Br H 2 SO 4. 1) NaOCH 3 2) dil. H 3 O + O CH 3 OH, H 2 SO 4. 1) LDA, -78 o C.
 Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
PLEASE DO NOT WRITE ON THE EXAM (EVEN YOUR NAME) UNTIL TOLD TO START! Student ID # CHEM 8A, Organic Chemistry EXAM 1 (300 points)
 PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
C h a p t e r N i n e: Addition Reactions of Alkenes
 C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
UCSC, Binder. Section TA. CHEM 108B Organic Chemistry II FINAL EXAM, Version B (400 points)
 UCSC, Binder Name Section TA SID CEM 108B rganic Chemistry II FINAL EXAM, Version B (400 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions
UCSC, Binder Name Section TA SID CEM 108B rganic Chemistry II FINAL EXAM, Version B (400 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions
EXAMINATION 2 Chemistry 3A
 000402 1 EXAMINATION 2 Chemistry 3A Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt April 11, 2017 GSI (if you are taking Chem 3AL): Please provide the following information
000402 1 EXAMINATION 2 Chemistry 3A Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt April 11, 2017 GSI (if you are taking Chem 3AL): Please provide the following information
Answers to Hour Examination #3, Chemistry 302X, 2006
 Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Section Practice Exam II Solutions
 Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems
 Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (OL 2007, 9, 385). OCH OCH 3
 ame Page 1 F.08.215FEP1 I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (L 2007, 9, 85). a l B. Provide the step-wise, curved arrow mechanism for the following (JAS 2002,
ame Page 1 F.08.215FEP1 I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (L 2007, 9, 85). a l B. Provide the step-wise, curved arrow mechanism for the following (JAS 2002,
CEM 852 Final Exam. May 6, 2010
 CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
UCSC, Binder. CHEM 8B Organic Chemistry II FINAL EXAM (300 points)
 UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Diastereomeric resolution directed towards chirality. determination focussing on gas-phase energetics of coordinated. sodium dissociation
 Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
UCSC, Binder. CHEM 8B Organic Chemistry II FINAL EXAM, Version A (300 points)
 UCSC, Binder TA Name SID CEM 8B rganic Chemistry II FINAL EXAM, Version A (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in
UCSC, Binder TA Name SID CEM 8B rganic Chemistry II FINAL EXAM, Version A (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
CHAPTER 23 HW: ENOLS + ENOLATES
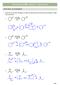 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
For each of the pairs shown below, choose the statements that can be used to describe the drawings and their relationship, if a relationship exists.
 I. ( points) ame Page or each of the pairs shown below, choose the statements that can be used to describe the drawings and their relationship, if a relationship exists. (a) (b) and : "Check" any/all descriptions
I. ( points) ame Page or each of the pairs shown below, choose the statements that can be used to describe the drawings and their relationship, if a relationship exists. (a) (b) and : "Check" any/all descriptions
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
MgBr 17 H 16 N 2 O 3 HO N. no partial. 4 each; can be either order -2 if steps unnumbered 1) CH 3 OH/TsOH. 1) NaH 2) CH 3 I
 I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
Midterm #1 Chem 3A - Fall 2013 Sept. 7, :00 8:30 pm. Name SID
 Midterm #1 Chem 3A - Fall 2013 Sept. 7, 2013 7:00 8:30 pm ame SID Including the title page, there should be 5 total questions spread over 7 pages (printed on both sides). The back of the last page may
Midterm #1 Chem 3A - Fall 2013 Sept. 7, 2013 7:00 8:30 pm ame SID Including the title page, there should be 5 total questions spread over 7 pages (printed on both sides). The back of the last page may
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
216 S10-Exam #1 Page 2. Name
 216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
Importance of Carbohydrates
 Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Problem Points Score GSI I 30 II 21 III 28 IV 30 V 31 Total 140
 hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Exam I 19 April 2004 Name:
 Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions:
 1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
"Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A.
 ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
Massachusetts Institute of Technology. Chemistry 5.43 February 28 th, Exam #1. Question 1a /4 points Question 2b /10 points
 Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below.
 hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Chemistry 14D Winter 2010 Exam 2 Page 1
 Chemistry 14D Winter 2010 Exam 2 Page 1 1. (2) Circle the best statement of Markovnikov s rule. (a) When X adds to an alkene, the hydrogen of X becomes bonded to the alkene carbon that bears the least
Chemistry 14D Winter 2010 Exam 2 Page 1 1. (2) Circle the best statement of Markovnikov s rule. (a) When X adds to an alkene, the hydrogen of X becomes bonded to the alkene carbon that bears the least
Initials: 1. Chem 633: Advanced Organic Chemistry 2011 Final Exam
 Initials: 1 ame: Chem 633: Advanced rganic Chemistry 2011 Final Exam Please answer the following questions clearly and concisely. In general, use pictures and less than 10 words in your answers. Write
Initials: 1 ame: Chem 633: Advanced rganic Chemistry 2011 Final Exam Please answer the following questions clearly and concisely. In general, use pictures and less than 10 words in your answers. Write
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
INTRODUCTORY ORGANIC CHEMISTRY II --- PROBLEM SET #2
 CHEM 222 Section 01 Fall 2007 Dr. C. Rogers Page 1 of 5 INTRDUCTRY RGANIC CHEMISTRY II --- PRBLEM SET #2 DISTRIBUTED: Thurs.Nov.15 th. CMPLETIN DEADLINE: Thurs.Nov.29 th @ 10:15am. INSTRUCTINS: Work through
CHEM 222 Section 01 Fall 2007 Dr. C. Rogers Page 1 of 5 INTRDUCTRY RGANIC CHEMISTRY II --- PRBLEM SET #2 DISTRIBUTED: Thurs.Nov.15 th. CMPLETIN DEADLINE: Thurs.Nov.29 th @ 10:15am. INSTRUCTINS: Work through
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
CHEM 330. Final Exam December 8, 2010 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Midterm Exam 2. Chem 3B, Fall 2016 Thursday, Nov. 3, :00 9:00 pm. Name
 Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
ORGANIC - BROWN 8E CH AMINO ACIDS AND PROTEINS.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO PROTEINS Proteins are polypeptides that have some biological function. Peptides are composed of polymers of monomeric units called α-amino acids The 20 most
!! www.clutchprep.com CONCEPT: INTRODUCTION TO PROTEINS Proteins are polypeptides that have some biological function. Peptides are composed of polymers of monomeric units called α-amino acids The 20 most
1 Answer. 2 Answer A B C D
 216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
Lecture 18. Oxidation and Reduction. Oxidation. Reduction O CH 4 CH 3 OH H C H. Chemistry 328N
 Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Design Synthesis and SAR of Non-peptidic Urotensin II Agonists
 MEDICIAL CEMISTY Design Synthesis and SA of on-peptidic Urotensin II Agonists C Val Cys Tyr Lys Trp 2 Glu Thr Pro Asp Cys Phe uman urotensin II (S) pec 50 7.49 () pec 50 5.84 FL-104 AC-7954 Fredrik Lehmann
MEDICIAL CEMISTY Design Synthesis and SA of on-peptidic Urotensin II Agonists C Val Cys Tyr Lys Trp 2 Glu Thr Pro Asp Cys Phe uman urotensin II (S) pec 50 7.49 () pec 50 5.84 FL-104 AC-7954 Fredrik Lehmann
Topics in the June 2009 Exam Paper for CHEM1102
 June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
CHEM J-2 June In the spaces provided, briefly explain the meaning of the following terms.
 CHEM1611 2013-J-2 June 2013 In the spaces provided, briefly explain the meaning of the following terms. Effective nuclear charge 4 The force of attraction experienced by the outer electrons of an atom.
CHEM1611 2013-J-2 June 2013 In the spaces provided, briefly explain the meaning of the following terms. Effective nuclear charge 4 The force of attraction experienced by the outer electrons of an atom.
1.5 h; 120 points please print clearly Dr. Kathleen Nolta Signature. Problem Points Score GSI I 20 II 27 III 25 IV 22 V 26 Total 120
 hemistry 0 First ame econd Examination Last ame. h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II 7 III IV V Total
hemistry 0 First ame econd Examination Last ame. h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II 7 III IV V Total
Alcohols: Contain a hydroxy group( OH) bonded to an sp 2 or sp 3 hybridized
 Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
*Assignments could be reversed. *
 Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Midterm Exam #1 /280 CHEM 6352 Fall 2011
 Midterm Exam #1 /280 CEM 6352 Fall 2011 ( %) Name Sept 30 th, 2011 18:00-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Midterm Exam #1 /280 CEM 6352 Fall 2011 ( %) Name Sept 30 th, 2011 18:00-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Chemistry 224 Bioorganic Chemistry Friday, Sept. 29, This Exam is closed book and closed notes. Please show all your work!
 page 1 of 6 hemistry 224 ame Bioorganic hemistry Friday, ept. 29, 2000 Exam 1 100 points This Exam is closed book and closed notes Please show all your work! tereochemistry counts as indicated! eatness
page 1 of 6 hemistry 224 ame Bioorganic hemistry Friday, ept. 29, 2000 Exam 1 100 points This Exam is closed book and closed notes Please show all your work! tereochemistry counts as indicated! eatness
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
Chemistry 216. First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh. Lab section. GSI name. Name Please print.
 Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Chapter 9:Nucleophiles & Substitution Reactions
 1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
