Exam 4. Name CHEM 210. PBr 3. HBr. 1) NaH 2) Br H 2 SO 4. 1) NaOCH 3 2) dil. H 3 O + O CH 3 OH, H 2 SO 4. 1) LDA, -78 o C.
|
|
|
- Gervais Wiggins
- 5 years ago
- Views:
Transcription
1 Exam 4 Name CEM (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where necessary. a. P 3 1) Na 2 S 4 b. 1) NaC 3 2) dil. 3 C 3, 2 S 4 c. 1) LDA, -78 o C 1) LDA, 25 o C d. 1) B 3 2) 2 2, K S 4 C 2 N 2 peracid
2 2. (8 pts) Mechanism conversion of R to R with P 3 with stereochemical inversion. In the space below show how this conversion occurs using 2-butanol as the starting material with curved arrows for the movement of electrons, proper Lewis structures and formal charges. Be sure to show how the stereochemistry is inverted. P 3 3. (6 pts) When the following cyclic ether is treated with methanol with a catalytic amount of 2 S 4, one of the two products shown is formed quantitatively with only a trace of the other. Which one is the major product? Propose a reaction mechanism to illustrate why. C 3, 2 S 4 C 3 C 3 4. What is the most likely product of the following reaction? b. II e. A racemic mixture of I and II
3 5. What is the most likely product of the following reaction? 6. What reagent(s) would be the best choice to complete the following reaction? a. 1. Na; 2. 3 d. 1. Na; 2. - b. 2 e. c. P 3 7. What is the most likely product of the following reaction?
4 8. What is the most likely product of the following reaction? 9. Which of the following compounds would not produce the same major product as the others when treated with Cl? a. A d. D b. B e. E c. C 10. What will be the major product of the reaction below? (Tf is like Ts or 2 S 4 ) a. d. b. e. c.
5 Exam 4 - KEY Name CEM (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where necessary. a. P 3 1) Na 2 S 4 major b. 3 C 1) NaC 3 2) dil. 3 C 3, 2 S 4 C 3 c. 1) LDA, -78 o C 1) LDA, 25 o C major d. 1) B 3 2) 2 2, K 2 2 S 4 2 C 2 N 2 peracid
6 12. (8 pts) Mechanism conversion of R to R with P 3 with stereochemical inversion. In the space below show how this conversion occurs using 2-butanol as the starting material with curved arrows for the movement of electrons, proper Lewis structures and formal charges. Be sure to show how the stereochemistry is inverted. P P P P 13. (6 pts) When the following cyclic ether is treated with methanol with a catalytic amount of 2 S 4, one of the two products shown is formed quantitatively with only a trace of the other. Which one is the major product? Propose a reaction mechanism to illustrate why. S 3 S 3 C 3 C 3 - C 3 2 o cation not resonance stabilized S 3 S 3 C 3 C 3 - C 3 2 o cation resonance stabilized lowers E A for this path 14. What is the most likely product of the following reaction? b. II e. A racemic mixture of I and II
7 15. What is the most likely product of the following reaction? 16. What reagent(s) would be the best choice to complete the following reaction? a. 1. Na; 2. 3 d. 1. Na; 2. - b. 2 e. c. P What is the most likely product of the following reaction?
8 18. What is the most likely product of the following reaction? 19. Which of the following compounds would not produce the same major product as the others when treated with Cl? a. A d. D b. B e. E c. C 20. What will be the major product of the reaction below? (Tf is like Ts or 2 S 4 ) a. d. b. e. c.
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
a) 1. O 3 2. (CH 3 ) 2 S
 Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
CHEM 8A Summer Student ID # Organic Chemistry FINAL EXAM (400 points)
 CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
C C H 2. O p-tsoh (catalytic) H 3 O +! O
 ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
Chemistry 250B Final Exam Answer Key December 19, 2008
 Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
California State Polytechnic University, Pomona Chem 315. Exam Points 1. Nomenclature (1) 30
 hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:!
 CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CHEM 302 Organic Chemistry I Exam III 12-December-2006 Answers
 CEM 302 rganic Chemistry I Exam III 12-December-2006 nswers 1) (18 pts) Compound, C 9 16, was found to be optically active. n catalytic reduction over a palladium catalyst, 2 equivalents of hydrogen were
CEM 302 rganic Chemistry I Exam III 12-December-2006 nswers 1) (18 pts) Compound, C 9 16, was found to be optically active. n catalytic reduction over a palladium catalyst, 2 equivalents of hydrogen were
Partial Periodic Table
 Printed ame: CEM 3371, Spring 2015 Professor Walba First our Exam March 10, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither given nor received unauthorized
Printed ame: CEM 3371, Spring 2015 Professor Walba First our Exam March 10, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither given nor received unauthorized
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
!!!! Organic Chemistry CHM 224. Exam I Questions
 ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
5. Draw the alkene that gives the product shown, and specify its stereochemistry. (2 points)
 JSPERSE HEM 350 TEST 3 VERSIN 1 h. 7 Structure and Synthesis of lkenes h. 8 Reactions of lkenes 1 1. How many elements of unsaturation are in the formula 6 H 9 N 2? (3 points) a. 0 b. 1 c. 2 d. 3 e. 4
JSPERSE HEM 350 TEST 3 VERSIN 1 h. 7 Structure and Synthesis of lkenes h. 8 Reactions of lkenes 1 1. How many elements of unsaturation are in the formula 6 H 9 N 2? (3 points) a. 0 b. 1 c. 2 d. 3 e. 4
Partial Periodic Table
 CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
1. Name the following compound. Use the IUPAC system and include stereochemical designations.
 Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Partial Periodic Table
 CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
First Name MIKE. Chem 30A Winter 2005 MIDTERM #2 (50 Min) Weds March 2nd
 Last Name First Name MI Student I Number: Total Score Circle the name of your TA: MIKE ROB iscussion Section ay: Time: / 100 Chem 30A Winter 2005 MITERM #2 (50 Min) Weds March 2nd INTERPRETATION OF THE
Last Name First Name MI Student I Number: Total Score Circle the name of your TA: MIKE ROB iscussion Section ay: Time: / 100 Chem 30A Winter 2005 MITERM #2 (50 Min) Weds March 2nd INTERPRETATION OF THE
More tutorial at
 Answer all the questions. 1) (12 pts) Assign (R) or (S) to all the chiral centers in the following molecules and mark the priority of each group around a chiral center (just the top chiral carbon in molecule
Answer all the questions. 1) (12 pts) Assign (R) or (S) to all the chiral centers in the following molecules and mark the priority of each group around a chiral center (just the top chiral carbon in molecule
Chemistry 3720, Spring 2004 Exam 3 Name:
 Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
HOMEWORK PROBLEMS: ALKYNES. 1. Provide the complete IUPAC name for the following compounds:
 CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
CM 241 Organic Chemistry Fall 1999
 M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
Chemistry Fall Semester 2007; Midterm 3 Exam
 1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
A. Provide reagents for the following transformations. T 6. Cl OH
 rganic Chemistry I est 3 Extra Synthesis Practice Problems Page 1: Synthesis Design Practice. Page 2+3: Predict the Product Practice (including some that involve stereochemistry). Page 4: Cis/trans Stereospecific
rganic Chemistry I est 3 Extra Synthesis Practice Problems Page 1: Synthesis Design Practice. Page 2+3: Predict the Product Practice (including some that involve stereochemistry). Page 4: Cis/trans Stereospecific
PLEASE DO NOT WRITE ON THE EXAM (EVEN YOUR NAME) UNTIL TOLD TO START! Student ID # CHEM 8A, Organic Chemistry EXAM 1 (300 points)
 PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
PLEASE D T WRITE TE EXAM (EVE YUR AME) UTIL TLD T START! UCSC, Binder ame Student ID # CEM 8A, rganic Chemistry EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry
216 S10-Exam #1 Page 2. Name
 216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
UCSC, Binder (First and Last) CHEM 8A, Organic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60)
 UCSC, Binder Name (First and Last) CEM 8A, rganic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60) 2 (65) 3 (60) 4 (70) 5 (75) 6 (70) Total (400) % In each of the following problems, use your knowledge
UCSC, Binder Name (First and Last) CEM 8A, rganic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60) 2 (65) 3 (60) 4 (70) 5 (75) 6 (70) Total (400) % In each of the following problems, use your knowledge
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Stereochemistry is EZ! Thanks to Jonah H. for the title.
 Stereochemistry is EZ! Thanks to Jonah. for the title. rganic Chemistry CE 310, Exam #3 100 painless points! November 17, 2017 Rules of the road: 1. There are 9 questions on 5 pages, plus a couple of extra
Stereochemistry is EZ! Thanks to Jonah. for the title. rganic Chemistry CE 310, Exam #3 100 painless points! November 17, 2017 Rules of the road: 1. There are 9 questions on 5 pages, plus a couple of extra
Partial Periodic Table
 EMITY 3331, pring 2005 Professor Walba Final Exam, May 3 U onor ode Pledge: n my honor, as a University of olorado at oulder tudent, I have neither given nor received unauthorized assistance. scores: 1)
EMITY 3331, pring 2005 Professor Walba Final Exam, May 3 U onor ode Pledge: n my honor, as a University of olorado at oulder tudent, I have neither given nor received unauthorized assistance. scores: 1)
Final Exam /340 ( CHEM 6352 Fall %) Name
 Final Exam /340 ( CEM 6352 Fall 2012 %) Name Dec. 15 th, 2012 9:30-13:45 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Final Exam /340 ( CEM 6352 Fall 2012 %) Name Dec. 15 th, 2012 9:30-13:45 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Organic Chemistry CHM 224
 rganic Chemistry CHM 224 Exam I Review Questions This set of questions is a compilation of old exams and additional questions, and does not represent the typical length of an exam - this is WAY longer!
rganic Chemistry CHM 224 Exam I Review Questions This set of questions is a compilation of old exams and additional questions, and does not represent the typical length of an exam - this is WAY longer!
(CHE 325) Organic Chemistry II Spring 2011 EXAM #4
 (CE 325) rganic Chemistry II Spring 2011 EXAM #4 ame: KEY ID#: Check your exam to be sure it is complete. There are eight questions in this exam. It is worth 100 points. To receive full credit for your
(CE 325) rganic Chemistry II Spring 2011 EXAM #4 ame: KEY ID#: Check your exam to be sure it is complete. There are eight questions in this exam. It is worth 100 points. To receive full credit for your
Chem 312 SS05 Page 1 of 6 Kantorowski. 17 August 2005 Name:
 Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Final Exam /415 ( CHEM 6352 Fall %) Name
 Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only
(4) (5) (6) a. b. H. explanation: explanation:
 ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
CHEMISTRY MIDTERM # 3 March 31, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
1. Complete the template below to show the stereochemistry of (3R,4R) 3-chloro-4,5,5- trimethyl-hexane-1,4-diol.
 Chemistry 51 Exam 1, Fall 2004 This is a closed book exam. The exam lasts 50 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer sheet
Chemistry 51 Exam 1, Fall 2004 This is a closed book exam. The exam lasts 50 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer sheet
Chemistry 52 Exam #1. Name: 22 January This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages.
 Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Chemistry 51 Summer 2007 Final Exam
 Chemistry 51 Summer 2007 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
Chemistry 51 Summer 2007 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
Sample Final Examination Organic Chemistry I
 Sample Final Examination rganic Chemistry I CEM 2423 Rofecoxib (Vioxx)- is a nonsteroidal anti-inflammatory drug (NSAID) developed by Merck & Co.to treat osteoarthritis, acute pain conditions, and dysmenorrhoea
Sample Final Examination rganic Chemistry I CEM 2423 Rofecoxib (Vioxx)- is a nonsteroidal anti-inflammatory drug (NSAID) developed by Merck & Co.to treat osteoarthritis, acute pain conditions, and dysmenorrhoea
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
CH 3 OCH 3. Question 4. Provide the missing reactants for the following Diels-Alder reaction. heat
 CHEMISTRY 234, MIDTERM #2 PRACTICE TEST #1-3 - NAME Question 3 For each reaction 1) Provide the missing reagents/conditions or major organic products as appropriate 2) State whether the OVERALL reaction
CHEMISTRY 234, MIDTERM #2 PRACTICE TEST #1-3 - NAME Question 3 For each reaction 1) Provide the missing reagents/conditions or major organic products as appropriate 2) State whether the OVERALL reaction
Partial Periodic Table
 CEM 33-00, Fall 203 Professor Walba First our Exam September 24, 203 scores: ) 20 2) 20 3) 20 4) 20 5) 20 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither
CEM 33-00, Fall 203 Professor Walba First our Exam September 24, 203 scores: ) 20 2) 20 3) 20 4) 20 5) 20 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, I have neither
Time: 3 hours (180 minutes) Marking Scheme For The Exam
 hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
Exam #1. Chemistry 334. Principles of Organic Chemistry II. Thursday October 5, 2006
 Exam #1 Chemistry 334 Principles of rganic Chemistry II Thursday ctober 5, 2006 ame: KEY. The exam is worth a total of 100 points; there are six questions. Please show all work to receive full credit for
Exam #1 Chemistry 334 Principles of rganic Chemistry II Thursday ctober 5, 2006 ame: KEY. The exam is worth a total of 100 points; there are six questions. Please show all work to receive full credit for
FALL 2018 CEM 251: Organic Chemistry I Sections Oct 23 rd, 2018
 FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
FALL 2018 CEM 251: Organic Chemistry I Sections 25-36 TUE-TU 8:00 9:20 AM Oct 23 rd, 2018 Name: Section: PID: TA: This is a closed book and note examination. If boxes are provided for your answers, only
Partial Periodic Table
 Easily Legible Printed Name: CEM 3351 (100), Fall 2015 Professor Walba econd our Exam ctober 20, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither given
Easily Legible Printed Name: CEM 3351 (100), Fall 2015 Professor Walba econd our Exam ctober 20, 2015 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither given
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Midterm Exam #1 /280 CHEM 6352 Fall 2011
 Midterm Exam #1 /280 CEM 6352 Fall 2011 ( %) Name Sept 30 th, 2011 18:00-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Midterm Exam #1 /280 CEM 6352 Fall 2011 ( %) Name Sept 30 th, 2011 18:00-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Chemistry 118 C Spring 2011 Second Midterm Fri. May 20 th, 2011 Instructor: Lievens. Name: Last First MI. Student ID. # T.A.
 Chemistry 118 C pring 2011 econd Midterm Fri. May 20 th, 2011 Instructor: Lievens This exam contains seven (7) pages and eight (8) problems. Please make sure that your copy contains all seven pages. If
Chemistry 118 C pring 2011 econd Midterm Fri. May 20 th, 2011 Instructor: Lievens This exam contains seven (7) pages and eight (8) problems. Please make sure that your copy contains all seven pages. If
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
CHEMISTRY 3331: Fundamentals of Organic Chemistry I Final Exam. Prof. Ognjen Š. Miljanić. Last 4 Digits of Student ID Number:
 CHEMISTRY 3331: Fundamentals of Organic Chemistry I Final Exam Prof. Ognjen Š. Miljanić December 7, 2011 Name: (print legibly) Last First Last 4 Digits of Student ID Number: Read all directions very carefully.
CHEMISTRY 3331: Fundamentals of Organic Chemistry I Final Exam Prof. Ognjen Š. Miljanić December 7, 2011 Name: (print legibly) Last First Last 4 Digits of Student ID Number: Read all directions very carefully.
Hour Examination # 2
 CHEM 347 Hour Examination # 2 Spring 2014 Page 1 of 8 CHEM 347 rganic Chemistry II (for Majors) Instructor: Paul J. Bracher Hour Examination # 2 Wednesday, March 5 th, 2014 5:30 8:30 p.m. Student Name
CHEM 347 Hour Examination # 2 Spring 2014 Page 1 of 8 CHEM 347 rganic Chemistry II (for Majors) Instructor: Paul J. Bracher Hour Examination # 2 Wednesday, March 5 th, 2014 5:30 8:30 p.m. Student Name
CHEM 2423 Unit 8 Homework Answers
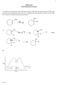 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
FINAL EXAM ANSWER KEY Winter Session 2011R
 ANSWE KEY Page 1 of 11 CEM 2220 rganic Chemistry II: eactivity and Synthesis Prof. P.G. ultin, Prof. T. egmann, Dr.. Luong FINAL EXAM ANSWE KEY Winter Session 2011 Monday April 25, 2011 9:00 am 12:00 pm
ANSWE KEY Page 1 of 11 CEM 2220 rganic Chemistry II: eactivity and Synthesis Prof. P.G. ultin, Prof. T. egmann, Dr.. Luong FINAL EXAM ANSWE KEY Winter Session 2011 Monday April 25, 2011 9:00 am 12:00 pm
Topics in the November 2007 Exam Paper for CHEM1904
 November 2007 Topics in the November 2007 Exam Paper for CEM1904 Click on the links for resources on each topic. 2007-N-2: 2007-N-3: 2007-N-4: 2007-N-5: 2007-N-6: 2007-N-7: Solubility Equilibrium Intermolecular
November 2007 Topics in the November 2007 Exam Paper for CEM1904 Click on the links for resources on each topic. 2007-N-2: 2007-N-3: 2007-N-4: 2007-N-5: 2007-N-6: 2007-N-7: Solubility Equilibrium Intermolecular
R or S? oxidation #: hybridization:
 Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
CHEM 231 (Davis) Organic Chemistry Exam III 19 April, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
Structure at the isoelectric point: The predominant form of this tripeptide at ph 1 has a net charge of: (circle one)
 ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
350 Organic Chemistry I Winona State University
 350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Partial Periodic Table
 Easily Legible Printed Name: CEM 3311 (300), Fall 2014 Professor Walba First our Exam September 23, 2014 scores: 1) 2) 3) 4) 5) CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CEM 3311 (300), Fall 2014 Professor Walba First our Exam September 23, 2014 scores: 1) 2) 3) 4) 5) CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student,
UCSC, Binder. CHEM 8B Organic Chemistry II EXAM 2A, Winter 2018 (200 points)
 UCSC, Binder Name CEM 8B rganic Chemistry II EXAM 2A, Winter 2018 (200 points) Use your knowledge of organic chemistry conventions to answer the questions in the proper manner. Be sure to read all questions
UCSC, Binder Name CEM 8B rganic Chemistry II EXAM 2A, Winter 2018 (200 points) Use your knowledge of organic chemistry conventions to answer the questions in the proper manner. Be sure to read all questions
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018
 CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1
 Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
I pledge my honor that I have neither given nor received aid on this examination
 Chemistry 220b, Section 1 Exam 1 (100 pts) Tuesday, February 3, 2015 Chapters 13, 15, 16 Name Write and sign the VU onor Pledge: I pledge my honor that I have neither given nor received aid on this examination
Chemistry 220b, Section 1 Exam 1 (100 pts) Tuesday, February 3, 2015 Chapters 13, 15, 16 Name Write and sign the VU onor Pledge: I pledge my honor that I have neither given nor received aid on this examination
= ( 3 ) + 2 ( 3 ) = ( ) = = ( ) = 20 H H
 Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
CEM 852 Final Exam. May 5, 2011
 CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
CHEM 203. Midterm Exam 2 November 13, 2014 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 2 ovember 13, 2014 Your name: ASWERS This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15 3.
CEM 203 Midterm Exam 2 ovember 13, 2014 Your name: ASWERS This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15 3.
Chem 2061 Final Exam. Fall Andy Aspaas, Instructor. Thursday, December 15, Instructions: Please print: Last name: First name:
 Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
CH2710-Exam #5 (Katz)
 C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
CHEM 330. Final Exam December 11, 2007 A N S W E R S. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
CHEM 8A Summer Student ID # Organic Chemistry EXAM 2 (300 points)
 CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry EXAM 2 (300 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the questions
CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry EXAM 2 (300 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the questions
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
2. 2D Lewis structure (large structure with possible formal charge) 20
 hem 201 Final Fall, 2017 Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 30 2. 2D Lewis structure (large structure with possible formal charge) 20 3. yclohexane
hem 201 Final Fall, 2017 Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 30 2. 2D Lewis structure (large structure with possible formal charge) 20 3. yclohexane
Homework Problem Set 11 Iverson CH320N Due Friday, May 5
 omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
+ HBr ΔH = OH CH CH 2. HgOAc
 radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
Exam I 19 April 2004 Name:
 Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
