Chem 150 Week 7 Handout 1 Thermochemistry (I) Energy used to move an object over some distance.
|
|
|
- Eric Houston
- 6 years ago
- Views:
Transcription
1 Chem 150 Week 7 Handout 1 Thermochemistry (I) Define Energy: The capacity to do work or to transfer heat. Work Energy used to move an object over some distance. w = F x d, where w is work, F is the force, and d is the distance over which the force is exerted. Heat Energy used to cause the temperature of an object to rise. Heat flows from warmer objects to cooler objects. System and Surroundings exchange of energy and matter can occur in an open system in a closed system energy can exchange but matter cannot in an isolated system neither heat nor matter can be exchanged with the surroundings First Law of Thermodynamics Energy is neither created nor destroyed; it is conserved. In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it U. By definition, the change in internal energy, U, is the final energy of the system minus the initial energy of the system: Changes in Internal Energy U = U final U initial When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). That is: U = q + w.
2 If U > 0 i.e. U final > U initial Therefore, the system absorbed energy from the surroundings. This energy change is called endothermic. If U < 0 i.e. U final < U initial Therefore, the system released energy to the surroundings. This energy change is called exothermic. State functions Usually we have no way of knowing the internal energy of a system; finding that value is simply too complex a problem. However, we do know that the internal energy of a system is independent of the path by which the system achieved that state. In the system above, the water could have reached room temperature from either direction. Therefore, internal energy is a state function. It depends only on the present state of the system, not on the path by which the system arrived at that state. And so, U depends only on U initial and U final.
3 However, q and w are not state functions. Enthalpy Whether the battery is shorted out or is discharged by running the fan, its U is the same. But q and w are different in the two cases. If a process takes place at constant pressure (as do the majority of processes we study) and the only work done is this pressure-volume work, we can account for heat flow during the process by measuring the enthalpy of the system. Enthalpy is the internal energy plus the product of pressure and volume: H = U + PV Since U = q + w and w = P V, we can substitute these into the enthalpy expression: H = U + P V H = (q+w) w H = q So, at constant pressure the change in enthalpy is the heat gained or lost. In other words the enthalpy of a reaction is the heat of a reaction at constant pressure. Enthalpies of Reaction The change in enthalpy, H, is the enthalpy of the products minus the enthalpy of the reactants and is called the enthalpy of reaction or the heat of reaction. H = H products H reactants
4 Brown, LeMay About Enthalpy: 1. Enthalpy is an extensive property (depends on amount of substance) 2. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. 3. H for a reaction depends on the state of the products and the state of the reactants. How different are H and U? Consider the following reaction: 2CO + O 2 2CO 2
5 Calorimetry As with U we cannot know the exact enthalpy of the reactants and products, but we can measure H through calorimetry, the measurement of heat flow. Heat Capacity and Specific Heat The amount of energy required to raise the temperature of a substance by 1 K (1 C) is its heat capacity. We define specific heat capacity (or simply specific heat) as the amount of energy required to raise the temperature of 1 g of a substance by 1 K. Specific heat = (quantity of heat transferred)/{(grams of substance)x(temprature change)}, c = q/(m ΔT) By carrying out a reaction in aqueous solution in a simple calorimeter one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Because the specific heat for water is well known (4.184 J/mol K), we can measure H for the reaction with this equation: q rxn = (specific heat of solution) x (grams of solution) T Hess s Law Hess s law states that If a reaction is carried out in a series of steps, H for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps, or simply: H total = H 1 + H H n Enthalpies of Formation An enthalpy of formation, f H, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. Standard enthalpies of formation, f H, are measured under standard conditions (298 K and 1.00 bar pressure). Many are tabulated and we can simply look them up.
6 Example to apply Hess law Calculation of H of reactions that cannot be measured (or carried out) like the formation of propane directly from the elements: If the reaction of hydrogen with carbon is carried out in practice a mixture of products is obtained and more importantly the reaction does not proceed quantitatively meaning there are still unreacted carbon and hydrogen left after equilibrium has established. Calculating the enthalpy change associated with this reaction can help us partly understand why this might be the case. 3C (graphite) + 4H 2 (g) C 3 H 8 (g) H =? What can we measure? We can measure the heat of combustion of propane: C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) H = kj/mol We can also measure the heat of formations of CO 2 and H 2 O from the elements C (graphite) + O 2 (g) CO 2 (g) H 2 (g) + 1/2 O 2 (g) H 2 O (l) H = kj/mol H = kj/mol Rearranging these equations can give us the heat of formation of propane 3 CO 2 (g) + 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) H = kj/mol 3 C (graphite) + 3 O 2 (g) 3 CO 2 (g) H = 3x( kj/mol) 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l) H = 4x( kj/mol) 3C (graphite) + 4H 2 (g) C 3 H 8 (g) H = kj/mol Definition: An enthalpy of formation, f H, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. (Arbitrary if the reaction can actually be carried out in this way or not- we get important information on the energy that is stored (ore used) in compounds relative to the elements it is made up from) We can use Hess s law in another way: H = n H f(products) - m H f(reactants) where n and m are the stoichiometric coefficients. Calculate the heat of combustion of sucrose (use data in the table from handout thermochemistry II) C 12 H 22 O 11 (s) + 12O 2 (g) 12CO 2 (g) + 11H 2 O (l)
7 H = n fh(products) - m fh(reactants) H = Energy in Foods and Fuels Tables from: Brown LeMay Chemistry the Central Science Most of the fuel in the food we eat comes from carbohydrates and fats. While most of the fuel consumed by society today comes from fossil fuels, natural gas, etc.
Lecture Presentation. Chapter 5. Thermochemistry Pearson Education, Inc.
 Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Chapter 5. Thermochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chapter 5. Thermochemistry
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO 2009, Prentice-Hall,
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO 2009, Prentice-Hall,
CHEM 103 CHEMISTRY I
 CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5. Thermochemistry. Energy. Potential Energy. aa
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO Energy Energy is the
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO Energy Energy is the
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Lecture Presentation. Chapter 5. Thermochemistry. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Lecture 0503 Calorimetry and Hess s Law
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Lecture 0503 Calorimetry and Hess s Law John D. Bookstaver St. Charles Community College Cottleville,
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Lecture 0503 Calorimetry and Hess s Law John D. Bookstaver St. Charles Community College Cottleville,
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Chem 121 G. Thermochemistry
 Chem 121 G. Thermochemistry Energy 1 st law Enthalpy, enthalpy of combustion, fuels Calorimetry Enthalpy of reaction Hess's Law, calculations Energy Energy: capacity to do work or transfer heat Matter
Chem 121 G. Thermochemistry Energy 1 st law Enthalpy, enthalpy of combustion, fuels Calorimetry Enthalpy of reaction Hess's Law, calculations Energy Energy: capacity to do work or transfer heat Matter
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Thermochemistry 14.notebook. November 24, Thermochemistry. Energy the capacity to do work or produce heat. translational.
 Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Enthalpy and Internal Energy
 Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Chemical Thermodynamics
 Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Enthalpy Chapter 5.3-4,7
 Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Chemistry 101 Chapter 10 Energy
 Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
To calculate heat (q) for a given temperature change: heat (q) = (specific heat) (mass) ( T) where T = T f T i
 Use your textbook or other resources available to answer the following questions General Information: Thermochemistry Phase Change A change in the physical form/state but not a change in the chemical identity
Use your textbook or other resources available to answer the following questions General Information: Thermochemistry Phase Change A change in the physical form/state but not a change in the chemical identity
Section 3.0. The 1 st Law of Thermodynamics. (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities Definitions and Concepts
 Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Chem 115 Class Notes 3/13/08 KF. THERMOCHEMISTRY (Ch.5)
 Chem 115 Class Notes 3/13/08 KF THERMOCHEMISTRY (Ch.5) - thermochemistry is the portion of thermodynamics that deals with the relationship between chemical reactions and the exchange of energy in the form
Chem 115 Class Notes 3/13/08 KF THERMOCHEMISTRY (Ch.5) - thermochemistry is the portion of thermodynamics that deals with the relationship between chemical reactions and the exchange of energy in the form
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Thermodynamics. Internal Energy. Study of energy and its transformations Thermochemistry
 Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Calorimetry. Chapter 5. Week 2 Unit 1. Calorimetry. Since we cannot know the of the reactants and products, we measure H through, the of.
 Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
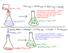 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
AAE THERMOCHEMISTRY BASICS
 5.4 THERMOCHEMISTRY BASICS Ch5 23 Energies in Chemical Reactions Enthalpy of Combustion (Reactions): Q CV H in = H reactant H out = H product REACTANTS Stoichiometric fuel-oxidizer (air) mixture at standard
5.4 THERMOCHEMISTRY BASICS Ch5 23 Energies in Chemical Reactions Enthalpy of Combustion (Reactions): Q CV H in = H reactant H out = H product REACTANTS Stoichiometric fuel-oxidizer (air) mixture at standard
THE ENERGY OF THE UNIVERSE IS CONSTANT.
 Chapter 6 Thermochemistry.notebook Chapter 6: Thermochemistry Jan 29 1:37 PM 6.1 The Nature of Energy Thermodynamics: The study of energy and its interconversions Energy: the capacity to do work or to
Chapter 6 Thermochemistry.notebook Chapter 6: Thermochemistry Jan 29 1:37 PM 6.1 The Nature of Energy Thermodynamics: The study of energy and its interconversions Energy: the capacity to do work or to
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Study Guide Chapter 5
 Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Most hand warmers work by using the heat released from the slow oxidation of iron: The amount your hand temperature rises depends on several factors:
 Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter 6. Thermochemistry
 Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Enthalpy. Enthalpy. Enthalpy. Enthalpy. E = q + w. Internal Energy at Constant Volume SYSTEM. heat transfer in (endothermic), +q
 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
THERMODYNAMICS. Dear Reader
 THERMODYNAMICS Dear Reader You are familiar with many chemical reactions which are accompanied by the absorption or release of energy. For example, when coal burns in air, a lot of heat is given out. Another
THERMODYNAMICS Dear Reader You are familiar with many chemical reactions which are accompanied by the absorption or release of energy. For example, when coal burns in air, a lot of heat is given out. Another
Chapter 11. Thermochemistry: Heat & Chemical Change
 Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Chapter 6. Heat Flow
 Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
2013, 2011, 2009, 2008 AP
 Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Lecture outline: Chapter 5
 Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Unit 7 Thermochemistry Chemistry 020, R. R. Martin
 Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
Energy Changes in Reactions p
 Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
