Chapter 5. Thermochemistry
|
|
|
- Pauline Jordan
- 6 years ago
- Views:
Transcription
1 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO 2009, Prentice-Hall, Inc.
2 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an object to rise is called heat.
3 Potential Energy Potential energy is energy an object possesses by virtue of its position or chemical composition.
4 Kinetic Energy Kinetic energy is energy an object possesses by virtue of its motion. 1 KE = mv 2 2
5 Units of Energy The SI unit of energy is the joule (J). 1 J = 1 An older, non-si unit is still in widespread use: the calorie (cal). 1 cal = J kg m2 s 2
6 Definitions: System and Surroundings The system includes the molecules we want to study (here, the hydrogen and oxygen molecules). The surroundings are everything else (here, the cylinder and piston).
7 Definitions: Work Energy used to move an object over some distance is work. w = F d where w is work, F is the force, and d is the distance over which the force is exerted.
8 Heat Energy can also be transferred as heat. Heat flows from warmer objects to cooler objects.
9 Conversion of Energy Energy can be converted from one type to another. For example, the cyclist above has potential energy as she sits on top of the hill.
10 Conversion of Energy As she coasts down the hill, her potential energy is converted to kinetic energy. At the bottom, all the potential energy she had at the top of the hill is now kinetic energy.
11 First Law of Thermodynamics Energy is neither created nor destroyed. In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa.
12 Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it E.
13 Internal Energy By definition, the change in internal energy, E, is the final energy of the system minus the initial energy of the system: E = E final E initial
14 Changes in Internal Energy If E > 0, E final > E initial Therefore, the system absorbed energy from the surroundings. This energy change is called endergonic.
15 Changes in Internal Energy If E < 0, E final < E initial Therefore, the system released energy to the surroundings. This energy change is called exergonic.
16 Changes in Internal Energy When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). That is, E = q + w.
17 E, q, w, and Their Signs
18 Exchange of Heat between System and Surroundings When heat is absorbed by the system from the surroundings, the process is endothermic.
19 Exchange of Heat between System and Surroundings When heat is absorbed by the system from the surroundings, the process is endothermic. When heat is released by the system into the surroundings, the process is exothermic.
20 State Functions Usually we have no way of knowing the internal energy of a system; finding that value is simply too complex a problem.
21 State Functions However, we do know that the internal energy of a system is independent of the path by which the system achieved that state. In the system below, the water could have reached room temperature from either direction.
22 State Functions Therefore, internal energy is a state function. It depends only on the present state of the system, not on the path by which the system arrived at that state. And so, E depends only on E initial and E final.
23 State Functions However, q and w are not state functions. Whether the battery is shorted out or is discharged by running the fan, its E is the same. But q and w are different in the two cases.
24 Work Usually in an open container the only work done is by a gas pushing on the surroundings (or by the surroundings pushing on the gas).
25 Work We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston. w = -P V
26 Enthalpy If a process takes place at constant pressure (as the majority of processes we study do) and the only work done is this pressure-volume work, we can account for heat flow during the process by measuring the enthalpy of the system. Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV
27 Enthalpy When the system changes at constant pressure, the change in enthalpy, H, is This can be written H = (E + PV) H = E + P V
28 Enthalpy Since E = q + w and w = -P V, we can substitute these into the enthalpy expression: H = E + P V H = (q+w) w H = q So, at constant pressure, the change in enthalpy is the heat gained or lost.
29 Endothermicity and Exothermicity A process is endothermic when H is positive.
30 Endothermicity and Exothermicity A process is endothermic when H is positive. A process is exothermic when H is negative.
31 Enthalpy of Reaction The change in enthalpy, H, is the enthalpy of the products minus the enthalpy of the reactants: H = H products H reactants
32 Enthalpy of Reaction This quantity, H, is called the enthalpy of reaction, or the heat of reaction.
33 The Truth about Enthalpy 1. Enthalpy is an extensive property. 2. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. 3. H for a reaction depends on the state of the products and the state of the reactants.
34 Calorimetry Since we cannot know the exact enthalpy of the reactants and products, we measure H through calorimetry, the measurement of heat flow.
35 Heat Capacity and Specific Heat The amount of energy required to raise the temperature of a substance by 1 K (1 C) is its heat capacity.
36 Heat Capacity and Specific Heat We define specific heat capacity (or simply specific heat) as the amount of energy required to raise the temperature of 1 g of a substance by 1 K.
37 Heat Capacity and Specific Heat Specific heat, then, is Specific heat = mass heat transferred temperature change s = m q T
38 Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter.
39 Constant Pressure Calorimetry Because the specific heat for water is well known (4.184 J/g-K), we can measure H for the reaction with this equation: q = m s T
40 Bomb Calorimetry Reactions can be carried out in a sealed bomb such as this one. The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction.
41 Bomb Calorimetry Because the volume in the bomb calorimeter is constant, what is measured is really the change in internal energy, E, not H. For most reactions, the difference is very small.
42 Hess s Law H is well known for many reactions, and it is inconvenient to measure H for every reaction in which we are interested. However, we can estimate H using published H values and the properties of enthalpy.
43 Hess s Law Hess s law states that [i]f a reaction is carried out in a series of steps, H for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps.
44 Hess s Law Because H is a state function, the total enthalpy change depends only on the initial state of the reactants and the final state of the products.
45 Enthalpies of Formation An enthalpy of formation, H f, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms.
46 Standard Enthalpies of Formation Standard enthalpies of formation, H f, are measured under standard conditions (25 C and 1.00 atm pressure).
47 Calculation of H C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) Imagine this as occurring in three steps: C 3 H 8 (g) 3 C (graphite) + 4 H 2 (g) 3 C (graphite) + 3 O 2 (g) 3 CO 2 (g) 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l)
48 Calculation of H C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) Imagine this as occurring in three steps: C 3 H 8 (g) 3 C (graphite) + 4 H 2 (g) 3 C (graphite) + 3 O 2 (g) 3 CO 2 (g) 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l)
49 Calculation of H C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) Imagine this as occurring in three steps: C 3 H 8 (g) 3 C (graphite) + 4 H 2 (g) 3 C (graphite) + 3 O 2 (g) 3 CO 2 (g) 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l)
50 Calculation of H C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) The sum of these equations is: C 3 H 8 (g) 3 C (graphite) + 4 H 2 (g) 3 C (graphite) + 3 O 2 (g) 3 CO 2 (g) 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l)
51 Calculation of H We can use Hess s law in this way: H = n H f products m H f reactants where n and m are the stoichiometric coefficients.
52 Calculation of H C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) H = [3( kj) + 4( kj)] [1( kj) + 5(0 kj)] = [( kj) + ( kj)] [( kj) + (0 kj)] = ( kj) ( kj) = kj
53 Energy in Foods Most of the fuel in the food we eat comes from carbohydrates and fats.
54 Energy in Fuels The vast majority of the energy consumed in this country comes from fossil fuels.
Chapter 5. Thermochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chapter 5. Thermochemistry. Energy. Potential Energy. aa
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO Energy Energy is the
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO Energy Energy is the
Lecture Presentation. Chapter 5. Thermochemistry Pearson Education, Inc.
 Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
CHEM 103 CHEMISTRY I
 CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
Chem 150 Week 7 Handout 1 Thermochemistry (I) Energy used to move an object over some distance.
 Chem 150 Week 7 Handout 1 Thermochemistry (I) Define Energy: The capacity to do work or to transfer heat. Work Energy used to move an object over some distance. w = F x d, where w is work, F is the force,
Chem 150 Week 7 Handout 1 Thermochemistry (I) Define Energy: The capacity to do work or to transfer heat. Work Energy used to move an object over some distance. w = F x d, where w is work, F is the force,
Lecture 0503 Calorimetry and Hess s Law
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Lecture 0503 Calorimetry and Hess s Law John D. Bookstaver St. Charles Community College Cottleville,
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Lecture 0503 Calorimetry and Hess s Law John D. Bookstaver St. Charles Community College Cottleville,
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Lecture Presentation. Chapter 5. Thermochemistry. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Thermodynamics. Internal Energy. Study of energy and its transformations Thermochemistry
 Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Calorimetry. Chapter 5. Week 2 Unit 1. Calorimetry. Since we cannot know the of the reactants and products, we measure H through, the of.
 Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Lecture 11 thermodynamics
 Lecture 11 thermodynamics We ll be dealing with the energy of chemical reactions How do you keep track of it? Where does it come from? 1 Energy Can come from a variety of sources: Ø Light (photochemistry)
Lecture 11 thermodynamics We ll be dealing with the energy of chemical reactions How do you keep track of it? Where does it come from? 1 Energy Can come from a variety of sources: Ø Light (photochemistry)
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
2013, 2011, 2009, 2008 AP
 Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Slide 2 / 118. Thermochemistry
 Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
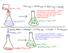 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Most hand warmers work by using the heat released from the slow oxidation of iron: The amount your hand temperature rises depends on several factors:
 Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
THERMODYNAMICS. Dear Reader
 THERMODYNAMICS Dear Reader You are familiar with many chemical reactions which are accompanied by the absorption or release of energy. For example, when coal burns in air, a lot of heat is given out. Another
THERMODYNAMICS Dear Reader You are familiar with many chemical reactions which are accompanied by the absorption or release of energy. For example, when coal burns in air, a lot of heat is given out. Another
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chemistry 101 Chapter 10 Energy
 Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Energy, Heat and Temperature. Introduction
 Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Thermochemistry 14.notebook. November 24, Thermochemistry. Energy the capacity to do work or produce heat. translational.
 Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
AP CHEMISTRY. Unit 5 Thermochemistry. Jeff Venables Northwestern High School
 AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Lecture Presentation. Chapter 5. Thermochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 5 James F. Kirby Quinnipiac University Hamden, CT Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work.
Lecture Presentation Chapter 5 James F. Kirby Quinnipiac University Hamden, CT Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work.
Chemical Thermodynamics
 Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Lecture 10. What is energy? Professor Hicks Inorganic Chemistry (CHE151) Ability to do work. Work means moving something against a force
 Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
THERMOCHEMISTRY -1. Dr. Sapna Gupta
 THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
CHEMISTRY. CHM201 Class #10 CHEMISTRY. Chapter 5. Particulate Review. Thermochemistry: Energy Changes in Reactions
 CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #10 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #10 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Ch. 7: Thermochemistry
 Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Unit #5- Chapter #6. Types of chemical reactions. Energy: its forms 10/15/2013. Thermodynamics
 Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 6. Energy Thermodynamics
 Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Study Guide Chapter 5
 Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Chapter 6. Heat Flow
 Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Chapter 6 Thermochemistry Heat Flow Heat (q): energy transferred from body at high T to body at low T Two definitions: System: part of universe we are interested in Surrounding: the rest of the universe
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS
 CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
Properties of Matter
 Properties of Matter Chapter 4 Hein and Arena Version 1.0 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons, Inc Properties of Substances 2 Properties of a Substance A property
Properties of Matter Chapter 4 Hein and Arena Version 1.0 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons, Inc Properties of Substances 2 Properties of a Substance A property
Chapter 5 THERMO. THERMO chemistry. 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation
 Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chapter 6: Thermochemistry
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
S = k log W 11/8/2016 CHEM Thermodynamics. Change in Entropy, S. Entropy, S. Entropy, S S = S 2 -S 1. Entropy is the measure of dispersal.
 Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
