Functional Identification of Spike-Processing Neural Circuits
|
|
|
- Emory Hines
- 5 years ago
- Views:
Transcription
1 Functional Identification of Spike-Processing Neural Circuits Aurel A. Lazar 1, Yevgeniy B. Slutskiy 1 1 Department of Electrical Engineering, Columbia University, New York, NY 127. Keywords: System identification, spiking neural circuits, biophysical models, phase response curves, RKHS Abstract We introduce a novel approach for a complete functional identification of biophysical spike-processing neural circuits. The circuits considered accept multidimensional spike trains as their input and are comprised of a multitude of temporal receptive fields and of conductance-based models of action potential generation. Each temporal receptive field describes the spatio-temporal contribution of all synapses between any two neurons and incorporates the (passive) processing carried out by the dendritic tree. The aggregate dendritic current produced by a multitude of temporal receptive fields is encoded into a sequence of action potentials by a spike generator modeled as a nonlinear dynamical system. Our approach builds on the observation that during any experiment, an entire neural circuit including its receptive fields and biophysical spike generators, is projected onto the space of stimuli. The projection is determined by the input signals used to identify the circuit. Employing the reproducing kernel Hilbert space (RKHS) of trigonometric polynomials to describe input stimuli, we quantitatively describe the relationship between underlying circuit parameters and their projections. We also derive experimental conditions under which these projections converge to the true parameters. In doing so, we achieve the mathematical tractability needed to (i) characterize the biophysical spike generator and (ii) identify the multitude of receptive fields. The algorithms obviate the need to repeat experiments in order to compute the neurons rate of response, rendering our methodology of interest to both experimental and theoretical neuroscientists. Neural Computation, accepted for publication (preprint, August 213). The authors names are alphabetically ordered. 1
2 1 Introduction Understanding how neural circuits perform computation is one of the most challenging problems in neuroscience. For the fruit fly (Drosophila), the advent of the genome sequence, coupled with extensive anatomical and electrophysiological studies, has led to an exponential growth in our knowledge about the organization of many neural circuits, including detailed knowledge about circuit connectivity, circuit inputs and outputs, as well as morphological and biophysical properties of individual neurons (Chiang et al., 211; Liang and Luo, 21; Yaksi and Wilson, 21; Chou et al., 21; Gouwens and Wilson, 29). Combined with the powerful genetic tools for visualizing, activating and deactivating specific circuit components, these advances raise the possibility of providing a comprehensive functional characterization of information processing within and between neurons. To this day, a number of methods have been proposed to quantify and model neuronal processing (see (Wu et al., 26) for an in-depth review). The majority of these methods assume that the input to a neural circuit is continuous and that the output is generated by a point process (e.g., Poisson process). In biological neural circuits, however, the inputs for most neurons are spike trains generated by presynaptic cells and the outputs are determined by a multi-dimensional dynamical system (Izhikevich, 27). Furthermore, the highly nonlinear nature of spike generation has been shown to produce interactions between stimulus features (Slee et al., 25; Hong et al., 27) that profoundly affect the estimation of receptive fields (Pillow and Simoncelli, 23). Hence, there is a fundamental need to develop tractable methods for identifying neural circuits that incorporate biophysical models of spike generation and receive multi-dimensional spike trains as input stimuli. Here we describe a new methodology for a complete neural circuit identification that takes the above-mentioned considerations into account. Our neural circuit models admit multidimensional spike trains as input stimuli. They also incorporate nonlinear dynamical system (Hodgkin-Huxley, Morris Lecar, hardthreshold IAF, etc.) models of the spike-generating mechanism. The nonlinear contribution of a dynamical system such as the Hodgkin-Huxley neuron model is stimulus-driven. It changes from one spike to the next and thus affects receptive field estimation if not properly taken into account. Our approach builds on the observation that during any experiment, a neural circuit is projected onto a particular space of input signals, with the circuit projection determined by how well the input space explores that circuit. Employing reproducing kernel Hilbert spaces (in particular, spaces of bandlimited functions) to model the input stimuli, we quantitatively describe the relationship between the underlying circuit parameters and their projections. We also derive conditions under which these projections converge to the true parameters. In doing so, we achieve the mathematical tractability needed to characterize biophysical spike generators and to identify the multitude of receptive fields in neural circuits with full connectivity. We estimate all model parameters directly from spike times produced by neurons and repeating the same stimulus is no longer necessary. 2
3 2 Problem Statement and Modeling Consider the simple neural circuit depicted in Fig. 1(a). The postsynaptic neuron shown in gray receives its feedforward spiking input from three cells that are highlighted in red, cyan and green. Presynaptic action potentials, depicted as spikes on the axon terminals, are processed by the dendritic tree of the postsynaptic cell and the resulting dendritic current is encoded into a single postsynaptic train of action potentials by the axon hillock. For the postsynaptic neuron in Fig. 1(a) we seek to (i) characterize the encoding carried out by the axon hillock and (ii) identify the dendritic processing of presynaptic spikes, assuming that both presynaptic and postsynaptic spike times are available to an observer. A block diagram representation of the postsynaptic neuron in Fig. 1(a) is shown in Fig. 1(b). Spikes from M N different neurons arrive at times (s m k ) k Z, s m k R, m = 1,..., M, forming M input spike trains s m. Since in biological neurons spikes typically arrive via multiple synapses at different locations on the dendritic tree, we model the processing of each spike train s m with a temporal receptive field h m that describes the combined spatio-temporal contributions of all synapses from neuron m, m = 1, 2,..., M. Such a temporal receptive field can capture not only the overall excitatory/inhibitory nature of synapses, but also the time-domain analog processing carried out by the dendritic tree. The aggregate dendritic current v = m vm, m = 1,..., M, produced in the tree is then encoded by a biophysical spike generator model (e.g., the Hodgkin-Huxley model) into a (s 1 k) (s 2 k) (s 3 k) (a) (t k) k Z (s 11 k ) k Z (s 1M k ) k Z Feedforward Input h 111 (t) h 11M (t) v 111 (t) v 11M (t) b 1 v 211 (t) v + 1 (t) v 221 (t) Back Propagation h 211 (t) Biophysical Spike Generator h 221 (t) (t 1 k) k Z (s 1 k) k Z (s M k ) k Z Feedforward Input h 1 (t) h M (t) v 1 (t) v M (t) v(t) + b Biophysical Spike Generator (t k) k Z (s 21 k ) k Z (s 2M k ) k Z Feedforward Input h 121 (t) h 12M (t) v 121 (t) v 12M (t) b 2 v 212 (t) v + 2 (t) v 222 (t) Lateral Input h 212 (t) Biophysical Spike Generator h 222 (t) Back Propagation (t 2 k) k Z (b) (c) Figure 1: Problem Setup (a) A simple neural circuit in which a neuron receives spiking input from multiple presynaptic cells. (b) Block diagram of the circuit in (a). Spatiotemporal dendritic processing of spike trains s m, m = 1,..., M, M N, is described by M temporal receptive fields with kernels h m. Action potentials are produced by a biophysical spike generator. (c) A canonical neural circuit model with a spiking feedforward input, lateral connections and feedback. 3
4 sequence of spike times (t k ) k Z. Generalizing the ideas above, one can consider more complex spiking neural circuits, in which (i) every neuron may receive not only feedforward inputs from a presynaptic layer, but also lateral inputs from neurons in the same layer and (ii) back-propagating action potentials (Waters et al., 25) may contribute to computations within the dendritic tree. A two-neuron circuit incorporating these considerations is shown in Fig. 1(c). The processing of lateral inputs in layer 2 is described by the temporal receptive fields (cross-feedback filters) h 212 and h 221, while various signals produced by back-propagating action potentials are modeled by the temporal receptive fields (feedback filters) h 211 and h 222. In what follows we show that the elementary building blocks of spiking neural circuits, including temporal receptive fields and spike generators, together with all inputs and outputs, can be naturally defined as elements of a reproducing kernel Hilbert space (RKHS), thereby making circuit identification mathematically tractable. Without loss of generality, we choose to work with the space of trigonometric polynomials H: Definition 1. The space of trigonometric polynomials H is the Hilbert space of complex-valued functions u(t) = L l= L u le l (t), where e l (t) = exp (jlωt/l) / T, l = L,..., L, is an orthonormal basis, u l C, t [, T ]. Here, T = 2πL/Ω is the period, Ω is the bandwidth and L is the order of the space. Endowed with the inner product u, w = T u(t)w(t)dt, H is a reproducing kernel Hilbert space (RKHS) with a reproducing kernel (RK) given by K(s, t) = L l= L e l(s)e l (t). Remark 1. The key property of an RKHS is the reproducing kernel property: for all u H and t [, T ]. u( ), K(, t) = u(t), 2.1 Modeling Dendritic Processing We assume that the precise shape and amplitude of action potentials received by the postsynaptic cell is of no particular significance in neural information processing. Although such information can be readily incorporated into the methods outlined below, it can be challenging to obtain in practice as it requires simultaneous intracellular (whole-cell) recordings from multiple presynaptic cells. Making a less stringent assumption that only the timing of action potentials is important, we take each spike arriving at a time s k to be a Dirac-delta function δ(t s k ), t R, so that the train of spikes s m from a presynaptic neuron m, m = 1,..., M, is given by s m (t) = k Z δ(t sm k ), t R (see Fig. 1). The spike times sm k correspond to peaks (or throughs) of action potentials and can be readily obtained by extracellular recordings. Given presynaptic spike trains s m, m = 1,..., M, and corresponding temporal receptive fields with kernels h m (Fig. 1(b)), the aggregate postsynaptic current amounts to v(t) = M m=1 (sm h m )(t) = M m=1 (s m h m ) denotes the convolution of s m with h m. k Z hm (t s m k ), where 4
5 Input Receptive Field Output δ(t) h(t) h(t) K(t, ) h(t) Ph(t) Figure 2: Modeling a single spike. (top row) A spike δ(t) at the input to a temporal receptive field produces a kernel h at its output. (bottom row) If a spike is replaced with the RK K(t, ) H, the receptive field output is the signal Ph(t) that closely approximates the output h produced by the spike δ(t) on t [, T ]. We model kernels h m as finite-energy functions with a finite temporal support (memory) on the interval [, S], i.e., h m belongs to the space H = { h L 2 (R) supp(h) [, S]}. Under quite natural conditions, each kernel h m can be approximated arbitrarily closely (in the L 2 norm (Grafakos, 28)) on [, S] by its projection Ph m in the space of trigonometric polynomials H (see Definition 1). The conditions for an arbitrarily-close L 2 approximation of the kernel h H by its projection Ph H are (i) T S and (ii) the bandwidth Ω and the order L of the space H are sufficiently high (Lazar and Slutskiy, 212). Thus, v (t) = K(t, s k ) h(t) (a) = (Ph)(t s k ) L 2 h(t s k ) (b) = δ(t s k ) h(t) = v(t), where (a) and (b) follow from the sampling properties of the RK and the Dirac-delta function, respectively. In other words, if an input spike δ(t s k ) is replaced with K(t, s k ), the output v of the temporal receptive field converges in the L 2 norm (with increasing bandwidth Ω and order L of the space H) to the output v elicitepd by the spike δ(t s k ). This is also illustrated in Fig. 2, where we compare the output of a temporal receptive field when stimulated with a delta function (top row) and an RK (bottom row). Example 1. Consider three arbitrarily chosen temporal receptive fields with kernels h m, m = 1, 2, 3, each with a temporal support of 1 ms and bandlimited to 1 Hz. Given three spike trains s 1, s 2, s 3, shown in red, green and blue in Fig. 3(a) and an appropriate choice of the space H (here Ω = 2π 16 rad/s and L = 4), we choose spikes in any window of length T (here T = 2πL/Ω =.25 s, shown in yellow) and replace every such spike s m k with the sampled reproducing kernel K(t, s m k ). We thus obtain three continuous signals Psm = 1 [,T ] s m K(t, ), m = 1,..., 3, that are periodic on the real line, as depicted in Fig. 3(b). Note that signals Ps m H with Ps m = L l= L Ps m, e l e l, m = 1,..., 3, l = L, L + 1,..., L. 5
6 (a) Input spike trains (s m ) 3 m =1 s 1 s 2 s 3 [,T] v (b) Projections of ( 1 [,T ] s m) 3 onto H with Ω = 2π 16 rad/s, L =4 m =1 (c) Aggregate dendritic current v = 3 m =1 sm h m and its estimate v = 3 m =1 Psm h m v [ ] c 3 [,T] m=1 supphm v v / max v [ ] c [,T] 3 m=1 supphm (d) Error between v and v P s 1 P s 2 P s 3 [,T] Figure 3: Modeling spiking input. (a) Spike streams (s m ) 3 m=1 (red, green, blue) at the input to a dendritic tree. Since streams have no beginning and end, we pick out spikes in a window (yellow) of length T. (b) Replacing spikes with the sampled RKs results in continuous signals (Ps m ) 3 m=1 (red, green, blue). (c) Passing signals (Ps m ) 3 m=1 through receptive fields (h m ) 3 m=1, we obtain the current v which is the same on t [, T ] [ 3 m=1 supp h m ] c (green) as the current v produced by the spiking input. (d) The error between v and v. Passing these three signals through the temporal receptive fields h m, m = 1, 2, 3, produces an aggregate dendritic current v = 3m=1 Psm h m. The latter is indistinguishable from the true dendritic current v = 3m=1 sm h m on the interval [, T ] [ 3 m=1 supp h m ] c (depicted in green in Fig. 3(c)). The error between v and v is shown in Fig. 3(d). Note that the approximation does not work in the time interval [, T ] [ 3 m=1 supp h m ] because the filters are causal and have memory, i.e., the spikes from both the present and the past affect the dendritic current. 2.2 Modeling Biophysical Spike Generators The model of action potential generation can be chosen from a wide class of spiking point neuron models, including nonlinear conductance-based models with stable limit cycles (e.g., Hodgkin-Huxley, Fitzhugh-Nagumo, Morris Lecar (Izhikevich, 27)), as well as simpler models such as the integrate-and-fire (IAF) or the threshold-and-fire neuron model. Although these models are deterministic in their original formulation, they can readily be extended to incorporate various noise sources in the form of, e.g., random thresholds (Lazar and Pnevmatikakis, 29) or stochastic gating variables (Lazar, 21) (see also section 3.3). Furthermore, 6
7 given the same input, even deterministic models typically do not produce the same output since their response fundamentally depends on the initial conditions of the dynamical system. Here we focus on conductance-based neuron models only. For convenience of the reader, we first briefly review the Hodgkin-Huxley point neuron model. We then present another model, called the reduced project-integrate-and-fire neuron with conditional phase response curves (reduced PIF-cPRC). This reduced model can be used to accurately capture response properties of many point neuron models, including Hodgkin-Huxley, Morris-Lecar, Fitzhugh-Nagumo and others. Furthermore, as discussed in section 3.1, the reduced PIF-cPRC model provides a simple way to faithfully characterize the spike generation process of biological neurons when the underlying neuron parameters are not known Conductance-Based Spike Generator Models The point neuron models considered here are described but the set of differential equations dx dt = f(x) + [I(t),,,..., ]T, (1) where the vector x describes the state of the point neuron, I(t), t R, is the aggregate dendritic current and x T denotes the transpose of x. A block diagram of a biophysical neuron with a single temporal receptive field is depicted in Fig. 4. Here the aggregate dendritic current is assumed to be of the form I(t) = v(t) + I b, where I b is a constant bias term and v(t) is the output of the temporal receptive field with the kernel h processing the continuous input stimulus u, i.e., v(t) = (u h)(t), t R. Example 2. The biophysics of action potential generation is captured by the four differential equations of the Hodgkin-Huxley neuron model (Johnston and Wu, 1994) with C dv dt = I b g Na m 3 h(v E Na ) g K n 4 (V E k ) g L (V E L ) dm dt = α m(v )(1 m) β m (V )m dh dt = α h(v )(1 m) β h (V )h dn dt = α n(v )(1 m) β n (V )n, α m (V ) =.1(25 V )/(e 25 V 1 1) β m (V ) = 4e V 18 α h (V ) =.7e V 2 β h (V ) = 1/(e 3 V 1 + 1) α n (V ) =.1(1 V )/(e 1 V 1 1) β n (V ) =.125e V 8, where V is the membrane potential, m, h and n are gating variables and I b R + is a constant input (bias) current. The original HH equations above can be compactly (2) 7
8 Biophysical Neuron Model u(t) h(t) v(t) + I(t) + ẋ(t) dt x Spike Detector (t k ) n k=1 I b f( ) Figure 4: Block diagram of a simple [RF]-[Biophysical Neuron] circuit. Aggregate current v produced by the receptive field is encoded by a nonlinear dynamical system, e.g., Hodgkin-Huxley neuron, that is described by a set of differential equations dx/dt = f(x). written as dx/dt = f(x), where x = [V, m, h, n] T is a vector comprised of the membrane voltage and sodium/potassium gating variables, while f = [f 1, f 2, f 3, f 4 ] T is the corresponding function vector. The sequence of spike times {t k } k Z is obtained by detecting the peaks of the action potentials of the first component of the vector x, i.e., the membrane potential x 1 = V Reduced Spike Generator Model Using non-linear perturbation analysis, it can be shown that for weak input stimuli the HH neuron (as well as many other conductance-based neuron models) are to a first order input/output (I/O)-equivalent to a reduced project-integrate-andfire (PIF) neuron (Lazar, 21). The PIF neuron is closely related to the ideal integrate-and-fire (IAF) neuron, with an additional step of projecting the external input current v(t) onto the (infinitesimal) phase response curve (PRC) (Izhikevich, 27) of the neuron: tk+1 t k ϕ 1 (s t k )v(s)ds = q k, (3) where q k = δ (t k+1 t k ) is the neuron s phase advance or delay, δ is the period of the neuron and ϕ 1 (t), t [, t k+1 t k ), is the PRC on a stable orbit. Eq. (3) is also known as the t-transform of the reduced PIF neuron (Lazar, 21). PRCs have been studied extensively in the neuroscience literature and simply describe the transient change in the cycle period of the neuron induced by a perturbation as a function of the phase at which that perturbation is received (Izhikevich, 27). For multidimensional models such as the Hodgkin-Huxley model, the function ϕ 1 is the first component of the vector-valued PRC ϕ = [ϕ 1, ϕ 2, ϕ 3, ϕ 4 ] T, corresponding to the membrane potential V. For strong input stimuli that introduce large perturbations into the dynamics of the neuronal response, the behavior of the neuron can be accurately described by the reduced PIF-cPRC neuron, the reduced project-integrate-and-fire neuron with conditional PRCs (Kim and Lazar, 211): tk+1 t k ϕ 1 k(s t k )v(s)ds = q k, (4) 8
9 Reduced PIF neuron with Conditional PRCs u(t) h(t) v(t) ϕ 1 k(t t k ) + + I(t) dt threshold reset V (t) δ k (t k ) n k=1 1 voltage reset to Figure 5: [RF]-Reduced PIF-cPRC neural circuit. Receptive field current v is encoded by a reduced PIF neuron with conditional PRCs. Both the PRC ϕ 1 k and the threshold δ k are stimulus-driven and change at spike times. where q k = δ k (t k+1 t k ) with δ k corresponding to the PRC ϕ 1 k. In this model the phase response curve is not frozen but conditioned on the input signal, as visualized in Fig. 5. The aggregate current v generated by the receptive field appears as an input to the PRC block. The latter depicts an entire family of PRCs and produces a PRC ϕ 1 k that is conditioned on v. The receptive field current is multiplied by the conditional PRC and the resulting signal v(t)ϕ 1 k (t t k), t [t k, t k+1 ), is encoded into a sequence of spikes by an integrate-and-fire type model, in which the threshold δ k is also conditioned on the input stimulus via the PRC. The subscript k points to the fact that the PRC ϕ 1 k (t) and the threshold δ k may change at each spike time, depending on the input signal v. 3 Identifying Biophysical Neuron Models In what follows we present a methodology for identifying neuron models consisting of receptive fields and spike generators. In section 3.1 we discuss the identification of the spike generator. Note that in the past literature the spike generator is called the point neuron. The identification of dendritic processing is presented in section 3.2. Finally, in section 3.3 the identification of noisy neurons is discussed. 3.1 Identifying the Spike Generator If parameters of the spike generator are not known a priori, we can use the reduced PIF neuron with conditional PRCs introduced above to derive a first-order equivalent model. In this case, identification of the spike generator calls for finding a family of PRCs. A number of different theoretical and experimental methods have been proposed to compute phase response curves both in model neurons (Izhikevich, 27) and in biological neurons (Netoff et al., 212). One of the most popular and widely used methods involves delivering a perturbation that is infinitesimallysmall in amplitude and duration and measuring its affect on the timing of subsequent spikes. Simple in its nature, this method requires delivering hundreds to thousands of precisely-timed pulses of current at different phases to map out the PRC. While easy to simulate in a model neuron, this procedure is a daunting task for any experimentalist working with biological neurons. Furthermore, 9
10 delivering perturbations that are infinitesimally small in duration is very difficult technically and the resulting perturbations are usually spread out in time. An alternative method, referred to as Malkin s approach or the adjoint method, involves linearizing the dynamical system about a stable limit cycle and solving the corresponding adjoint equations (Malkin, 1949; Ermentrout and Chow, 22; Izhikevich, 27). The drawback of this method is that the differential equations describing the dynamical system need to be known a priori. Below we introduce a new method for estimating PRCs that does not require knowing parameters of the dynamical system or delivering pulses of current at different phases of the oscillation cycle. Instead, our method is based on injecting a random current waveform and estimating the PRC from the information contained in the spike train at the output of the neuron. Although similar in spirit to what has been suggested in (Izhikevich, 27) and (Netoff et al., 212), our approach is different in that (i) it does not use white noise stimuli and (ii) it provides strong insight into how the perturbation signal affects the estimated PRC. It is also worth pointing out that injecting truly white noise signals is not possible in an experimental setting since all electrodes have finite bandwidths. We demonstrate that if the bandwidth of the injected current is not taken into account, the estimated PRC can be substantially different from the underlying PRC of the neuron. Finally, when compared to standard pulse methods for estimating the PRC, our approach is more immune to spike generation jitter since stimulus fluctuations are spread over the entire oscillation cycle as opposed to being concentrated at a particular moment in time (Izhikevich, 27). Consider a point neuron model on a stable limit cycle with a period δ that is generated by an input bias current I b = const. Given a weak random input signal u(t), t R, the response of the point neuron is faithfully captured by the reduced PIF neuron Eq. (4) as t k+1 t k ϕ 1 (t t k )u(t)dt = q k. For u H with a period T = 2πL/Ω, we get tk+1 t k T ϕ 1 (t)u(t + t k )dt = tk+1 t k T ϕ 1 (t) tk+1 t k u(s) ϕ 1 (t)k(t, s t k )dtds ( ) = T u(s)k(s, t + t k )dsdt = u(s)pϕ 1 (s t k )ds, where Pϕ 1 H is the PRC projection onto H and ( ) holds, provided that t k+1 t k T, since ϕ 1 (t) = for t > t k+1 t k for most neurons, including the Hodgkin- Huxley neuron (Goel and Ermentrout, 22). The inequality t k+1 t k T can be easily satisfied by an appropriate choice of the space H. By the Riesz representation theorem (Berlinet and Thomas-Agnan, 24) it follows that the right hand side of (5) is a linear functional and T u(s)pϕ 1 (s t k )ds = L(Pϕ 1 ) = Pϕ 1, φ k, where φ k H. In other words, spikes time perturbations due to the weak random input u H can be interpreted as measurements of the projection Pϕ 1. To reconstruct Pϕ 1 from these measurements, we have the following result. (5) 1
11 Theorem 1. Let {u i u i H} N i=1 be a collection of N linearly independent weak currents perturbing the Hodgkin Huxley neuron on a stable limit cycle with a period δ k. If the total number of spikes n = N i=1 ni generated by the neuron satisfies n 2L + N + 1, then the PRC projection Pϕ 1 can be identified from a collection of I/O pairs {(u i, T i )} N i=1 as (Pϕ 1 )(t) = L ψ l e l (t), (6) l= L where ψ l = [ψ] l, l = L, L + 1,..., L, and ψ = Φ + q. Furthermore, Φ = [Φ 1 ; Φ 2 ;... ; Φ N ], q = [q 1 ; q 2 ;... ; q N ] and [q i ] k = qk i with each Φi of size (n i 1) (2L + 1) and q i of size (n i 1) 1. The elements of matrices Φ i are given by [ [Φ i ] kl = 1 T L m= L, m l u i ml[e l+m (t i k+1 ) e l+m(t i k )] j(l + m)ω + u i l(t i k+1 t i k) for all k = 1, 2,..., n 1, l = L, L + 1,..., L, and i = 1, 2,..., N. Proof: Since Pϕ 1 H, it can be written as (Pϕ 1 )(t) = L l= L ψ le l (t). Furthermore, since the stimuli are linearly independent, the measurements (qk i 1 )ni k=1 provided by the PIF neuron are distinct. Writing (5) for a stimulus u i, we obtain ] (7) qk i = L Pϕ 1, φ i k = ψ l φ i l,k, (8) l= L or q i = Φ i ψ, with [q i ] k = q i k, [Φi ] kl = φ i l,k and [ψ] l = ψ l. Repeating for all i = 1,..., N, we get q = Φψ with Φ = [Φ 1 ; Φ 2 ;... ; Φ N ] and q = [q 1 ; q 2 ;... ; q N ]. This system of linear equations can be solved for ψ, provided that the rank r(φ) of the matrix Φ is r(φ) = 2L + 1. A necessary condition for the latter is that the total number n = N i=1 ni of spikes generated in response to all N signals satisfies n 2L + N + 1. Then the solution can be computed as ψ = Φ + q. To find the coefficients φ i l,k, we note that φi l,k = Li k (e l). Remark 2. Theorem 1 shows that only the PRC projection Pϕ 1 can be recovered from the recorded spike train. Note that Pϕ 1 is given by the projection of the PRC ϕ 1 onto the space of stimuli H and is, in general, quite different from the underlying PRC. In a practical setting, H is determined by the choice of stimuli in the experimental setup. Clearly, the bandwidth of the electrode/neuron seal plays a critical role in the PRC estimate. Remark 3. The random current waveforms {u i } N i=1 can be delivered either in separate experiments or in a single experimental trial. Since the effects of a perturbation can last longer than a single cycle (Netoff et al., 212), each current 11
12 Current, [μa/cm 2 ] Voltage, [mv] (a) Injected current I (t). Ω = 2π 524 rad/s, L = (b) HH neuron response (c) Output sequence (t k ) I b =7 μa/cm 2 Membrane potential All spikes Stable orbit spikes Perturbed orbit spikes All t k Stable orbit t k Perturbed orbit t k Potassium gating variable n (d) HHneuronresponseintheV-nplane.45 Stable orbit, δ k =7.627 ms Perturbed orbit.4 All spikes Stable spikes Perturbed spikes Membrane potential V, [mv] (e) Original PRC ϕ 1 vs. the identified PRC Pϕ 1.1 ϕ 1, MSE(P ϕ 1,ϕ 1 ) =-37.7dB P ϕ 1, MSE(P ϕ 1, P ϕ 1 ) =-38.2dB P ϕ π/2 π 3π/2 2π Phase θ, [rad] Figure 6: Estimating a single PRC of a HH neuron, I b = 7 µa/cm 2. (a) Injected spike-triggered random current waveform. (b) Corresponding membrane potential of the neuron. Stable and perturbed orbit spikes are shown in green and red, respectively. (c) Spike times used for the PRC identification. (d) HH neuron response (blue trajectory) in the V-n phase plane. The stable orbit is shown in pink. (e) The original PRC ϕ 1, its projection Pϕ 1 (Eq. (6))) onto the input current space and the identified PRC Pϕ 1 are shown in black, blue, and red, respectively. waveform may be followed by a quiescent period to ensure that one perturbation does not influence the neuronal response to another perturbation. The resulting spike-triggered random injected current waveform protocol minimizes interactions between consecutive current waveforms and allows one to efficiently measure the PRC projection Pϕ 1. Example 3. The proposed PRC identification procedure and its performance for a Hodgkin-Huxley neuron are illustrated in Fig. 6. First, a constant bias current I b = 7µA/cm 2 injected into the neuron (Fig. 6a) places the state of the neuron onto a stable limit cycle. The period of the oscillation δ on that limit cycle can be computed by recording stable spikes, produced in response to the constant current and highlighted in green color in Fig. 6b. Next, a sequence of random current waveforms with bandwidth Ω = 2π 524 rad/s and order L = 4 (Fig. 6a) is injected into the neuron and the perturbed spikes (highlighted in red, Fig. 6b) are recorded. In this example, the waveforms are delivered at every other spike in order to minimize the effect of one perturbation on the neuronal response to a subsequent perturbation, (see also Remark 3). We demonstrate the perturbation experienced by the dynamical system in Fig. 6d, where the Hodgkin-Huxley neuron response is 12
13 1 MSE between the original and identified PRC vs. stimulus bandwidth.15.1 ϕ 1 P ϕ 1 P ϕ ϕ 1 P ϕ 1 P ϕ 1 MSE(ϕ 1, P ϕ 1 ), [db] ϕ 1 P ϕ 1 P ϕ 1.1 π /2 π 3π /2 2π Phase θ,[rad] π /2 π 3π /2 2π Phase θ,[rad] 3.1 π /2 π 3π /2 2π Phase θ,[rad].15 ϕ 1.1 P ϕ 1 P ϕ π /2 π 3π /2 2π Phase θ,[rad] Stimulus Bandwidth, [Hz] Figure 7: PRC estimation is stimulus dependent. Mean-squared error (in decibels) between the original and identified PRC is plotted as a function of the stimulus bandwidth. plotted in a two dimensional V-n phase-plane. The stable limit cycle produced by the current I b is shown in pink and the perturbed trajectory around that limit cycle is plotted in blue. The oscillation period on the stable limit cycle was found to be δ = ms. Using δ together with the injected current waveforms and produced spike times (Fig. 6c), we identify the PRC projection Pϕ 1 and plot it together with the theoretical value of the projection Pϕ 1 and the underlying PRC ϕ 1 in Fig. 6d. Note that there is essentially no difference between the three waveforms in the latter plot. Remark 4. Note that the identification error in Fig. 7 decreases with increasing bandwidth and eventually levels off, providing us with a measurement of the PRC bandwidth. This is something that cannot be determined using the traditional white noise and pulse methods. Furthermore, glass electrodes traditionally used in the PRC estimation naturally impose a bandlimitation. Traditional methods do not take that fact into account and simply declare that the estimated PRC is the underlying PRC of the neuron. Our results suggest that in reality the experimenter often finds only the PRC projected onto the space determined by the input stimuli employed and the electrode properties (bandwidth). As a result, different results may be obtained in day-to-day experiments due to the variability in electrodes and groups using different types of electrodes may come up with different estimates. Example 4. In the above example, the bandwidth of the stimulus was Ω = 13
14 Potassium Gating Variable n (a) Limit cycles of the HH neuron in the V-n plane.4 x o, I b =1μA/cm 2 x o, I b =7μA/cm 2 Zero phase Membrane Potential V, [mv] Period δk, [ms] (b) Period of a HH neuron on a stable limit cycle δ k(i b) δ k, I b =1μA/cm 2 δ k, I b =7μA/cm Bias current I b,[μa/cm 2 ] (c) PRC family of the HH neuron (d) PRC family of the HH neuron PRC ϕ 1,[rad].5 I b =1μA/cm 2 I b =7μA/cm 2 PRC ϕ 1,[rad] Phase θ, [rad] I b,[μa/cm 2 ] 2 5 Time, [ms] 1 15 Figure 8: A family of Hodgkin-Huxley PRCs computed using the method described in this section. 2π 524 rad/s and the PRC ϕ 1 was identified with a very high precision. In general however, the projection Pϕ 1 is stimulus (bandwidth) dependent. This dependency is demonstrated in Fig. 7, where the mean-squared error between the identified PRC projection Pϕ 1 and the original PRC ϕ 1 as a function of the stimulus bandwidth Ω is depicted. Several identification examples are shown as insets in the plot. Note that the identified functions in the first two examples are very different from the PRC estimated in Fig. 6d. Example 5. An entire family of PRCs that was estimated using the above method for 63 different limit cycles is shown in Fig. 8. As the input bias current I b increases, the limit cycle x of the neuron shrinks (from blue to green Fig. 8a), in the V n plane. At the same time, the period of the oscillation δ k decreases from 17.2 ms to 7.6 ms (Fig. 8b). As a result, the temporal support of each PRC decreases as well (Fig. 8d), requiring higher-bandwidth currents for estimating the underlying PRC at high spike rates. The entire family of PRCs as a function of phase θ [, 2π] and time t [, δ k ] is shown in Fig. 8c and Fig. 8d, respectively. 3.2 Identifying Dendritic Processing Once the biophysical spike generator has been characterized and the family of its phase response curves computed, one can begin identifying the temporal receptive fields describing the analog processing of incoming spikes by the dendritic tree. In 14
15 what follows we use the notation s i = (s 1i, s 2i,..., s Mi ) to describe M spike train sequences that stimulate the neuron on the i th trial, i = 1, 2,..., N. Theorem 2. Channel Identification Machine (CIM) Let {s i } N i=1 be a collection of spike train M-tuples at the input to a neuron with M temporal receptive fields h m H, m = 1,..., M. Furthermore, let the family of conditional phase response curves ϕ 1, corresponding to the membrane voltage V be known and let (t i k ) k Z, i = 1,..., N, be the sequence of spikes produced by the neuron. Given a space H with T S and sufficiently high order L and bandwidth Ω, the filter projections Ph m can be identified from a collection of input and output spike trains {s i } N i=1 and {t i } N i=1 as (Ph m )(t) = L l= L hm l e l (t), m = 1,..., M. Here the coefficients h m l are given by h = Φ + q with q = [q 1, q 2,..., q N ] T, [q i ] k = qk i and h = [h 1 L,..., h1 L, h2 L,..., h2 L,..., hm L,..., hm L ]T, provided that the matrix Φ has rank r(φ) = M(2L + 1). The i th row of matrix Φ is given by [Φ 1i, Φ 2i,..., Φ Mi ], i = 1,..., N, with [Φ mi ] kl = T s mi l and the column index l = L,..., L. t i k+1 t i k ϕ 1 (t t i k )e l(t)dt, where s mi l = Ps mi, e l Proof: Since each Ph m H, it can be written as (Ph m )(t) = L l= L hm l e l (t). Hence, for the m th component of the spike-train M-tuple Ps i we have (Ps mi e l (t) and the aggregate current produced by the den- T L l= L hm l s mi l e l (t), with v i H. Substituting the h m )(t) = T L l= L hm l s mi l dritic tree is v i (t) = M m=1 M m=1 (a) = L i k (vi ) (b) = v i, φk i = last expression into the t-transform (4), we obtain qk i L l= L T h m l s mi l φ i l,k, where (a) and (b) follow from the Riesz representation theorem (Berlinet and Thomas-Agnan, 24) with φ i k = L l= L φi l,k e l(t). In matrix form, we have q i = [Φ 1i, Φ 2i,..., Φ Mi ]h with h = [h 1 L,..., h1 L, h2 L,..., h2 L,..., hm L,..., h M L ]T, [q i ] k = q i k and [Φmi ] kl = T s mi l φ i l,k. Repeating for all M-tuples Psi, i = 1,..., N, we obtain q = Φh. This system of equations can be solved for h, provided that the matrix rank r(φ) = M(2L + 1). To find the coefficients φ i l,k, we note that φ i l,k = Li k (e l) = t i k+1 t i k ϕ 1 (t t i k )e l(t)dt. Remark 5. To satisfy the condition r(φ) = M(2L + 1) of the CIM algorithm, the neuron must produce a total of at least M(2L + 1) + N spikes in response to all N spike-train M-tuples. If each M-tuple is of duration T, this condition can be met by increasing the duration N T of the experimental recording. Example 6. Identification results for the circuit of Fig. 1(b) are presented in Fig. 9. Here, a single neuron receives two spiking inputs, the analog processing of which is described by two temporal receptive fields. Both temporal receptive fields were chosen arbitrarily and had a positive mean in order to generate an excitatory current when stimulated with spikes. Multiple recorded input spike trains s i = (s 1i, s 2i ) were used together with the output spike trains to identify the receptive fields. Two spike trains from the first experimental trial i = 1 are shown in red and green in Fig. 9(a). The aggregate current produced in the dendritic tree (Fig. 15
16 Current, [μa/cm 2 ] Voltage, [mv] (b) (a) Input spike trains (s 1m ) 2 m =1 s 11 k s 12 k 1[,T ] Aggregate dendritic current v 1 (t) +I b (c) HH neuron response v 1 (t) +I b Bias I b = Membrane potential Spike times t 1 k (d) Output spike train (t 1 k ) Spike times t 1 k 1 [,T ] [ 2 m=1 supp hm ] c Potassium gating variable n (e) HHneuronresponseintheV-nplane Perturbed orbit Zero phase Membrane Potential, [mv] (f ) Original filter h 1 vs. the identified filter P h 1 h 1, MSE(P h 1,h 1 ) =-26.9dB P h 1 (g ) Original filter h 2 vs. the identified filter P h 2 h 2, MSE(P h 2,h 2 ) =-26.8dB P h Figure 9: Identifying receptive fields in cascade with a HH neuron. (a) Feedforward input spike trains s m1, m = 1, 2. (b) Induced aggregate dendritic current v 1 (t). (c) Membrane potential of the HH neuron as a function of time. Red dots denote the peaks of action potentials. (d) Corresponding spike times (t 1 k ) k Z. (e) HH neuron response in the V-n phase plane. (f-g) Identified receptive field projections Ph m, m = 1, 2. 9(b)) was then encoded into a sequence of action potentials (Fig. 9(c)) by the Hodgkin-Huxley model with a bias I b =. The corresponding sequence of spike times (t 1 k ) k Z as measured, e.g., in extracellular recordings, is shown in Fig. 9(d). For clarity, the response of the HH neuron is also visualized in the V -n phase plane in Fig. 9(e). Even for large perturbations of the dynamical system, the presented algorithm allows one to faithfully identify the two dendritic processing filters, shown in red and green in Fig. 9(f-g). The mean squared error between the original and identified kernels is 27 db. 3.3 Extension to Spike Generators with Stochastic Conductances Since all currents flowing through the neuronal cell membrane are generated by ion channels, the opening and closing of which are probabilistic in nature (Johnston and Wu, 1994), it is natural to introduce stochastic conductances into the differential equations describing a conductance-based spike generator model. This allows one to incorporate the intrinsic noise observed in neuronal responses. For example, a Hodgkin-Huxley neuron with stochastic conductances and an additively coupled input current I(t) can be described by the following equations (Lazar, 21): 16
17 dx = f(x)dt + [I(t),,, ] T dt + [, dw 2, dw 3, dw 4 ] T, where X = [X 1 (t), X 2 (t), X 3 (t), X 4 (t)] T is a stochastic process and W = [, W 2 (t), W 3 (t), W 4 (t)] T is a vector of independent Brownian motions. This neuron is to a first order I/O-equivalent to a project-integrate-and-fire (PIF) neuron with random thresholds, for which the t-transform is given by tk+1 t k ϕ 1 k(t t k )I(t)dt = q k + ε k, where ϕ 1 k and q k are provided by (4) and ε k is the error term introduced by stochastic conductances and effectively describes the spike jitter due to noise. Example 7. Multiple histograms of inter-spike intervals produced by a Hodgkin- Huxley neuron with stochastic conductances are shown in Fig. 1. Stationary independent increments W i (t) W i (s), i = 2,..., 4, of Brownian motions followed a normal distribution with a mean µ = and variance σ 2 = t s = 1 6. When driven by an input bias current I b = const, the neuron produced interspike intervals that followed a normal distribution, whose variance decreased with increasing values of the current I b. The spike-time jitter was as big as 1 ms for I b = 12 µa/cm 2. Estimating the PRC Family from Noisy Measurements The methodology employed in Section 3.1 can be extended within an appropriate mathematical setting to biophysical neuron models with stochastic conductances. Since inter-spike intervals of a Hodgkin-Huxley neuron with stochastic conductances follow a normal distribution, we assume that ε k N (, σ 2 ), k = 1, 2,..., n 1, are i.i.d.. In the presence of noise we can identify an estimate Pϕ 1 of Pϕ 1 that is optimal for an appropriately defined cost function. For example, we can formulate a bi-criterion Tikhonov regularization problem min Pϕ 1 H N n 1 ( Pϕ 2 1, φ i k qk) i + λ Pϕ 1 i=1 k=1 2 H, (9) where the scalar λ > provides a trade-off between the faithfulness of the identified PRC projection Pϕ 1 to measurements (q k ) n 1 k=1 and its norm Pϕ 1 H. Theorem 3. Problem (9) can be explicitly solved in analytical form. The optimal solution is achieved by ( Pϕ L 1 )(t) = ψ l e l (t), (1) l= L where ψ l = [ψ] l, l = L, L + 1,..., L, with ψ = (Φ H Φ + λi) 1 Φ H q, Φ = [Φ 1 ; Φ 2 ;... ; Φ N ] and Φ i, i = 1, 2,..., N, as defined in (7). 17
18 I, [μa/cm 2 ] Injected Current.5 1 V, [mv] 1 5 HH Response 2 4 n Phase Portrait 5 1 Density 2 1 ISI Jitter I, [μa/cm 2 ] V, [mv] n Density I, [μa/cm 2 ] V, [mv] n Density I, [μa/cm 2 ] V, [mv] Time, [ms] n V, [mv] Density Time, [ms] Figure 1: Spike timing jitter due to stochastic conductances (σ = 1 3 ). Proof: Since the minimizer Pϕ 1 is in H, it is of the form given in (1). Substituting this into (9), we obtain min Φψ q 2 ψ C 2L+1 R + λ n 1 ψ 2 C, 2L+1 where Φ = [Φ 1 ; Φ 2 ;... ; Φ N ] with Φ i, i = 1, 2,..., N, as defined in (7). This quadratic optimization problem can be analytically solved by expressing the objective as a convex quadratic function J(ψ) = ψ H Φ H Φψ 2q H Φψ+q H q+λψ H ψ with H denoting the conjugate transpose. A vector ψ minimizes J if and only if J = 2(Φ H Φ + λi)ψ 2Φ H q =, i.e., ψ = (Φ H Φ + λi) 1 Φ H q. Example 8. PRC identification results for a Hodgkin-Huxley neuron with stochastic conductances are shown in Fig. 11. As before, stationary independent increments W i (t) W i (s), i = 2,..., 4, of Brownian motions followed a normal distribution with mean µ = and variance σ 2 = t s = 1 6. Estimating Temporal Receptive Fields from Noisy Measurements Similarly, the CIM methodology (Theorem 2) for identifying temporal kernels describing the dendritic processing of incoming spikes can be can be extended to the case when the spike times produced by the neuron are noisy. For each temporal receptive field h m, m = 1,..., M, min Ph m H N n 1 ( ) 2 Ph m, φ i k qk i + λ Ph m 2, (11) H i=1 k=1 18
19 Potassium Gating Variable n (a) Limit cycles of the HH neuron in the V-n plane.4 x o, I b =1μA/cm 2 x o, I b =7μA/cm 2 Zero phase Membrane Potential V, [mv] Period δk, [ms] (b) Period of a HH neuron on a stable limit cycle δ k(i b) δ k, I b =1μA/cm 2 δ k, I b =7μA/cm Bias current I b,[μa/cm 2 ] (c) PRC family of the HH neuron (d) PRC family of the HH neuron PRC ϕ 1,[rad].5 I b =1μA/cm 2 I b =7μA/cm 2 PRC ϕ 1,[rad] Phase θ, [rad] I b,[μa/cm 2 ] Time, [ms] 15 Figure 11: Identifying PRC from noisy measurements (σ = 1 3 ). where the scalar λ > provides a trade-off between the faithfulness of the identified filter projection Ph m to measurements (q k ) n 1 k=1 and its norm Ph m H. Theorem 4. Problem (11) can be solved explicitly in analytical form. The optimal solution is achieved by L ( Ph m )(t) = h m l e l (t), (12) l= L with h m = (Φ H Φ+λI) 1 Φ H q, Φ = [Φ 1 ; Φ 2 ;... ; Φ N ] and [Φ i ] kl = T s mi t i k+1 l ϕ 1 (t t i k t i k )e l(t)dt, i = 1, 2,..., N. Proof: Essentially similar to the proof of Theorem 3. Example 9. Identification of two temporal receptive fields in the presence of spike generation jitter is shown in Fig. 12. The inset in plot Fig. 12(e) shows the effect of stochastic conductances on the subthreshold behavior of the neuron. Although a significant amount of noise is introduced into the system, we can identify optimal temporal receptive field estimates Ph 1 and Ph 2 that are very close to the underlying kernels h 1 and h 2. The MSE of identification is on the order of 24 db as seen in Fig. 12(f-g). 19
20 Current, [μa/cm 2 ] Voltage, [mv] (b) (a) Input spike trains (s 1m ) 2 m =1 s 11 k s 12 k 1 [,T ] Aggregate dendritic current v 1 (t) +I b (c) HH neuron response v 1 (t) +I b Bias I b = Membrane potential Spike times t 1 k (d) Output spike train (t 1 k ) Spike times t 1 k 1 [,T ] [ 2 m=1 supp hm ] c Potassium gating variable n (e) HHneuronresponseintheV-nplane Perturbed orbit Zero phase Membrane Potential, [mv] (f ) Original filter h 1 vs. the identified filter P h 1 h 1, MSE(P h 1,h 1 ) =-24.6dB P h 1 (g ) Original filter h 2 vs. the identified filter P h 2 h 2, MSE(P h 2,h 2 ) =-23.2dB P h Figure 12: Identifying temporal receptive fields in a noisy circuit (σ = 1 3 ). 4 Identifying Spiking Neural Circuits The methods presented in the previous section can be extended in two important directions. First, very few neurons in any organism function in isolation from each other, unless they are confined to the very sensory periphery. Most neurons upstream of the periphery are usually part of a larger neural circuit and receive, in addition to feedforward inputs, lateral inputs from other neurons in the same layer. It is desirable to be able to take such inputs into account. Second, there is increasing evidence that the processing of feedforward and lateral inputs by the dendritic tree of a neuron depends on the spiking activity of the neuron itself and that there is an interaction between external inputs and the back-propagating action potential of the neuron (Stuart et al., 1997; Waters et al., 25; Rózsa et al., 28; Casale and McCormick, 211). It is instructive to be able to take such interactions into account by introducing a feedback filter describing the effects of action potential generation on the activity within the dendritic tree. Such a filter can also capture the effects of various adaptation mechanisms observed in all biological neurons. As adaptation may take place on many different timescales (from milliseconds to several seconds), the temporal support of the feedback filter need not be limited to a single cycle of oscillation. In section 4.1 we present the identification methodology and simulation results for circuits with feedforward and lateral spiking inputs. Finally, in section 4.2 feedback is present in the system. Two cases are distinguished: (i) the feedback is 2
21 fast and essentially confined to a single inter-spike interval, and (ii) the feedback effects are spread over multiple consecutive interspike intervals. In the former, we demonstrate how such short-lived feedback can be captured by the family of phase response curves. In the latter, we show how to identify the kernel in the feedback path. 4.1 Circuits with Lateral Connectivity Without loss of generality, consider the neural circuit in Fig. 13. It is comprised of two second-layer neurons, each receiving multiple feedforward spiking inputs (s jm k ) k Z from the first layer. Here index j = 1, 2 labels the neurons in layer 2 and index m = 1,..., M, with M N, labels the input number. The processing of these feedforward inputs is described by M temporal receptive fields with kernels h 1jm, where j = 1, 2 and m = 1,..., M as before, and the first index 1 labels the input of the second layer. For simplicity, we assume that each neuron in Layer 1 output (s 11 k ) k Z (s 1M k ) k Z (s 21 k ) k Z (s 2M k ) k Z Feedforward Input h 111 (t) h 11M (t) Feedforward Input h 121 (t) h 12M (t) v 111 (t) v 11M (t) b 1 v 121 (t) v 12M (t) Layer 2 v + 1 (t) b 2 v 221 (t) v 212 (t) + v2 (t) Biophysical Spike Generator h 221 (t) Lateral Input h 212 (t) Biophysical Spike Generator Layer 2 output (t 1 k) k Z (t 2 k) k Z Figure 13: A simple circuit with lateral connectivity. layer 2 receives feedforward inputs only from layer 1 and that both neurons receive the same number of feedforward inputs M. In biological circuits, the number of layers and the number of inputs from these layers would be specified by the anatomy and prior knowledge about the circuit. In addition to feedforward spiking inputs, each neuron receives a lateral spiking input from the other neuron located in the same layer. The effects of these lateral inputs are described by kernels h 221 and h 212, where the first index indicates the output of layer number 2 and the last two indices specify the origin and destination of spikes, respectively. The aggregate dendritic currents v 1 and v 2, produced by all feedforward temporal receptive fields and cross-feedback, are encoded into sequences of action potentials by biophysical spike generation models. The spike times (t 1 k ) k Z, (t 2 k ) k Z comprise the output of the second layer. To summarize, each neuron receives M inputs from a presynaptic layer and 1 input from its own layer. Using the recorded spike times (s jm k ) k Z and (t j k ) k Z, j = 1, 2, m = 1,..., M we would like to identify a total of 2M + 2 temporal receptive fields. To that end, we have the following result: Corollary 1. Let {s ji } N i=1, j = 1, 2, be collections of spike train M-tuples at the input of two Hodgkin-Huxley neurons with feedforward temporal receptive fields h 1jm H, j = 1, 2, m = 1,..., M, and lateral receptive fields h 212, h 221. Let (t 1 k ) k Z and (t 2 k ) k Z be sequences of spike times produced by the two neurons. Given 21
Encoding Natural Scenes with Neural Circuits with Random Thresholds
 Encoding Natural Scenes with Neural Circuits with Random Thresholds Aurel A. Lazar, Eftychios A. Pnevmatikakis and Yiyin Zhou Department of Electrical Engineering Columbia University, New York, NY 10027
Encoding Natural Scenes with Neural Circuits with Random Thresholds Aurel A. Lazar, Eftychios A. Pnevmatikakis and Yiyin Zhou Department of Electrical Engineering Columbia University, New York, NY 10027
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
Massively Parallel Neural Circuits for Stereoscopic Color Vision: Encoding, Decoding and Identification
 Massively Parallel Neural Circuits for Stereoscopic Color Vision: Encoding, Decoding and Identification Aurel A. Lazar a,1,, Yevgeniy B. Slutskiy a,1, Yiyin Zhou a,1 a Department of Electrical Engineering,
Massively Parallel Neural Circuits for Stereoscopic Color Vision: Encoding, Decoding and Identification Aurel A. Lazar a,1,, Yevgeniy B. Slutskiy a,1, Yiyin Zhou a,1 a Department of Electrical Engineering,
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Research Article Channel Identification Machines
 Hindawi Pulishing Corporation Computational Intelligence and Neuroscience Volume 22, Article ID 2959, 2 pages doi:55/22/2959 Research Article Channel Identification Machines Aurel A Lazar and Yevgeniy
Hindawi Pulishing Corporation Computational Intelligence and Neuroscience Volume 22, Article ID 2959, 2 pages doi:55/22/2959 Research Article Channel Identification Machines Aurel A Lazar and Yevgeniy
Memory and hypoellipticity in neuronal models
 Memory and hypoellipticity in neuronal models S. Ditlevsen R. Höpfner E. Löcherbach M. Thieullen Banff, 2017 What this talk is about : What is the effect of memory in probabilistic models for neurons?
Memory and hypoellipticity in neuronal models S. Ditlevsen R. Höpfner E. Löcherbach M. Thieullen Banff, 2017 What this talk is about : What is the effect of memory in probabilistic models for neurons?
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
Math 345 Intro to Math Biology Lecture 20: Mathematical model of Neuron conduction
 Math 345 Intro to Math Biology Lecture 20: Mathematical model of Neuron conduction Junping Shi College of William and Mary November 8, 2018 Neuron Neurons Neurons are cells in the brain and other subsystems
Math 345 Intro to Math Biology Lecture 20: Mathematical model of Neuron conduction Junping Shi College of William and Mary November 8, 2018 Neuron Neurons Neurons are cells in the brain and other subsystems
Single-Compartment Neural Models
 Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1
 Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Compartmental Modelling
 Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Topics in Neurophysics
 Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Decoding. How well can we learn what the stimulus is by looking at the neural responses?
 Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Neural Excitability in a Subcritical Hopf Oscillator with a Nonlinear Feedback
 Neural Excitability in a Subcritical Hopf Oscillator with a Nonlinear Feedback Gautam C Sethia and Abhijit Sen Institute for Plasma Research, Bhat, Gandhinagar 382 428, INDIA Motivation Neural Excitability
Neural Excitability in a Subcritical Hopf Oscillator with a Nonlinear Feedback Gautam C Sethia and Abhijit Sen Institute for Plasma Research, Bhat, Gandhinagar 382 428, INDIA Motivation Neural Excitability
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Single-Cell and Mean Field Neural Models
 1 Single-Cell and Mean Field Neural Models Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 The neuron
1 Single-Cell and Mean Field Neural Models Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 The neuron
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
BME 5742 Biosystems Modeling and Control
 BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Neural Spike Train Analysis 1: Introduction to Point Processes
 SAMSI Summer 2015: CCNS Computational Neuroscience Summer School Neural Spike Train Analysis 1: Introduction to Point Processes Uri Eden BU Department of Mathematics and Statistics July 27, 2015 Spikes
SAMSI Summer 2015: CCNS Computational Neuroscience Summer School Neural Spike Train Analysis 1: Introduction to Point Processes Uri Eden BU Department of Mathematics and Statistics July 27, 2015 Spikes
Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses
 Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses Jonathan Pillow HHMI and NYU http://www.cns.nyu.edu/~pillow Oct 5, Course lecture: Computational Modeling of Neuronal Systems
Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses Jonathan Pillow HHMI and NYU http://www.cns.nyu.edu/~pillow Oct 5, Course lecture: Computational Modeling of Neuronal Systems
3.3 Simulating action potentials
 6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators
 Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators Ranjeetha Bharath and Jean-Jacques Slotine Massachusetts Institute of Technology ABSTRACT This work explores
Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators Ranjeetha Bharath and Jean-Jacques Slotine Massachusetts Institute of Technology ABSTRACT This work explores
Dynamical Systems in Neuroscience: Elementary Bifurcations
 Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
Dynamical systems in neuroscience. Pacific Northwest Computational Neuroscience Connection October 1-2, 2010
 Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Encoding of Multivariate Stimuli with MIMO Neural Circuits
 20 IEEE International Symposium on Information Theory Proceedings Encoding of Multivariate Stimuli with MIMO Neural Circuits Aurel A. Lazar Department of Electrical Engineering Columbia University, New
20 IEEE International Symposium on Information Theory Proceedings Encoding of Multivariate Stimuli with MIMO Neural Circuits Aurel A. Lazar Department of Electrical Engineering Columbia University, New
Single neuron models. L. Pezard Aix-Marseille University
 Single neuron models L. Pezard Aix-Marseille University Biophysics Biological neuron Biophysics Ionic currents Passive properties Active properties Typology of models Compartmental models Differential
Single neuron models L. Pezard Aix-Marseille University Biophysics Biological neuron Biophysics Ionic currents Passive properties Active properties Typology of models Compartmental models Differential
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
Stochastic Oscillator Death in Globally Coupled Neural Systems
 Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Phenomenological Models of Neurons!! Lecture 5!
 Phenomenological Models of Neurons!! Lecture 5! 1! Some Linear Algebra First!! Notes from Eero Simoncelli 2! Vector Addition! Notes from Eero Simoncelli 3! Scalar Multiplication of a Vector! 4! Vector
Phenomenological Models of Neurons!! Lecture 5! 1! Some Linear Algebra First!! Notes from Eero Simoncelli 2! Vector Addition! Notes from Eero Simoncelli 3! Scalar Multiplication of a Vector! 4! Vector
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Synchrony in Neural Systems: a very brief, biased, basic view
 Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Neural Modeling and Computational Neuroscience. Claudio Gallicchio
 Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
We observe the model neuron s response to constant input current, studying the dependence of:
 BioE332A Lab 2, 21 1 Lab 2 December 23, 29 A Spiking Neuron Like biological neurons, the model neuron we characterize in this lab has a repertoire of (model) ion-channel populations. Here we focus on the
BioE332A Lab 2, 21 1 Lab 2 December 23, 29 A Spiking Neuron Like biological neurons, the model neuron we characterize in this lab has a repertoire of (model) ion-channel populations. Here we focus on the
Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model
 Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model Jonathan W. Pillow, Liam Paninski, and Eero P. Simoncelli Howard Hughes Medical Institute Center for Neural Science New York
Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model Jonathan W. Pillow, Liam Paninski, and Eero P. Simoncelli Howard Hughes Medical Institute Center for Neural Science New York
Linearization of F-I Curves by Adaptation
 LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model
 1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
FRTF01 L8 Electrophysiology
 FRTF01 L8 Electrophysiology Lecture Electrophysiology in general Recap: Linear Time Invariant systems (LTI) Examples of 1 and 2-dimensional systems Stability analysis The need for non-linear descriptions
FRTF01 L8 Electrophysiology Lecture Electrophysiology in general Recap: Linear Time Invariant systems (LTI) Examples of 1 and 2-dimensional systems Stability analysis The need for non-linear descriptions
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits
 Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Simple models of neurons!! Lecture 4!
 Simple models of neurons!! Lecture 4! 1! Recap: Phase Plane Analysis! 2! FitzHugh-Nagumo Model! Membrane potential K activation variable Notes from Zillmer, INFN 3! FitzHugh Nagumo Model Demo! 4! Phase
Simple models of neurons!! Lecture 4! 1! Recap: Phase Plane Analysis! 2! FitzHugh-Nagumo Model! Membrane potential K activation variable Notes from Zillmer, INFN 3! FitzHugh Nagumo Model Demo! 4! Phase
Coupling in Networks of Neuronal Oscillators. Carter Johnson
 Coupling in Networks of Neuronal Oscillators Carter Johnson June 15, 2015 1 Introduction Oscillators are ubiquitous in nature. From the pacemaker cells that keep our hearts beating to the predator-prey
Coupling in Networks of Neuronal Oscillators Carter Johnson June 15, 2015 1 Introduction Oscillators are ubiquitous in nature. From the pacemaker cells that keep our hearts beating to the predator-prey
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
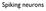 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks
 Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks Himadri Mukhopadhyay Bucknell
Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks Himadri Mukhopadhyay Bucknell
Chapter 24 BIFURCATIONS
 Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
NON-MARKOVIAN SPIKING STATISTICS OF A NEURON WITH DELAYED FEEDBACK IN PRESENCE OF REFRACTORINESS. Kseniia Kravchuk and Alexander Vidybida
 MATHEMATICAL BIOSCIENCES AND ENGINEERING Volume 11, Number 1, February 214 doi:1.3934/mbe.214.11.xx pp. 1 xx NON-MARKOVIAN SPIKING STATISTICS OF A NEURON WITH DELAYED FEEDBACK IN PRESENCE OF REFRACTORINESS
MATHEMATICAL BIOSCIENCES AND ENGINEERING Volume 11, Number 1, February 214 doi:1.3934/mbe.214.11.xx pp. 1 xx NON-MARKOVIAN SPIKING STATISTICS OF A NEURON WITH DELAYED FEEDBACK IN PRESENCE OF REFRACTORINESS
Electrophysiology of the neuron
 School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
From neuronal oscillations to complexity
 1/39 The Fourth International Workshop on Advanced Computation for Engineering Applications (ACEA 2008) MACIS 2 Al-Balqa Applied University, Salt, Jordan Corson Nathalie, Aziz Alaoui M.A. University of
1/39 The Fourth International Workshop on Advanced Computation for Engineering Applications (ACEA 2008) MACIS 2 Al-Balqa Applied University, Salt, Jordan Corson Nathalie, Aziz Alaoui M.A. University of
Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops
 Math. Model. Nat. Phenom. Vol. 5, No. 2, 2010, pp. 67-99 DOI: 10.1051/mmnp/20105203 Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops J. Ma 1 and J. Wu 2 1 Department of
Math. Model. Nat. Phenom. Vol. 5, No. 2, 2010, pp. 67-99 DOI: 10.1051/mmnp/20105203 Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops J. Ma 1 and J. Wu 2 1 Department of
Information Theory and Neuroscience II
 John Z. Sun and Da Wang Massachusetts Institute of Technology October 14, 2009 Outline System Model & Problem Formulation Information Rate Analysis Recap 2 / 23 Neurons Neuron (denoted by j) I/O: via synapses
John Z. Sun and Da Wang Massachusetts Institute of Technology October 14, 2009 Outline System Model & Problem Formulation Information Rate Analysis Recap 2 / 23 Neurons Neuron (denoted by j) I/O: via synapses
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
DYNAMICS OF CURRENT-BASED, POISSON DRIVEN, INTEGRATE-AND-FIRE NEURONAL NETWORKS
 DYNAMICS OF CURRENT-BASED, POISSON DRIVEN, INTEGRATE-AND-FIRE NEURONAL NETWORKS KATHERINE A. NEWHALL, GREGOR KOVAČIČ, PETER R. KRAMER, DOUGLAS ZHOU, AADITYA V. RANGAN, AND DAVID CAI Abstract. Synchronous
DYNAMICS OF CURRENT-BASED, POISSON DRIVEN, INTEGRATE-AND-FIRE NEURONAL NETWORKS KATHERINE A. NEWHALL, GREGOR KOVAČIČ, PETER R. KRAMER, DOUGLAS ZHOU, AADITYA V. RANGAN, AND DAVID CAI Abstract. Synchronous
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS
 MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
+ + ( + ) = Linear recurrent networks. Simpler, much more amenable to analytic treatment E.g. by choosing
 Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
Data Mining Part 5. Prediction
 Data Mining Part 5. Prediction 5.5. Spring 2010 Instructor: Dr. Masoud Yaghini Outline How the Brain Works Artificial Neural Networks Simple Computing Elements Feed-Forward Networks Perceptrons (Single-layer,
Data Mining Part 5. Prediction 5.5. Spring 2010 Instructor: Dr. Masoud Yaghini Outline How the Brain Works Artificial Neural Networks Simple Computing Elements Feed-Forward Networks Perceptrons (Single-layer,
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Neuronal Dynamics. From Single Neurons to Networks and Models of Cognition
 Neuronal Dynamics From Single Neurons to Networks and Models of Cognition Wulfram Gerstner, Werner M. Kistler, Richard Naud and Liam Paninski Cambridge Univ. Press, Cambridge, 2014 Preprint-draft, Chapter
Neuronal Dynamics From Single Neurons to Networks and Models of Cognition Wulfram Gerstner, Werner M. Kistler, Richard Naud and Liam Paninski Cambridge Univ. Press, Cambridge, 2014 Preprint-draft, Chapter
DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT
 Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Neural variability and Poisson statistics
 Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring. Elementary neuron models -- conductance based -- modelers alternatives
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Bo Deng University of Nebraska-Lincoln UNL Math Biology Seminar
 Mathematical Model of Neuron Bo Deng University of Nebraska-Lincoln UNL Math Biology Seminar 09-10-2015 Review -- One Basic Circuit By Kirchhoff's Current Law 0 = I C + I R + I L I ext By Kirchhoff s Voltage
Mathematical Model of Neuron Bo Deng University of Nebraska-Lincoln UNL Math Biology Seminar 09-10-2015 Review -- One Basic Circuit By Kirchhoff's Current Law 0 = I C + I R + I L I ext By Kirchhoff s Voltage
Limitations of the Hodgkin-Huxley Formalism: Effects of Single Channel Kinetics on Transmembrane Voltage Dynamics
 ARTICLE Communicated by Idan Segev Limitations of the Hodgkin-Huxley Formalism: Effects of Single Channel Kinetics on Transmembrane Voltage Dynamics Adam F. Strassberg Computation and Neural Systems Program,
ARTICLE Communicated by Idan Segev Limitations of the Hodgkin-Huxley Formalism: Effects of Single Channel Kinetics on Transmembrane Voltage Dynamics Adam F. Strassberg Computation and Neural Systems Program,
Modeling Action Potentials in Cell Processes
 Modeling Action Potentials in Cell Processes Chelsi Pinkett, Jackie Chism, Kenneth Anderson, Paul Klockenkemper, Christopher Smith, Quarail Hale Tennessee State University Action Potential Models Chelsi
Modeling Action Potentials in Cell Processes Chelsi Pinkett, Jackie Chism, Kenneth Anderson, Paul Klockenkemper, Christopher Smith, Quarail Hale Tennessee State University Action Potential Models Chelsi
1 R.V k V k 1 / I.k/ here; we ll stimulate the action potential another way.) Note that this further simplifies to. m 3 k h k.
 1. The goal of this problem is to simulate a propagating action potential for the Hodgkin-Huxley model and to determine the propagation speed. From the class notes, the discrete version (i.e., after breaking
1. The goal of this problem is to simulate a propagating action potential for the Hodgkin-Huxley model and to determine the propagation speed. From the class notes, the discrete version (i.e., after breaking
Neurophysiology of a VLSI spiking neural network: LANN21
 Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
5.4 Modelling ensembles of voltage-gated ion channels
 5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
Computability Theory for Neuroscience
 Computability Theory for Neuroscience by Doug Rubino Abstract Neuronal circuits are ubiquitously held to be the substrate of computation in the brain, information processing in single neurons is though
Computability Theory for Neuroscience by Doug Rubino Abstract Neuronal circuits are ubiquitously held to be the substrate of computation in the brain, information processing in single neurons is though
Frequency Adaptation and Bursting
 BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
Biological Modeling of Neural Networks
 Week 3 part 1 : Rection of the Hodgkin-Huxley Model 3.1 From Hodgkin-Huxley to 2D Biological Modeling of Neural Netorks - Overvie: From 4 to 2 dimensions - MathDetour 1: Exploiting similarities - MathDetour
Week 3 part 1 : Rection of the Hodgkin-Huxley Model 3.1 From Hodgkin-Huxley to 2D Biological Modeling of Neural Netorks - Overvie: From 4 to 2 dimensions - MathDetour 1: Exploiting similarities - MathDetour
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback. Carter L. Johnson
 Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Chapter 5 Experimentally Estimating Phase Response Curves of Neurons: Theoretical and Practical Issues
 Chapter 5 Experimentally Estimating Phase Response Curves of Neurons: Theoretical and Practical Issues Theoden Netoff, Michael A. Schwemmer, and Timothy J. Lewis Abstract Phase response curves (PRCs) characterize
Chapter 5 Experimentally Estimating Phase Response Curves of Neurons: Theoretical and Practical Issues Theoden Netoff, Michael A. Schwemmer, and Timothy J. Lewis Abstract Phase response curves (PRCs) characterize
Processing of Time Series by Neural Circuits with Biologically Realistic Synaptic Dynamics
 Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
RADIO SYSTEMS ETIN15. Lecture no: Equalization. Ove Edfors, Department of Electrical and Information Technology
 RADIO SYSTEMS ETIN15 Lecture no: 8 Equalization Ove Edfors, Department of Electrical and Information Technology Ove.Edfors@eit.lth.se Contents Inter-symbol interference Linear equalizers Decision-feedback
RADIO SYSTEMS ETIN15 Lecture no: 8 Equalization Ove Edfors, Department of Electrical and Information Technology Ove.Edfors@eit.lth.se Contents Inter-symbol interference Linear equalizers Decision-feedback
Stochastic Wilson-Cowan equations for networks of excitatory and inhibitory neurons II
 Stochastic Wilson-Cowan equations for networks of excitatory and inhibitory neurons II Jack Cowan Mathematics Department and Committee on Computational Neuroscience University of Chicago 1 A simple Markov
Stochastic Wilson-Cowan equations for networks of excitatory and inhibitory neurons II Jack Cowan Mathematics Department and Committee on Computational Neuroscience University of Chicago 1 A simple Markov
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics
 J Comput Neurosci (212) 32:55 72 DOI 1.17/s1827-11-339-7 Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics Yi Sun Aaditya V. Rangan Douglas Zhou David Cai Received:
J Comput Neurosci (212) 32:55 72 DOI 1.17/s1827-11-339-7 Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics Yi Sun Aaditya V. Rangan Douglas Zhou David Cai Received:
Characterization of Nonlinear Neuron Responses
 Characterization of Nonlinear Neuron Responses Final Report Matt Whiteway Department of Applied Mathematics and Scientific Computing whit822@umd.edu Advisor Dr. Daniel A. Butts Neuroscience and Cognitive
Characterization of Nonlinear Neuron Responses Final Report Matt Whiteway Department of Applied Mathematics and Scientific Computing whit822@umd.edu Advisor Dr. Daniel A. Butts Neuroscience and Cognitive
MANY scientists believe that pulse-coupled neural networks
 IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 499 Class 1 Neural Excitability, Conventional Synapses, Weakly Connected Networks, and Mathematical Foundations of Pulse-Coupled Models Eugene
IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 499 Class 1 Neural Excitability, Conventional Synapses, Weakly Connected Networks, and Mathematical Foundations of Pulse-Coupled Models Eugene
