Chapter 1 Survey of Biomolecular Hydrodynamics
|
|
|
- Kory Ford
- 5 years ago
- Views:
Transcription
1 On-Line Biophysics Textbook Volume: Separations and Hydrodynamics Todd M. Schuster, editor Chapter 1 Survey of Biomolecular Hydrodynamics Victor A. Bloomfield Department of Biochemistry, Molecular Biology, and Biophysics University of Minnesota 1479Gortner Avenue Saint Paul, Minnesota victor@tc.umn.edu September 9, Introduction This chapter surveys modern methods for measuring the hydrodynamic properties of biological macromolecules and for calculating the hydrodynamic properties of useful models for biomolecules. Hydrodynamic properties are quantities like sedimentation and diffusion coefficient, rotational relaxation time, intrinsic viscosity, and viscoelastic relaxation time. They enable one to determine how rapidly molecules translate and rotate in solution, and how they influence the steady-state and time-dependent viscosity of solutions. These measured properties, in turn, when interpreted in terms of suitable models, give us information about the molecular weight, size, hydration, shape, flexibility, conformation, and degree of association of biological macromolecules. Like so much of science, hydrodynamics has a long history, but has been dramatically changed by recent advances in experiment, theory, and computation. Classic textbooks tell of the experimental work of Fick in 1855 on diffusion [1], Svedberg in the 1920s on sedimentation [2], and Poiseuille in 1846 on viscosity [3]. Theories for simple shapes such as spheres and ellipsoids were worked out by Stokes (1847) [4], Einstein (1906) [5, 6], and Perrin (1936) [7, 8]. However, recent experimental developments such as dynamic laser light scattering, nanosecond fluorescence depolarization measurements, fluorescence recovery after photobleaching, fluorescence correlation spectroscopy, pulsed field gradient NMR, and improved analytical ultracentrifuge design have made measurements of hydrodynamic properties more convenient, precise, and refined. At the same time, development of new theoretical and computational tools have enabled calculation of the properties of complex, realistic molecular models. Copyright 2000 by the author 1
2 2 2 FRICTIONAL COEFFICIENTS In this chapter we present these new techniques, along with the older but still useful ones, in a way that we hope is suitable for an audience of students and researchers in biophysics in biochemistry. We forego the heavier experimental and mathematical details and focus on physical principles, results, and applications. We also provide links to Web-based resources and computer programs that can be used to calculate hydrodynamic properties of atomic-level structures. 2 Frictional coefficients When a particle moves in a fluid, either under the influence of an applied force or torque, or due to Brownian motion, it experiences frictional resistance. The proportionality between particle velocity and frictional resistance is the frictional coefficient. The translational frictional coefficient f t is the proportionality constant between the velocity ˇwith which a particle is moving, and the frictional force F f which resists that motion: F f = f t v, (1) where the minus sign arises because the frictional force opposes the particle motion. The motion is produced by an applied force (e.g. centrifugation or electrophoresis) or by a concentration gradient, as in diffusion. Likewise, the rotational frictional coefficient f r is the constant of proportionality between the angular velocity ω of the molecule, and the frictional torque T r resisting rotation: T r = f r ω, (2) where the rotation may be produced by an applied electric or hydrodynamic flow field, or by diffusion of molecular axes. R Ff v ω Tr Figure 1: Frictional force and torque exerted on a translating and rotating body 2.1 Viscosity of water All frictional coefficients depend on the viscosity of the medium in which the particles are immersed. The viscosity η is defined as the force per unit area needed to maintain unit velocity gradient between two parallel surfaces moving relative to one another in the fluid. The cgs units of η are therefore dyne-sec/cm 2,orpoise in honor of Poiseuille who investigated the fundamental laws of viscous flow in tubes [3]. Most biopolymer experiments are conducted in water or dilute aqueous buffers. The viscosity of water is a strongly decreasing function of temperature. Hydrodynamic results are commonly
3 2.2 Spheres 3 adjusted to 20 C, at which temperature the viscosity of water is poise, or centipoise (cp). Values of η for water at other temperatures are tabulated in the CRC Handbook of Chemistry and Physics and are closely approximated by the equations 1301 log 10 η = (3) (t 20) (t 20) 2 in the range 0 t 20 C, and η (20 t) (t 20)2 log 10 = η 20 t in the range 20 t 100 C, where t is the Celsius temperature. (4) 2.2 Spheres We will discuss in the following sections the experiments by which these frictional coefficients are measured, but it will be helpful now to have a sense of how they depend on molecular size and shape for the simplest shape, a sphere, and for other model shapes. A sphere has radius R and volume V =(4/3)πR 3. It has translational and rotational frictional coefficients f t =6πηR (5) and f r =8πηR 3, (6) Eq. 5 is known as Stokes law, and the hydrodynamic radius R is often called the Stokes radius. Note that translational friction varies only as the first power of the molecular size, and therefore is much less sensitive to molecular dimensions than is rotational friction, which varies as R 3 or molecular volume. 2.3 Ellipsoids of revolution and cylinders Other rigid models of macromolecules, being nonspherical, have more complex properties than spheres. The ellipsoid of revolution model is widely used because its properties can be calculated exactly [7, 8]. This is the generalization of a sphere, obtained by rotating an ellipsoid about one of its axes. The axis about which rotation occurs is called the symmetry axis, and its half-length (analogous to the radius of a sphere) is a. The half-lengths of the two other axes are equal to b. The axial ratio a/b = p and the volume V =(4/3)πab 2, an obvious generalization of the result for a sphere. Oblate ellipsoids are pancake-shaped, with p<1. Prolate ellipsoids of revolution are football-shaped, with p>1. They are often used as models for cylindrical rods, giving reasonably accurate results if length and volume of the two objects are equated (2a = L, b ell = 3/2b cyl ). However, since cylinders do not have gradually narrowing ends, their frictional resistance, especially to rotational motion, is somewhat different from that of ellipsoids. Therefore, numerically accurate equations for cylinders have been obtained. Translational and rotational friction coefficients for ellipsoids and cylinders are listed in Table 1. They are expressed as ratios relative to the frictional coefficients of spheres of the same volume. That is, F t = f t /6πηR e (7)
4 4 2 FRICTIONAL COEFFICIENTS and F r = f t /8πηR 3 e. (8) The equivalent radii R e of the spheres of equal volume are given in the first row of Table 1. For ellipsoids, q =1/p; for cylinders, p = L/2b where 2b is the diameter. In translation, the frictional coefficients are different for movement with the long axes parallel or perpendicular to the direction of motion. However, since what is almost invariably measured is the average over all orientations, only the average is given for F t. In rotation, F r (i) is the frictional coefficient for rotation around the ith axis (i = a or b). Table 1: Translational and rotational frictional coefficients of ellipsoids and cylindrical rods relative to spheres of the same volume [7 11] Prolate ellipsoid Oblate ellipsoid Cylinder R e (ab 2 ) 1/3 (ab 2 ) 1/3 (3/2p 2 ) 1/3 (L/2) F t 1 q 2 q 2/3 ln 1+ 1 q 2 F r (a) F r (b) q 2 1 q 2/3 arctan q 2 1 q 4(1 q 2 ) 4(1 q 2 ) 3(2 2q 4/3 /F t 4(1 q 4 ) 4(1 q 4 ) 3q 2 [2q 2/3 (2 q 2 )/F t 2] 3q 2 [2q 2/3 (2 q 2 )/F t 2] (2p 2 /3) 1/3 3(2 2q 4/3 /F t 0.64 ln p+γ,γ = ( ) p p 2 2p 2 9(ln p+δ p p 2,δ= p p Random coils A random coil has on average a spherical domain, so it is not surprising that it behaves hydrodynamically like a sphere with effective hydrodynamic radius closely related to its radius of gyration [12]. For a random coil with N bonds of length b, that is not swollen by excluded volume, f t =6πη 3 π 8 ( ) b 2 N =6πη <R 2 g 6 >1/2, (9) which shows that the effective hydrodynamic radius is about 2/3 of the radius of gyration <R 2 g > 1/2. A similar relation holds for coils with excluded volume, but the numerical factor varies slowly with the excluded volume. Because of its flexibility, a random coil does not undergo a defined (rigid) rotational motion. The equivalent of a rotational relaxation time is the relaxation time τ 1 for the longest normal mode of internal motion of the coil. 2.5Oligomeric arrays of spheres Many proteins are effectively modeled as compact arrays of spheres. A tabulation of translational and rotational friction coefficients for various geometries is given in Table 2.
5 5 Table 2: Translational and rotational friction coefficients for arrays of n identical spheres in the indicated geometries, relative to f t and f r for the spherical monomer. 33 corresponds to rotation around the axis of highest symmetry, and 11 is for either axis perpendicular to it. [13] n Geometry F t F r (11) F r (33) 1 Sphere Dimer Triangle Linear Square Tetrahedron Linear Pentagon Bipyramid Hexagon Octahedron Trigonal prism Cube Experimental determination of hydrodynamic properties 3.1 Sedimentation coefficient Of the various hydrodynamic techniques, the most widely used is sedimentation. Another term is centrifugation; and if the centrifuge operates at high speeds, ultracentrifugation [14]. We distinguish two types of sedimentation experiments: those in which the velocity of molecular motion is measured, and those in which the centrifuge runs until equilibrium is reached and one measures the unchanging concentration distribution. In this chapter we consider only sedimentation velocity, since it depends on the translational frictional coefficient. In a sedimentation velocity experiment, one measures the velocity of the macromolecular solute, dissolved in solvent, in response to a centrifugal field. It is conventional in a multicomponent system to denote water as component 1, polymer solute as component 2, and other small molecular solvent components (salts, buffer, etc.) as components 3,... Consider a particle of mass m, spun with angular velocity ω at a distance r from the axis of rotation. The molecular weight of the polymer is M 2,som = M 2 /N A,whereN A is Avogadro s number. If the specific volume (more rigorously, the partial specific volume) of the polymer is v 2 cc/g, and the density of the solvent is ρ g/cc, each polymer molecule displaces a mass of solvent mv 2 ρ, so its effective buoyant mass is M 2 (1 v 2 ρ)/n A. The angular acceleration is ω 2 r (sometimes expressed a a multiple of g, the gravitational acceleration, equal to 980 cm/sec 2 ), so the centrifugal force on the particle is F cent = M 2 N A (1 v 2 ρ)ω 2 r. (10)
6 6 3 EXPERIMENTAL DETERMINATION OF HYDRODYNAMIC PROPERTIES This produces a velocity v = dr/dt, which is resisted by a frictional force F fr = f t v = f t dr dt. (11) In the steady state, the sum of these forces is zero, so one can define the sedimentation coefficient S as the velocity per unit acceleration: S = dr/dt ω 2 r = M 2(1 v 2 ρ) N A f t. (12) The first of these equalities allows determination of S in terms of measurable quantities. The second enables determination, from S and the buoyancy factor, of the quotient of molecular quantities M 2 /f t. The units of S are seconds (s). Generally values of S are in the range to s. To eliminate these small numbers, a sedimentation coefficient of sisgiventhevalueof1svedberg (S), after the inventor of the ultracentrifuge. The proper unit of angular velocity (ω) is rad/s, but centrifuge speeds are most commonly stated in revolutions per minute, rpm. The conversion factor is 1 rpm = 60/2π rad/s. When doing calculations, it is important to work in consistent units. The sedimentation coefficient is determined experimentally by measuring the position, as a function of time, of the band of solute material initially layered on top of the buffer solution, or of the midpoint of the boundary formed by redistribution of the components of the initially uniform solution in the centrifugal field. Rearrangement of the first equality in eq. 12 leads to dr m r m = Sω 2 dt (13) which when integrated yields ln(r m /r 0 )=Sω 2 t, (14) where r m is to be interpreted as the midpoint of band or boundary at time t, andr o is the starting position at t =0. ThusS is obtained from the slope of a plot of ln r m vs t, divided by ω 2. Various means are available to measure concentration distributions in sedimentation. In the modern generation of analytical ultracentrifuges, the concentration across the cell is monitored optically during the course of the run. For nucleic acids, which absorb light strongly at 260 nm, measurement of A 260 as a function of r allows determination of the concentration distribution at quite low concentrations. For proteins, measurement around 280 nm or in the absorption band of some chromophore also allows sensitive detection. In older instruments, and even today for some purposes, refractive index measurements using schlieren or Rayleigh interference fringe techniques were employed. The concentration distribution in a preparative ultracentrifuge can be determined after a run is complete, by withdrawing and measuring solution from successive layers in the centrifuge tube. In this case, if the polymer has been radioactively labelled, or if it has some enzymatic or biological activity, very low concentrations can be detected. There are numerous details, inappropriate for discussion here, that are important to the successful execution and interpretation of sedimentation experiments. They concern matters such as diffusional broadening, concentration dependence, and interaction of charged molecules. These are treated in monographs, manufacturer s literature, and Web sites.
7 3.2 Translational diffusion coefficient Translational diffusion coefficient Molecules in solution undergo brownian motion under thermal bombardment from their surroundings, which causes them to move even when not subject to an external force. This thermal motion, called diffusion, is random in magnitude and direction, but can be measured by the techniques described below. Diffusion is also observed when a concentration gradient is set up in solution or when a barrier between solutions at two different concentrations is removed. The tendency toward equalization of concentrations is attributable at the macroscopic level to a gradient of concentration or chemical potential; but at the molecular level it can also be understood as the random motion of molecules, with more molecules in the concentrated region of solution available to move randomly into the more dilute region. The rate of brownian motion or evening-out of concentration gradients is proportional to the translational diffusion coefficient D t, which in turn is inversely proportional to f t. In this section we summarize the basic laws characterizing translational diffusion, and describe some of the most important modern techniques whereby D t can be measured. We note that molecules also undergo rotational brownian motion. This will be discussed later Fick s laws of diffusion At the macroscopic level, two basic equations describe the change of concentration as a function of position and time under the influence of translational diffusion. The first is Fick s first law, which states that the flux J of matter across an imaginary surface per unit area and per unit time is proportional to the concentration gradient and the diffusion coefficient: [ ] c(x, t) J(x, t) = D t, (15) x t where we have written the one-dimensional equation for simplicity. Note that this equation states that a positive flux occurs from high to low concentration. J(x) A J(x+dx) x x+dx Figure 2: Fluxes into and out of an infinitesimal volume element. If we consider an infinitesimal volume element dv in solution of cross-sectional area A and thickness dx located at x, conservation of mass requires that the difference between the flow of matter into the element and the flow out must equal the amount accumulated within the element: [ ] [ ] J(x, t) c(x, t) [J(x, t) J(x + dx, t)] A = Adx = dv, (16) x t t x
8 8 3 EXPERIMENTAL DETERMINATION OF HYDRODYNAMIC PROPERTIES where the middle equality comes from expanding J(x + dx) in a Taylor s series about x. Since dv = Adx, we may substitute eq. (15) into eq. (16) to obtain Fick s second law: [ ] c(x, t) t x [ 2 ] c(x, t) = D t x 2, (17) t wherewehaveassumedthatd t is independent of c, and hence independent of x. The solution of eq. (17) for the initial condition in which there is a very narrow (delta function) spike of material c 0 at x = x 0, and zero concentration elsewhere, is c(x, t)= c 0 2 (x x 0 ) 4πDt t e 4D t t. (18) c(x,t) 10 s 100 s 1000 s x. cm Figure 3: Concentration profile from an initial spike at x = 0 after 10, 100, and 1000 s, for a protein with typical diffusion coefficient D = cm 2 /s Frictional resistance and brownian motion Given its physical meaning, it seems reasonable that D t should increase as the thermal energy of the solution increases, and decrease with the frictional resistance to translational motion. In fact, as Einstein showed in 1906, the relation is D t = k BT f t. (19) An important physical consequence of Fick s second law relates the mean-square distance diffused in time t to the diffusion coefficient: <x 2 >=2D t t. (20)
9 3.2 Translational diffusion coefficient 9 This equation can be derived from the solution to Fick s second law for an initial sharp spike of concentration located at x 0 = 0 (see eq. (18)), or by probabilistic considerations of molecules in regions of different concentrations moving randomly along the x-axis. The two- and threedimensional analogs of eq. (20) are <ρ 2 >=4D t t (21) and <r 2 >=6D t t, (22) respectively. The dependence of average distance traveled on the square root of the time means that diffusion is an efficient way of traversing short distances, on the order of a membrane thickness or the interior of a small cell, but is very inefficient for traversing longer distances Diffusion across a porous barrier A classical method of determining D t, particularly useful for small molecules such as drugs, is to place different concentrations c 1 and c 2 on opposite sides of a porous barrier of thickness x and area A. The molecules can either be radioactively labeled or have some optical property that enables measurement of their concentrations as a function of time. The solutions on the two sides of the barrier are stirred to disrupt stagnant boundary layers and make concentrations uniform on each side. The amount of material m that flows across the barrier in unit time is given by Fick s first law, eq. (15) in the finite difference form m = JA = AD t c x (23) where c = c 2 c 1. Since A and x are generally not measurable accurately, the apparatus is calibrated with a compound of known D t to obtain A/ x. Then measurement of m for the unknown enables calculation of its diffusion coefficient Broadening of sedimentation boundaries In a sedimentation velocity experiment, the boundary will broaden with time due to diffusion as it moves down the cell. Exact analysis of the broadening is complicated by the radial sector shape of the cell and by the concentration dependence of D t (an effect not treated in this chapter). However, to a reasonable approximation the width of the boundary (approximately the standard deviation of a Gaussian profile) will be given by eq. (20), <x 2 >=2D t t Dynamic laser light scattering Much of the modern determination of diffusion coefficients of macromolecules is done by dynamic laser light scattering (also called quasielastic light scattering or photon correlation spectroscopy) [15, 16]. As molecules diffuse under the influence of Brownian motion, the electric field E s (t) and intensity I s (t) = E s (t) 2 scattered from them fluctuate with time. The time variation thus gives information on particle motions. There are three types of fluctuations to be considered in dynamic light scattering experiments.
10 10 3 EXPERIMENTAL DETERMINATION OF HYDRODYNAMIC PROPERTIES 1. Occupation number fluctuations are due to fluctuations in the number of particles N in the scattering volume. From Poisson statistics, we know that these fluctuations are inversely proportional to N. To have fluctuations at the 1% level, N must be 10 4 or less in a scattering volume typically 1 mm on a side. This is a very low concentration, hence only very strong scatterers, such as whole cells, will produce measurable occupation number fluctuations. These are not generally important for macromolecules. Further, since particles must diffuse a distance of about 1 mm to enter or exit the scattering volume, occupation number fluctuations are slow, typically on the order of seconds. 2. Amplitude fluctuations result from rotation and internal motions of molecules. In order to be observable, these motions must have a characteristic length that is not too small compared to the wavelength λ. Such internal fluctuations are readily observed, if not always readily interpreted, in the dynamic scattering from large DNA molecules and protein polymers. 3. Most important, phase fluctuations arise from translation of molecule over a distance comparable to the wavelength λ. These fluctuations are rapid and observable from molecules over a very wide range of sizes. The characteristic time scale for phase fluctuations due to translational diffusion can be estimated from eq. (20). The distance travelled to produce a measurable change in the phase of the scattered light (i.e. a change in amplitude which is a significant fraction of the peak-to-trough amplitude) is on the order of λ/10. Thus, eq. (20) yields t (λ/10) 2 /2D t.withλ 0 about 5000 Å in vacuo, corresponding to about cm in aqueous solution with refractive index n =1.33, and a typical D t of 10 7 cm 2 /s, this gives t seconds. The microsecond to millisecond range for dynamic light scattering fluctuations is observed for most biological macromolecules. Thus dynamic light scattering can be used for very rapid measurement of D t. The dynamic light scattering equations are derived in a more complete and systematic fashion in numerous sources (e.g. Berne & Pecora, 1976; Bloomfield & Lim, 1978; Schmitz, 1990; Chu, 1991). The important result is that the autocorrelation function g 2 (t) of the photocurrent resulting from the scattered light is g 2 (t) =1+βe 2q2 D tt (24) where β is an instrumental constant and q is the scattering vector, (4πn/λ 0 )sin(θ/2) where θ is the scattering angle. This equation showns that 2q 2 D t is the slope of a semilogarithmic plot of g 2 (t) 1vst. Sinceq 2 is known, D t can be determined Fluorescence photobleaching recovery A useful method for determining 2-dimensional diffusion in cell membranes or in concentrated solutions is fluorescence photobleaching recovery (FPR), sometimes called fluorescence recovery after photobleaching (FRAP). The simplest realization of the method, which is all we will discuss here, is to briefly illuminate a cross-section of fluorescent molecules with a narrow, intense laser beam under a microscope. The fluorophore may be a protein such as green fluorescent protein, GFP, perhaps in a genetically engineered construct with another protein of interest. Or it may be a fluorescent label bound to a protein, nucleic acid, or lipid. If the laser wavelength is in the absorption band of the fluorophore, some of the molecules exposed to the beam will be bleached (i.e., their fluorescence will be permanently quenched by a photochemical reaction). Then if the
11 3.3 Rotation 11 fluorescence intensity F from the bleached region is monitored by a probe or reading laser beam (usually the same as the bleaching beam, but greatly attenuated so as not to cause additional photobleaching), it will initially be found to be less than F before the bleach. However, as time proceeds, molecules from adjacent regions will diffuse into the bleached region, increasing F until it eventually recovers its initial value. (If the fluorophores are constrained by attachment to a cytoskeletal or membrane network, full recovery may not be achieved. This makes FPR a useful indicator of such attachment in cell biology research.) In the most common case the laser beam has a Gaussian profile of radius w (the radius at which the intensity has decreased to e 2 of its central maximum intensity). Then the time-dependence of the fractional recovery of fluorescence is determined by the function θ(t) = where the characteristic recovery time τ D is (see eq. (21)) Pulsed field gradient NMR 1 1+t/τ D, (25) τ D = w2 4D t. (26) With the increasing availability of high-field NMR spectrometers, the use of pulsed field gradient NMR to measure translational diffusion of both small molecules and macromolecules has become common. The basic idea is that if a magnetic field gradient G is applied to the sample in the NMR probe, in the same direction as the dominant magnetic field H 0, the magnetization M will change as the molecules diffuse to different regions of the gradient. In the most common realization of the experiment [17], Fourier transform pulse techniques are used. A four-step sequence is used: pulse; 2. linear field gradient pulse of duration δ; pulse after time τ; 4. second, identical, field gradient pulse at time after the first. The resulting magnetization, relative to that with no field gradient, is then M(G) [ ] M(0) =exp γ 2 G 2 δ 2 ( δ/3)d t, (27) where γ is the magnetogyric ratio. 3.3 Rotation We see by comparing eqs. (5) and (6) that rotational frictional resistance varies as R 3 rather than R; that is, rotation is much more sensitive to small changes in molecular size. This makes it a very useful probe of small conformational changes. Thanks largely to advances in lasers and electronics, which can measure processes on the nanosecond to microsecond time scale, methods that measure
12 12 3 EXPERIMENTAL DETERMINATION OF HYDRODYNAMIC PROPERTIES the rotational motion of proteins and nucleic acids have become important in characterizing their structures in solution. Detection of rotational motion generally depends on differences in optical properties of biomolecules along their principal molecular axes Rotational diffusion coefficients and relaxation times For simplicity we shall assume that the orientation of the molecule of interest can be described by a single angle θ. Under the influence of randomizing Brownian motion, a function f(θ, t) of the orientation of a molecular axis will exponentially decay, or relax, with a characteristic time constant: f(θ, t) =f(θ, 0)e t/τr. (28) This rotational relaxation time is denoted τ r, and is inversely proportional to the rotational diffusion coefficient D r, which has units of reciprocal seconds, s 1. The relation between D r and f r the rotational frictional coefficient, is the same for rotation as for translation: D r = k BT f r. (29) There are two commonly defined rotational relaxation times, depending on the experiment being performed. The first, τ r (1), is obtained by experiments, such as dielectric relaxation, that directly measure the orientation, f (1) (θ) =cos(θ), of the molecular axis with respect to an external field. The second type of relaxation time, τ r (2), is obtained in experiments (more common in contemporary practice) such as fluorescence anisotropy, electric dichroism, and NMR relaxation, that measure the orientation function f (2) (θ) = 1 2 (3 cos2 (θ) 1). One can show that the relaxation time of axis a by rotational diffusion about axes b and c is 1/τ a (1) = D r,b + D r,c. For a sphere, with three equivalent axes, τ r (1) = 1 = f r 2D r 2k B T. (30) Also for a sphere, τ (2) r = 1 6D r = f r 6k B T. (31) Combining this with eq. (6) and the volume V = 4 3 πr3,wegettheelegantlysimpleresult τ (2) r = ηv k B T (32) Since this result was first obtained by Debye, it is sometimes known as the Debye relaxation time. Results for molecular models of other shapes, such as ellipsoids of revolution and rigid rods, are given later in this chapter. In all cases it is found that τ r varies roughly according to V or R 3, where R is some characteristic linear dimension Photoselection: Fluorescence and Triplet State Anisotropy Decay Techniques that measure rotational motion can be divided into two basic classes: those that orient molecules by application of an external field, and those that use polarized light to select molecules
13 3.3 Rotation 13 that are already oriented along the polarization direction. The most widely used of the photoselection techniques is fluorescence anisotropy decay, also known as fluorescence depolarization, which probes motions in the nanosecond time regime. A thorough discussion is given by Cantor and Schimmel (1980, pp ). To understand photoselection techniques requires the concept of the electronic transition dipole moment µ a, which can be qualitatively understood as the dipole produced by the electronic motion under the influence of the electric field of the incident light. Fluorescence involves first the absorption and then the emission of light. If the exciting light is vertically plane-polarized with electric field E along the z-axis, then only those molecules with a component of their absorption transition dipole moment µ a in the same direction as E will be able to absorb. The amplitude of E along µ a is E µ a = Eµ a cos θ, and the intensity of absorption, proportional to the square of the amplitude, variesascos 2 θ. Thus those molecules with cos θ near 1 will be preferentially excited. Assume initially that the system is rigid, so that the molecule does not rotate between excitation and emission, because it is very large or because the solution is effectively frozen. The fluorescence emission is polarized along the direction of the emission transition dipole moment µ e. In the simplest and most common case, µ a and µ e are coincident. If the amplitude of the fluorescence electric field is F, then the component of the intensity of a single emission event in the direction of the excitation direction will be (Fµ e ) 2 cos 2 θ. This is multiplied by the probability that the molecule was excited in the first place, cos 2 θ. Thus the total intensity of fluorescence along the excitation direction, F, will be proportional to < cos 4 θ>where the angular brackets denote an average over the whole population of fluorescent molecules. Likewise, the probability of emission perpendicular to the excitation direction, F, will be proportional to < cos 2 θ sin 2 θcos 2 φ>where φ is the azimuthal angle and sin θ cos φ is the projection of µ e on the x-axis. The values of these averages are F =1/5 andf =1/15. Experimentally, one defines the fluorescence anisotropy as the difference between fluorescence intensity emitted parallel and perpendicular to the exciting field, normalized by the total emitted intensity (there are one parallel and two perpendicular directions of emission): A = F F F +2F. (33) The maximum possible value of this quantity, assuming that the molecules are randomly oriented at excitation, have not rotated at all during the interval between excitation and fluorescence, and that the absorption and emission transition dipoles are coincident, is calculated as A 0 =2/5 from the values of F and F for a rigid system. In the more general case, bfmu e and µ a may not be the same, if intersystem crossing has occurred during excitation and vibrational relaxation. If the angle between µ a and µ e is ξ, the value of the anisotropy for a rigid system is calculated to be A 0 =(3cos 2 ξ 1)/5. Now consider the case in which the molecules rotate in the time between excitation and emission. This period is typically a few picoseconds to a few nanoseconds, during which the electronic excitation energy is redistributed within the molecule (primarily by transfer into vibrational modes) and/or transferred to surrounding solvent, and then emitted as a red-shifted photon as the molecule drops from excited singlet S 1 to ground state singlet S 0. The orientations of the molecules change as they rotate, so the fluorescence anisotropy and polarization will change. In the limiting case that rotation is very fast compared to emission, all initial orientation information will be lost, so A will decay to zero.
14 14 3 EXPERIMENTAL DETERMINATION OF HYDRODYNAMIC PROPERTIES In the more general (and more useful) case, rotational relaxation time τ r and fluorescence lifetime τ F will be comparable. Then the parallel and perpendicular components of the emission will decay with time for two reasons: the fraction of molecules in the excited state decays according to exp( t/τ F ), and the orientation according to exp( t/τ r (2) ). The result (Cantor and Schimmel, p. 461) is A(t) =(2/5) exp( t/τ r (2) )[(3 cos 2 ξ 1)/2]. (34) Thus the time-dependence of the anisotropy can be used to determine τ r (2), and thereby provide information on molecular size and shape. If the experiments are done in the steady-state rather than in a time-resolved manner, then one must average separately over the time dependence of components F and F before computing the steady-state anisotropy <A>. The result is conveniently written in reciprocal form as <A> 1 = A 1 0 ( 1+ τ ) F τ r (2) ( ) = A 1 k B T 0 1+τ F ηv Another commonly used measure of anisotropy, particularly in the older literature, is the polarization P, defined as P = F F. (36) F + F which has maximum value P 0 =1/2 for a rigid system. Algebraic rearrangement shows that (35) ( 1 P 1 ) 1 = 3 A, (37) 3 2 so the equation for the steady-state polarization is ( 1 P 1 ) ( 1 = 1 ) ( 1+ τ ) ( F 1 3 P 0 3 τ r (2) = 1 )( ) k B T 1+τ F P 0 3 ηv (38) These are known as the Perrin equations (Perrin, 1934). Thus the molecular volume V can be obtained, if τ F is known by independent measurement, by measuring 1/ <A>or 1/ <P >as a function of T/η. In these experiments, the viscosity is generally varied by changing the temperature (for water, η changes by 2% for each centigrade degree) or by adding an inert compound such as sucrose or glycerol to the solution. The equations in this section are strictly valid only for rigid, spherical molecules. If the molecule is substantially nonspherical, the expressions become significantly more complicated, owing to the contributions from rotations about the nonequivalent axes. In the limit, as many as five relaxation times may enter into the decay of the anisotropy, though it is rare that more than two can be resolved experimentally. Another complexity arises if the chromophore has substantial independent mobility within the macromolecule. The measured rotational relaxation time will then be much shorter than that corresponding to the macromolecular dimensions. On the other hand, approximate equality of measured and calculated relaxation times indicates that the chromophore moves rigidly with the macromolecule.
15 3.4 Intrinsic viscosity Intrinsic viscosity The viscosity of a fluid reflects its resistance to flow under an applied force. Viscosity is useful in the study of biopolymers because the addition of large molecules to a solvent increases its viscosity; the increase depends on the concentration, size, and structure of the polymer. Motion in one layer of a fluid causes motion in adjoining layers. To move layers with different relative velocities requires a force: the more viscous, the more force. The quantitative description of this relation was first enunciated by Newton. Suppose two parallel planes in the fluid each have area A, and are separated by distance h. If the upper plane moves with velocity v relative to the lower, then the shearing force F needed to maintain this velocity differential is F = η Av h. (39) The coefficient of proportionality, η, is called the viscosity. It can be seen to have units of dyne/cm 2 - s, or poise, in honor of the 19th C French physician Poiseuille, who investigated the flow of liquids in tubes. Water at 20 C has a viscosity of 0.01 poise, or 1 centipoise. Two factors influence F : the strength of the force between layers (greater in molasses than in water, and in water than in air), and the linear extent of the interactions. A cell extract is so viscous because the entangled network of macromolecules extends from one side of the container to the other, and the motion of one part of the fluid, e.g. in pouring or stirring, is retarded by interactions with molecules far away (in regions with different velocities) mediated by intervening molecular interactions. This suggests that viscosity will decrease as polymer concentration decreases, since the probability of intermolecular interactions and the spans of intermolecular complexes will decrease. This molecular picture predicts that bigger polymers, when dissolved in solution, will raise the viscosity more than smaller molecules since their molecular domain has a greater extent. This concept was made quantitative by Einstein, who showed that for spheres occupying volume fraction φ in dilute solution, the solution viscosity relative to solvent viscosity ho is ( η = η ) 2 φ. (40) The volume fraction (cc polymer/cc solution) can be expressed as the volume occupied by one gram of hydrated polymer, v h (cc polymer/g polymer), times the weight concentration of polymer c 2 (g polymer/cc solution). Thus eq. (40) can be rearranged to yield η η 0 η 0 c 2 = 5 2 v h. (41) It is customary to measure η at several concentrations and extrapolate to c 2 = 0. This yields the intrinsic viscosity [η]: η η 0 [η] = lim = 5 c2 0 η 0 c 2 2 v h (42) for spheres. For non-spherical particles, the factor 5 2 is replaced by a parameter ν > 5 2. Since intrinsic viscosity is rarely measured these days, we do not give these results, but refer the reader to the literature.
16 16 REFERENCES References [1] A. Fick (1855) Ann. Physik 23: 59. [2] Svedberg 1920s [3] J.L.M. Poiseuille (1846) Mémoirés présentés par divers savants á l academie royale des sciences de l institute de France 9: 433. [4] Stokes 1847 [5] Einstein 1906 [6] A. Einstein (1985) Investigations on the Theory of the Brownian Movement, Dover, New York. [7] Perrin, F. (1934) Mouvement brownien d un ellipsoide (I). Dispersion dielectrique pour des molecules ellipsoidales, J. Phys. Radium [7] 5, [8] Perrin, F. (1936) Mouvement brownien d un ellipsoide (II). Rotation libre et depolarisation des fluorescences. Translation et diffusion de moleculesellipsoidales, J. Phys. Radium [7]7, [9] Koenig, S. (1975) Brownian motion of an ellipsoid. A correction to Perrin s results, Biopolymers 14, [10] Tirado, M.M. and J. Garca de la Torre (1979) Translational friction coefficients of rigid, symmetric top macromolecules. Application to circular cylinders, J. Chem Phys. 71: [11] Tirado, M.M. and J. Garca de la Torre (1980) Rotational diffusion of rigid, symmetric top macromolecules. Application to circular cylinders, J. Chem Phys. 73, [12] Kirkwood, J.G. and J. Riseman (1956) in Eirich, F., ed. Rheology, Vol. 1, p. 495, Academic Press, New York. [13] García de la Torre, J. and V. A. Bloomfield Hydrodynamic properties of complex, rigid, biological macromolecules: Theory and applications. Q. Rev. Biophys.. 14: [14] Schuster, T.M. and T.M. Laue, eds. (1994) Modern Analytical Ultracentrifugation : Acquisition and Interpretation of Data for Biological and Synthetic Polymer Systems, Springer-Verlag, New York. [15] Berne, B. J. and R. Pecora. (1976). Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics, Wiley-Interscience, New York. [16] Santos, N.C. and M.A.R.B. Castanho (1996) Teaching Light Scattering Spectroscopy: The Dimension and Shape of Tobacco Mosaic Virus, Biophys. J. 71(3): (A.pdf version is available in the Biophysical Society On-line Textbook at [17] T.L. James and G.G. McDonald (1973) J. Mag. Res. 11: 58. [18] T.L. James (1975) Nuclear Magnetic Resonance in Biochemistry: Principles and Applications, Academic Press, New York. Chapter 5.8.
Hydrodynamics: Viscosity and Diffusion Hydrodynamics is the study of mechanics in a liquid, where the frictional drag of the liquid cannot be ignored
 Hydrodynamics: Viscosity and Diffusion Hydrodynamics is the study of mechanics in a liquid, where the frictional drag of the liquid cannot be ignored First let s just consider fluid flow, where the fluid
Hydrodynamics: Viscosity and Diffusion Hydrodynamics is the study of mechanics in a liquid, where the frictional drag of the liquid cannot be ignored First let s just consider fluid flow, where the fluid
Measuring the size and shape of macromolecules. Hydrodynamics: study of the objects in water How do the move? Translation Rotation
 Measuring the size and shape of macromolecules Hydrodynamics: study of the objects in water How do the move? Translation Rotation 1) Movement with no external forcefree diffusion 2) Movement under the
Measuring the size and shape of macromolecules Hydrodynamics: study of the objects in water How do the move? Translation Rotation 1) Movement with no external forcefree diffusion 2) Movement under the
Solution structure and dynamics of biopolymers
 Solution structure and dynamics of biopolymers Atomic-detail vs. low resolution structure Information available at different scales Mobility of macromolecules in solution Brownian motion, random walk,
Solution structure and dynamics of biopolymers Atomic-detail vs. low resolution structure Information available at different scales Mobility of macromolecules in solution Brownian motion, random walk,
Introduction to techniques in biophysics.
 Introduction to techniques in biophysics. Goal : resolve the structure and functions of biological objects applying physical approaches 1 At molecular level 1.1 Long-range goal: 1.1.1 complete structure
Introduction to techniques in biophysics. Goal : resolve the structure and functions of biological objects applying physical approaches 1 At molecular level 1.1 Long-range goal: 1.1.1 complete structure
Viscometry. - neglect Brownian motion. CHEM 305
 Viscometry When a macromolecule moves in solution (e.g. of water), it induces net motions of the individual solvent molecules, i.e. the solvent molecules will feel a force. - neglect Brownian motion. To
Viscometry When a macromolecule moves in solution (e.g. of water), it induces net motions of the individual solvent molecules, i.e. the solvent molecules will feel a force. - neglect Brownian motion. To
Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diffusion
 Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diusion I. Introduction A. There are a group o biophysical techniques that are based on transport processes. 1. Transport processes
Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diusion I. Introduction A. There are a group o biophysical techniques that are based on transport processes. 1. Transport processes
Biomolecular hydrodynamics
 Biomolecular hydrodynamics Chem 341, Fall, 2014 1 Frictional coefficients Consider a particle moving with velocity v under the influence of some external force F (say a graviational or electrostatic external
Biomolecular hydrodynamics Chem 341, Fall, 2014 1 Frictional coefficients Consider a particle moving with velocity v under the influence of some external force F (say a graviational or electrostatic external
Rotational Brownian motion; Fluorescence correlation spectroscpy; Photobleaching and FRET. David A. Case Rutgers, Spring 2009
 Rotational Brownian motion; Fluorescence correlation spectroscpy; Photobleaching and FRET David A. Case Rutgers, Spring 2009 Techniques based on rotational motion What we studied last time probed translational
Rotational Brownian motion; Fluorescence correlation spectroscpy; Photobleaching and FRET David A. Case Rutgers, Spring 2009 Techniques based on rotational motion What we studied last time probed translational
How DLS Works: Interference of Light
 Static light scattering vs. Dynamic light scattering Static light scattering measures time-average intensities (mean square fluctuations) molecular weight radius of gyration second virial coefficient Dynamic
Static light scattering vs. Dynamic light scattering Static light scattering measures time-average intensities (mean square fluctuations) molecular weight radius of gyration second virial coefficient Dynamic
Biomolecular hydrodynamics
 Biomolecular hydrodynamics David Case Rutgers, Spring, 2009 February 1, 2009 Why study hydrodynamical methods? The methods we study in this section are low resolution ones one studies diffusional motion
Biomolecular hydrodynamics David Case Rutgers, Spring, 2009 February 1, 2009 Why study hydrodynamical methods? The methods we study in this section are low resolution ones one studies diffusional motion
Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm. 161 Noyes Laboratory
 Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm 161 Noyes Laboratory Determination of the Stokes Radius by measuring the Rotational Diffusion Coefficient: D rot D
Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm 161 Noyes Laboratory Determination of the Stokes Radius by measuring the Rotational Diffusion Coefficient: D rot D
Fluorescence Workshop UMN Physics June 8-10, 2006 Quantum Yield and Polarization (1) Joachim Mueller
 Fluorescence Workshop UMN Physics June 8-10, 2006 Quantum Yield and Polarization (1) Joachim Mueller Quantum yield, polarized light, dipole moment, photoselection, dipole radiation, polarization and anisotropy
Fluorescence Workshop UMN Physics June 8-10, 2006 Quantum Yield and Polarization (1) Joachim Mueller Quantum yield, polarized light, dipole moment, photoselection, dipole radiation, polarization and anisotropy
Biomolecular hydrodynamics
 Biomolecular hydrodynamics David Case Rutgers, Spring, 2014 January 29, 2014 Why study hydrodynamical methods? The methods we study in this section are low resolution ones one studies diffusional motion
Biomolecular hydrodynamics David Case Rutgers, Spring, 2014 January 29, 2014 Why study hydrodynamical methods? The methods we study in this section are low resolution ones one studies diffusional motion
Correlation Spectroscopy in Polymer Physics Methodenseminar im Wahlpflichtfach Basics diffusion and brownian motion correlations functions
 Correlation Spectroscopy in Polymer Physics Methodenseminar im Wahlpflichtfach 3 1. Basics diffusion and brownian motion correlations functions 2. Dynamic light scattering (DLS) DLS on cellulose solutions
Correlation Spectroscopy in Polymer Physics Methodenseminar im Wahlpflichtfach 3 1. Basics diffusion and brownian motion correlations functions 2. Dynamic light scattering (DLS) DLS on cellulose solutions
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2008
 Lecture /1/8 University of Washington Department of Chemistry Chemistry 45 Winter Quarter 8 A. Analysis of Diffusion Coefficients: Friction Diffusion coefficients can be measured by a variety of methods
Lecture /1/8 University of Washington Department of Chemistry Chemistry 45 Winter Quarter 8 A. Analysis of Diffusion Coefficients: Friction Diffusion coefficients can be measured by a variety of methods
Polymer dynamics. Course M6 Lecture 5 26/1/2004 (JAE) 5.1 Introduction. Diffusion of polymers in melts and dilute solution.
 Course M6 Lecture 5 6//004 Polymer dynamics Diffusion of polymers in melts and dilute solution Dr James Elliott 5. Introduction So far, we have considered the static configurations and morphologies of
Course M6 Lecture 5 6//004 Polymer dynamics Diffusion of polymers in melts and dilute solution Dr James Elliott 5. Introduction So far, we have considered the static configurations and morphologies of
Fluorescence polarisation, anisotropy FRAP
 Fluorescence polarisation, anisotropy FRAP Reminder: fluorescence spectra Definitions! a. Emission sp. b. Excitation sp. Stokes-shift The difference (measured in nm) between the peak of the excitation
Fluorescence polarisation, anisotropy FRAP Reminder: fluorescence spectra Definitions! a. Emission sp. b. Excitation sp. Stokes-shift The difference (measured in nm) between the peak of the excitation
Principles of Physical Biochemistry
 Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Introduction to Dynamic Light Scattering with Applications. Onofrio Annunziata Department of Chemistry Texas Christian University Fort Worth, TX, USA
 Introduction to Dynamic Light Scattering with Applications Onofrio Annunziata Department of Chemistry Texas Christian University Fort Worth, TX, USA Outline Introduction to dynamic light scattering Particle
Introduction to Dynamic Light Scattering with Applications Onofrio Annunziata Department of Chemistry Texas Christian University Fort Worth, TX, USA Outline Introduction to dynamic light scattering Particle
EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December Suggested resolution
 page 1 of 7 EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December 2013 Suggested resolution Exercise 1. [total: 25 p] a) [t: 5 p] Describe the bonding [1.5 p] and the molecular orbitals [1.5 p] of the ethylene
page 1 of 7 EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December 2013 Suggested resolution Exercise 1. [total: 25 p] a) [t: 5 p] Describe the bonding [1.5 p] and the molecular orbitals [1.5 p] of the ethylene
By: Ashley and Christine Phy 200 Professor Newman 4/13/12
 By: Ashley and Christine Phy 200 Professor Newman 4/13/12 What is it? Technique used to settle particles in solution against the barrier using centrifugal acceleration Two Types of Centrifuges Analytical
By: Ashley and Christine Phy 200 Professor Newman 4/13/12 What is it? Technique used to settle particles in solution against the barrier using centrifugal acceleration Two Types of Centrifuges Analytical
8.6 Drag Forces in Fluids
 86 Drag Forces in Fluids When a solid object moves through a fluid it will experience a resistive force, called the drag force, opposing its motion The fluid may be a liquid or a gas This force is a very
86 Drag Forces in Fluids When a solid object moves through a fluid it will experience a resistive force, called the drag force, opposing its motion The fluid may be a liquid or a gas This force is a very
Chem728 Spr. 12 page 1 2/29/12. Assignment 2 Estimates of Hydrodynamic Radii from Dynamic Light Scattering Data. ANSWERS
 Chem728 Spr. 12 page 1 2/29/12 Assignment 2 Estimates of Hydrodynamic Radii from Dynamic Light Scattering Data. ANSWERS Objective: Analyze experimental correlation functions of scattered light to determine
Chem728 Spr. 12 page 1 2/29/12 Assignment 2 Estimates of Hydrodynamic Radii from Dynamic Light Scattering Data. ANSWERS Objective: Analyze experimental correlation functions of scattered light to determine
Light scattering Small and large particles
 Scattering by macromolecules E B Incident light Scattered Light particle Oscillating E field from light makes electronic cloud oscillate surrounding the particle Intensity: I E Accelerating charges means
Scattering by macromolecules E B Incident light Scattered Light particle Oscillating E field from light makes electronic cloud oscillate surrounding the particle Intensity: I E Accelerating charges means
DEVELOPMENT OF NANO PARTICLE SIZING SYSTEM USING FLUORESCENCE POLARIZATION
 XX IMEKO World Congress Metrology for Green Growth September 9 14, 2012, Busan, Republic of Korea DEVELOPMENT OF NANO PARTICLE SIZING SYSTEM USING FLUORESCENCE POLARIZATION Terutake Hayashi, Masaki Michihata,
XX IMEKO World Congress Metrology for Green Growth September 9 14, 2012, Busan, Republic of Korea DEVELOPMENT OF NANO PARTICLE SIZING SYSTEM USING FLUORESCENCE POLARIZATION Terutake Hayashi, Masaki Michihata,
Static and dynamic light scattering. Cy Jeffries EMBL Hamburg
 Static and dynamic light scattering. Cy Jeffries EMBL Hamburg Introduction. The electromagnetic spectrum. visible 10-16 10-10 10-8 10-4 10-2 10 4 (l m) g-rays X-rays UV IR micro wave Long radio waves 400
Static and dynamic light scattering. Cy Jeffries EMBL Hamburg Introduction. The electromagnetic spectrum. visible 10-16 10-10 10-8 10-4 10-2 10 4 (l m) g-rays X-rays UV IR micro wave Long radio waves 400
Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics
 for resubmission Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics Aram Davtyan, 1 Gregory A. Voth, 1 2, a) and Hans
for resubmission Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics Aram Davtyan, 1 Gregory A. Voth, 1 2, a) and Hans
1. Transition dipole moment
 1. Transition dipole moment You have measured absorption spectra of aqueous (n=1.33) solutions of two different chromophores (A and B). The concentrations of the solutions were the same. The absorption
1. Transition dipole moment You have measured absorption spectra of aqueous (n=1.33) solutions of two different chromophores (A and B). The concentrations of the solutions were the same. The absorption
Polymer Dynamics and Rheology
 Polymer Dynamics and Rheology 1 Polymer Dynamics and Rheology Brownian motion Harmonic Oscillator Damped harmonic oscillator Elastic dumbbell model Boltzmann superposition principle Rubber elasticity and
Polymer Dynamics and Rheology 1 Polymer Dynamics and Rheology Brownian motion Harmonic Oscillator Damped harmonic oscillator Elastic dumbbell model Boltzmann superposition principle Rubber elasticity and
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray
 CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
Part 8. Special Topic: Light Scattering
 Part 8. Special Topic: Light Scattering Light scattering occurs when polarizable particles in a sample are placed in the oscillating electric field of a beam of light. The varying field induces oscillating
Part 8. Special Topic: Light Scattering Light scattering occurs when polarizable particles in a sample are placed in the oscillating electric field of a beam of light. The varying field induces oscillating
Fluorescence Depolarization. Ben Berger and Ben Berger
 Fluorescence Depolarization Ben Berger and Ben Berger Small molecules rotate quickly Large molecules rotate slowly There is a time delay from the moment a molecule is excited to the time it fluoresces
Fluorescence Depolarization Ben Berger and Ben Berger Small molecules rotate quickly Large molecules rotate slowly There is a time delay from the moment a molecule is excited to the time it fluoresces
Order of Magnitude Astrophysics - a.k.a. Astronomy 111. Photon Opacities in Matter
 1 Order of Magnitude Astrophysics - a.k.a. Astronomy 111 Photon Opacities in Matter If the cross section for the relevant process that scatters or absorbs radiation given by σ and the number density of
1 Order of Magnitude Astrophysics - a.k.a. Astronomy 111 Photon Opacities in Matter If the cross section for the relevant process that scatters or absorbs radiation given by σ and the number density of
Laboratory 9: The Viscosity of Liquids
 Laboratory 9: The Viscosity of Liquids Introduction The essential difference between solids and fluids lies in the nature of their response to the socalled shearing stress. In solids, an elastic force
Laboratory 9: The Viscosity of Liquids Introduction The essential difference between solids and fluids lies in the nature of their response to the socalled shearing stress. In solids, an elastic force
Particles, drops, and bubbles. Lecture 3
 Particles, drops, and bubbles Lecture 3 Brownian Motion is diffusion The Einstein relation between particle size and its diffusion coefficient is: D = kt 6πηa However gravitational sedimentation tends
Particles, drops, and bubbles Lecture 3 Brownian Motion is diffusion The Einstein relation between particle size and its diffusion coefficient is: D = kt 6πηa However gravitational sedimentation tends
Hydrodynamic Characterisation
 Hydrodynamic Characterisation Viscometry SEC-MALLs Analytical Ultracentrifugation Stephen Harding, NCMH University of Nottingham NCMH at Nottingham: An International Facility for characterising sizes/shapes
Hydrodynamic Characterisation Viscometry SEC-MALLs Analytical Ultracentrifugation Stephen Harding, NCMH University of Nottingham NCMH at Nottingham: An International Facility for characterising sizes/shapes
Measuring S using an analytical ultracentrifuge. Moving boundary
 Measuring S using an analytical ultracentrifuge Moving boundary [C] t = 0 t 1 t 2 0 top r bottom 1 dr b r b (t) r b ω 2 = S ln = ω 2 S (t-t dt r b (t o ) o ) r b = boundary position velocity = dr b dt
Measuring S using an analytical ultracentrifuge Moving boundary [C] t = 0 t 1 t 2 0 top r bottom 1 dr b r b (t) r b ω 2 = S ln = ω 2 S (t-t dt r b (t o ) o ) r b = boundary position velocity = dr b dt
What s important: viscosity Poiseuille's law Stokes' law Demo: dissipation in flow through a tube
 PHYS 101 Lecture 29x - Viscosity 29x - 1 Lecture 29x Viscosity (extended version) What s important: viscosity Poiseuille's law Stokes' law Demo: dissipation in flow through a tube Viscosity We introduced
PHYS 101 Lecture 29x - Viscosity 29x - 1 Lecture 29x Viscosity (extended version) What s important: viscosity Poiseuille's law Stokes' law Demo: dissipation in flow through a tube Viscosity We introduced
AP PHYSICS 2 FRAMEWORKS
 1 AP PHYSICS 2 FRAMEWORKS Big Ideas Essential Knowledge Science Practices Enduring Knowledge Learning Objectives ELECTRIC FORCE, FIELD AND POTENTIAL Static Electricity; Electric Charge and its Conservation
1 AP PHYSICS 2 FRAMEWORKS Big Ideas Essential Knowledge Science Practices Enduring Knowledge Learning Objectives ELECTRIC FORCE, FIELD AND POTENTIAL Static Electricity; Electric Charge and its Conservation
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Lecture 5. Anisotropy decay/data analysis. Enrico Gratton
 Lecture 5. Anisotropy decay/data analysis Enrico Gratton Anisotropy decay Energy-transfer distance distributions Time resolved spectra Excited-state reactions Basic physics concept in polarization The
Lecture 5. Anisotropy decay/data analysis Enrico Gratton Anisotropy decay Energy-transfer distance distributions Time resolved spectra Excited-state reactions Basic physics concept in polarization The
Protein Dynamics, Allostery and Function
 Protein Dynamics, Allostery and Function Lecture 3. Protein Dynamics Xiaolin Cheng UT/ORNL Center for Molecular Biophysics SJTU Summer School 2017 1 Obtaining Dynamic Information Experimental Approaches
Protein Dynamics, Allostery and Function Lecture 3. Protein Dynamics Xiaolin Cheng UT/ORNL Center for Molecular Biophysics SJTU Summer School 2017 1 Obtaining Dynamic Information Experimental Approaches
Fluorescence Spectroscopy
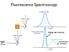 Fluorescence Spectroscopy Raleigh light scattering light all freqs Fluorescence emission Raleigh Scattering 10 nm Raleigh light scattering Fluorescence emission 400 nm Scattering - TWO particle 10 nm Particle
Fluorescence Spectroscopy Raleigh light scattering light all freqs Fluorescence emission Raleigh Scattering 10 nm Raleigh light scattering Fluorescence emission 400 nm Scattering - TWO particle 10 nm Particle
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2013
 Lecture 1 3/13/13 University of Washington Department of Chemistry Chemistry 53 Winter Quarter 013 A. Definition of Viscosity Viscosity refers to the resistance of fluids to flow. Consider a flowing liquid
Lecture 1 3/13/13 University of Washington Department of Chemistry Chemistry 53 Winter Quarter 013 A. Definition of Viscosity Viscosity refers to the resistance of fluids to flow. Consider a flowing liquid
Modern Optical Spectroscopy
 Modern Optical Spectroscopy With Exercises and Examples from Biophysics and Biochemistry von William W Parson 1. Auflage Springer-Verlag Berlin Heidelberg 2006 Verlag C.H. Beck im Internet: www.beck.de
Modern Optical Spectroscopy With Exercises and Examples from Biophysics and Biochemistry von William W Parson 1. Auflage Springer-Verlag Berlin Heidelberg 2006 Verlag C.H. Beck im Internet: www.beck.de
NMR, the vector model and the relaxation
 NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
Particle Characterization Laboratories, Inc.
 Analytical services Particle size analysis Dynamic Light Scattering Static Light Scattering Sedimentation Diffraction Zeta Potential Analysis Single Point Titration Isoelectric point determination Aqueous
Analytical services Particle size analysis Dynamic Light Scattering Static Light Scattering Sedimentation Diffraction Zeta Potential Analysis Single Point Titration Isoelectric point determination Aqueous
Added topics: depend on time/rate - Skipped
 VIa- 1 Added topics: depend on time/rate - Skipped - 2012 a) diffusion rate material moves in medium faster motion more friction affect equilibrium separations like electrophoresis HPLC pharmacological
VIa- 1 Added topics: depend on time/rate - Skipped - 2012 a) diffusion rate material moves in medium faster motion more friction affect equilibrium separations like electrophoresis HPLC pharmacological
Contents. xiii. Preface v
 Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
Part I.
 Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
UltraScan Workshop/Bioc5083 Hydrodynamic Methods
 UltraScan Workshop/Bioc5083 Hydrodynamic Methods Borries Demeler, Ph.D. Department of Biochemistry May/June 2013 Analytical Ultracentrifugation Background What can be learned from AUC? Excellent method
UltraScan Workshop/Bioc5083 Hydrodynamic Methods Borries Demeler, Ph.D. Department of Biochemistry May/June 2013 Analytical Ultracentrifugation Background What can be learned from AUC? Excellent method
Entanglements. M < M e. M > M e. Rouse. Zero-shear viscosity vs. M (note change of slope) Edwards degennes Doi. Berry + Fox, slope 3.4.
 Entanglements Zero-shear viscosity vs. M (note change of slope) M < M e Rouse slope 3.4 M > M e Edwards degennes Doi slope 1 Berry + Fox, 1968 Question: Which factors affect the Me: T, P, M, flexibility,
Entanglements Zero-shear viscosity vs. M (note change of slope) M < M e Rouse slope 3.4 M > M e Edwards degennes Doi slope 1 Berry + Fox, 1968 Question: Which factors affect the Me: T, P, M, flexibility,
In Situ Imaging of Cold Atomic Gases
 In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
CPS Instruments Europe P.O. Box 180, NL-4900 AD Oosterhout, The Netherlands T: +31 (0) F: +31 (0) E:
 Introduction to Differential Sedimentation Differential Centrifugal Sedimentation, or DCS (sometimes also called "two-layer" sedimentation) is a widely used analysis method that produces extremely high
Introduction to Differential Sedimentation Differential Centrifugal Sedimentation, or DCS (sometimes also called "two-layer" sedimentation) is a widely used analysis method that produces extremely high
Lecture 0. NC State University
 Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Singlet. Fluorescence Spectroscopy * LUMO
 Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
C C C C 2 C 2 C 2 C + u + v + (w + w P ) = D t x y z X. (1a) y 2 + D Z. z 2
 This chapter provides an introduction to the transport of particles that are either more dense (e.g. mineral sediment) or less dense (e.g. bubbles) than the fluid. A method of estimating the settling velocity
This chapter provides an introduction to the transport of particles that are either more dense (e.g. mineral sediment) or less dense (e.g. bubbles) than the fluid. A method of estimating the settling velocity
Fluorescence Polarization Anisotropy FPA
 Fluorescence Polarization Anisotropy FPA Optics study of light Spectroscopy = light interacts the study of the interaction between matter & electro-magnetic radiation matter Spectroscopy Atomic Spectroscopy
Fluorescence Polarization Anisotropy FPA Optics study of light Spectroscopy = light interacts the study of the interaction between matter & electro-magnetic radiation matter Spectroscopy Atomic Spectroscopy
Supporting Information
 Supporting Information Wu et al. 1.173/pnas.15492112 SI Text S1) Derivation of the Wind-Chime Model Each pulse generates an angular momentum on each MoS 2 flake that makes them rotate unless they develop
Supporting Information Wu et al. 1.173/pnas.15492112 SI Text S1) Derivation of the Wind-Chime Model Each pulse generates an angular momentum on each MoS 2 flake that makes them rotate unless they develop
Time-Dependent Statistical Mechanics 1. Introduction
 Time-Dependent Statistical Mechanics 1. Introduction c Hans C. Andersen Announcements September 24, 2009 Lecture 1 9/22/09 1 Topics of concern in the course We shall be concerned with the time dependent
Time-Dependent Statistical Mechanics 1. Introduction c Hans C. Andersen Announcements September 24, 2009 Lecture 1 9/22/09 1 Topics of concern in the course We shall be concerned with the time dependent
Chapter 6: The Rouse Model. The Bead (friction factor) and Spring (Gaussian entropy) Molecular Model:
 G. R. Strobl, Chapter 6 "The Physics of Polymers, 2'nd Ed." Springer, NY, (1997). R. B. Bird, R. C. Armstrong, O. Hassager, "Dynamics of Polymeric Liquids", Vol. 2, John Wiley and Sons (1977). M. Doi,
G. R. Strobl, Chapter 6 "The Physics of Polymers, 2'nd Ed." Springer, NY, (1997). R. B. Bird, R. C. Armstrong, O. Hassager, "Dynamics of Polymeric Liquids", Vol. 2, John Wiley and Sons (1977). M. Doi,
Measuring particle aggregation rates by light scattering
 Measuring particle aggregation rates by light scattering Gregor Trefalt, Istvan Szilagyi, Michal Borkovec Email. gregor.trefalt@unige.ch, istvan.szilagyi@unige.ch, michal.borkovec@unige.ch Introduction
Measuring particle aggregation rates by light scattering Gregor Trefalt, Istvan Szilagyi, Michal Borkovec Email. gregor.trefalt@unige.ch, istvan.szilagyi@unige.ch, michal.borkovec@unige.ch Introduction
What is spectroscopy?
 Absorption Spectrum What is spectroscopy? Studying the properties of matter through its interaction with different frequency components of the electromagnetic spectrum. With light, you aren t looking directly
Absorption Spectrum What is spectroscopy? Studying the properties of matter through its interaction with different frequency components of the electromagnetic spectrum. With light, you aren t looking directly
van Quantum tot Molecuul
 10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
Determination of Molecular Weight and Its Distribution of Rigid-Rod Polymers Determined by Phase-Modulated Flow Birefringence Technique
 Determination of Molecular Weight and Its Distribution of Rigid-Rod Polymers Determined by Phase-Modulated Flow Birefringence Technique YUM RAK OH, YOUNG SIL LEE, MOO HYUN KWON, O OK PARK Department of
Determination of Molecular Weight and Its Distribution of Rigid-Rod Polymers Determined by Phase-Modulated Flow Birefringence Technique YUM RAK OH, YOUNG SIL LEE, MOO HYUN KWON, O OK PARK Department of
What the Einstein Relations Tell Us
 What the Einstein Relations Tell Us 1. The rate of spontaneous emission A21 is proportional to υ 3. At higher frequencies A21 >> B(υ) and all emission is spontaneous. A 21 = 8π hν3 c 3 B(ν) 2. Although
What the Einstein Relations Tell Us 1. The rate of spontaneous emission A21 is proportional to υ 3. At higher frequencies A21 >> B(υ) and all emission is spontaneous. A 21 = 8π hν3 c 3 B(ν) 2. Although
Lab Week 4 Module α 3. Polymer Conformation. Lab. Instructor : Francesco Stellacci
 3.014 Materials Laboratory Dec. 9 th Dec.14 th, 2004 Lab Week 4 Module α 3 Polymer Conformation Lab. Instructor : Francesco Stellacci OBJECTIVES 9 Review random walk model for polymer chains 9 Introduce
3.014 Materials Laboratory Dec. 9 th Dec.14 th, 2004 Lab Week 4 Module α 3 Polymer Conformation Lab. Instructor : Francesco Stellacci OBJECTIVES 9 Review random walk model for polymer chains 9 Introduce
University of Washington Department of Chemistry Chemistry 355 Spring Quarter 2005
 University of Washington Department of Chemistry Chemistry 55 Spring Quarter 5 Homework Assignment 7: Due no later than 5: pm on 5/14/4 8., 8.6, 8.1 8.) According to text equation 16, after N jumps the
University of Washington Department of Chemistry Chemistry 55 Spring Quarter 5 Homework Assignment 7: Due no later than 5: pm on 5/14/4 8., 8.6, 8.1 8.) According to text equation 16, after N jumps the
Lecture 15: Optoelectronic devices: Introduction
 Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
LABORATORY OF ELEMENTARY BIOPHYSICS
 LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
and another with a peak frequency ω 2
 Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer
 CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
single-molecule fluorescence resonance energy transfer
 single-molecule fluorescence resonance energy transfer (2) determing the Förster radius: quantum yield, donor lifetime, spectral overlap, anisotropy michael börsch 26/05/2004 1 fluorescence (1) absorbance
single-molecule fluorescence resonance energy transfer (2) determing the Förster radius: quantum yield, donor lifetime, spectral overlap, anisotropy michael börsch 26/05/2004 1 fluorescence (1) absorbance
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION
 ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
SEC MALLs and AUC. 2. Conformation and flexibility Viscometry,
 1. Molecular weight distribution analysis SEC MALLs and AUC 2. Conformation and flexibility Viscometry, AUC, Light scattering Lecture 4. Analytical l Ultracentrifugation t ti I: Molecular weight and conformation
1. Molecular weight distribution analysis SEC MALLs and AUC 2. Conformation and flexibility Viscometry, AUC, Light scattering Lecture 4. Analytical l Ultracentrifugation t ti I: Molecular weight and conformation
Rotational Kinematics and Dynamics. UCVTS AIT Physics
 Rotational Kinematics and Dynamics UCVTS AIT Physics Angular Position Axis of rotation is the center of the disc Choose a fixed reference line Point P is at a fixed distance r from the origin Angular Position,
Rotational Kinematics and Dynamics UCVTS AIT Physics Angular Position Axis of rotation is the center of the disc Choose a fixed reference line Point P is at a fixed distance r from the origin Angular Position,
XV 74. Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go?
 XV 74 Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go? 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet S = 0 could be any of them
XV 74 Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go? 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet S = 0 could be any of them
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay
 Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 15 Laser - I In the last lecture, we discussed various
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 15 Laser - I In the last lecture, we discussed various
Shell Balances in Fluid Mechanics
 Shell Balances in Fluid Mechanics R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University When fluid flow occurs in a single direction everywhere in a system, shell
Shell Balances in Fluid Mechanics R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University When fluid flow occurs in a single direction everywhere in a system, shell
EQUATIONS FOR ROTATIONAL AND TRANSLATIONAL DIFFUSION CONSTANTS
 A NUMERICAL INVERSION OF THE PERRIN EQUATIONS FOR ROTATIONAL AND TRANSLATIONAL DIFFUSION CONSTANTS BY ITERATIVE TECHNIQUES A. KENT WRIGHT and JOHN E. BAXTER From the Department of Biochemistry, University
A NUMERICAL INVERSION OF THE PERRIN EQUATIONS FOR ROTATIONAL AND TRANSLATIONAL DIFFUSION CONSTANTS BY ITERATIVE TECHNIQUES A. KENT WRIGHT and JOHN E. BAXTER From the Department of Biochemistry, University
SRI LANKAN PHYSICS OLYMPIAD COMPETITION 2008
 SRI LANKAN PHYSICS OLYMPIAD COMPETITION 008 Time Allocated : 0 Hours Calculators are not allowed to use. Date of Examination : 1 07 008 Index No. :. Time : 9.30 a.m. - 11.30 a.m. INSTRUCTIONS Answer all
SRI LANKAN PHYSICS OLYMPIAD COMPETITION 008 Time Allocated : 0 Hours Calculators are not allowed to use. Date of Examination : 1 07 008 Index No. :. Time : 9.30 a.m. - 11.30 a.m. INSTRUCTIONS Answer all
1 Fundamentals. 1.1 Overview. 1.2 Units: Physics 704 Spring 2018
 Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
12. Spectral diffusion
 1. Spectral diffusion 1.1. Spectral diffusion, Two-Level Systems Until now, we have supposed that the optical transition frequency of each single molecule is a constant (except when we considered its variation
1. Spectral diffusion 1.1. Spectral diffusion, Two-Level Systems Until now, we have supposed that the optical transition frequency of each single molecule is a constant (except when we considered its variation
Biophysical Chemistry: NMR Spectroscopy
 Spin Dynamics & Vrije Universiteit Brussel 25th November 2011 Outline 1 Pulse/Fourier Transform NMR Thermal Equilibrium Effect of RF Pulses The Fourier Transform 2 Symmetric Exchange Between Two Sites
Spin Dynamics & Vrije Universiteit Brussel 25th November 2011 Outline 1 Pulse/Fourier Transform NMR Thermal Equilibrium Effect of RF Pulses The Fourier Transform 2 Symmetric Exchange Between Two Sites
Sem /2007. Fisika Polimer Ariadne L. Juwono
 Chapter 8. Measurement of molecular weight and size 8.. End-group analysis 8.. Colligative property measurement 8.3. Osmometry 8.4. Gel-permeation chromatography 8.5. Ultracentrifugation 8.6. Light-scattering
Chapter 8. Measurement of molecular weight and size 8.. End-group analysis 8.. Colligative property measurement 8.3. Osmometry 8.4. Gel-permeation chromatography 8.5. Ultracentrifugation 8.6. Light-scattering
SPRING 2011 Phys 450 Solution set 2
 SPRING 011 Phys 450 Solution set Problem 1 (a) Estimate the diameter of a ater molecule from its self-diffusion coefficient, and using the Stokes-Einstein relation, assuming that it is a spherical molecule.
SPRING 011 Phys 450 Solution set Problem 1 (a) Estimate the diameter of a ater molecule from its self-diffusion coefficient, and using the Stokes-Einstein relation, assuming that it is a spherical molecule.
Single-Molecule Methods I - in vitro
 Single-Molecule Methods I - in vitro Bo Huang Macromolecules 2014.03.10 F 1 -ATPase: a case study Membrane ADP ATP Rotation of the axle when hydrolyzing ATP Kinosita group, 1997-2005 Single Molecule Methods
Single-Molecule Methods I - in vitro Bo Huang Macromolecules 2014.03.10 F 1 -ATPase: a case study Membrane ADP ATP Rotation of the axle when hydrolyzing ATP Kinosita group, 1997-2005 Single Molecule Methods
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy
 Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Biophysical Chemistry: NMR Spectroscopy
 Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
Introduction to Differential Sedimentation
 Introduction to Differential Sedimentation Differential Centrifugal Sedimentation, or DCS (sometimes also called "two-layer" sedimentation) is a widely used analysis method that produces extremely high
Introduction to Differential Sedimentation Differential Centrifugal Sedimentation, or DCS (sometimes also called "two-layer" sedimentation) is a widely used analysis method that produces extremely high
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction...
 Transport phenomena 1/16 Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction... Flux of mass, charge, momentum, heat,...... J = amount (of quantity) transported
Transport phenomena 1/16 Transport (kinetic) phenomena: diffusion, electric conductivity, viscosity, heat conduction... Flux of mass, charge, momentum, heat,...... J = amount (of quantity) transported
Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas )
 Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas ) Yasutomo ISHII and Andrei SMOLYAKOV 1) Japan Atomic Energy Agency, Ibaraki 311-0102, Japan 1) University
Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas ) Yasutomo ISHII and Andrei SMOLYAKOV 1) Japan Atomic Energy Agency, Ibaraki 311-0102, Japan 1) University
Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis)
 Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis) 2 2. Circular dichroism (optical activity) CD / ORD 3 3. Fluorescence spectroscopy and energy transfer Electromagnetic Spectrum Electronic Molecular
Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis) 2 2. Circular dichroism (optical activity) CD / ORD 3 3. Fluorescence spectroscopy and energy transfer Electromagnetic Spectrum Electronic Molecular
Laser Types Two main types depending on time operation Continuous Wave (CW) Pulsed operation Pulsed is easier, CW more useful
 What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
The dynamics of small particles whose size is roughly 1 µmt or. smaller, in a fluid at room temperature, is extremely erratic, and is
 1 I. BROWNIAN MOTION The dynamics of small particles whose size is roughly 1 µmt or smaller, in a fluid at room temperature, is extremely erratic, and is called Brownian motion. The velocity of such particles
1 I. BROWNIAN MOTION The dynamics of small particles whose size is roughly 1 µmt or smaller, in a fluid at room temperature, is extremely erratic, and is called Brownian motion. The velocity of such particles
Medical Biophysics II. Final exam theoretical questions 2013.
 Medical Biophysics II. Final exam theoretical questions 2013. 1. Early atomic models. Rutherford-experiment. Franck-Hertz experiment. Bohr model of atom. 2. Quantum mechanical atomic model. Quantum numbers.
Medical Biophysics II. Final exam theoretical questions 2013. 1. Early atomic models. Rutherford-experiment. Franck-Hertz experiment. Bohr model of atom. 2. Quantum mechanical atomic model. Quantum numbers.
Rate of change of velocity. a=dv/dt. Acceleration is a vector quantity.
 9.7 CENTRIFUGATION The centrifuge is a widely used instrument in clinical laboratories for the separation of components. Various quantities are used for the description and the calculation of the separation
9.7 CENTRIFUGATION The centrifuge is a widely used instrument in clinical laboratories for the separation of components. Various quantities are used for the description and the calculation of the separation
