Spontaneous action potentials and neural coding in un-myelinated axons
|
|
|
- Audra Fields
- 5 years ago
- Views:
Transcription
1 Spontaneous action potentials and neural coding in un-myelinated axons Cian O Donnell 1,2 and Mark C.W. van Rossum 1, August 14, Institute for Adaptive and Neural Computation, School of Informatics, University of Edinburgh, UK 2 Current address: Computational Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla CA, USA Corresponding author: mvanross@inf.ed.ac.uk Abstract The voltage-gated Na and K channels in neurons are responsible for action potential generation. Because ion-channels open and close in a stochastic fashion, spontaneous (ectopic) action potentials can result even in the absence of stimulation. While spontaneous action potentials have been studied in detail in single compartment models, studies on spatially extended processes have been limited. The simulations and analysis presented here show that spontaneous rate in un-myelinated axon depends non-monotonically on the length of the axon, that the spontaneous activity has sub-poisson statistics, and that neural coding can be hampered by the spontaneous spikes by reducing the probability of transmitting the first spike in a train. Introduction Action potentials rely crucially on the voltage-gated channels in the neural membrane. The classic Hodgkin-Huxley model describes how small depolarizations of the membrane lead to increased opening of the Na channels, the flux 1
2 through which further depolarizes the membrane. Once this process reaches a point of no return, an action potential is generated which is terminated by the opening of the hyper-polarizing K channels that follow the membrane potential more sluggishly than the Na channels. As the opening of the channels is a stochastic process, the action potentials have a stochastic character as well. This is most noticeable in the initiation phase of the spike when only few channels are open. During the later phase, so many channels open that stochastic fluctuations are largely averaged out. The stochastic character of ion channels has been studied extensively in both experimental and modeling literature. A number of modeling studies has considered single compartment models, in which a cell is lumped together in one iso-potential sphere. For spheres with small membrane areas, stochastic opening and closing of channels can lead to spontaneous action potentials, even in the absence of any stimulation. For large membrane areas, the stochastic character disappears and the system is well described by the deterministic Hodgkin-Huxley equations. In particular, the rate of spontaneous action potentials decreases exponentially with the membrane area (see below and Chow and White, 1996). Similarly, for un-myelinated axons the spontaneous action potential rate increases as the diameter of the axon thins. It has been argued using simulations that in 1mm long axons thinner than.1µm, spontaneous firing rates become so substantial that they severely interfere with the communication of stimulus-driven action potentials (Faisal, White, and Laughlin, 25). This effect might therefore impose a limit on the minimal diameter of axons, but length dependence has not been examined. In this study we examine in simulations the relation the spontaneous rate and the axon length. Interestingly, depending on diameter, the spontaneous rate is non-monotonic in length, it is high for both short and long axons, but lowest for intermediate length axons. Our subsequent reduced model allows to explore these effects exactly and identifies various regimes each with its particular characteristics. Next, we show how spontaneous spikes interfere with information coding. The spontaneous spikes have a peculiar characteristics different from any other sources of noise studied before in neurons, that can hamper coding but can also be used to identify spontaneous spiking activity experimentally. 2
3 Results We consider a uniform, un-myelinated axon with sealed ends and use the standard Hodgkin-Huxley model to describe the active properties of the axon. The only difference in the standard parameters is that we vary the diameter and length of the axon. While the HH model at 6.3C can hardly be called a realistic model for mammalian neurons, we use it because the full characterization of ion channels in mammalian un-myelinated axons is incomplete. Furthermore, using the HH model has the advantage that both its deterministic and stochastic variants have been studied extensively. The stochastic simulation were done using both the PSICS simulator (Cannon, O Donnell, and Nolan, 21), and the stochastic module in NEURON (Carnevale and Hines, 26). Rate vs length We first study the spontaneous rate in axons of arbitrary diameter and length, Fig.1A. For a given diameter the spontaneous rate is non-monotonic in the length: it is high for very short and very long axons, but lower for intermediate lengths. This behavior can be understood as follows: When the axon is very long, it can be seen as a collection of many weakly coupled compartments, each of which can generate spikes. Yet once a spike is generated, it will travel along the whole axon (unless it collides with another spontaneous spike, see below). As the number of such compartments and thus the number of independent initiation zones increases with axon length, the rate increases with length for long enough axons. In contrast, very short axons can be collapsed into a single compartment if they are much shorter than the electrotonic length (or space-constant) Λ. In this case the dependence of the spontaneous rate on area and thus length is well understood (Chow and White, 1996). In analogy with a Kramer s escape problem the spontaneous firing rate f behaves as f exp( c/σ 2 V ) (1) where σv 2 is variance in the membrane voltage and c is some constant. Under the assumption of small fluctuations, the variance in the voltage equals σ 2 V = S I (f) Z(f) 2 df, where S I (f) is the power-spectrum of the noise current, and Z(f) the membrane impedance. This voltage variance is in general a complicated expression, as the channel noise has a colored power-spectrum and the membrane impedance in the HH model has a resonance (O Donnell and van 3
4 Rossum, 214). However, here we only need the dependence on the membrane area. Assuming that the voltage is approximately constant, the channels are independent and the fluctuations in the current are proportional to the number of channels and hence proportional to the membrane area S I (f) A. However, the impedance is inversely proportional to area, Z(f) 1/A. So that we find that σv 2 1/A and log f A L. This exponential dependence on length for short axons together with the behavior for long axons determines the shape of Fig.1A: the spontaneous rate increases for both very short and very long axons. Effect of diameter and spike initiation zones While spikes can be generated anywhere in the axon, Fig.1D, the initiation is not uniform. A histogram of the initiation location shows that most spikes are generated at the ends of the axon, Fig.1B. This can be understood using an argument similar to the one above explaining the area dependence in single compartments. The variance of the noise currents scales with the number of channels in the local neighborhood. There are twice as many neighboring channels in the middle of the axon as there are at the end. However, the impedance at the end is twice that in the middle of the axon as the current can only flow in one instead of two directions (or equivalently, the current threshold is lower). Thus at the axon ends the variance in the voltage is double that in the middle of the axon (Tuckwell and Walsh, 1983), making it the preferred initiation site. Because of the exponential dependence of spike rate on voltage variance, Eq.1, many more spikes will be produced at the axon ends than in the middle, despite the noise only being double. Also note that the preferred initiation zone extends only little into the axon, much less than the electrotonic length Λ, which is about 8µm (for a.1µm diameter HH axon at rest, including the contributions of Na and K channels). The reason is that the channel noise, which contains high frequencies, is filtered within a much shorter distance than the electrotonic length Λ, which describes the DC decay in a cable. The total spontaneous generation rate in an axon of arbitrary length is by definition a sum of the generation rate at the ends and the generation rate anywhere else. We define ρ e as the generation rate at one end (measured in Hz, taken within 4µm) and ρ m as the generation rate anywhere else (measured in 4
5 A 5 B Spont rate (Hz) Axon length (µm) Spikes generated (Hz) Distance along axon (µm) C 2 D Rate (Hz) 15 1 Initiation rate at ends Initiation elsewhere (per mm) Fits Diameter (µm) Figure 1: Spontaneous action potentials in axons of arbitrary length and width. A) Spontaneous action potential rate as function of axon length for diameters (top to bottom curve):.1,.11,.12,.13,.14,.15,.2 and.25µm. B) The spike generation rate along an axon. Compartments at the ends are hot spots that generate many more spikes per compartment than the rest of the axon. Axon of 5µm length and.1µm diameter. C) The spontaneous spike initiation rates at the ends (ρ e, in Hz) and in the rest of the axon (ρ m, in Hz per mm) versus the diameter. Extracted from a simulation of an axon of 1mm length. Thin lines indicates fits to theory (see text). D) Short segment of a simulation showing the generation of two spontaneous spikes (arrows). 5
6 Hz/mm), so that f created = 2ρ e + Lρ m (2) where we assume the axon is long enough (L Λ) so that we can ignore the length dependence of ρ e in the left portion of Fig.1A. These spike initiation rates, ρ e and ρ m, are compared as a function of diameter in Fig.1C. It shows that both decrease for wider diameters. A larger diameter couples a larger part of the axon and hence collects more noise current, however, the impedance is lower as well, so that the voltage variance is smaller for wider axons. The effective area equals A dλ d 3/2. Consistent with this, we find that the simulations are well fitted to log(ρ e ) (d/d ) 3/2, while log(ρ m ) 2(d/d ) 3/2, with a fit parameter d.1µm. The factor 2 expresses the effect of the end of the axon. Due to the exponential dependence of the rate on the fluctuations, the ratio of these generation rates is not constant, but shifts in the favor of end-generation for thicker axons, explaining why the curves in Fig.1A change shape as the diameter is varied. We define the critical length L c as the length at which the two rates are comparable, L c = 2ρ e /ρ m. It increases approximately exponentially with diameter in our simulations, Fig. 2B. Collisions So far we have ignored another important effect for long axons: Two spikes traveling in opposite direction in the axon will collide. When this happens, the spikes annihilate as the membrane on either side of the collision will be in a refractory state and further propagation fails. The probability that spikes collide increases in longer axons: First, the rate increases with length as we have seen in Eq. 2. Secondly, it takes longer for the spikes to propagate the whole length. The speed of propagation in the HH model is about 2µm/ms for a diameter of.1µm, and increases with diameter as d. Thus while for short axons with low rates all initiated spikes travel the full axon, due to collisions the observed spontaneous rate in long axons is lower than the initiation rate. (It is easy to see from that the observed rate is independent of the observation point, whether at any end of the axon or anywhere in the middle). With every collision two spikes merge into one. The observed spontaneous rate, f obs, thus equals the number of spikes generated at the ends, plus the 6
7 A Rate (Hz) Generated at end B Generated elsewhere Collisions Lcrit (µm) L c (end vs rest of axon) L coll (collisions) 25 Total observed 5 1 Axon length (µm) Diameter (µm) Figure 2: A) Split of the various contributions to the observed spontaneous rate as a function of axon length (axon with.1µm diameter). Top: rate of spikes generated at the axon ends. Middle: rate of spikes generated in the rest of the axon and the rate of collisions of spikes in the axon. Bottom: The resulting observed firing rate (the sum of the two generation rates minus the collision rate). B) The two characteristic length parameters as a function of axon diameter: Solid line (L c ), for axons shorter than this length, most spikes are generated at the ends; for axons longer than this length, most spikes are generated in the rest of the axon. Dashed line (L coll ) for axons longer than this length, collisions of spikes significantly limit the observed spontaneous rate. 7
8 spikes generated anywhere else, minus the collision rate ρ colls, f obs = 2ρ e + Lρ m ρ colls In Fig.2A we plot these terms as extracted from the simulations. For the shortest axons, most spikes are generated at the ends. With longer axons the rate of spikes generated anywhere else increases linearly with the length, but the collision rate also increases linearly, and for very long the spontaneous rate is independent of axon length. Collisions become significant if it is likely that there are multiple spikes propagating simultaneously in the axon. This happens when fτ 1, where τ is the time for a spike to propagate from end to end τ = L/v. With Eq.2 this gives the axon length for which collisions become important, L coll, as L coll = 1 ( ρ e + ) ρ ρ 2 e + ρ m v m This is plotted in Fig.2B. For the HH system this length is always larger than the length for which spikes generated in the rest of the axon are dominant. Thus when collisions start to appear, the spontaneous rate is dominated by the spikes generated in the rest of the axon, and so L coll v/ρ m. As explored in detail below, when L L coll the firing rate becomes independent of the axon length, as can be seen in Fig.1A and Fig.2A. In summary, there are 4 regimes in the HH axon that explain the shape of the graph of spontaneous rate versus axon length, Fig.1A: 1. L Λ: the spontaneous spikes are mainly generated at the ends with a rate that decreases with length, no collisions occur, 2. Λ L L c : the spontaneous spikes are mainly generated at the ends, no collisions occur, the rate is roughly constant, 3. L c L L coll : the spontaneous spikes are mainly generated in the rest of the axon, no collisions occur, the spontaneous rate increases linearly with length, 4. L L coll : the spontaneous spikes are mainly generated in the rest of the axons, most spikes collide, and the rate is independent of length. 8
9 This is an idealized description that is meant to aid understanding, in practice the regimes will overlap. Changes in the ion channels properties and densities as well as the geometry can further alter the classification and ordering of regimes. For instance, if the propagation speed is slower, collisions will be more likely (L coll decreases). Or, as another example, if a large soma is attached at one axon end or it receives strong inhibition, no spontaneous spikes are expected to originate there. While in contrast, local depolarization can increase rates. Effect of collisions in simplified models In order to better understand the effect of collisions in long axons, we develop an abstract model of the action potential generation and propagation. Propagation failure is assumed to be negligible, indeed we never observed it in our Hodgkin-Huxley simulations. Spike generation probability is a function of the position along the axon. A simple model is to have a rate ρ e at both ends, and a rate per length ρ m along the rest of the axon, i.e. the Poisson rate density is ρ(x) = [δ(x) + δ(x L)]ρ e + ρ m. This system is simulated with an cellular automaton using a discretized axon, using 3 rules: It 1) creates spike according to a Poisson process according to density ρ(x); 2) it propagates the generated spikes towards left and right from the initiation point; 3) it annihilates colliding spikes. Generators on axon ends only We first analyze the idealized case when spikes are generated at the ends only. We assume that the axon length is long enough so that ρ e is constant. As above we define the propagation time τ = L/v as the time it takes to travel from one end of the axon to the other. The only dimensionless parameter is ρ e τ. We find (Appendix) that due to collisions the observed rate becomes f obs = ρ e (1 + ) ρ e τ For low rates collisions should not occur, hence f obs = 2ρ e. However, at high rates (or long axons) every spike generated on one end will annihilate with one from the other end, hence f obs = ρ e. The characteristic length for the switch-over is v/ρ e. 9 (3)
10 A i ii iii a δl 2δl B 6 Observed, HH 1mm Prediction, infinite length Time Rate (Hz) Diameter (µm) Figure 3: A) Cartoon of: i) Unhindered propagation of a single spontaneous spike, ii) Illustration of two spikes colliding, the third spike does not collide, iii) Diagram used for the calculation of the spontaneous rate in the limit of very long axons or high generation rates. B) Predicted spontaneous rate for axon of infinite length (dashed line, Eq. 6) and the rate extracted from HH simulations with 1mm length (solid). We extracted the propagation speed v and the generation rate ρ m from the simulation. 1
11 Uniform generation along the axon Next, we assume uniform generation along the axon constant in time (a socalled Poisson sheet). In the absence of collisions, the spontaneous rate obeys f obs = ρ m L, Eq.2. The first correction will be the occasional collision of two spikes, see Fig. 3A.ii, the probability for such events is proportional to the rate squared. It can be calculated exactly (see Appendix) [ f obs = ρ m L 1 1 ] 3 ρ ml 2 /v + O(ρ 2 m) (4) While in principle one can try to systematically expand to include higher order corrections, this quickly becomes unwieldy. Fig. 3A.ii illustrates why this calculation is hard: if the top left spike (2) had not occurred, the top-right spike (3) would have collided with the bottom-left spike (1). In other words, the collision pattern can depend on the full spike history. But while a general expression of spontaneous rate in terms of ρ m seems out of reach, the output rate becomes independent of the length of the axon in the limit of long axons (L L coll ). This can seen as follows: when the rates are high, most spikes will not propagate along the full axon, instead, they will collide. Apart from the ends, the axon behaves uniformly. Because the probability for collision is approximately uniform and constant, the spikes lengths l will be distributed exponentially P (l) = 1 exp( l/λ) (5) λ where λ is the mean distance a spike travels before it collides. Next, we wish to know the dependence of this mean distance on the generation rate. It follows from a self-consistency argument: Consider the spike labeled a in Fig.3A.iii. The collision process can be seen as a hazard-process, where the hazard for a collision is given by the presence of other spikes. The hazard probability h is the probability for the presence of another spike in infinitesimal length δl. Consider the shaded strip in Fig.3A.iii. In the limit of small δl the colliding spikes present in this strip can be described by a binary random process with an on-rate 2ρ m δl and an off-rate v/λ. The factor 2 in the on-rate is a geometrical factor, Fig.3A.iii. As required by self-consistency, the length of this colliding spike according to this process is exponentially distributed and the mean length is λ. 11
12 The hazard is thus h = 2ρ mδl 2ρ 2ρ m δl+v/λ mλδl/v. This hazard will reduce the survival probability of the original spike according to P (l + δl) = P (l) hp (l), so that P (l) exp( lh/δl). Identification with Eq. 5, gives the 2ρ m λ/v = 1/λ, from which the spike-length as a function of initiation rate follows as λ = v/2ρ m. The final step is to compute the output rate from the average spike length. Imagine that one observes the spike rate at a given location in the axon. The average number of observed spikes will be product of the total number of initiations (ρ m L), the probability to have propagated to that location (λ/l), and finally a factor 2 because every initiation leads to two spikes traveling in opposite direction. So that f obs = 2λρ m = 2vρ m (6) This matches the simulations of the reduced model, but also the full HH model well, Fig.3D. Note, that because L coll L c for the HH axon, the uniform generation assumption is valid. Due to refractory effects (at high rates) and the inability to simulate infinitely long axons (for very wide axons), the theory slightly overestimates the observed spontaneous rate. Variability of spontaneous spikes In single compartments and axons much shorter than L coll the spontaneous spikes in the reduced model will follow Poisson statistics and thus the interspike intervals have an exponential interval distribution. However, in longer axons the spontaneous spikes have more regular statistics. The intuition is that when by chance at some time many spikes are generated, the collision rate will increase as well, thus smoothing out some of the variability in the rate. To study this separately from the HH dynamics, including refractoriness, and mainly for reasons of efficiency we use the simplified model, with a length L = 1L coll with a spontaneous rate of about 1Hz, assuming that spikes are generated uniformly throughout the axon (ρ(x) = ρ m ). Due to collisions the inter-spike interval distribution is more regular than a Poisson process, Fig.4 (top left panel). This can be further analyzed by sorting the spikes according to their direction of travel. In particular, while the probability for a leftward-going spike to be followed by another leftward-going spike approximately resembles a Poisson process. Yet, a leftward-going spike is un- 12
13 Measured halfway Measured at end 2 All spikes Count 2 Same direction 2 Alternating Spike interval (s) Figure 4: Inter-spike interval distribution in an axon with length 1L coll. Top: counting all intervals; middle: events where the subsequent spikes travel in the same direction; bottom: events where the subsequent spikes travel in opposite direction. The variability is sub-poisson (CV isi.67) in the middle of the axon (left column), but more Poisson-like at the end of the axon (CV isi.78, right column). This due to the fact that most spikes there travel in the same direction (towards the end). Virtually no events have alternating directions at the end of the axon (bottom right panel). 13
14 likely to be immediately followed by a rightward traveling spike, as the second spike is likely to collide with a recent spike traveling in the opposite direction. As a result the probability for intervals where the spikes travel in opposite directions is small for short intervals (bottom left panel, numerically resembling a gamma-distribution with shape parameter k = 2). When measuring the interval statistics irrespective of the propagation directions, the mix of these two processes leads to a process of sub-poisson variability with a interspike interval coefficient of variation (CV) of about.67. While away from the ends, the rate of left-going and right-going spikes is equal, near the ends of the axon only few spontaneous spike travel towards the middle. As a result the interval distribution is dominated by spikes traveling in the same direction and is more Poisson-like near the ends and has a higher CV, Fig.4 (right panels). Effect on signal transmission Finally, we consider a situation where the neuron tries to send informative spikes down the axon. We analyze the spikes arriving at the other end of the axon where one could image a synaptic terminal to be present. We consider the transmission of a short, periodic spike train through the axon and measure whether or not the spikes arrive at the terminal at the expected time, again using the abstract model, Fig.5A. As one can observe the first spike has a only a limited probability to be transmitted, while later spikes are more likely to be transmitted, Fig.5B. The reason is that the first spike has a high probability to encounter a spontaneously generated spike and thus be annihilated. The first spikes act as a snowplow, clearing the way for the subsequent spikes. The probability that a colliding spike is present, is proportional to the area of the striped triangle in Fig.4C, which equals ρ m Lτ = (L/L coll ) 2. Under a Poisson assumption, the probability for transmission without collision equals exp[ (L/L coll ) 2 ]. For a spike much later in the train the collision probability is equal to the slice P = ρ m Lt isi, where t isi is the time between the spikes in the train, which is much smaller (hatched region in Fig.4B). The value transmission probabilities for Fig.4B are accordingly.37 for the first spike and.8 for the later spikes (train at 5Hz), which matches the simulations reasonably well. The higher the frequency of the train, the less probable it is that a spontaneous spike occurs between the spikes, hence the reliability increases with the frequency of the train, Fig.4B. This is different from most other 14
15 A C Driven end Output end Time (s) Time B Transmission reliability f=1 f=5 f= Index of spike in the train D Output rate (Hz) Long axon Very short axon Stimulus rate (Hz) Figure 5: A) Raster plot of a short simulation where the spike trains are generated at one end of the axon (top) and received at the other end of the axon (bottom). The short dashed bars in the lower row indicate when spikes would arrive if had not collided. In particular the first spikes in the train are vulnerable to transmission failure. Some spontaneous spikes can be observed at either end. B) The transmission probability of a spike from one end of the axon to the other for various stimulus frequencies. Due to collisions the first spike has a low transmission probability, but later ones encounter only few spontaneous spikes on their way and have a high transmission probability, in particular at high stimulus frequencies. C) Diagram to calculate the transmission probabilities. The first (left-most) spike in the train has a high collision probability (shaded traingle region), but later spikes have a lower one (hashed region). D) The output rate as a function of stimulus rate in a long axon (solid line). Low stimulus frequencies hardly increase the output rate. In contrast, in a single compartment model with spontaneous Poisson spikes, the output rate is simply the sum of spontaneous and driven rates (dashed line). Axon of length L = L coll, 1Hz spontaneous rate. 15
16 types of noise in spiking, as usually the first spike is the most reliable, while subsequent ones are either jittered or unreliable (e.g. Mainen and Sejnowski, 1995; van Rossum, O Brien, and Smith, 23). In addition to the spike times of the arriving train one can study the total spike count. We measure the output rate as a function of the driving rate for a long train. The spontaneous activity will cause output spikes in the absence of somatically driven spikes, abscissa in Fig.4D. However, unlike a single compartment model, the output rate is not simply the sum of driven and output spikes. Instead, the driven spikes partly collide with the spontaneous spikes. As a result of this replacement noise, the resulting rate resembles the soft-max of spontaneous and drives rates, Fig.4D, and the Signal-to-Noise ratio for small stimuli will be particularly poor. Discussion Some effects of the effect of ion channel noise has previously been studied in dendrites and axons. For instance, as the spike propagates, the speed varies, resulting in jitter of the spike arrival times at the axon terminals (Verveen and Derksen, 1968; Lass and Abeles, 1975; Horikawa, 1991; Faisal and Laughlin, 27). We have considered spontaneous spike generation in axons. In thin axons the spontaneous rates can be substantial. The spontaneous spikes are preferably generated at the ends of the axons, with a much lower rate in the rest of the axon. However, for long axons (L L c ) the contribution of the rest of the axon dominates. As a result we find a non-monotonic relation between the spontaneous rate and the length of the axon. Because the minimal firing rate occurs at a length of about 1mm, the length used in Faisal, White, and Laughlin (25), our results put even more stringent bounds on the diameter than suggested there. In particular long axons might need to be somewhat thicker. For even longer axons (L L coll ), the spontaneous rate saturates as spikes will start to collide with each other. Our theory predicts when this will happen and what the expected spike rate will be. Many questions are left about the collision regime, even in the simplified model: What is the firing rate for intermediate length axon, between Eq.4 and Eq.6? What if And what is the exact interval distribution for such axons? The most prominent question is whether spontaneous spikes caused by stochastic processes occur in biology and at what rate. If there is strong 16
17 evolutionary pressure to reduce wiring diameter, as has been suggested (Faisal, White, and Laughlin, 25), it is not unlikely that the nervous system operates in a regime where some spontaneous spikes occur. Related to that question is our choice of model system, namely the standard Hodgkin-Huxley model. The simulation presented in Faisal, White, and Laughlin (25) show that the spontaneous rates in HH and axon models tuned to mammalian fibers are comparable. Nevertheless, the exact characterization of the voltage-gated channels and its densities in long axons required to make quantitative predictions is far from complete and the spontaneous rate s exponential dependence on the fluctuations, Eq.1, makes it sensitive to precise model details. Whether spontaneous spikes are mainly generated throughout the axon or at the ends is similarly difficult to determine. In this light, the current study should be seen as demarcation of the various possible regimes. However, our results on the interval statistics and transmission might be used to test whether axons are in the collision regime. The conduction speed in mammalian C-fibers is on the order of 1 m/s (cf. to.2m/s for the HH model), so that for spontaneous rates of 1Hz, the collision length is L coll 1m. Acknowledgments Discussions with Sue Fleetwood-Walker, Jean-Marc Luck and Eleni Vasilaki are gratefully acknowledged. COD was supported by EPSRC/BBSRC/MRC through the Doctoral Training Centre in Neuroinformatics. Appendix: Analytic approach Spontaneous rate for generation at ends only To calculate the spontaneous rate when spikes are generated at the ends of the axon only, we use the following approach. We assume that during a time T there is total of n spikes generated of which m are on one side and n m at the other side. Both m and n m are independently Poisson distributed. In this case each spike can at most collide with one other spike. We calculate the probability that a given spike at the top end collides with any of the n m spikes from the other end. The probability for a given top-end spike with a bottomend spike to collide is 2τ/T, Fig.6top. Because the events are independent thus the probability for colliding with any spike is p = 1 (1 2τ/T ) n m. 17
18 L 2τ Time L Figure 6: Top: When spikes are generated at the ends only, a second spike generated at the bottom on the indicated interval 2τ will collide with the top spike. Right: note, that if the first top spike has collided, a second top spike propagates freely. Bottom: When spikes are generated throughout the axon, a second spike will collide with the indicated one if it falls anywhere in the shaded triangle region. We ignore effects at the start and end of the time, which is allowed as we will take the limit T. When a collision happened, the collided spikes should be removed from the pool and subsequent collision probability changes. For instance, if m = 2, there are two possible ways that just one collision takes place: 1) The first spike does not collide (probability q 1 p ), and the second does (probability p ), and 2) the first spike collides p, and the second does not. However in the second case we have to update the collision probability as there is one spike less to collide with. The probability for the second spike not colliding becomes q 1 = 1 p 1, where p i = 1 (1 2τ/T ) n m i 1 exp[ 2(n m i)τ/t ] is the probability for collision after i spikes have collided already and have thus been removed. The probability for k collisions is p(k m, n) = ( k 1 ) p l l= m k i o,i 1...i k = l i l=m k [ k The term in the round brackets corresponds to the probability of k spikes colliding, while the sum term corresponds to all configurations of other spikes not colliding. The sum is restricted so that the sum of indices l i l = m k, it ensures that there are exactly m k spikes that do not collide. The average l= q i l l ] (7) 18
19 observed rate follows as f obs = (n k )/T. Eq. 7 is a polynomial in τ, that quickly grows in complexity with increases k and m. Despite having an explicit expression for p(k), a systematic expansion for k in the limit of large n, m, T is hard. Using Mathematica we numerically expanded the mean k wrt to τ/t and find that the terms behave as k = m(1 2mτ + 4m 2 τ ), consistent with the relation found above, Eq.3. Perturbation expansion for spontaneous rate for uniform generation Here we derive Eq.4, the collision rate in the first order in the limit of low rates assuming a uniform generation throughout the axon with a rate ρ m. We assume that this rate is constant along the axon and in time, although the formalism can in principle be extended to varying rates. The mean number of spikes generated during a time T is n = ρ m LT. So that the observed rate will be ρ m L if there are no collisions. The first correction corresponds to two spike colliding so that only one spike remains. Consider a first spike generated somewhere, sometime in the axon, Fig. 6bottom. As soon as the second spike is generated in either shaded triangular region, it will collide with the first spike. Given a spike generated at a time t 1 and location x 1, the probability for a collision with the second spike (x 2, t 2 ) is given by the integral over these shaded regions divided by the total area, LT. This can be formally expressed using integrals with the Heaviside function H() as p(x 1, t 1 ) = 1 LT ˆ L ˆ x1 x 1 dx 2 ˆ T ˆ T dx 2 dt 2 H(x 1 /v + t 1 x 2 /v t 2 )H(x 1 /v t 1 x 2 /v + t 2 ) + dt 2 H( x 1 /v t 1 + x 2 /v + t 2 )H( x 1 /v + t 1 + x 2 /v t 2 ) Averaged over all possible positions of the first spike and in the limit of T, p = 1 LT = 2 τ 3 T ˆ L ˆ T dx 1 dt 1 p(x 1, t 1 ) This is collision probability per pair. As there are n 2 /2 pairs, the collision rate is f coll = n 2 τ/3 = ρ 2 ml 3 T/3v, which leads directly to Eq.4. The same arguments can be followed for the possible collisions with three 19
20 spikes. Obviously, two spikes can collide, while a third one propagates independently (order ρ 2 m). However, the interaction of three spikes (order ρ 3 m) can lead to either one or two collisions, depending on the precise configuration of the initiation points. These effects make higher order expansion complicated and cumbersome. References Cannon, R. C, C O Donnell, and M. F Nolan (21). Stochastic ion channel gating in dendritic neurons: morphology dependence and probabilistic synaptic activation of dendritic spikes. PLoS computational biology 6(8). Carnevale, N. T and M. L Hines (26). The NEURON Book. Cambridge University Press, Cambridge. Chow, C. C and J. A White (1996). Spontaneous action potentials due to channel fluctuations. Biophys. J. 71: Faisal, A. A and S. B Laughlin (27). Stochastic simulations on the reliability of action potential propagation in thin axons. PLoS computational biology 3(5): e79. Faisal, A. A, J. A White, and S. B Laughlin (25). Ion-channel noise places limits on the miniaturization of the brain s wiring. Current biology : CB 15(12): Horikawa, Y (1991). Noise effects on spike propagation in the stochastic Hodgkin-Huxley models. Biological cybernetics 66(1): Lass, Y and M Abeles (1975). Transmission of information by the axon. i. noise and memory in the myelinated nerve fiber of the frog. Biol Cybern 19(2): Mainen, Z. F and T. J Sejnowski (1995). Reliability of Spike timing in neocortical neurons. Science 268: O Donnell, C and M. C. W Rossum (214). Systematic analysis of the contributions of stochastic voltage gated channels to neuronal noise (submitted). 2
21 Tuckwell, H. C and J. B Walsh (1983). Random currents through nerve membranes. Biol. Cybern. 49: van Rossum, M. C. W, B. J O Brien, and R. G Smith (23). The effects of noise on the timing precision of retinal ganglion cells. J. Neurophysiol. 89: Verveen, A and H Derksen (1968). Fluctuation phenomena in nerve membrane. Proceedings of the IEEE 56(6):
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Probabilistic Models in Theoretical Neuroscience
 Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Title. Author(s)Yanagita, T. CitationPhysical Review E, 76(5): Issue Date Doc URL. Rights. Type.
 Title Input-output relation of FitzHugh-Nagumo elements ar Author(s)Yanagita, T. CitationPhysical Review E, 76(5): 5625--5625-3 Issue Date 27- Doc URL http://hdl.handle.net/25/32322 Rights Copyright 27
Title Input-output relation of FitzHugh-Nagumo elements ar Author(s)Yanagita, T. CitationPhysical Review E, 76(5): 5625--5625-3 Issue Date 27- Doc URL http://hdl.handle.net/25/32322 Rights Copyright 27
GENERAL ARTICLE Noisy Neurons
 Noisy Neurons Hodgkin Huxley Model and Stochastic Variants Shruti Paranjape Shruti Paranjape is a fourth year student at IISER Pune, majoring in Physics. This is an article based on what she worked on
Noisy Neurons Hodgkin Huxley Model and Stochastic Variants Shruti Paranjape Shruti Paranjape is a fourth year student at IISER Pune, majoring in Physics. This is an article based on what she worked on
/639 Final Solutions, Part a) Equating the electrochemical potentials of H + and X on outside and inside: = RT ln H in
 580.439/639 Final Solutions, 2014 Question 1 Part a) Equating the electrochemical potentials of H + and X on outside and inside: RT ln H out + zf 0 + RT ln X out = RT ln H in F 60 + RT ln X in 60 mv =
580.439/639 Final Solutions, 2014 Question 1 Part a) Equating the electrochemical potentials of H + and X on outside and inside: RT ln H out + zf 0 + RT ln X out = RT ln H in F 60 + RT ln X in 60 mv =
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Ch. 5. Membrane Potentials and Action Potentials
 Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Frontiers in Computational Neuroscience 1 INTRODUCTION. Jolla CA, USA
 Frontiers in Computational Neuroscience Research Article 19 August 214 Systematic analysis of the contributions of stochastic voltage gated channels to neuronal noise Cian O Donnell 1,2,, Mark C W van
Frontiers in Computational Neuroscience Research Article 19 August 214 Systematic analysis of the contributions of stochastic voltage gated channels to neuronal noise Cian O Donnell 1,2,, Mark C W van
STUDENT PAPER. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters Education Program 736 S. Lombard Oak Park IL, 60304
 STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Lecture 10 : Neuronal Dynamics. Eileen Nugent
 Lecture 10 : Neuronal Dynamics Eileen Nugent Origin of the Cells Resting Membrane Potential: Nernst Equation, Donnan Equilbrium Action Potentials in the Nervous System Equivalent Electrical Circuits and
Lecture 10 : Neuronal Dynamics Eileen Nugent Origin of the Cells Resting Membrane Potential: Nernst Equation, Donnan Equilbrium Action Potentials in the Nervous System Equivalent Electrical Circuits and
Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters
 Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters Christina M. Weaver 1,2,3*, Susan L. Wearne 1,2,3* 1 Laboratory of Biomathematics, Mount Sinai School of Medicine, New York, New
Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters Christina M. Weaver 1,2,3*, Susan L. Wearne 1,2,3* 1 Laboratory of Biomathematics, Mount Sinai School of Medicine, New York, New
Transmission of Nerve Impulses (see Fig , p. 403)
 How a nerve impulse works Transmission of Nerve Impulses (see Fig. 12.13, p. 403) 1. At Rest (Polarization) outside of neuron is positively charged compared to inside (sodium ions outside, chloride and
How a nerve impulse works Transmission of Nerve Impulses (see Fig. 12.13, p. 403) 1. At Rest (Polarization) outside of neuron is positively charged compared to inside (sodium ions outside, chloride and
DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT
 Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Learning and Memory in Neural Networks
 Learning and Memory in Neural Networks Guy Billings, Neuroinformatics Doctoral Training Centre, The School of Informatics, The University of Edinburgh, UK. Neural networks consist of computational units
Learning and Memory in Neural Networks Guy Billings, Neuroinformatics Doctoral Training Centre, The School of Informatics, The University of Edinburgh, UK. Neural networks consist of computational units
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Action Potential Propagation
 Action Potential Propagation 2 Action Potential is a transient alteration of transmembrane voltage (or membrane potential) across an excitable membrane generated by the activity of voltage-gated ion channels.
Action Potential Propagation 2 Action Potential is a transient alteration of transmembrane voltage (or membrane potential) across an excitable membrane generated by the activity of voltage-gated ion channels.
Topics in Neurophysics
 Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies
 Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies Introduction Since work on epiretinal electrical stimulation
Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies Introduction Since work on epiretinal electrical stimulation
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
Channel Noise in Excitable Neuronal Membranes
 Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo
 An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo Hamish Meffin Anthony N. Burkitt David B. Grayden The Bionic Ear Institute, 384-388 Albert
An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo Hamish Meffin Anthony N. Burkitt David B. Grayden The Bionic Ear Institute, 384-388 Albert
Evolution of the Average Synaptic Update Rule
 Supporting Text Evolution of the Average Synaptic Update Rule In this appendix we evaluate the derivative of Eq. 9 in the main text, i.e., we need to calculate log P (yk Y k, X k ) γ log P (yk Y k ). ()
Supporting Text Evolution of the Average Synaptic Update Rule In this appendix we evaluate the derivative of Eq. 9 in the main text, i.e., we need to calculate log P (yk Y k, X k ) γ log P (yk Y k ). ()
An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding
 NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
Resting Distribution of Ions in Mammalian Neurons. Outside Inside (mm) E ion Permab. K Na Cl
 Resting Distribution of Ions in Mammalian Neurons Outside Inside (mm) E ion Permab. K + 5 100-81 1.0 150 15 +62 0.04 Cl - 100 10-62 0.045 V m = -60 mv V m approaches the Equilibrium Potential of the most
Resting Distribution of Ions in Mammalian Neurons Outside Inside (mm) E ion Permab. K + 5 100-81 1.0 150 15 +62 0.04 Cl - 100 10-62 0.045 V m = -60 mv V m approaches the Equilibrium Potential of the most
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model
 All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Nervous System: Part II How A Neuron Works
 Nervous System: Part II How A Neuron Works Essential Knowledge Statement 3.E.2 Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and
Nervous System: Part II How A Neuron Works Essential Knowledge Statement 3.E.2 Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
A FINITE STATE AUTOMATON MODEL FOR MULTI-NEURON SIMULATIONS
 A FINITE STATE AUTOMATON MODEL FOR MULTI-NEURON SIMULATIONS Maria Schilstra, Alistair Rust, Rod Adams and Hamid Bolouri Science and Technology Research Centre, University of Hertfordshire, UK Department
A FINITE STATE AUTOMATON MODEL FOR MULTI-NEURON SIMULATIONS Maria Schilstra, Alistair Rust, Rod Adams and Hamid Bolouri Science and Technology Research Centre, University of Hertfordshire, UK Department
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Neurophysiology of a VLSI spiking neural network: LANN21
 Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
2401 : Anatomy/Physiology
 Dr. Chris Doumen Week 6 2401 : Anatomy/Physiology Action Potentials NeuroPhysiology TextBook Readings Pages 400 through 408 Make use of the figures in your textbook ; a picture is worth a thousand words!
Dr. Chris Doumen Week 6 2401 : Anatomy/Physiology Action Potentials NeuroPhysiology TextBook Readings Pages 400 through 408 Make use of the figures in your textbook ; a picture is worth a thousand words!
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 5 Μοντέλο Hodgkin-Huxley (Hodgkin-Huxley Model) Response to Current Injection 2 Hodgin & Huxley Sir Alan Lloyd
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 5 Μοντέλο Hodgkin-Huxley (Hodgkin-Huxley Model) Response to Current Injection 2 Hodgin & Huxley Sir Alan Lloyd
Chapter 9: The Perceptron
 Chapter 9: The Perceptron 9.1 INTRODUCTION At this point in the book, we have completed all of the exercises that we are going to do with the James program. These exercises have shown that distributed
Chapter 9: The Perceptron 9.1 INTRODUCTION At this point in the book, we have completed all of the exercises that we are going to do with the James program. These exercises have shown that distributed
Neuron. Detector Model. Understanding Neural Components in Detector Model. Detector vs. Computer. Detector. Neuron. output. axon
 Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Compartmental Modelling
 Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Neural Modeling and Computational Neuroscience. Claudio Gallicchio
 Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
In#uence of dendritic topologyon "ring patterns in model neurons
 Neurocomputing 38}40 (2001) 183}189 In#uence of dendritic topologyon "ring patterns in model neurons Jacob Duijnhouwer, Michiel W.H. Remme, Arjen van Ooyen*, Jaap van Pelt Netherlands Institute for Brain
Neurocomputing 38}40 (2001) 183}189 In#uence of dendritic topologyon "ring patterns in model neurons Jacob Duijnhouwer, Michiel W.H. Remme, Arjen van Ooyen*, Jaap van Pelt Netherlands Institute for Brain
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
General Appendix A Transmission Line Resonance due to Reflections (1-D Cavity Resonances)
 A 1 General Appendix A Transmission Line Resonance due to Reflections (1-D Cavity Resonances) 1. Waves Propagating on a Transmission Line General A transmission line is a 1-dimensional medium which can
A 1 General Appendix A Transmission Line Resonance due to Reflections (1-D Cavity Resonances) 1. Waves Propagating on a Transmission Line General A transmission line is a 1-dimensional medium which can
A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy
 A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy Neocortical Pyramidal Cells Can Send Signals to Post-Synaptic Cells Without Firing Erin
A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy Neocortical Pyramidal Cells Can Send Signals to Post-Synaptic Cells Without Firing Erin
Subthreshold Voltage Noise Due to Channel Fluctuations in Active Neuronal Membranes
 Journal of Computational Neuroscience 9, 133 148, 2000 c 2000 Kluwer Academic Publishers. Manufactured in The Netherlands. Subthreshold Voltage Noise Due to Channel Fluctuations in Active Neuronal Membranes
Journal of Computational Neuroscience 9, 133 148, 2000 c 2000 Kluwer Academic Publishers. Manufactured in The Netherlands. Subthreshold Voltage Noise Due to Channel Fluctuations in Active Neuronal Membranes
BIOL Week 5. Nervous System II. The Membrane Potential. Question : Is the Equilibrium Potential a set number or can it change?
 Collin County Community College BIOL 2401 Week 5 Nervous System II 1 The Membrane Potential Question : Is the Equilibrium Potential a set number or can it change? Let s look at the Nernst Equation again.
Collin County Community College BIOL 2401 Week 5 Nervous System II 1 The Membrane Potential Question : Is the Equilibrium Potential a set number or can it change? Let s look at the Nernst Equation again.
Single-Compartment Neural Models
 Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators
 Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators Ranjeetha Bharath and Jean-Jacques Slotine Massachusetts Institute of Technology ABSTRACT This work explores
Nonlinear Observer Design and Synchronization Analysis for Classical Models of Neural Oscillators Ranjeetha Bharath and Jean-Jacques Slotine Massachusetts Institute of Technology ABSTRACT This work explores
Introduction to Neural Networks. Daniel Kersten. Lecture 2. Getting started with Mathematica. Review this section in Lecture 1
 Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
The Nervous System and the Sodium-Potassium Pump
 The Nervous System and the Sodium-Potassium Pump 1. Define the following terms: Ion: A Student Activity on Membrane Potentials Cation: Anion: Concentration gradient: Simple diffusion: Sodium-Potassium
The Nervous System and the Sodium-Potassium Pump 1. Define the following terms: Ion: A Student Activity on Membrane Potentials Cation: Anion: Concentration gradient: Simple diffusion: Sodium-Potassium
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Naseem Demeri. Mohammad Alfarra. Mohammad Khatatbeh
 7 Naseem Demeri Mohammad Alfarra Mohammad Khatatbeh In the previous lectures, we have talked about how the difference in permeability for ions across the cell membrane can generate a potential. The potential
7 Naseem Demeri Mohammad Alfarra Mohammad Khatatbeh In the previous lectures, we have talked about how the difference in permeability for ions across the cell membrane can generate a potential. The potential
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Modelling stochastic neural learning
 Modelling stochastic neural learning Computational Neuroscience András Telcs telcs.andras@wigner.mta.hu www.cs.bme.hu/~telcs http://pattern.wigner.mta.hu/participants/andras-telcs Compiled from lectures
Modelling stochastic neural learning Computational Neuroscience András Telcs telcs.andras@wigner.mta.hu www.cs.bme.hu/~telcs http://pattern.wigner.mta.hu/participants/andras-telcs Compiled from lectures
A Three-dimensional Physiologically Realistic Model of the Retina
 A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Information processing. Divisions of nervous system. Neuron structure and function Synapse. Neurons, synapses, and signaling 11/3/2017
 Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Information Theory. Mark van Rossum. January 24, School of Informatics, University of Edinburgh 1 / 35
 1 / 35 Information Theory Mark van Rossum School of Informatics, University of Edinburgh January 24, 2018 0 Version: January 24, 2018 Why information theory 2 / 35 Understanding the neural code. Encoding
1 / 35 Information Theory Mark van Rossum School of Informatics, University of Edinburgh January 24, 2018 0 Version: January 24, 2018 Why information theory 2 / 35 Understanding the neural code. Encoding
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Topological target patterns and population oscillations in a network with random gap junctional coupling
 0 0 0 0 Neurocomputing }0 (00) } Topological target patterns and population oscillations in a network with random gap junctional coupling Timothy J. Lewis*, John Rinzel Center for Neural Science and Courant
0 0 0 0 Neurocomputing }0 (00) } Topological target patterns and population oscillations in a network with random gap junctional coupling Timothy J. Lewis*, John Rinzel Center for Neural Science and Courant
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
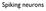 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Channels can be activated by ligand-binding (chemical), voltage change, or mechanical changes such as stretch.
 1. Describe the basic structure of an ion channel. Name 3 ways a channel can be "activated," and describe what occurs upon activation. What are some ways a channel can decide what is allowed to pass through?
1. Describe the basic structure of an ion channel. Name 3 ways a channel can be "activated," and describe what occurs upon activation. What are some ways a channel can decide what is allowed to pass through?
Tactics Box 23.1 Using Kirchhoff's Loop Law
 PH203 Chapter 23 solutions Tactics Box 231 Using Kirchhoff's Loop Law Description: Knight/Jones/Field Tactics Box 231 Using Kirchhoff s loop law is illustrated Learning Goal: To practice Tactics Box 231
PH203 Chapter 23 solutions Tactics Box 231 Using Kirchhoff's Loop Law Description: Knight/Jones/Field Tactics Box 231 Using Kirchhoff s loop law is illustrated Learning Goal: To practice Tactics Box 231
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Problem Set Number 01, MIT (Winter-Spring 2018)
 Problem Set Number 01, 18.377 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Thursday, March 8, 2018. Turn it in (by 3PM) at the Math.
Problem Set Number 01, 18.377 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Thursday, March 8, 2018. Turn it in (by 3PM) at the Math.
Application of density estimation methods to quantal analysis
 Application of density estimation methods to quantal analysis Koichi Yoshioka Tokyo Medical and Dental University Summary There has been controversy for the quantal nature of neurotransmission of mammalian
Application of density estimation methods to quantal analysis Koichi Yoshioka Tokyo Medical and Dental University Summary There has been controversy for the quantal nature of neurotransmission of mammalian
Balance of Electric and Diffusion Forces
 Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing:
 Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
2. SPECTRAL ANALYSIS APPLIED TO STOCHASTIC PROCESSES
 2. SPECTRAL ANALYSIS APPLIED TO STOCHASTIC PROCESSES 2.0 THEOREM OF WIENER- KHINTCHINE An important technique in the study of deterministic signals consists in using harmonic functions to gain the spectral
2. SPECTRAL ANALYSIS APPLIED TO STOCHASTIC PROCESSES 2.0 THEOREM OF WIENER- KHINTCHINE An important technique in the study of deterministic signals consists in using harmonic functions to gain the spectral
Synaptic Input. Linear Model of Synaptic Transmission. Professor David Heeger. September 5, 2000
 Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Nerve Excitation. L. David Roper This is web page
 Nerve Excitation L. David Roper http://arts.bev.net/roperldavid This is web page http://www.roperld.com/science/nerveexcitation.pdf Chapter 1. The Action Potential A typical action potential (inside relative
Nerve Excitation L. David Roper http://arts.bev.net/roperldavid This is web page http://www.roperld.com/science/nerveexcitation.pdf Chapter 1. The Action Potential A typical action potential (inside relative
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
The Axonal Plexus. A description of the behavior of a network of neurons connected by gap junctions. Erin Munro, 4/4/2007
 The Axonal Plexus A description of the behavior of a network of neurons connected by gap junctions Erin Munro, 4/4/2007 Why are we interested in studying the axonal plexus? Gap junctions are indicated
The Axonal Plexus A description of the behavior of a network of neurons connected by gap junctions Erin Munro, 4/4/2007 Why are we interested in studying the axonal plexus? Gap junctions are indicated
Neural variability and Poisson statistics
 Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Noise-assisted spike propagation in myelinated neurons
 PHYSICAL REVIEW E 7, Noise-assisted spike propagation in myelinated neurons Anna Ochab-Marcinek,, Gerhard Schmid, Igor Goychuk, and Peter Hänggi Institut für Physik, Universität Augsburg, Universitätsstra
PHYSICAL REVIEW E 7, Noise-assisted spike propagation in myelinated neurons Anna Ochab-Marcinek,, Gerhard Schmid, Igor Goychuk, and Peter Hänggi Institut für Physik, Universität Augsburg, Universitätsstra
The homogeneous Poisson process
 The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Controllingthe spikingactivity in excitable membranes via poisoning
 Physica A 344 (2004) 665 670 www.elsevier.com/locate/physa Controllingthe spikingactivity in excitable membranes via poisoning Gerhard Schmid, Igor Goychuk, Peter Hanggi Universitat Augsburg, Institut
Physica A 344 (2004) 665 670 www.elsevier.com/locate/physa Controllingthe spikingactivity in excitable membranes via poisoning Gerhard Schmid, Igor Goychuk, Peter Hanggi Universitat Augsburg, Institut
A Universal Model for Spike-Frequency Adaptation
 A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
1 Balanced networks: Trading speed for noise
 Physics 178/278 - David leinfeld - Winter 2017 (Corrected yet incomplete notes) 1 Balanced networks: Trading speed for noise 1.1 Scaling of neuronal inputs An interesting observation is that the subthresold
Physics 178/278 - David leinfeld - Winter 2017 (Corrected yet incomplete notes) 1 Balanced networks: Trading speed for noise 1.1 Scaling of neuronal inputs An interesting observation is that the subthresold
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
