An Experiment to Determine Whether Electromagnetic Waves have Mass by Mark A. Newstead and Stephen C. Newstead
|
|
|
- Jessie Hodges
- 5 years ago
- Views:
Transcription
1 An Experiment to Determine Whether Electromagnetic Waves have Mass by Mark A. Newstead and Stephen C. Newstead Submitted: 6 June 2011 In previous papers we have proposed that the mass of an electromagnetic wave is dependent upon its speed. This relationship is such that when the wave is travelling at the speed of light it has no mass, but its mass monotonically increases as the wave slows down (i.e. the wave has mass when it is passing through a medium). In this paper we propose an experiment that would be able to determine whether electromagnetic waves have mass when they are not travelling at the speed of light. Also the experiment would be able to determine whether the wave s frequency affects the wave s mass for a given speed. 1 Introduction We have previously proposed that not only do electromagnetic waves have mass, but also that their mass changes inversely proportional to their velocity [9, 10]. This relationship between mass (m) and velocity (v) is given by m = hf 1 v2 c 2 c, (1) 2 where h is Planck s constant, c is the speed of light (in a vacuum) and f is the frequency of the wave. Thus the mass of an electromagnetic wave is zero when it is travelling at the speed of light, but monotonically increases as the wave slows down, until it stops (i.e. impacts something) when the maximum mass is obtained. Although many experiments have already been done to determine what the mass of an electromagnetic wave is, they have all considered the wave as a particle that may have mass [11]. Thus, if the wave did have mass then it 1
2 would have a rest mass, which would then increase with velocity, in the same way the mass of the particle increases [2, 4]. Some of these experiments (observations) have been extra-terrestrial, i.e. looking for the effects a wave with mass would have on objects or the dispersion of starlight [11]. However from our definition of the mass of an electromagnetic wave, it would be zero since the waves were travelling in a vacuum, which correlates with the results. The terrestrial experiments are measuring waves within air and/or using radio waves [11]. From our description these waves would only produce extremely minute amounts of mass, due to their lower frequency and/or their speed of propagation, which again correlates with the experimental results. Hence in this paper we propose a different experiment, which uses high frequency waves travelling at very slow speeds. Thus according to our description these should produce a detectable amount of mass, assuming sufficient number of waves are used simultaneously. 2 The Experiment We have stated that mass and velocity are inversely proportional to each other, as described by equation (1). Therefore in order to best detect whether electromagnetic waves have mass, we should slow the waves down as much as possible, as this generates more mass. In fact over the last couple of decades scientists have been able to purposely slow electromagnetic waves to several miles an hour in various experiments [7, 5]. For example the Rowland Institute for Science slowed these waves down to 38 mph in Even though scientists can significantly slow down the wave speed, the mass of a bunch of electromagnetic waves is still minute. Therefore we propose that in order to measure this minute mass, a very sensitive balance scale is used, as shown in Figure 1. Moreover, since the mass of the wave is proportional to frequency then gamma rays should be used. These will then cause the greatest unbalancing of the scales for a given wave speed. 2
3 Figure 1: A schematic diagram for an experiment that would be able to test whether electromagnetic waves have mass as we have proposed, or not. Moreover, it should be able to determine whether the mass of an electromagnetic wave is proportional to its frequency and inversely proportional to its velocity. Figure 1 shows a schematic diagram of an experiment that should be able to test whether electromagnetic waves have mass. On the left hand side of the balance are two closed tubes (represented by the white and grey circles) that the gamma rays will pass through. The ball on the right hand side is the counterbalance weight, which is sufficiently heavy to balance the scales when no gamma rays (or any other electromagnetic waves) are passing through either of the left hand tubes. The reason for having two tubes on the left hand side is that they contain different mediums. The top white tube contains a vacuum, and thus when the waves pass through it, the balance scale should remain level. We note that the balance will only remain level if a very thin material, which has a minimal affect on the speed of electromagnetic waves, is used on the two ends of the tube. Otherwise the mass generated by the gamma rays as they pass through the ends of the tub will be sufficient to unbalance the scales. The bottom grey circle (tube) on the left hand side contains the medium that 3
4 significantly slows electromagnetic waves down. Hence by only changing which tube the electromagnetic waves pass through, determines whether the balance is balanced (i.e. in the case of the vacuum tube) or not (i.e. in the case of the grey tube). Also some of the waves maybe absorbed by the medium in the grey tube, as they pass it producing heat which may affect the results. Therefore we propose that as much as possible of the tube is covered in a reflective surface, such that the minimum amount of energy is lost externally, along the tube. This reflective surface may require multiple layers to retain the maximum amount of energy. These reflective surface(s) are represented by the black circle, which surrounds the grey one. Additionally, as this is such a fine experiment, we have added a laser to the top of the balance beam. Therefore any movement in the beam is amplified by the movement of the laser dot on the screen (shown on the far right hand side). Moreover, the further the screen is from the balance the greater the amplification of any movement, allowing the minute mass generated by the electromagnetic waves to be detected. Furthermore we note that gamma rays can also destroy atomic nuclei and may therefore cause the apparatus to lose mass, since the nuclei will be heavier than the resulting electromagnetic waves [3, 1, 6, 8]. However as the mass of the electromagnetic waves should instantly become non-zero as they enter the medium slowing the wave down, then the experiment should be able to detect the change in weight almost immediately. Thus the experiment should not need to run for a long time, which has the side effect of limiting the number of atoms destroyed by the gamma rays. Overall though, if the experiment is set up carefully enough, then it should be able to detect the mass of an electromagnetic waves, as well as having the possibility of measuring it. 3 Conclusions In this paper we have proposed a new experiment to detect whether electromagnetic waves have mass or not. This new experiment is based upon our previous 4
5 proposals that the mass of an electromagnetic wave is proportional to its frequency and inversely proportional to its velocity, such that at the speed of light the wave has no mass [9, 10]. Moreover, all current experiments correlate with our proposal, since they have returned results implying that the mass of an electromagnetic wave is zero [11]. However these experiments have tried to measure the mass of a wave that is travelling at or very close to the speed of light. This new experiment though would be able to detect the minute mass of a high frequency, slow travelling wave. It should also be able to determine whether the wave s mass is proportional to its frequency and inversely proportional to its velocity, as we have proposed. References [1] F. Close. Antimatter. OUP Oxford, January [2] A. Einstein and R. W. Lawson. Relativity: The Special and General Theory. Dover Publications, March [3] G. Fraser. Antimatter: The Ultimate Mirror. Cambridge University Press, 1st edition, July [4] M. P. Hobson, G. Efetathiou, and A. N. Lasenby. General relativity: an introduction for physicists. Cambridge University Press, Feb [5] P.-C. Ku, F. Sedgwick, C. Chang-Hasnain, P. Palinginis, T. Li, H. Wang, S.-W. Chang, and S.-L. Chuang. Optics Letters, (19). [6] E. P. Liang, S. C. Wilks, and M. Tabak. Pair production by ultraintense lasers. Physical Review Letters, 81(22): , [7] C. Liu, Z. Dutton, C. H. Behroozi, and L. V. Hau. Observation of coherent optical information storage in an atomic medium using halted light pulses. Letter to Nature, 409: , [8] M. A. Newstead and S. C. Newstead. The Atom Uncovered. Baldr Limited, [9] M. A. Newstead and S. C. Newstead. Gravity and Electromagnetic Waves. Baldr Limited, [10] M. A. Newstead and S. C. Newstead. Mass of an electromagnetic wave. vixra, [11] L.-C. Tu, J. Luo, and G. T. Gilles. The mass of the photon. Reports on Progress in Physics, 68:77 130, Acknowledgements We would like to thank E. Waltham for his editing assistance and general comments. 5
Mass of an Electromagnetic Wave by Mark A. Newstead and Stephen C. Newstead
 Mass of an Electromagnetic Wave by Mark A. Newstead and Stephen C. Newstead email: mark.newstead@alumni.lboro.ac.uk Submitted: 27 May 2011 In this paper we investigate whether an electromagnetic wave can
Mass of an Electromagnetic Wave by Mark A. Newstead and Stephen C. Newstead email: mark.newstead@alumni.lboro.ac.uk Submitted: 27 May 2011 In this paper we investigate whether an electromagnetic wave can
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5. Past and Proposed Experiments Detecting Absolute Motion
 Chapter 5 Past and Proposed Experiments Detecting Absolute Motion In this Chapter I gave different interpretations for the results of some famous past experiments. My interpretations are based on the following
Chapter 5 Past and Proposed Experiments Detecting Absolute Motion In this Chapter I gave different interpretations for the results of some famous past experiments. My interpretations are based on the following
Stopped Light With Storage Times Greater than 1 second using Electromagnetically Induced Transparency in a Solid
 Stopped Light With Storage Times Greater than 1 second using Electromagnetically Induced Transparency in a Solid J.J Londell, E. Fravel, M.J. Sellars and N.B. Manson, Phys. Rev. Lett. 95 063601 (2005)
Stopped Light With Storage Times Greater than 1 second using Electromagnetically Induced Transparency in a Solid J.J Londell, E. Fravel, M.J. Sellars and N.B. Manson, Phys. Rev. Lett. 95 063601 (2005)
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
Quantum Model Einstein s Hypothesis: Photoelectric Effect
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT Quantum Model Einstein s Hypothesis: Photoelectric Effect The photoelectric effect was discovered by Hertz in 1887 as he confirmed Maxwell s electromagnetic
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT Quantum Model Einstein s Hypothesis: Photoelectric Effect The photoelectric effect was discovered by Hertz in 1887 as he confirmed Maxwell s electromagnetic
The Sine Wave. You commonly see waves in the environment. Light Sound Electricity Ocean waves
 The Sine Wave Mathematically, a function that represents a smooth oscillation For example, if we drew the motion of how the weight bobs on the spring to the weight we would draw out a sine wave. The Sine
The Sine Wave Mathematically, a function that represents a smooth oscillation For example, if we drew the motion of how the weight bobs on the spring to the weight we would draw out a sine wave. The Sine
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 6. Quantum Theory and the Electronic Structure of Atoms Part 1
 Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
6. Atomic and Nuclear Physics
 6. Atomic and Nuclear Physics Chapter 6.2 Radioactivity From IB OCC, prepared by J. Domingues based on Tsokos Physics book Warm Up Define: nucleon atomic number mass number isotope. Radioactivity In 1896,
6. Atomic and Nuclear Physics Chapter 6.2 Radioactivity From IB OCC, prepared by J. Domingues based on Tsokos Physics book Warm Up Define: nucleon atomic number mass number isotope. Radioactivity In 1896,
Being a Physicist Unit 5. Summary Sheets. Gleniffer High School
 Being a Physicist Unit 5 Summary Sheets Gleniffer High School 0 Experiences & Outcomes I can explain how sound vibrations are carried by waves through air, water and other materials SCN 2-11a By exploring
Being a Physicist Unit 5 Summary Sheets Gleniffer High School 0 Experiences & Outcomes I can explain how sound vibrations are carried by waves through air, water and other materials SCN 2-11a By exploring
De Broglie Wavelength and McMahon field theory Abstract Theory: McMahon, C.R. (2015)
 Copyright Version: 2 nd February, 2015, updated 24 th September, 2018 Page: 1 of 11 De Broglie Wavelength and McMahon field theory Abstract: Here, I use the concepts of the McMahon field theory to explain
Copyright Version: 2 nd February, 2015, updated 24 th September, 2018 Page: 1 of 11 De Broglie Wavelength and McMahon field theory Abstract: Here, I use the concepts of the McMahon field theory to explain
GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES
 Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. FRIDAY, 25 May 2012 1½ hours ADDITIONAL MATERIALS In addition to this paper,
Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. FRIDAY, 25 May 2012 1½ hours ADDITIONAL MATERIALS In addition to this paper,
Electromagnetic Radiation
 Electromagnetic Radiation aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves). Sometimes it behaves like billiard balls (particles).
Electromagnetic Radiation aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves). Sometimes it behaves like billiard balls (particles).
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!)
 NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
SPH4U UNIVERSITY PHYSICS
 SPH4U UNIVERSITY PHYSICS REVOLUTIONS IN MODERN PHYSICS:... L Photons & Momentum (P.624-626) By 1916, Robert Millikan had established that the magnitude of the charge on an electron was 1.60 x 10-19 C.
SPH4U UNIVERSITY PHYSICS REVOLUTIONS IN MODERN PHYSICS:... L Photons & Momentum (P.624-626) By 1916, Robert Millikan had established that the magnitude of the charge on an electron was 1.60 x 10-19 C.
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space.
 Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Did something ν just break the speed limit?
 Did something ν just break the speed limit? Amol Dighe Department of Theoretical Physics Tata Institute of Fundamental Research Chai-and-Why, Prithvi Theatre, Nov 6, 2011 Did something ν break the speed
Did something ν just break the speed limit? Amol Dighe Department of Theoretical Physics Tata Institute of Fundamental Research Chai-and-Why, Prithvi Theatre, Nov 6, 2011 Did something ν break the speed
Wavelength (λ)- Frequency (ν)- Which of the following has a higher frequency?
 Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
5.3. Physics and the Quantum Mechanical Model
 Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
History of the Atomic Model
 Chapter 5 Lecture Chapter 5 Electronic Structure and Periodic Trends 5.1 Electromagnetic Radiation Learning Goal Compare the wavelength, frequency, and energy of electromagnetic radiation. Fifth Edition
Chapter 5 Lecture Chapter 5 Electronic Structure and Periodic Trends 5.1 Electromagnetic Radiation Learning Goal Compare the wavelength, frequency, and energy of electromagnetic radiation. Fifth Edition
Time In Gravitational Fields & Gravitational Red Shift By Michael Spears.
 Time In Gravitational Fields & Gravitational Red Shift By Michael Spears. serious_spears@yahoo.com.au Abstract: In the course of this paper I will seek to give a new explanation for two phenomena predicted
Time In Gravitational Fields & Gravitational Red Shift By Michael Spears. serious_spears@yahoo.com.au Abstract: In the course of this paper I will seek to give a new explanation for two phenomena predicted
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
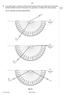 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
CHEMISTRY Matter and Change
 CHEMISTRY Matter and Change Chapter 5: Electrons in Atoms 5 Section 5.1 Section Section 5.3 Table Of Contents Light and Quantized Energy Electron Configuration Compare the wave and particle natures of
CHEMISTRY Matter and Change Chapter 5: Electrons in Atoms 5 Section 5.1 Section Section 5.3 Table Of Contents Light and Quantized Energy Electron Configuration Compare the wave and particle natures of
Chapter 10: Wave Properties of Particles
 Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
12.2 Photons and the Quantum Theory of Light
 12.2 Photons and the Quantum Theory of Light Lasers are used everywhere, from concert light shows to grocery store checkout lines to cutting-edge research labs (Figure 1). Although classical physics says
12.2 Photons and the Quantum Theory of Light Lasers are used everywhere, from concert light shows to grocery store checkout lines to cutting-edge research labs (Figure 1). Although classical physics says
TITLE: AN EXPERIMENT AGAINST RELATIVITY INTERACTIVE DEPENDENCY
 Page:1 TITLE: AN EXPERIMENT AGAINST RELATIVITY INTERACTIVE DEPENDENCY (A NEW STORY OF THE PRESENT PHYSICS - THE FAILURE OF RELATIVITY LEADS THE PRESENT PHYSICS FROM TIME DILATION TO NO TIME.) AUTHER: BANDARU
Page:1 TITLE: AN EXPERIMENT AGAINST RELATIVITY INTERACTIVE DEPENDENCY (A NEW STORY OF THE PRESENT PHYSICS - THE FAILURE OF RELATIVITY LEADS THE PRESENT PHYSICS FROM TIME DILATION TO NO TIME.) AUTHER: BANDARU
MODERN OPTICS. P47 Optics: Unit 9
 MODERN OPTICS P47 Optics: Unit 9 Course Outline Unit 1: Electromagnetic Waves Unit 2: Interaction with Matter Unit 3: Geometric Optics Unit 4: Superposition of Waves Unit 5: Polarization Unit 6: Interference
MODERN OPTICS P47 Optics: Unit 9 Course Outline Unit 1: Electromagnetic Waves Unit 2: Interaction with Matter Unit 3: Geometric Optics Unit 4: Superposition of Waves Unit 5: Polarization Unit 6: Interference
FIBER OPTICS. Prof. R.K. Shevgaonkar. Department of Electrical Engineering. Indian Institute of Technology, Bombay. Lecture: 15. Optical Sources-LASER
 FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 15 Optical Sources-LASER Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical
FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 15 Optical Sources-LASER Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical
Mr Casey Ray McMahon, B.Sci (Hons), B.MechEng (Hons) Copyright Version: 17 th May, 2015 Page: 1 of 8 String theory explained via McMahon field theory.
 Copyright Version: 17 th May, 2015 Page: 1 of 8 String theory explained via McMahon field theory. Abstract: String theory can easily be explained in a way that can be understood with McMahon field theory
Copyright Version: 17 th May, 2015 Page: 1 of 8 String theory explained via McMahon field theory. Abstract: String theory can easily be explained in a way that can be understood with McMahon field theory
Outline Chapter 9 The Atom Photons Photons The Photoelectron Effect Photons Photons
 Outline Chapter 9 The Atom 9-1. Photoelectric Effect 9-3. What Is Light? 9-4. X-rays 9-5. De Broglie Waves 9-6. Waves of What? 9-7. Uncertainty Principle 9-8. Atomic Spectra 9-9. The Bohr Model 9-10. Electron
Outline Chapter 9 The Atom 9-1. Photoelectric Effect 9-3. What Is Light? 9-4. X-rays 9-5. De Broglie Waves 9-6. Waves of What? 9-7. Uncertainty Principle 9-8. Atomic Spectra 9-9. The Bohr Model 9-10. Electron
I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited
 NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
WAVE NATURE OF LIGHT
 WAVE NATURE OF LIGHT Light is electromagnetic radiation, a type of energy composed of oscillating electric and magnetic fields. The fields oscillate perpendicular to each other. In vacuum, these waves
WAVE NATURE OF LIGHT Light is electromagnetic radiation, a type of energy composed of oscillating electric and magnetic fields. The fields oscillate perpendicular to each other. In vacuum, these waves
Realization of Bose-Einstein Condensation in dilute gases
 Realization of Bose-Einstein Condensation in dilute gases Guang Bian May 3, 8 Abstract: This essay describes theoretical aspects of Bose-Einstein Condensation and the first experimental realization of
Realization of Bose-Einstein Condensation in dilute gases Guang Bian May 3, 8 Abstract: This essay describes theoretical aspects of Bose-Einstein Condensation and the first experimental realization of
Bannerman High School Physics Department. Making Accurate Statements. Higher Physics. Quanta and Waves
 Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
Physics 107: Ideas of Modern Physics
 Physics 107: Ideas of Modern Physics Exam 3 Nov. 30, 2005 Name ID # Section # On the Scantron sheet, 1) Fill in your name 2) Fill in your student ID # (not your social security #) 3) Fill in your section
Physics 107: Ideas of Modern Physics Exam 3 Nov. 30, 2005 Name ID # Section # On the Scantron sheet, 1) Fill in your name 2) Fill in your student ID # (not your social security #) 3) Fill in your section
Being a Chemist. Summary Sheets. Gleniffer High School
 Being a Chemist Summary Sheets Gleniffer High School 0 State that the light year is a measure of astronomical distance State the speed at which light travels Give examples of the relative distance between
Being a Chemist Summary Sheets Gleniffer High School 0 State that the light year is a measure of astronomical distance State the speed at which light travels Give examples of the relative distance between
GOZO COLLEGE. Half Yearly Examinations for Secondary Schools FORM 4 PHYSICS TIME: 1h 30min
 GOZO COLLEGE Track 3 Half Yearly Examinations for Secondary Schools 2011 FORM 4 PHYSICS TIME: 1h 30min Name: Class: Answer all questions. All working must be shown. The use of a calculator is allowed.
GOZO COLLEGE Track 3 Half Yearly Examinations for Secondary Schools 2011 FORM 4 PHYSICS TIME: 1h 30min Name: Class: Answer all questions. All working must be shown. The use of a calculator is allowed.
Electromagnetic spectrum Electromagnetic radiation
 Chapter 4 Section 1 Electromagnetic spectrum includes all the different wave lengths of radiation. Electromagnetic radiation is a form of energy that exhibits wave like behavior as it travels through space.
Chapter 4 Section 1 Electromagnetic spectrum includes all the different wave lengths of radiation. Electromagnetic radiation is a form of energy that exhibits wave like behavior as it travels through space.
A Level. A Level Physics. Quantum Physics (Answers) AQA, Edexcel. Name: Total Marks: /30
 Visit http://www.mathsmadeeasy.co.uk/ for more fantastic resources. AQA, Edexcel A Level A Level Physics Quantum Physics (Answers) Name: Total Marks: /30 Maths Made Easy Complete Tuition Ltd 2017 1. Numerous
Visit http://www.mathsmadeeasy.co.uk/ for more fantastic resources. AQA, Edexcel A Level A Level Physics Quantum Physics (Answers) Name: Total Marks: /30 Maths Made Easy Complete Tuition Ltd 2017 1. Numerous
P5 Revision Questions
 P5 Revision Questions Part 2 Question 1 How can microwaves be used to communicate? Answer 1 Sent from transmitter, received and amplified by satellite in space, re-transmitted back to earth and picked
P5 Revision Questions Part 2 Question 1 How can microwaves be used to communicate? Answer 1 Sent from transmitter, received and amplified by satellite in space, re-transmitted back to earth and picked
Extreme Light Infrastructure - Nuclear Physics ELI - NP
 Extreme Light Infrastructure - Nuclear Physics ELI - NP Nicolae-Victor Zamfir National Institute for Physics and Nuclear Engineering (IFIN-HH) Bucharest-Magurele, Romania www.eli-np.ro Bucharest-Magurele
Extreme Light Infrastructure - Nuclear Physics ELI - NP Nicolae-Victor Zamfir National Institute for Physics and Nuclear Engineering (IFIN-HH) Bucharest-Magurele, Romania www.eli-np.ro Bucharest-Magurele
Atomic Physics Worksheet. between 4000 and 5000 Angstroms ( nanometers): between 6500 and 7500 Angstroms ( nanometers):
 Atomic Physics Worksheet 1. Which of the gas samples shows an emission line with a wavelength between 4000 and 5000 Angstroms (400-500 nanometers): between 6500 and 7500 Angstroms (650-750 nanometers):
Atomic Physics Worksheet 1. Which of the gas samples shows an emission line with a wavelength between 4000 and 5000 Angstroms (400-500 nanometers): between 6500 and 7500 Angstroms (650-750 nanometers):
Write the electron configuration for Chromium (Cr):
 Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Rules of electromagnetic induction -Explained using McMahon field theory. Abstract: Theory: McMahon, C.R. (2015)
 Updated 10 th April, 2015 Page: 1 of 11 Rules of electromagnetic induction -Explained using McMahon field theory. Abstract: In this paper, I use the McMahon field theory to explain electromagnetic induction,
Updated 10 th April, 2015 Page: 1 of 11 Rules of electromagnetic induction -Explained using McMahon field theory. Abstract: In this paper, I use the McMahon field theory to explain electromagnetic induction,
CHAPTER 12 TEST REVIEW
 IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
Physics and the Quantum Mechanical Model
 chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
AS COMPETITION PAPER 2011 SOLUTIONS Total Mark/50
 AS COMPETITION PAPER 011 SOLUTIONS Total Mark/50 011 AS Competition Paper Mark Scheme and Notes for Teachers Thank you for entering the 011 British Physics Olympiad AS Competition. Please note the following
AS COMPETITION PAPER 011 SOLUTIONS Total Mark/50 011 AS Competition Paper Mark Scheme and Notes for Teachers Thank you for entering the 011 British Physics Olympiad AS Competition. Please note the following
Electrons in Atoms. Section 5.1 Light and Quantized Energy Section 5.2 Quantum Theory and the Atom Section 5.3 Electron Configuration
 Electrons in Atoms Section 5.1 Light and Quantized Energy Section 5.2 Quantum Theory and the Atom Section 5.3 Electron Configuration Click a hyperlink or folder tab to view the corresponding slides. Exit
Electrons in Atoms Section 5.1 Light and Quantized Energy Section 5.2 Quantum Theory and the Atom Section 5.3 Electron Configuration Click a hyperlink or folder tab to view the corresponding slides. Exit
Selected "Phacts" for the Physics Regents Exam You Should Know
 Selected "Phacts" for the Physics Regents Exam You Should Know I. Mechanics Study Hard! 1. Mass and inertia are the same thing. (Mass actually measures inertia in kilograms Much as monetary resources measures
Selected "Phacts" for the Physics Regents Exam You Should Know I. Mechanics Study Hard! 1. Mass and inertia are the same thing. (Mass actually measures inertia in kilograms Much as monetary resources measures
Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
UNIVERSITY OF TECHNOLOGY Laser & Opto-Electronic Eng. Dept rd YEAR. The Electromagnetic Waves
 Spectroscopy Interaction of electromagnetic radiation with matter yields that energy is absorbed or emitted by matter in discrete quantities (quanta). Measurement of the frequency or (wave length) of the
Spectroscopy Interaction of electromagnetic radiation with matter yields that energy is absorbed or emitted by matter in discrete quantities (quanta). Measurement of the frequency or (wave length) of the
Properties of Light and Atomic Structure. Chapter 7. So Where are the Electrons? Electronic Structure of Atoms. The Wave Nature of Light!
 Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
AS COMPETITION PAPER 2009
 AS COMPETITION PAPER 2009 Name School Town & County Total Mark/50 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
AS COMPETITION PAPER 2009 Name School Town & County Total Mark/50 Time Allowed: One hour Attempt as many questions as you can. Write your answers on this question paper. Marks allocated for each question
Experiment 9. Emission Spectra. measure the emission spectrum of a source of light using the digital spectrometer.
 Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002
 Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
Chapter 27: Light. What is light?
 Chapter 27: Light What is light? Scientists first theorized light was a wave as it behaved with a wave properties, i.e. diffraction and interference. In 1905, Einstein realized that light was behaving
Chapter 27: Light What is light? Scientists first theorized light was a wave as it behaved with a wave properties, i.e. diffraction and interference. In 1905, Einstein realized that light was behaving
Abstract: Here, I use the basic principles of the McMahon field theory to explain the strong force and the weak force, as described for atoms.
 Copyright Version: 2 nd March, 2015, updated 10 th April, 2015 Page: 1 of 8 The Strong and weak forces- explained via McMahon field theory Abstract: Here, I use the basic principles of the McMahon field
Copyright Version: 2 nd March, 2015, updated 10 th April, 2015 Page: 1 of 8 The Strong and weak forces- explained via McMahon field theory Abstract: Here, I use the basic principles of the McMahon field
Atomic Theory. Contribution to Modern Atomic Theory
 Alief High School Chemistry STAAR Review Reporting Category 2: Atomic Structure and Nuclear Chemistry C.6.A Understand the experimental design and conclusions used in the development of modern atomic theory,
Alief High School Chemistry STAAR Review Reporting Category 2: Atomic Structure and Nuclear Chemistry C.6.A Understand the experimental design and conclusions used in the development of modern atomic theory,
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Energy of Waves. What is the relationship between l, n and Energy?!
 Chapter 5 Part 2 c = ln Practice! What is the wavelength of a microwave that has a frequency of 1.56 x 10 9 Hz? The red-colored light in a fireworks display might be produced when Strontium salts are heated.
Chapter 5 Part 2 c = ln Practice! What is the wavelength of a microwave that has a frequency of 1.56 x 10 9 Hz? The red-colored light in a fireworks display might be produced when Strontium salts are heated.
Unit 3 Part 1: Quantum Physics. introduce the idea of quanta as a new way of looking at light and sub atomic physical behaviour
 In this lesson you will Unit 3 Part 1: Quantum Physics consider and list some of the properties of light and sub atomic particles that were at odds with the classical wave theory of electromagnetic radiation
In this lesson you will Unit 3 Part 1: Quantum Physics consider and list some of the properties of light and sub atomic particles that were at odds with the classical wave theory of electromagnetic radiation
progressive electromagnetic wave
 LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
LECTURE 11 Ch17 A progressive electromagnetic wave is a self-supporting, energy-carrying disturbance that travels free of its source. The light from the Sun travels through space (no medium) for only 8.3
Q1 and Q2 Review large CHEMISTRY
 Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
PE summary from last class:
 PH300 Modern Physics SP11 Today: Finish photoelectric effect Photons Next Week: Atomic spectra/balmer series Lasers learned very early the difference between knowing the name of something and knowing something.!
PH300 Modern Physics SP11 Today: Finish photoelectric effect Photons Next Week: Atomic spectra/balmer series Lasers learned very early the difference between knowing the name of something and knowing something.!
Fizeau s Interference Experiment with Moving Water Contradicts the Special Theory of Relativity.
 Fizeau s Interference Experiment with Moving Water Contradicts the Special Theory of Relativity. Gennady Sokolov, Vitali Sokolov gennadiy@vtmedicalstaffing.com The interference experiment with moving water
Fizeau s Interference Experiment with Moving Water Contradicts the Special Theory of Relativity. Gennady Sokolov, Vitali Sokolov gennadiy@vtmedicalstaffing.com The interference experiment with moving water
General Chemistry by Ebbing and Gammon, 8th Edition
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 26-Mar-2009 Chapter 7: Quantum Theory of the Atom These Notes are to SUPPLIMENT the Text, They do NOT Replace
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 26-Mar-2009 Chapter 7: Quantum Theory of the Atom These Notes are to SUPPLIMENT the Text, They do NOT Replace
Electromagnetic Waves
 4/15/12 Chapter 26: Properties of Light Field Induction Ok, so a changing magnetic field causes a current (Faraday s law) Why do we have currents in the first place? electric fields of the charges Changing
4/15/12 Chapter 26: Properties of Light Field Induction Ok, so a changing magnetic field causes a current (Faraday s law) Why do we have currents in the first place? electric fields of the charges Changing
You are about to start an exciting series of lessons on physical science. God s Design for the Physical World
 Table of of Contents Unit 1 - Forms of Energy Lesson 1 Forms of Energy 8 Lesson 2 Mechanical Energy 12 Lesson 3 Chemical Energy 16 Lesson 4 Nuclear Energy 18 Lesson 5 Nuclear Weapons 21 Special Feature
Table of of Contents Unit 1 - Forms of Energy Lesson 1 Forms of Energy 8 Lesson 2 Mechanical Energy 12 Lesson 3 Chemical Energy 16 Lesson 4 Nuclear Energy 18 Lesson 5 Nuclear Weapons 21 Special Feature
ATOMIC PHYSICS. history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS
 ATOMIC PHYSICS http://www.aip.org/ history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS What We Will Study Basics of electromagnetic radiation - The AC generator, again
ATOMIC PHYSICS http://www.aip.org/ history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS What We Will Study Basics of electromagnetic radiation - The AC generator, again
E = mc 2. Inertial Reference Frames. Inertial Reference Frames. The Special Theory of Relativity. Slide 1 / 63. Slide 2 / 63.
 Slide 1 / 63 The Special Theory of Relativity E = mc 2 Inertial Reference Frames Slide 2 / 63 Newton's laws are only valid in inertial reference frames: n inertial reference frame is one which is not accelerating
Slide 1 / 63 The Special Theory of Relativity E = mc 2 Inertial Reference Frames Slide 2 / 63 Newton's laws are only valid in inertial reference frames: n inertial reference frame is one which is not accelerating
Physics 280 Week 04 In-Class Problems Summer 2016
 Physics 80 Week 04 In-Class Problems Summer 016 1. Consider the interface of water with class (say at the bottom of a beaker filled with water). Given: The index of refraction for water is n w = 1.33 and
Physics 80 Week 04 In-Class Problems Summer 016 1. Consider the interface of water with class (say at the bottom of a beaker filled with water). Given: The index of refraction for water is n w = 1.33 and
Energy - the ability to do work or cause change. 1 point
 Energy and Waves Energy - the ability to do work or cause change Work - the transfer of energy Work = Force X Distance Power - the rate at which work is done Power = Work Time Kinetic Energy - the energy
Energy and Waves Energy - the ability to do work or cause change Work - the transfer of energy Work = Force X Distance Power - the rate at which work is done Power = Work Time Kinetic Energy - the energy
Unit 4. Electrons in Atoms
 Unit 4 Electrons in Atoms When were most of the subatomic particles discovered? Who discovered densely packed nucleus surrounded by fast moving electrons? Rutherford s Model Major development Lacked detail
Unit 4 Electrons in Atoms When were most of the subatomic particles discovered? Who discovered densely packed nucleus surrounded by fast moving electrons? Rutherford s Model Major development Lacked detail
Physics 3204 UNIT 3 Test Matter Energy Interface
 Physics 3204 UNIT 3 Test Matter Energy Interface 2005 2006 Time: 60 minutes Total Value: 33 Marks Formulae and Constants v = f λ E = hf h f = E k + W 0 E = m c 2 p = h λ 1 A= A T 0 2 t 1 2 E k = ½ mv 2
Physics 3204 UNIT 3 Test Matter Energy Interface 2005 2006 Time: 60 minutes Total Value: 33 Marks Formulae and Constants v = f λ E = hf h f = E k + W 0 E = m c 2 p = h λ 1 A= A T 0 2 t 1 2 E k = ½ mv 2
2) The number of cycles that pass through a stationary point is called A) wavelength. B) amplitude. C) frequency. D) area. E) median.
 Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
PHYSICAL SCIENCES GRADE 10 P1 JUNE 2016 PRE-TEST MEMO
 PHYSICAL SCIENCES GRADE 10 P1 JUNE 2016 PRE-TEST MEMO QUESTION 1 1.1 B 1.2 D 1.3 A 1.4 B 1.5 D 1.6 A 1.7 B 1.8 B 1.9 B 1.10 D [20] QUESTION 2 2.1.1 It is the maximum displacement of the particles of the
PHYSICAL SCIENCES GRADE 10 P1 JUNE 2016 PRE-TEST MEMO QUESTION 1 1.1 B 1.2 D 1.3 A 1.4 B 1.5 D 1.6 A 1.7 B 1.8 B 1.9 B 1.10 D [20] QUESTION 2 2.1.1 It is the maximum displacement of the particles of the
Electromagnetic Radiation. Physical Principles of Remote Sensing
 Electromagnetic Radiation Physical Principles of Remote Sensing Outline for 4/3/2003 Properties of electromagnetic radiation The electromagnetic spectrum Spectral emissivity Radiant temperature vs. kinematic
Electromagnetic Radiation Physical Principles of Remote Sensing Outline for 4/3/2003 Properties of electromagnetic radiation The electromagnetic spectrum Spectral emissivity Radiant temperature vs. kinematic
Mansoor Sheik-Bahae. Class meeting times: Mondays, Wednesdays 17:30-18:45 am; Physics and Astronomy, Room 184
 Mansoor Sheik-Bahae Office: Physics & Astronomy Rm. 1109 (North Wing) Phone: 277-2080 E-mail: msb@unm.edu To see me in my office, please make an appointment (call or email). Class meeting times: Mondays,
Mansoor Sheik-Bahae Office: Physics & Astronomy Rm. 1109 (North Wing) Phone: 277-2080 E-mail: msb@unm.edu To see me in my office, please make an appointment (call or email). Class meeting times: Mondays,
Electrons in Atoms. Section 5.1 Light and Quantized Energy
 Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
An Analysis of Einstein s Second Postulate to his Special Theory of Relativity
 An Analysis of Einstein s Second Postulate to his Special Theory of Relativity Edward G. Lake* Independent Researcher (Dated: April 11, 2017) detect@outlook.com Abstract: An analysis of books, presentations
An Analysis of Einstein s Second Postulate to his Special Theory of Relativity Edward G. Lake* Independent Researcher (Dated: April 11, 2017) detect@outlook.com Abstract: An analysis of books, presentations
Lesmahagow High School AHChemistry Inorganic and Physical Chemistry Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Electromagnetic Radiation and Atomic Spectra 1 Electromagnetic Radiation Radiation such as light, microwaves,
Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Electromagnetic Radiation and Atomic Spectra 1 Electromagnetic Radiation Radiation such as light, microwaves,
The coldest place in Ireland
 Snapshots of Doctoral Research at University College Cork 2011 Laura Russell Physics Department, UCC Introduction Did you know that the Quantum Optics Laboratory in Tyndall National Institute is the coldest
Snapshots of Doctoral Research at University College Cork 2011 Laura Russell Physics Department, UCC Introduction Did you know that the Quantum Optics Laboratory in Tyndall National Institute is the coldest
Astro-2: History of the Universe
 Astro-2: History of the Universe Lecture 13; May 30 2013 Previously on astro-2 Energy and mass are equivalent through Einstein s equation and can be converted into each other (pair production and annihilations)
Astro-2: History of the Universe Lecture 13; May 30 2013 Previously on astro-2 Energy and mass are equivalent through Einstein s equation and can be converted into each other (pair production and annihilations)
Properties of Light. Arrangement of Electrons in Atoms. The Development of a New Atomic Model. Electromagnetic Radiation CHAPTER 4
 CHAPTER 4 Arrangement of Electrons in Atoms The Development of a New Atomic Model The Rutherford model was a great improvement over the Thomson model of the atom. But, there was one major question that
CHAPTER 4 Arrangement of Electrons in Atoms The Development of a New Atomic Model The Rutherford model was a great improvement over the Thomson model of the atom. But, there was one major question that
Senior Design Proposal
 Senior Design Proposal Kathleen E Gesterling April 10, 2008 1 Introduction The following report describes the analysis for finding the speed of an extensive air shower produced by an ulta-high energy cosmic
Senior Design Proposal Kathleen E Gesterling April 10, 2008 1 Introduction The following report describes the analysis for finding the speed of an extensive air shower produced by an ulta-high energy cosmic
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere.
 Chapter 29 and 30 Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere. Explain how sunspots are related to powerful magnetic fields on the sun.
Chapter 29 and 30 Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere. Explain how sunspots are related to powerful magnetic fields on the sun.
Atomic Structure. Part 3: Wave-Mechanical Model of the Atom. Key Question: How does the wave mechanical model explain the location of electrons?
 Name Chemistry Atomic Structure Essential Question: How was the structure of the atom determined? Vocabulary: bright-line spectrum electron configuration excited state ground state orbital wave-mechanical
Name Chemistry Atomic Structure Essential Question: How was the structure of the atom determined? Vocabulary: bright-line spectrum electron configuration excited state ground state orbital wave-mechanical
What can laser light do for (or to) me?
 What can laser light do for (or to) me? Phys 1020, Day 15: Questions? Refection, refraction LASERS: 14.3 Next Up: Finish lasers Cameras and optics 1 Eyes to web: Final Project Info Light travels more slowly
What can laser light do for (or to) me? Phys 1020, Day 15: Questions? Refection, refraction LASERS: 14.3 Next Up: Finish lasers Cameras and optics 1 Eyes to web: Final Project Info Light travels more slowly
Light at a Standstill Tim Kuis June 13, 2008
 Light at a Standstill Tim Kuis June 13, 008 1. Introduction There is something curious about the seed of light. It is the highest obtainable seed. Nothing can travel faster. But how slow can light go?
Light at a Standstill Tim Kuis June 13, 008 1. Introduction There is something curious about the seed of light. It is the highest obtainable seed. Nothing can travel faster. But how slow can light go?
Modern Physics Part 1: Quantization & Photons
 Modern Physics Part 1: Quantization & Photons Last modified: 15/12/2017 Contents Links Contents Introduction Classical Physics Modern Physics Quantization Definition & Examples Photons Black Body Radiation
Modern Physics Part 1: Quantization & Photons Last modified: 15/12/2017 Contents Links Contents Introduction Classical Physics Modern Physics Quantization Definition & Examples Photons Black Body Radiation
Electromagnetic waves
 Lecture 21 Electromagnetic waves Atomic Physics Atomic Spectra Lasers Applications Electromagnetic Waves Electromagnetic Waves composed of electric and magnetic fields can be created by an oscillating
Lecture 21 Electromagnetic waves Atomic Physics Atomic Spectra Lasers Applications Electromagnetic Waves Electromagnetic Waves composed of electric and magnetic fields can be created by an oscillating
Table of Contents Electrons in Atoms > Light and Quantized Energy > Quantum Theory and the Atom > Electron Configuration
 Electrons in Atoms October 20, 2014 Table of Contents Electrons in Atoms > Light and Quantized Energy > Quantum Theory and the Atom > Electron Configuration 1 Electromagnetic Spectrum Electromagnetic radiation
Electrons in Atoms October 20, 2014 Table of Contents Electrons in Atoms > Light and Quantized Energy > Quantum Theory and the Atom > Electron Configuration 1 Electromagnetic Spectrum Electromagnetic radiation
Lecture 10: General Relativity I
 Lecture 10: General Relativity I! Einstein Tower Experiment! Gravitational redshifting! Strong Equivalence Principal! Read Chapter 8! Due to snow and confusion the mid-term is delayed to Thursday March
Lecture 10: General Relativity I! Einstein Tower Experiment! Gravitational redshifting! Strong Equivalence Principal! Read Chapter 8! Due to snow and confusion the mid-term is delayed to Thursday March
AN OVERVIEW OF LIGO Adapted from material developed by Brock Wells Robert L. Olds Junior High School, Connell, WA August 2001
 AN OVERVIEW OF LIGO Adapted from material developed by Brock Wells Robert L. Olds Junior High School, Connell, WA August 2001 The purpose of this guide is to provide background about the LIGO project at
AN OVERVIEW OF LIGO Adapted from material developed by Brock Wells Robert L. Olds Junior High School, Connell, WA August 2001 The purpose of this guide is to provide background about the LIGO project at
Chapter 22: Cosmology - Back to the Beginning of Time
 Chapter 22: Cosmology - Back to the Beginning of Time Expansion of Universe implies dense, hot start: Big Bang Future of universe depends on the total amount of dark and normal matter Amount of matter
Chapter 22: Cosmology - Back to the Beginning of Time Expansion of Universe implies dense, hot start: Big Bang Future of universe depends on the total amount of dark and normal matter Amount of matter
 Radioactive Decay What is Radioactivity? http://explorecuriocity.org/explore/articleid/3033 http://explorecuriocity.org/explore/articleid/3035 http://explorecuriocity.org/explore/articleid/2160 Quick Review
Radioactive Decay What is Radioactivity? http://explorecuriocity.org/explore/articleid/3033 http://explorecuriocity.org/explore/articleid/3035 http://explorecuriocity.org/explore/articleid/2160 Quick Review
