Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse
|
|
|
- Alfred Bryant
- 5 years ago
- Views:
Transcription
1 March 1, 2013 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1
2 Foothold principles: Newton s Laws Newton 0: An object responds only to the forces it feels and only at the instant it feels them. Newton 1: An object that feels a net force of 0 keeps moving with the same velocity (which may = 0). Newton 2: An object that is acted upon by other objects changes its velocity according to the rule Newton 3: When two objects interact the forces they exert on each other are equal and opposite.! a A =! F type A!B! F A net m A = " F! type B!A 2
3 Kinetic Energy and Work Newton s laws tell us how velocity changes. The Work-Energy theorem tells us how speed (independent of direction) changes. Kinetic energy = Work done by a force = (part of force to displacement) Work-energy theorem: 1 mv 2 i 3 2 F x!x or F!!r!( 1 2 mv 2 ) = F! net f!r (small step)!( 1 2 mv 2 net ) = " F! dr (any size step)
4 Potential Energy The work done by some forces only depends on the change in position. Then it can be written U is called a potential energy. For gravity, U gravity = mgh For a spring, U spring = ½ kx 2 For electric force, U electric = k C Q 1 Q 2 /r 12! F! " r! = #"U Potential to force:! F =! "U " r! =! $ #U % & #x î + #U #y ĵ + #U #z ' ˆk ( ) =! *U! The force associated with a PE at a given place points downhill in the direction where the PE falls the fastest. 4
5 Energy Kinds of energy (macro) Kinetic Potential Thermal Chemical Kinds of energy (micro)? First law of thermodynamics Conservation of total energy Energy needed to add internal energy at constant pressure (Enthalpy) Internal energy Thermal energy entering!u = Q " W Work done on the rest of the world!h =!U + p!v 5
6 Inter-atomic interactions The interaction between atoms arises from the combination of the electrical forces of its components (electrons and nuclei). It can be quite complex and involve electron sharing and chemical bonds. The complexity arises from the quantum character of electrons. Despite this complexity, a simple potential model summarizes many features of a two-atom interaction. 6
7 Inter-atomic potentials The interaction between neutral atoms includes an attraction at long-range that arises from the fluctuating charge distribution in each atom; the PE behaves like 1/r 6. When the atoms are pressed close, they repel each other strongly; both because the +nuclei repel and because of the Pauli principle (two electrons cannot be in the same state). Two commonly used models are: The Lennard-Jones potential (A/r 12 -B/r 6 ) The Morse potential (exponentials) 7
8 Foothold principles: Randomness Matter is made of of molecules in constant motion and interaction. This motion moves stuff around. If the distribution of a chemical is non-uniform, the randomness of molecular motion will tend to result in molecules moving from more dense regions to less. This is not directed but is an emergent phenomenon arising from the combination of random motion and non-uniform concentration. 8
9 Thermal Equilibrium & Equipartition Degrees of freedom where energy can reside in a system. Thermodynamic equilibrium is dynamic. Changes keep happening, but equal amounts in both directions. Equipartition At equilibrium, the same energy density in all space and in all DoFs. 9
10 Microstate and macrostates A microstate is a specific distribution of energy telling how much is in each DoF. A macrostate is a statement about some average properties of a state (pressure, temperature, density,...). A given macrostate corresponds to many microstates. If the system is sufficiently random, each microstate is equally probable. As a result, the probability of seeing a given macrostate depends on how many microstates it corresponds to. 10
11 Thermal Equilibrium & Equipartition Degrees of freedom where energy can reside in a system. Thermodynamic equilibrium is dynamic Changes keep happening, but equal amounts in both directions. Equipartition At equilibrium, the same energy density in all space and in all DoFs. 11
12 Entropy Entropy an extensive measure of how well energy is spread in an object. Entropy measures The number of microstates in a given macrostate The amount that the energy of a system is spread among the various degrees of freedom Change in entropy upon heat flow S = k B ln(w )!S = Q T 12
13 The Second Law of Thermodynamics Systems composed of a large number of particles spontaneously move toward the thermodynamic (macro) state that correspond to the largest possible number of particle arrangements (microstates). The 2 nd law is probabilistic. Systems show fluctuations violations that get proportionately smaller as N gets large. Systems that are not in thermodynamic equilibrium will spontaneously transform so as to increase the entropy. The entropy of any particular system can decrease as long as the entropy of the rest of the universe increases more. The universe tends towards states of increasing chaos and uniformity. (Is this contradictory?) 13
14 Transforming energy Internal energy: thermal plus chemical Enthalpy: internal plus amount needed to make space at constant p!u!h =!U + p!v!g =!H " T!S Gibbs free energy: enthalpy minus amount associated with raising entropy of the rest of the universe due to energy dumped A process will go spontaneously if ΔG < 0. 14
15 Spontaneity ΔG = ΔH TΔS -TΔS total -TΔS surroundings TΔS system The sign of the Gibbs Free Energy change indicates spontaneity! ΔG < 0 à ΔS total > 0 spontaneous ΔG > 0 à ΔS total < 0 not spontaneous 15
16 Energy distribution Due to the randomness of thermal collisions, even in (local) thermal equilibrium a range of energy is found in each degree of freedom. The probability of finding an energy E is proportional to the Boltzmann factor P(E)! e "E k B T (for one DoF) P(E)! e "E RT (for one mole) At 300 K, k B T ~ 1/40 ev N A k B T = RT ~ 2.4 kj/mol 16
17 Charge A hidden property of matter Matter is made up of two kinds of electrical matter (positive and negative) that usually cancel very precisely. Like charges repel, unlike charges attract. Bringing an unbalanced charge up to neutral matter polarizes it, so both kinds of charge attract neutral matter The total amount of charge (pos neg) is constant. 17
18 Conductors and Insulators Insulators In some matter, the charges they contain are bound and cannot move around freely. Excess charge put onto this kind of matter tends to just sit there (like spreading peanut butter). Conductors In some matter, charges in it can move around throughout the object. Excess charge put onto this kind of matter redistributes itself or flows off (if there is a conducting path to ground). 18
19 Foothold idea: Coulomb s Law All objects attract each other with a force whose magnitude is given by!! F = " F = k CqQ q!q Q!q r qq 2 ˆr q!q k C is put in to make the units come out right. k C = 9!10 9 N-m 2 / C 2 19
20 Energies between charge clusters Atoms and molecules are made up of charges. The potential energy between two charges is U elec 12 = k Q Q C 1 2 r 12 The potential energy between many charges is elec U 12...N = N! i< j=1 k C Q i Q j r ij 20
21 Foothold idea: Fields Test particle We pay attention to what force it feels. We assume it does not have any affect on the source particles. Source particles We pay attention to the forces they exert and assume they do not move. Physical field We consider what force a test particle would feel if it were at a particular point in space and divide by its coupling strength to the force. This gives a vector at each point in space.! g = 1 m!! 1 W E!m E = q! F all charges!q V = 1 q U elec all charges!q 21
22 Electric potential energy and potential The potential energy between two charges is The potential energy of many charges is The potential energy added by adding a test charge q is!u q elec = N " i=1 k C qq i r iq = qv elec U 12...N U elec 12 = k Q Q C = N! i< j=1 r 12 k C Q i Q j r ij = the voltage at the position of the test charge
23 Units Gravitational field units of g = Newtons/kg Electric field units of E = Newtons/C Electric potential units of V = Joules/C = Volts Energy = qv so eδv = the energy gained by an electron (charge e = 1.6 x C) in moving through a change of ΔV volts. 1 ev = 1.6 x J 23
24 Electric charges in materials The electric field inside the body of a static conductor (no moving charges) is zero. The entire body of a static conductor (no charges moving through it) is at the same potential. The average electric field in an insulator is reduced (due to the polarization of the material by the field) by a factor that is a property of the material: the dielectric constant, κ. (Sometimes written in biology as ε) Since κ is the ratio of two fields, it is dimensionless. 24
25 !V = E!x = Ed Foothold ideas: Capacitors Q E = 4"k C # = 4"k C A $ Q = % A ( & ' 4"k C ) * E Q = Q = C!V % A ( & ' 4"k C d ) *!V C = "# A 0 d Energy stored = 1 2 Q!V What does this Q stand for? If plates are separated by a material 25
Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse
 March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
Review Materials Midterm 1
 March 1, 2013 Physics 132 W. Losert Review Materials Midterm 1 Intro Materials Thermodynamics Electrostatic Charges 3/1/13 1 Physics 132 Intro Material 3/1/13 Physics 132 2 Foothold principles: Newton
March 1, 2013 Physics 132 W. Losert Review Materials Midterm 1 Intro Materials Thermodynamics Electrostatic Charges 3/1/13 1 Physics 132 Intro Material 3/1/13 Physics 132 2 Foothold principles: Newton
Building your toolbelt
 March 3, 2017 Physics 132 Prof. E. F. Redish Theme Music: Take the A Train Duke Ellington Cartoon: Fox Trot Bill Amend 3/3/17 Physics 132 1 Building your toolbelt Using math to make meaning in the physical
March 3, 2017 Physics 132 Prof. E. F. Redish Theme Music: Take the A Train Duke Ellington Cartoon: Fox Trot Bill Amend 3/3/17 Physics 132 1 Building your toolbelt Using math to make meaning in the physical
Outline. March 2 is the day of the first midterm Heads up! Recap of electric forces Fields Examples. 2/17/17 Physics 132 1
 Outline March 2 is the day of the first midterm Heads up! Recap of electric forces Fields Examples 2/17/17 Physics 132 1 The Electric Field!!!! Fq ( r ) E (r ) = q 2/17/17 2 Physics 132 Foothold idea:
Outline March 2 is the day of the first midterm Heads up! Recap of electric forces Fields Examples 2/17/17 Physics 132 1 The Electric Field!!!! Fq ( r ) E (r ) = q 2/17/17 2 Physics 132 Foothold idea:
Phys /7/13. November 7, 2013 Physics 131 Prof. E. F. Redish. Theme Music: Duke Ellington Take the A Train. Cartoon: Bill Amend FoxTrot
 November 7, 2013 Physics 131 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Bill Amend FoxTrot 1 Tension: The Spring A spring changes its length in response to pulls (or pushes)
November 7, 2013 Physics 131 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Bill Amend FoxTrot 1 Tension: The Spring A spring changes its length in response to pulls (or pushes)
Theme Music: Robert Alda Luck be a lady Cartoon: Bill Amend FoxTrot. Foothold ideas: Inter-atomic interactions
 February 1, 2013 Prof. E. F. Redish Theme Music: Robert Alda Luck be a lady Cartoon: Bill Amend FoxTrot From Guys & Dolls original cast recording 1 Inter-atomic interactions The interaction between atoms
February 1, 2013 Prof. E. F. Redish Theme Music: Robert Alda Luck be a lady Cartoon: Bill Amend FoxTrot From Guys & Dolls original cast recording 1 Inter-atomic interactions The interaction between atoms
Theme Music: Take the A Train Duke Ellington Cartoon: FoxTrot Bill Amend
 November 11, 2016 Prof. E. F. Redish Theme Music: Take the A Train Duke Ellington Cartoon: FoxTrot Bill Amend 1 Basic Principles of Motion Prof. Redish Description of motion v = Δ r vector displacement
November 11, 2016 Prof. E. F. Redish Theme Music: Take the A Train Duke Ellington Cartoon: FoxTrot Bill Amend 1 Basic Principles of Motion Prof. Redish Description of motion v = Δ r vector displacement
Quiz 2 Info. Average 5.06 St. Dev Frequency. 2/20/2014 Physics 132
 Quiz 2 Info Average 5.06 St. Dev 1.85 30 25 20 Frequency 15 10 5 0 0 1 2 3 4 5 6 7 8 9 10 2/20/2014 Physics 132 1 1. (3 pts) The internal energy of an object at atmospheric pressure is observed to increase.
Quiz 2 Info Average 5.06 St. Dev 1.85 30 25 20 Frequency 15 10 5 0 0 1 2 3 4 5 6 7 8 9 10 2/20/2014 Physics 132 1 1. (3 pts) The internal energy of an object at atmospheric pressure is observed to increase.
Week 4. Outline Review electric Forces Review electric Potential
 Week 4 Outline Review electric Forces Review electric Potential Electric Charge - A property of matter Matter is made up of two kinds of electric charges (positive and negative). Like charges repel, unlike
Week 4 Outline Review electric Forces Review electric Potential Electric Charge - A property of matter Matter is made up of two kinds of electric charges (positive and negative). Like charges repel, unlike
Physics 132- Fundamentals of Physics for Biologists II. Statistical Physics and Thermodynamics
 Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Electric Potential Energy Conservative Force
 Electric Potential Energy Conservative Force Conservative force or field is a force field in which the total mechanical energy of an isolated system is conserved. Examples, Gravitation, Electrostatic,
Electric Potential Energy Conservative Force Conservative force or field is a force field in which the total mechanical energy of an isolated system is conserved. Examples, Gravitation, Electrostatic,
Chapter 19 Electric Potential and Electric Field
 Chapter 19 Electric Potential and Electric Field The electrostatic force is a conservative force. Therefore, it is possible to define an electrical potential energy function with this force. Work done
Chapter 19 Electric Potential and Electric Field The electrostatic force is a conservative force. Therefore, it is possible to define an electrical potential energy function with this force. Work done
Electric Field of a uniformly Charged Thin Spherical Shell
 Electric Field of a uniformly Charged Thin Spherical Shell The calculation of the field outside the shell is identical to that of a point charge. The electric field inside the shell is zero. What are the
Electric Field of a uniformly Charged Thin Spherical Shell The calculation of the field outside the shell is identical to that of a point charge. The electric field inside the shell is zero. What are the
Theme Music: Duke Ellington Take the A Train Cartoon: Bill Amend FoxTrot
 May18, 2011 Physics 122 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Bill Amend FoxTrot 1 Review sheets for Final Exam Material from two previous exams plus Electric currents
May18, 2011 Physics 122 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Bill Amend FoxTrot 1 Review sheets for Final Exam Material from two previous exams plus Electric currents
Professor: Arpita Upadhyaya Physics 131
 Physics 131- Fundamentals of Physics for Biologists I Professor: Arpita Upadhyaya Energy Chemical bonding Intermolecular Interactions 1 Physics 131 Quiz 11 60 50 Q 1.1 11 40 30 20 10 0 A B C D 2 18 Q 1.2
Physics 131- Fundamentals of Physics for Biologists I Professor: Arpita Upadhyaya Energy Chemical bonding Intermolecular Interactions 1 Physics 131 Quiz 11 60 50 Q 1.1 11 40 30 20 10 0 A B C D 2 18 Q 1.2
Before the Quiz. Make sure you have SIX pennies
 Before the Quiz Make sure you have SIX pennies If you have more than 6, please share with your neighbors There are some additional pennies in the baskets up front please be sure to return them after class!!!
Before the Quiz Make sure you have SIX pennies If you have more than 6, please share with your neighbors There are some additional pennies in the baskets up front please be sure to return them after class!!!
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chemical thermodynamics the area of chemistry that deals with energy relationships
 Chemistry: The Central Science Chapter 19: Chemical Thermodynamics Chemical thermodynamics the area of chemistry that deals with energy relationships 19.1: Spontaneous Processes First law of thermodynamics
Chemistry: The Central Science Chapter 19: Chemical Thermodynamics Chemical thermodynamics the area of chemistry that deals with energy relationships 19.1: Spontaneous Processes First law of thermodynamics
10th week Lectures March Chapter 12
 Electric charge. 10th week Lectures March 20. 2017. Chapter 12 Conductors and Insulators Coulomb law Electric field Electric Potential 3/20/2017 Physics 214 Spring 2017 1 Electric charge an atom has a
Electric charge. 10th week Lectures March 20. 2017. Chapter 12 Conductors and Insulators Coulomb law Electric field Electric Potential 3/20/2017 Physics 214 Spring 2017 1 Electric charge an atom has a
Chapter 16. Electric Energy and Capacitance
 Chapter 16 Electric Energy and Capacitance Electric Potential Energy The electrostatic force is a conservative force It is possible to define an electrical potential energy function with this force Work
Chapter 16 Electric Energy and Capacitance Electric Potential Energy The electrostatic force is a conservative force It is possible to define an electrical potential energy function with this force Work
Quiz 3 Info. Average 6.40 St. Dev Quiz 3 Scores. 2/25/2014 Physics 132 1
 Quiz 3 Info Quiz 3 Scores Average 6.40 St. Dev 2.62 35 30 25 20 15 10 5 0 0 1 2 3 4 5 6 7 8 9 10 2/25/2014 Physics 132 1 1. (3 pts) A particular reaction has a negative enthalpy change AND a negative entropy
Quiz 3 Info Quiz 3 Scores Average 6.40 St. Dev 2.62 35 30 25 20 15 10 5 0 0 1 2 3 4 5 6 7 8 9 10 2/25/2014 Physics 132 1 1. (3 pts) A particular reaction has a negative enthalpy change AND a negative entropy
c. They have electric charges that move freely d. Electrons are added to the rod a. charges are of unlike signs b. charges are of like signs
 Physics Review Chapter 17 & 18 Name: Date: Period: 1. What sentence best characterizes electron conductors? a. They have low mass density b. They have high tensile strength c. They have electric charges
Physics Review Chapter 17 & 18 Name: Date: Period: 1. What sentence best characterizes electron conductors? a. They have low mass density b. They have high tensile strength c. They have electric charges
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
Uniform Electric Fields and Potential Difference Forces and Fields 8
 Uniform Electric Fields and Potential Difference Forces and Fields 8 POS Checklist compare, qualitatively, gravitational potential energy and electric potential energy. define electric potential difference
Uniform Electric Fields and Potential Difference Forces and Fields 8 POS Checklist compare, qualitatively, gravitational potential energy and electric potential energy. define electric potential difference
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen
 Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Nicholas J. Giordano. Chapter 18. Electric Potential. Marilyn Akins, PhD Broome Community College
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 18 Electric Potential Marilyn Akins, PhD Broome Community College Electric Potential Electric forces can do work on a charged object Electrical
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 18 Electric Potential Marilyn Akins, PhD Broome Community College Electric Potential Electric forces can do work on a charged object Electrical
PHYSICS - Electrostatics
 PHYSICS - Electrostatics Electrostatics, or electricity at rest, involves electric charges, the forces between them, and their behavior in materials. 22.1 Electrical Forces and Charges The fundamental
PHYSICS - Electrostatics Electrostatics, or electricity at rest, involves electric charges, the forces between them, and their behavior in materials. 22.1 Electrical Forces and Charges The fundamental
Electric potential energy The concept of electric potential and potential difference Motion of charges in electric field
 In this chapter, you will learn: Electric potential energy The concept of electric potential and potential difference Motion of charges in electric field 2.1 Electric potential energy When a charged particle
In this chapter, you will learn: Electric potential energy The concept of electric potential and potential difference Motion of charges in electric field 2.1 Electric potential energy When a charged particle
the electrical nature of matter is inherent in its atomic structure E & M atoms are made up of p+, n, and e- the nucleus has p+ and n
 Electric Forces and Fields E & M the electrical nature of matter is inherent in its atomic structure atoms are made up of p+, n, and e- a.k.a Electricity and Magnetism the nucleus has p+ and n surrounding
Electric Forces and Fields E & M the electrical nature of matter is inherent in its atomic structure atoms are made up of p+, n, and e- a.k.a Electricity and Magnetism the nucleus has p+ and n surrounding
Electric Force and Charges. Conceptual Physics 11 th Edition. What are Atoms Made of?
 Conceptual Physics 11 th Edition Electrical Forces and Charges Conservation of Charge Coulomb s Law Conductors and Insulators Chapter 22: ELECTROSTATICS Charging Charge Polarization Electric Field Electric
Conceptual Physics 11 th Edition Electrical Forces and Charges Conservation of Charge Coulomb s Law Conductors and Insulators Chapter 22: ELECTROSTATICS Charging Charge Polarization Electric Field Electric
Objects can be charged by rubbing
 Electrostatics Objects can be charged by rubbing Charge comes in two types, positive and negative; like charges repel and opposite charges attract Electric charge is conserved the arithmetic sum of the
Electrostatics Objects can be charged by rubbing Charge comes in two types, positive and negative; like charges repel and opposite charges attract Electric charge is conserved the arithmetic sum of the
3/5/2009 PHYS202 SPRING Lecture notes Electrostatics
 PHYS0 SPRING 009 Lecture notes Electrostatics 1 What is Electricity Static effects known since ancient times. Static charges can be made by rubbing certain materials together. Described by Benjamin Franklin
PHYS0 SPRING 009 Lecture notes Electrostatics 1 What is Electricity Static effects known since ancient times. Static charges can be made by rubbing certain materials together. Described by Benjamin Franklin
Physics is time symmetric Nature is not
 Fundamental theories of physics don t depend on the direction of time Newtonian Physics Electromagnetism Relativity Quantum Mechanics Physics is time symmetric Nature is not II law of thermodynamics -
Fundamental theories of physics don t depend on the direction of time Newtonian Physics Electromagnetism Relativity Quantum Mechanics Physics is time symmetric Nature is not II law of thermodynamics -
Ch. 19 Entropy and Free Energy: Spontaneous Change
 Ch. 19 Entropy and Free Energy: Spontaneous Change 19-1 Spontaneity: The Meaning of Spontaneous Change 19-2 The Concept of Entropy 19-3 Evaluating Entropy and Entropy Changes 19-4 Criteria for Spontaneous
Ch. 19 Entropy and Free Energy: Spontaneous Change 19-1 Spontaneity: The Meaning of Spontaneous Change 19-2 The Concept of Entropy 19-3 Evaluating Entropy and Entropy Changes 19-4 Criteria for Spontaneous
Physics 132- Fundamentals of Physics for Biologists II
 Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics It s all about energy Classifying Energy Kinetic Energy Potential Energy Macroscopic Energy Moving baseball
Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics It s all about energy Classifying Energy Kinetic Energy Potential Energy Macroscopic Energy Moving baseball
Theme Music: Earth, Wind, & Fire Magnetic Cartoon: Cantu & Castellanos Baldo
 May 4, 2011 Physics 122 Prof. E. F. Redish Theme Music: Earth, Wind, & Fire Magnetic Cartoon: Cantu & Castellanos Baldo 1 Outline Go over Q10 Recap: Capacitors Dielectrics Magnetism basics Magnetism and
May 4, 2011 Physics 122 Prof. E. F. Redish Theme Music: Earth, Wind, & Fire Magnetic Cartoon: Cantu & Castellanos Baldo 1 Outline Go over Q10 Recap: Capacitors Dielectrics Magnetism basics Magnetism and
(3.5.1) V E x, E, (3.5.2)
 Lecture 3.5 Capacitors Today we shall continue our discussion of electrostatics and, in particular, the concept of electrostatic potential energy and electric potential. The main example which we have
Lecture 3.5 Capacitors Today we shall continue our discussion of electrostatics and, in particular, the concept of electrostatic potential energy and electric potential. The main example which we have
Joy of Science Discovering the matters and the laws of the universe
 Joy of Science Discovering the matters and the laws of the universe Key Words Universe, Energy, Quantum mechanics, Chemical reaction, Structure of matter Unless otherwise noted, copied pictures are taken
Joy of Science Discovering the matters and the laws of the universe Key Words Universe, Energy, Quantum mechanics, Chemical reaction, Structure of matter Unless otherwise noted, copied pictures are taken
Energy Stored in Capacitors
 Energy Stored in Capacitors U = 1 2 qv q = CV U = 1 2 CV 2 q 2 or U = 1 2 C 37 Energy Density in Capacitors (1) We define the, u, as the electric potential energy per unit volume Taking the ideal case
Energy Stored in Capacitors U = 1 2 qv q = CV U = 1 2 CV 2 q 2 or U = 1 2 C 37 Energy Density in Capacitors (1) We define the, u, as the electric potential energy per unit volume Taking the ideal case
Now for something totally (?) different
 Now for something totally (?) different OUR FIRST REAL FORCE LAW: F = -G m M / r 2 Universal gravitational force (Newton) Acting between any two masses Proportional to both of these masses Inversely proportional
Now for something totally (?) different OUR FIRST REAL FORCE LAW: F = -G m M / r 2 Universal gravitational force (Newton) Acting between any two masses Proportional to both of these masses Inversely proportional
Ch 7 Electric Potential
 Ch 7 Electric Potential Electric Energy, Electric Potential Energy concepts are going to be extremely important to us as we consider the behavior of charges in electric fields. How do energy concepts help
Ch 7 Electric Potential Electric Energy, Electric Potential Energy concepts are going to be extremely important to us as we consider the behavior of charges in electric fields. How do energy concepts help
PHY102 Electricity Course Summary
 TOPIC 1 ELECTOSTTICS PHY1 Electricity Course Summary Coulomb s Law The magnitude of the force between two point charges is directly proportional to the product of the charges and inversely proportional
TOPIC 1 ELECTOSTTICS PHY1 Electricity Course Summary Coulomb s Law The magnitude of the force between two point charges is directly proportional to the product of the charges and inversely proportional
Chapter 21 Electric Potential
 Chapter 21 Electric Potential Chapter Goal: To calculate and use the electric potential and electric potential energy. Slide 21-1 Chapter 21 Preview Looking Ahead Text: p. 665 Slide 21-2 Review of Potential
Chapter 21 Electric Potential Chapter Goal: To calculate and use the electric potential and electric potential energy. Slide 21-1 Chapter 21 Preview Looking Ahead Text: p. 665 Slide 21-2 Review of Potential
Welcome to PHYS2002!
 Welcome to PHYS00! Physics I Done! We are now all experts in mechanics. Mechanics Mass M Interaction: mm F = G r 1 G = 6.67 10 Nm/ kg r M 11 1 We never said what mass is, only how it behaves. New Semester
Welcome to PHYS00! Physics I Done! We are now all experts in mechanics. Mechanics Mass M Interaction: mm F = G r 1 G = 6.67 10 Nm/ kg r M 11 1 We never said what mass is, only how it behaves. New Semester
12/15/2015. Newton per Coulomb N/C. vector. A model of the mechanism for electrostatic interactions. The Electric Field
 Chapter 15 Lecture The Electric Field A model of the mechanism for electrostatic interactions A model for electric interactions, suggested by Michael Faraday, involves some sort of electric disturbance
Chapter 15 Lecture The Electric Field A model of the mechanism for electrostatic interactions A model for electric interactions, suggested by Michael Faraday, involves some sort of electric disturbance
Thermodynamics: Free Energy and Entropy. Suggested Reading: Chapter 19
 Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
7/19/2011. Models of Solution. State of Equilibrium. State of Equilibrium Chemical Reaction
 Models of Solution Chemistry- I State of Equilibrium A covered cup of coffee will not be colder than or warmer than the room temperature Heat is defined as a form of energy that flows from a high temperature
Models of Solution Chemistry- I State of Equilibrium A covered cup of coffee will not be colder than or warmer than the room temperature Heat is defined as a form of energy that flows from a high temperature
Chapter 21. Electric Charge and Electric Field
 1.1 Electric Charge Chapter 1 Electric Charge and Electric Field Only varieties of electric charges exist in nature; positive and negative charges. Like charges repel each other, while opposite charges
1.1 Electric Charge Chapter 1 Electric Charge and Electric Field Only varieties of electric charges exist in nature; positive and negative charges. Like charges repel each other, while opposite charges
3/30/2017. Section 17.1 Spontaneous Processes and Entropy Thermodynamics vs. Kinetics. Chapter 17. Spontaneity, Entropy, and Free Energy
 Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
Chapter 16 Electrical Energy Capacitance. HW: 1, 2, 3, 5, 7, 12, 13, 17, 21, 25, 27 33, 35, 37a, 43, 45, 49, 51
 Chapter 16 Electrical Energy Capacitance HW: 1, 2, 3, 5, 7, 12, 13, 17, 21, 25, 27 33, 35, 37a, 43, 45, 49, 51 Electrical Potential Reminder from physics 1: Work done by a conservative force, depends only
Chapter 16 Electrical Energy Capacitance HW: 1, 2, 3, 5, 7, 12, 13, 17, 21, 25, 27 33, 35, 37a, 43, 45, 49, 51 Electrical Potential Reminder from physics 1: Work done by a conservative force, depends only
CHAPTER 15 ELECTRIC FORCE & FIELDS
 CHAPTER 15 ELECTRIC FORCE & FIELDS We will look at the basic properties of electric charge. Electric charge comes in discrete units The total charge in the universe remains constant The force law that
CHAPTER 15 ELECTRIC FORCE & FIELDS We will look at the basic properties of electric charge. Electric charge comes in discrete units The total charge in the universe remains constant The force law that
Chemistry Terms. atomic number The atomic number of an element is the number of protons in the nucleus of each atom.
 Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name. Circle the vector quantities below and underline the scalar quantities below
 AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
Chapter 18 Electric Force and Electric Fields. Sections
 Chapter 18 Electric Force and Electric Fields Sections 18.1 18.6 Objectives: After finishing this unit, you should be able to: Explain and demonstrate the First law of electrostatics and discuss charging
Chapter 18 Electric Force and Electric Fields Sections 18.1 18.6 Objectives: After finishing this unit, you should be able to: Explain and demonstrate the First law of electrostatics and discuss charging
47 CHARGE. 1. What are the basic particles of charge?
 47 CHARGE 1. What are the basic particles of charge? 2. There are three variables for charge listed to the right. Tell the typical circumstances when each is used. 3. Charge What are the units of charge?
47 CHARGE 1. What are the basic particles of charge? 2. There are three variables for charge listed to the right. Tell the typical circumstances when each is used. 3. Charge What are the units of charge?
Ch 25 Electric Potential
 Ch 25 Electric Potential Electric Energy, Electric Potential Energy concepts are going to be extremely important to us as we consider the behavior of charges in electric fields. How do energy concepts
Ch 25 Electric Potential Electric Energy, Electric Potential Energy concepts are going to be extremely important to us as we consider the behavior of charges in electric fields. How do energy concepts
Chapter 17 & 18. Electric Field and Electric Potential
 Chapter 17 & 18 Electric Field and Electric Potential Electric Field Maxwell developed an approach to discussing fields An electric field is said to exist in the region of space around a charged object
Chapter 17 & 18 Electric Field and Electric Potential Electric Field Maxwell developed an approach to discussing fields An electric field is said to exist in the region of space around a charged object
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Now for something totally (?) different
 Now for something totally (?) different OUR FIRST REAL FORCE LAW: F = G m M / r 2 Universal gravitational force (Newton) Acting between any two masses Proportional to both of these masses Inversely proportional
Now for something totally (?) different OUR FIRST REAL FORCE LAW: F = G m M / r 2 Universal gravitational force (Newton) Acting between any two masses Proportional to both of these masses Inversely proportional
COLLEGE PHYSICS Chapter 19 ELECTRIC POTENTIAL AND ELECTRIC FIELD
 COLLEGE PHYSICS Chapter 19 ELECTRIC POTENTIAL AND ELECTRIC FIELD Electric Potential Energy and Electric Potential Difference It takes work to move a charge against an electric field. Just as with gravity,
COLLEGE PHYSICS Chapter 19 ELECTRIC POTENTIAL AND ELECTRIC FIELD Electric Potential Energy and Electric Potential Difference It takes work to move a charge against an electric field. Just as with gravity,
MTE1 results. Mean 75% = 90/120
 MTE1 results Mean 75% = 90/120 Scores available at Learn@UW, your TAs have exams If your score is an F or a D, talk to us and your TAs for suggestions on how to improve From last times Electric charges
MTE1 results Mean 75% = 90/120 Scores available at Learn@UW, your TAs have exams If your score is an F or a D, talk to us and your TAs for suggestions on how to improve From last times Electric charges
Electric Force and Charges. Conceptual Physics 11 th Edition. Electric Force and Charges
 Conceptual Physics 11 th Edition Central rule of electricity Opposite charges attract one another; like charges repel. Chapter 22: ELECTROSTATICS This lecture will help you understand: Electrical Forces
Conceptual Physics 11 th Edition Central rule of electricity Opposite charges attract one another; like charges repel. Chapter 22: ELECTROSTATICS This lecture will help you understand: Electrical Forces
Molecular Modeling -- Lecture 15 Surfaces and electrostatics
 Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
PHYS 2426 Brooks INTRODUCTION. Physics for Scientists and Engineers, with Modern Physics, 4 th edition Giancoli
 PHYS 2426 Brooks INTRODUCTION http://iws.ccccd.edu/mbrooks Physics for Scientists and Engineers, with Modern Physics, 4 th edition Giancoli Chapter 21 Electric Charge and Electric Field Static Electricity;
PHYS 2426 Brooks INTRODUCTION http://iws.ccccd.edu/mbrooks Physics for Scientists and Engineers, with Modern Physics, 4 th edition Giancoli Chapter 21 Electric Charge and Electric Field Static Electricity;
Electrostatics so far
 Electrostatics so far F = 1 2 1 2 2 Electric Force b/n q and q : qq 1 2 kq Electric Field E due to q : E = 1 1 r 2 kq q r q e = 1.6 x10-19 C k = 9 x 10 9 Nm 2 /C 2 Tesla Envy http://www.youtube.com/watch?v=jl
Electrostatics so far F = 1 2 1 2 2 Electric Force b/n q and q : qq 1 2 kq Electric Field E due to q : E = 1 1 r 2 kq q r q e = 1.6 x10-19 C k = 9 x 10 9 Nm 2 /C 2 Tesla Envy http://www.youtube.com/watch?v=jl
General Physics II. Electric Charge, Forces & Fields
 General Physics II Electric Charge, Forces & Fields Electric Charge Recall that fundamental particles carry something called electric charge protons have exactly one unit of positive charge +1.602 x 10-19
General Physics II Electric Charge, Forces & Fields Electric Charge Recall that fundamental particles carry something called electric charge protons have exactly one unit of positive charge +1.602 x 10-19
Electricity
 Electricity Electric Charge There are two fundamental charges in the universe. Positive (proton) has a charge of +1.60 x 10-19 C Negative (electron) has a charge of 1.60 x 10-19 C There is one general
Electricity Electric Charge There are two fundamental charges in the universe. Positive (proton) has a charge of +1.60 x 10-19 C Negative (electron) has a charge of 1.60 x 10-19 C There is one general
Gibbs Free Energy. Evaluating spontaneity
 Gibbs Free Energy Evaluating spontaneity Predicting Spontaneity An increase in entropy; Changing from a more structured to less structured physical state: Solid to liquid Liquid to gas Increase in temperature
Gibbs Free Energy Evaluating spontaneity Predicting Spontaneity An increase in entropy; Changing from a more structured to less structured physical state: Solid to liquid Liquid to gas Increase in temperature
Sharpen thinking about connections among electric field, electric potential difference, potential energy
 PHYS 2015 -- Week 6 Sharpen thinking about connections among electric field, electric potential difference, potential energy Apply the ideas to capacitance and the parallel plate capacitor For exclusive
PHYS 2015 -- Week 6 Sharpen thinking about connections among electric field, electric potential difference, potential energy Apply the ideas to capacitance and the parallel plate capacitor For exclusive
Some differences: Some basic similarities: Charges. Electrons vs. Protons 3/25/12. Chapters 22-25: Electromagnetism!
 Chapters 22-25: Electromagnetism! Electric Force vs. Gravitational Force What properties does the gravitational force depend on? What properties does the electric force depend on? F grav = G*m 1 *m 2 /d
Chapters 22-25: Electromagnetism! Electric Force vs. Gravitational Force What properties does the gravitational force depend on? What properties does the electric force depend on? F grav = G*m 1 *m 2 /d
ISLAMABAD ACADEMY PHYSICS FOR 10TH CLASS (UNIT # 15)
 PHYSICS FOR 10TH CLASS (UNIT # 15) SHORT QUESTIONS Define the term If in the presence of a charged body, an insulated Electrostatic induction? conductor has like charges at one end and unlike charges at
PHYSICS FOR 10TH CLASS (UNIT # 15) SHORT QUESTIONS Define the term If in the presence of a charged body, an insulated Electrostatic induction? conductor has like charges at one end and unlike charges at
Atoms, electrons and Solids
 Atoms, electrons and Solids Shell model of an atom negative electron orbiting a positive nucleus QM tells that to minimize total energy the electrons fill up shells. Each orbit in a shell has a specific
Atoms, electrons and Solids Shell model of an atom negative electron orbiting a positive nucleus QM tells that to minimize total energy the electrons fill up shells. Each orbit in a shell has a specific
33 Electric Fields and Potential. An electric field is a storehouse of energy.
 An electric field is a storehouse of energy. The space around a concentration of electric charge is different from how it would be if the charge were not there. If you walk by the charged dome of an electrostatic
An electric field is a storehouse of energy. The space around a concentration of electric charge is different from how it would be if the charge were not there. If you walk by the charged dome of an electrostatic
iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed?
 1 iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed? q A: C->2 C0 B: C-> C0 C: C-> C0/2 D: C->- C0 E: C->-2 C0 2 iclicker A metal ball of
1 iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed? q A: C->2 C0 B: C-> C0 C: C-> C0/2 D: C->- C0 E: C->-2 C0 2 iclicker A metal ball of
= 16! = 16! W A = 3 = 3 N = = W B 3!3!10! = ΔS = nrln V. = ln ( 3 ) V 1 = 27.4 J.
 Answer key: Q1A Both configurations are equally likely because the particles are non-interacting (i.e., the energy does not favor one configuration over another). For A M = 16 N = 6 W A = 16! 0.9 101 =
Answer key: Q1A Both configurations are equally likely because the particles are non-interacting (i.e., the energy does not favor one configuration over another). For A M = 16 N = 6 W A = 16! 0.9 101 =
PH 202-1E Fall Electric Forces and Electric Fields. Lectures 1-4. Chapter 18 (Cutnell & Johnson, Physics 6 th edition)
 PH 202-1E Fall 2006 Electric Forces and Electric Fields Lectures 1-4 Chapter 18 (Cutnell & Johnson, Physics 6 th edition) 1 Electric Force and Electric Charge Qualitatively, a force is any push or pull
PH 202-1E Fall 2006 Electric Forces and Electric Fields Lectures 1-4 Chapter 18 (Cutnell & Johnson, Physics 6 th edition) 1 Electric Force and Electric Charge Qualitatively, a force is any push or pull
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
PHYS102 - Gauss s Law.
 PHYS102 - Gauss s Law. Dr. Suess February 2, 2007 PRS Questions 2 Question #1.............................................................................. 2 Answer to Question #1......................................................................
PHYS102 - Gauss s Law. Dr. Suess February 2, 2007 PRS Questions 2 Question #1.............................................................................. 2 Answer to Question #1......................................................................
Unit 12. Thermochemistry
 Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
PHYSICS. Curriculum Standard One: The student will understand that Newton s laws predict the motion of most objects.
 Science Motion and Forces 11-12 Curriculum Standard One: The student will understand that Newton s laws predict the motion of most objects. *1A. The student will demonstrate an problems involving constant
Science Motion and Forces 11-12 Curriculum Standard One: The student will understand that Newton s laws predict the motion of most objects. *1A. The student will demonstrate an problems involving constant
Physics 9 Spring 2012 Midterm 1 Solutions
 Physics 9 Spring 22 NAME: TA: Physics 9 Spring 22 Midterm s For the midterm, you may use one sheet of notes with whatever you want to put on it, front and back. Please sit every other seat, and please
Physics 9 Spring 22 NAME: TA: Physics 9 Spring 22 Midterm s For the midterm, you may use one sheet of notes with whatever you want to put on it, front and back. Please sit every other seat, and please
Part I Electrostatics. 1: Charge and Coulomb s Law July 6, 2008
 Part I Electrostatics 1: Charge and Coulomb s Law July 6, 2008 1.1 What is Electric Charge? 1.1.1 History Before 1600CE, very little was known about electric properties of materials, or anything to do
Part I Electrostatics 1: Charge and Coulomb s Law July 6, 2008 1.1 What is Electric Charge? 1.1.1 History Before 1600CE, very little was known about electric properties of materials, or anything to do
Electrostatics. Typeset by FoilTEX 1
 Electrostatics Typeset by FoilTEX 1 Question 1 A plastic rod is rubbed and touched to a small metal ball. After this the rod is observed to repel the ball. Which of the following is correct? 1. The force
Electrostatics Typeset by FoilTEX 1 Question 1 A plastic rod is rubbed and touched to a small metal ball. After this the rod is observed to repel the ball. Which of the following is correct? 1. The force
Chemistry and the material world Unit 4, Lecture 4 Matthias Lein
 Chemistry and the material world 123.102 Unit 4, Lecture 4 Matthias Lein Gibbs ree energy Gibbs ree energy to predict the direction o a chemical process. Exergonic and endergonic reactions. Temperature
Chemistry and the material world 123.102 Unit 4, Lecture 4 Matthias Lein Gibbs ree energy Gibbs ree energy to predict the direction o a chemical process. Exergonic and endergonic reactions. Temperature
Chapter 19. Chemical Thermodynamics. Chemical Thermodynamics
 Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Electric Fields 05/16/2008. Lecture 17 1
 lectric Charge The lectric Force lectric Charge lectric Fields lectron Beams Recall that fundamental particles carry something called electric charge protons have exactly one unit of positive charge electrons
lectric Charge The lectric Force lectric Charge lectric Fields lectron Beams Recall that fundamental particles carry something called electric charge protons have exactly one unit of positive charge electrons
Physics 132- Fundamentals of Physics for Biologists II
 Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics A confession Temperature Object A Object contains MANY atoms (kinetic energy) and interactions (potential energy)
Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics A confession Temperature Object A Object contains MANY atoms (kinetic energy) and interactions (potential energy)
Electromagnetism. Electricity Electromagnetism Magnetism Optics. In this course we are going to discuss the fundamental concepts of electromagnetism:
 Electromagnetism Electromagnetism is one of the fundamental forces in nature, and the the dominant force in a vast range of natural and technological phenomena The electromagnetic force is solely responsible
Electromagnetism Electromagnetism is one of the fundamental forces in nature, and the the dominant force in a vast range of natural and technological phenomena The electromagnetic force is solely responsible
Ch 16: Electric Charge and Electric Field. Opposites attract by Paula Abdul
 Ch 16: Electric Charge and Electric Field Opposites attract by Paula Abdul Static Electricity A neutral object rubbed with another object can acquire a charge due to friction. It is said to posses a net
Ch 16: Electric Charge and Electric Field Opposites attract by Paula Abdul Static Electricity A neutral object rubbed with another object can acquire a charge due to friction. It is said to posses a net
Chapter 21 Electric Charge and Electric Field
 Chapter 21 Electric Charge and Electric Field 21-1 Static Electricity; Electric Charge and Its Conservation Objects can be charged by rubbing 21-1 Static Electricity; Electric Charge and Its Conservation
Chapter 21 Electric Charge and Electric Field 21-1 Static Electricity; Electric Charge and Its Conservation Objects can be charged by rubbing 21-1 Static Electricity; Electric Charge and Its Conservation
ELECTRIC FORCES AND ELECTRIC FIELDS
 CHATER 18 ELECTRIC FORCES AND ELECTRIC FIELDS CONCETUAL QUESTIONS 1. REASONING AND SOLUTION In Figure 18.9, the grounding wire is removed first, followed by the rod, and the sphere is left with a positive
CHATER 18 ELECTRIC FORCES AND ELECTRIC FIELDS CONCETUAL QUESTIONS 1. REASONING AND SOLUTION In Figure 18.9, the grounding wire is removed first, followed by the rod, and the sphere is left with a positive
Plasma Astrophysics Chapter 1: Basic Concepts of Plasma. Yosuke Mizuno Institute of Astronomy National Tsing-Hua University
 Plasma Astrophysics Chapter 1: Basic Concepts of Plasma Yosuke Mizuno Institute of Astronomy National Tsing-Hua University What is a Plasma? A plasma is a quasi-neutral gas consisting of positive and negative
Plasma Astrophysics Chapter 1: Basic Concepts of Plasma Yosuke Mizuno Institute of Astronomy National Tsing-Hua University What is a Plasma? A plasma is a quasi-neutral gas consisting of positive and negative
Lecture 24 Chapter 22 Electrostatics II Electric Field & Potential. Chapter 23 Electric Current. From last time--
 Lecture 24 Chapter 22 Electrostatics II Electric Field & Potential Chapter 23 Electric Current 21-Oct-10 From last time-- Electric charge (q), measured in Coulombs (C) Positive and negative charge Electric
Lecture 24 Chapter 22 Electrostatics II Electric Field & Potential Chapter 23 Electric Current 21-Oct-10 From last time-- Electric charge (q), measured in Coulombs (C) Positive and negative charge Electric
2: What is the magnitude of the electric charge of an electron? 3: What is the law of conservation of electric charge?
 Chapter 18 Discussion January-03-15 8:58 PM Electric Forces and Electric Fields Reading Review 1: What is the SI unit of electric charge? 2: What is the magnitude of the electric charge of an electron?
Chapter 18 Discussion January-03-15 8:58 PM Electric Forces and Electric Fields Reading Review 1: What is the SI unit of electric charge? 2: What is the magnitude of the electric charge of an electron?
Electrostatics. 3) positive object: lack of electrons negative object: excess of electrons. Particle Mass Electric Charge. m e = 9.
 Electrostatics 1) electric charge: 2 types of electric charge: positive and negative 2) charging by friction: transfer of electrons from one object to another 3) positive object: lack of electrons negative
Electrostatics 1) electric charge: 2 types of electric charge: positive and negative 2) charging by friction: transfer of electrons from one object to another 3) positive object: lack of electrons negative
Chapter 21 Electric Charge and the Electric Field
 Chapter 21 Electric Charge and the Electric Field 1 Electric Charge Electrostatics is the study of charges when they are stationery. Figure 1: This is Fig. 21.1 and it shows how negatively charged objects
Chapter 21 Electric Charge and the Electric Field 1 Electric Charge Electrostatics is the study of charges when they are stationery. Figure 1: This is Fig. 21.1 and it shows how negatively charged objects
Objects usually are charged up through the transfer of electrons from one object to the other.
 1 Part 1: Electric Force Review of Vectors Review your vectors! You should know how to convert from polar form to component form and vice versa add and subtract vectors multiply vectors by scalars Find
1 Part 1: Electric Force Review of Vectors Review your vectors! You should know how to convert from polar form to component form and vice versa add and subtract vectors multiply vectors by scalars Find
Chapter Assignment Solutions
 Chapter 20-21 Assignment Solutions Table of Contents Page 558 #22, 24, 29, 31, 36, 37, 40, 43-48... 1 Lightning Worksheet (Transparency 20-4)... 4 Page 584 #42-46, 58-61, 66-69, 76-79, 84-86... 5 Chapter
Chapter 20-21 Assignment Solutions Table of Contents Page 558 #22, 24, 29, 31, 36, 37, 40, 43-48... 1 Lightning Worksheet (Transparency 20-4)... 4 Page 584 #42-46, 58-61, 66-69, 76-79, 84-86... 5 Chapter
Physics 12 ELECTROSTATICS
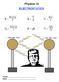 Physics 12 ELECTROSTATICS F = kq 1Q 2 r2 E = V d V = kq r E p = kq 1Q 2 r F = qe V = E p Q 1 000 000 Volts 1 000 000 Volts NAME: Block: Text References 3 rd Ed. Giancolli Pg. 416-30 4 th Ed. Giancolli
Physics 12 ELECTROSTATICS F = kq 1Q 2 r2 E = V d V = kq r E p = kq 1Q 2 r F = qe V = E p Q 1 000 000 Volts 1 000 000 Volts NAME: Block: Text References 3 rd Ed. Giancolli Pg. 416-30 4 th Ed. Giancolli
