A comparison of the value of viscosity for several water models using Poiseuille flow in a nano channel. Abstract
|
|
|
- Ashlyn Jemima Harris
- 5 years ago
- Views:
Transcription
1 A comparison of the value of viscosity for several water models using Poiseuille flow in a nano channel A. P. Markesteijn 1, Remco Hartkamp 2, S. Luding 2, and J. Westerweel 1 1 Laboratory for Aero & Hydrodynamics, Delft University of Technology, Leeghwaterstraat 21, 2628 CA Delft, The Netherlands and 2 Multi Scale Mechanics, MESA+ Institute for Nanotechnology, University of Twente, P.O.Box 217, 7500 AE Enschede, The Netherlands (Dated: 8th February 2012) Abstract The viscosity-temperature relation is determined for the water models SPC/E, TIP4P, TIP4P/Ew, and TIP4P/2005 by considering Poiseuille flow inside a nano channel using molecular dynamics. The viscosity is determined by fitting the resulting velocity profile (away from the walls) to the continuum solution for a Newtonian fluid and then compared to experimental values. The results show that the TIP4P/2005 model gives the best prediction of the viscosity for the complete range of temperatures for liquid water, and thus it is the preferred water model of these considered here for simulations where the magnitude of viscosity is crucial. On the other hand, with the TIP4P model the viscosity is severely underpredicted, and overall the model performed worst, whereas, the SPC/E and TIP4P/Ew models perform moderately. 1
2 I. INTRODUCTION Viscosity plays an important role in many physical transport processes, and hence it is important to specify it accurately in computer simulations in which these processes are investigated. Of special interest is water. When simulating water with molecular dynamics (MD) it is vital to select a water model that correctly predicts the process investigated. Many water models have been developed, differing in parameter values and number of charge sites, and each having different success in predicting the correct value of a certain physical parameter. However, only few references give a complete set of the viscosity versus temperature of a certain water model, which makes it difficult to select the appropriate water model. In this study, the viscosity-temperature relation of four water models is reported. These are the popular 3-point charge SPC/E water model [1], and several variants of the 4-point charge models; TIP4P [2], TIP4P/Ew [3], and the recent TIP4P/2005 [4]. There are several ways to obtain the values of the viscosity by means of MD simulations and most of them require a long simulation time in order to obtain statistically meaningful results. The most frequently used methods are the Green-Kubo method [5, 6] and the Stokes-Einstein method [7]. Both methods are based on the auto-correlation function of the stress tensor, which is computationally expensive to obtain. Furthermore, there are problems in interpretation of the Green-Kubo expressions of the transport coefficients [8, 9]. Another way of finding the viscosity is by simulating Couette shear flow, i.e. computation of the ratio of shear stress and strain rate. The quality of viscosity obtained from these methods strongly depends on the accuracy of the stress, which in turn strongly depends on the used cut-off radius and method used to compute the long-range interactions [10]. Alternatively, the periodic perturbation method [11] can be used, where the viscosity can be calculated from a steady-state velocity profile generated by a periodic external force applied to the system. Compared to the stress, the velocity profile is straightforward to extract from a MD simulation and requires fewer data points to be collected, i.e. less simulation time, in order to obtain statistically meaningful results. Previous calculations of the viscosity for different water models showed that there can be a large difference between the calculated value and the experimental value, as discussed next. Most research concentrated on the SPC/E water model. For example Balasubramanian et al. [12] showed how the viscosity of SPC/E is calculated for T = K, using both equilibrium and non-equilibrium molecular dynamics. The value they found is about 18% less than the experimental value, which is similar to the error that Gou et al. [13] found. Hess et al. [10] used periodic shear flow to calculate the viscosity of the SPC/E water model at T = 300K and found a value about 30% lower than the experimental value. 2
3 Recently, this has also been verified by Chen et al. [14] using the Green-Kubo method. Wu et al. [15] simulated shear flow at T = 298.5K and found an error of about 23% for SPC/E. On the other hand, Bordat et al. [16], using a reverse non-equilibrium molecular dynamics simulation, calculated the value of the viscosity for the SPC/E water model at T = 300K, which was almost the same as the experimental value (within 5% error). Less research is done on the other water models. For example, Yongli et al. [17] calculated the value of the viscosity at several liquid water temperatures for several water models (including the TIP4P model) using the Stokes-Einstein relation and reported errors between 30.3% and 52.3% between experimental values and calculated values. Wensink et al. [18] calculated the value of the viscosity for the TIP4P model at T = K and found an error of approximately 48% compared to the experimental data. Very recently, Song et al. [19] performed non-equilibrium molecular dynamics simulations using the periodic perturbation method to simulate the shear viscosity of five commonly used water models (including the SPC/E and TIP4P model). The value they found for the viscosity of the SPC/E model at T = 300K is 15% less than the experimental value, while the value for the TIP4P model was 41% less than the experimental value. For the TIP4P/Ew water model no data for the viscosity is known to us, while for the TIP4P/2005 only very recently [20] the dependency of the value of the viscosity on the pressure at three different temperatures were calculated using the Green-Kubo method. The conclusion was that, at least at these temperatures, the value of the viscosity is very well predicted (slightly less than 5% error) with the TIP4P/2005 water model. Guevara-Carrion et al. [21] compared the SPC, SPC/E, TIP4P and TIP4P/2005 model for the prediction of several transport properties of pure liquid water and mixtures with methanol and ethanol. They found that the TIP4P/2005 model performed better than the other models over the whole range of properties. However, they note that the TIP4P/2005 model does not predict the properties of the saturated vapor phase correctly. Furthermore, in other recent papers [22 24] it was shown how the TIP4P/2005 water model predicts also other material properties with high accuracy and therefore is a promising water model. In this study, several of the water models (SPC/E, TIP4P, TIP4P/Ew, and TIP4P/2005) are tested on their ability to model the viscosity of liquid water between the temperatures T = 273K and 373K. Especially, it is shown how Poiseuille flow generated inside a nano-sized channel can be used to extract the values of viscosity versus temperature for these models very efficiently. The flow is generated by a constant body force on each of the water molecules inside the channel and the resulting velocity profile is used to calculate the viscosity. This technique was used before to investigate the viscosity of simple 3
4 Figure 1: The MD model of the nano channel. In total 2048 water molecules are placed between two solid atomistic walls each consisting of 648 silicon atoms in four layers. The distance between the centres of the two walls is 4.3 nm. Poiseuille flow is generated by a body force f bx in the x-direction. fluids, like argon [25], and has several benefits compared to the other methods. For example, the reported simulation times needed in order to obtain sufficiently converged statistics to calculate the viscosity using the Green-Kubo method, the Stokes-Einstein method, or the Couette shear flow method are 10, 20, or 60 ns, respectively. Water simulations commonly use a time step of 1 or 2 fs, meaning that several tens of million time steps are required in these simulations and therefore imply a considerable computational effort. Although the periodic perturbation method performs better, where only 2 4 ns of simulation time are needed to obtain the results, good statistics for the velocity profile can be obtained within only 1.2 ns of steady state flow. However, similar to the periodic perturbation method, the viscosity is not obtained in a shear-free situation. Therefore, care must be taken that the shear inside the nano channel does not become too strong. II. METHODS AND SIMULATION DETAILS A. Poiseuille flow in a nano-sized channel The value of the viscosity is calculated for the different water models by examining Poiseuille flow inside a nano-sized channel illustrated in Figure 1. The Poiseuille flow is generated by a constant body force f bx in the x-direction, on each molecule. The channel itself is created by modelling two parallel 4
5 solid atomistic lattice walls at a certain distance from each other in the z-direction. Between the two walls, the water molecules are placed. The boundary conditions in the x and y-direction are periodic, while in the z-direction the water molecules are constricted by the walls. The equation for (Poiseuille) flow inside a channel in this case is: ( d μ du ) x = ρf bx (II.1) dz dz where u x is the (macroscopic) velocity in the x-direction inside the channel, ρ is the liquid density, and μ is the unknown viscosity. The velocity profile and density can be extracted from the MD simulation, while the applied force is known. This gives the possibility to relate the resulting Poiseuille flow to the viscosity of the used water model [26]. However, care must be taken, since for example, Bitsanis et al. [27] showed that, at least for simple liquids like argon, the effective viscosity inside the channel can increase considerably in very narrow channels (with a height smaller than 5 molecular diameters). Similarly, Li et al. [28] found experimentally that the viscosity of water in a subnanometer gap can be several orders of magnitude larger than the viscosity of bulk water at the same phase point. One reason for this is the wall-fluid interaction, which results in a layering effect of atoms near the wall and this effect only gradually disappears away from the wall [29]. However, despite this, approximately quadratic velocity profiles can be obtained for simple liquids confined to channels only 10 molecular diameters in height [30]. For more complex liquids, like water simulated in this study, the same is true, as shown below. Furthermore, in Section III, also the effect of the confinement of the liquid, i.e. the height of the channel, on the value of the viscosity is studied. In order to extract the viscosity from the velocity profile, it is important that only the part of the results are used that show the expected continuum behaviour. To examine this, an equilibrium MD simulation (i.e. without applied flow) is carried out, where the variations near the wall and extent of the variations are studied, especially the density and charge distribution profiles. The density profile should show a region with a constant value, i.e. the expected continuum value, in the middle of the channel, while layering of molecules can be visible near the walls. The charge distribution profile is of interest, because this indirectly gives information about the orientation of the water molecules. However, note that in this study, the wall atoms are deliberately not charged. This means that the well-known phenomenon of the electric double layer [31] is not taken into account and the resulting velocity profile is only due to Poiseuille flow. 5
6 L OH q 1 a) b) q L 1 OH q 2 θ φ q 2 θ q 1 L OD q 1 Table I: The parameters of water models for which the value of viscosity is determined. The meaning of the parameters are schematically illustrated in the figure. SPC/E TIP4P TIP4P/Ew TIP4P/2005 B. Water models Type a b b b ɛ OO ( kj /mol) ( ) σ OO Å q 1 (e) q 2 (e) ( ) L OH Å ( ) L OD Å θ = θ HOH ( o ) ϕ ( o ) All water models that are investigated in this paper have in common that they solve for the following potential energy equation between molecules i and j: [ (σoo ) 12 U =4ɛ OO r OO ( σoo r OO ) ] 6 N + α=1 β=1 N q iα q jβ 4ɛ 0 r iα,jβ Lennard-Jones interaction between the water molecules is only considered between the O-atoms of each molecule i and j, where r OO is the distance between the two atoms. The Lennard-Jones parameters, ɛ OO and σ OO are the interaction energy strength and the distance at which the potential energy is zero, respectively. The Coulombic interaction of the water molecules is computed using a total of N charge sites associated to each water molecule, where q iα is the charge of the α th charge site of molecule i and ɛ 0 is the electrical permittivity of vacuum. The water models investigated in this article use N =3or N =4. The bond lengths and angles are fixed using the SHAKE algorithm [32]. The water models differ in the values of the parameters they use. Table I gives an overview of the used parameters in the four models compared here. 6 (II.2)
7 C. Simulation details For each water model, several MD simulations are performed in a (canonical) NVT ensemble. The temperature in the system is controlled by a Nose-Hoover thermostat [33, 34] and the simulated temperatures range from 273 to 373 K. The walls of the nano channel are placed approximately 4.3 nm apart, measured from the centre of the bottom wall lattice to the centre of the top wall lattice. Each of the atomistic walls consists of four layers of solid atoms, placed and fixed in a fcc lattice, i.e. in a so-called ABAB stacking. The chosen material properties of the wall are based on silicon, which has a density of ρ wall = 2329 kg /m 3 at the initialisation temperature of the MD system, T init = 293 K. Each wall consists of 648 wall atoms, which interact (only) with the oxygen atoms according to the Lennard-Jones 12-6 potential. The Lennard-Jones parameters for the wall in the case of Silicon are: σ wall O = σ Si O =3.24Å and ɛ wall O = ɛ Si O =1.274 kj /mol [35]. These values are the initial/standard values and are used in all simulations, unless stated otherwise. The total number of water molecules between the two walls is These are also initialised in a fcc lattice, with an initial density of ρ init = kg /m 3, and are allowed to melt during the equilibration process [36], which takes 0.3 ns. The value of the body force f bx is chosen such that the typical maximum velocity inside the channel never exceeds 20 m /s. This was done to prevent the shear rate inside the nano channel becoming too large. This maximum velocity is selected since it provides a high signal-to-noise ratio. It was verified that the value of the viscosity was not greatly affected by the amount of generated shear in the channel by performing simulations with different values of f bx. The integration of Newton s equation of motion is performed with the Verlet algorithm [37], while the electrostatic interactions are treated by the Particle-Particle and Particle-Mesh (PPPM) method [38]. However, the MD domain only has periodic boundary conditions specified in two dimensions, the PPPMmethod must be adapted to prevent erroneous summation in the direction perpendicular to the walls. The method adopted here is the 2D-Slab method with an added (vacuum) space at both sides of the wall [39], and includes the ELC term to prevent slab-slab interactions [40, 41]. The grid size for the PPPM method is [ ] with a splitting parameter β =0.305, while the cut-off value for the Lennard-Jones interaction is r c =1.0nm, which is approximately 3σ OO. In order to verify whether the cut-off radius and the used grid size for the PPPM method are sufficient for obtaining the (correct) velocity profile, several simulations are performed using different quantities. This effectively controls the accuracy in obtaining the Lennard-Jones interactions and the electrostatic interactions. The results will show to what degree the velocity profile is influenced by this change. 7
8 density (kg/m 3 ) charge distribution (e/nm) z-position (nm) z-position (nm) Figure 2: The density profile (top) and charge distribution profile (bottom) obtained for the nano channel from the equilibrium MD simulation (ρ init = kg /m 3, T init = 293 K, using the SPC/E water model) For each MD simulation, the total simulation time is 1.5 ns, with a MD time step of 1.0 fs. All results are determined from the last 1.2 ns of the simulation and are obtained by binning the different macroscopic values in 500 bins, which are equally distributed across the z-direction. This relatively small simulation time was verified to be sufficient to obtain consistent values for the viscosity. A test simulation with a total simulation time of 4.2 ns was compared to the shorter simulation. The calculated value of viscosity did not change more than 1% and the statistical error of the data from the longer simulation decreased slightly. III. RESULTS AND DISCUSSION A. Variations near the channel walls First the equilibrium MD simulation is carried out in order to study the variations near the wall. The water model SPC/E is employed for this simulation, for which the parameters can be found in table I. During the simulation, the density and charge distribution values are collected in the bins. Figure 2 (top) shows the density profile while the bottom figure shows the charge distribution across the channel. The density profile shows large variations near the wall around a more or less constant value 8
9 in the middle of the channel. These variations are the result of the interaction of the water molecules with the solid atoms of the wall and can also be observed experimentally [42]. Water molecules spend more time inside certain layers parallel to the walls, where the interaction between the wall and the surrounding liquid is closer to equilibrium than elsewhere. These layers are situated at the peaks visible in the density profile and come from the fact that, due to the strong LJ repulsion, O-atoms have a minimal distance in z-direction, but can be disordered (inside the layer) in x-y direction. On the other hand, because of the dense layer of water molecules, the remaining molecules rarely come too close to this layer because of the strong repulsion at close range. On either side of a dense layer, fewer molecules are present on average, which are the troughs in the density profile. Only when the water molecules are far enough from the wall, the interaction between the surrounding water molecules becomes isotropic due to increased disorder, and the result is that more or less constant density is reached. The charge distribution profile shows that the orientation of the water molecules is also constricted near the walls. The first strong positive peak of the profile indicates that more H-atoms than O-atoms can be found near the wall, while the first trough after the peak shows a strong negative charge indicating a layer of mostly O-atoms coinciding with the first peak of the density profile. The second peak and trough show something similar. On the other hand, in the middle of the channel the average charge is zero, indicating random distribution and orientation of the water molecules. Note that the total charge averaged across the entire profile equals zero, e.g. the water inside the channel is neutral. Although in the simulation the electrokinetic effect caused by charges of the wall and counter-ions in the water, i.e. the electric double layer, was deliberately not simulated, the orientation of the water molecules itself can create a charge distribution in a direction normal to the wall. Near-bulk or continuum conditions are established 1.2 nm away from the walls, indicating the possibility to extract the value of the viscosity. However, the value of the density in the middle of the channel is about 1035 kg /m 3, while the averaged density across the complete density profile is exactly the same as the initialised value, ρ init = kg /m 3. The reason for the higher value of density measured in the middle of the channel is the combination of the Lennard-Jones parameters for the wall-fluid interaction and the resulting interaction with the water molecules. In order to compare the value of the viscosity of each water model to the experimental value of the viscosity, each simulation should be performed with a predefined value of the density in the middle of the channel. The value of the density that is aimed for, is the value at 1 bar for the different temperatures, i.e. ρ (T ) p=const and can be found for example in the book of Bird et al. [43]. There are several ways how 9
10 this can be accomplished. One possibility is to change the distance between the two walls of the channel accordingly. This is comparable to what is done in an NPT-simulation, where the pressure is kept constant by changing the size of the MD simulation box, i.e. effectively changing the volume. Another possibility is to change the number of water molecules between the walls, i.e. effectively changing the mass of fluid. However, we chose to keep the volume and mass constant and used an alternative method to change the bulk density. Namely, the correct density in the middle of the channel is obtained by adjustment of the wall-fluid interaction parameter of the wall, σ wall O. If this value is decreased, water molecules are able to move closer to the wall and therefore, on average, have access to a larger volume between the two walls. This results in a lower density in the middle of the channel. The following simulations are therefore performed with a variable value of the wall-fluid interaction parameter σ wall O. The (macroscopic) density can easily be sampled and does not require a long simulation time before a meaningful value is obtained. Therefore, the correct value of σ wall O can be obtained within the equilibration process (i.e. within 0.3 ns of simulation time) and the remainder of the simulation is performed with this obtained value. Typical values of σ wall O resulting from such a simulation ranged from 0.82 to 1.05 times the nominal value. B. Viscosity of the water models The viscosity will be determined for the four different water models, SPC/E, TIP4P, TIP4P/Ew and TIP4P/2005 for a temperature range of T = 273K 373K by simulating Poiseuille flow in the nano channel. As noted before, the value of the wall-fluid interaction parameter of the wall, σ wall O is changed such that the density in the middle of the channel is equal to the (experimental) value of the density of water at a pressure of 1 bar. Furthermore, the interaction strength between the wall and the water molecules is changed/increased to: ɛ wall O =3ɛ Si O =3.822 kj /mol. This was done in order to reduce any significant slip developing near the wall. Figure 3 shows a typical velocity profile obtained from such a simulation. As expected, the velocity profile is similar to a Poiseuille flow velocity profile, with only minor differences very near the wall. In order to determine the viscosity from the velocity profile, equation II.1 is rewritten to: μ = ρf bx d 2 u x/dz 2 Effectively, this means that the second derivative of the velocity profile needs to be measured and the easiest way to do this is to curve fit the data points of the velocity profile from the MD simulation with 10
11 points for fit velocity (m/s) z-position (nm) Figure 3: A typical velocity profile from one of the simulations of water inside a nanochannel (using TIP4P/2005). An illustration of the fraction of the velocity profile used for the curve fit is given. Table II: The values of viscosity from the four water models as obtained from the fitted velocity profile and the errors in percent between the calculated and experimental values of the viscosity of water. T (K) SPC/E TIP4P TIP4P/Ew TIP4P/2005 Experiment μ(mpas) (μ μ exp) μ exp μ(mpas) (μ μ exp) μ exp μ(mpas) (μ μ exp) μ exp μ(mpas) (μ μ exp) μ exp μ exp (mpas) ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % ± % a parabola. From this fitted velocity profile, the (analytical) second derivative with respect to the height of the channel can be taken easily. In detail, the data points, e.g. as shown in Figure 3, are fitted to a function described by u = u 0 + a (z z 0 ) 2, where the fitting parameters u 0 and a need to be determined and the parameter z 0 is taken as the middle of the channel. Doing so, means the obtained velocity profile is assumed to be symmetric, while the fitting parameter a is directly related to the second derivative and 11
12 therefore the viscosity by: μ = ρf bx/2a. However, because this value is sensitive to the actual fit, multiple symmetric curve fits of different (continuum-like) sections of the (same) velocity profile of the MD results are taken. The sections of the velocity profile that are used for the fit, are described by the fraction of the total velocity profile, where the value of 1.0 means the obtained velocity profile from wall to wall is used. In total 10 fits for each MD velocity profile are performed. The fractions for the set of curve fits are: 0.80, 0.75, 0.70, 0.65, 0.60, 0.55, 0.50, 0.45, 0.40, and This means that for each simulation a set of ten values of a is obtained including ten fit errors. Therefore, the final value for the viscosity is obtained by averaging the resulting set of fits and the error of the fitted value is estimated using the corresponding 95% confidence bounds of the set of fits. Therefore, note that the error described in the results is the fitting error of the curve rather than the statistical error resulting from the MD simulation. However, before the results of the value of viscosity for several water models are discussed, first the method is compared to results obtained using different methods. As mentioned in the introduction, the method to obtain the viscosity using the velocity profile in a MD simulation was used by Koplik et al. [25] for argon. The value of viscosity they computed was very similar to the value obtained using other methods, while the cut-off radius they used was (only) 2.5σ. It is expected that in the case when water is simulated, the same method to determine the viscosity can be used, however first it must be determined whether the velocity profile, and therefore the computed value of the viscosity, is not highly influenced by simulation parameters like the confinement of the water molecules between the two walls, the used cut-off radius, and the accuracy of the evaluation of the electrostatic interactions. To test this, four independent simulations are performed where one of the parameters is changed and the velocity profile and viscosity are compared to one reference simulation. The reference simulation uses the simulation parameters as specified in Section II C. All these simulations are done with the TIP4P water model at T = K, for which at least three different values of the viscosity can be found in literature. For example, Wensink et al. [18] calculated the value of the viscosity for the TIP4P model at T = K and found the values μ =0.464 ± mpas (48.2% error) and μ =0.479 ± mpas (46.5% error) using the periodic perturbation method. Song et al. [19] used the same method and found the values μ =0.505 ± mpas (43.6% error) and μ =0.506 ± mpas (43.5% error) for the TIP4P model at T = 300K. Finally, Yongli et al. [17] used the Stokes-Einstein relation and reported only errors, which are between 30.3% and 52.3% between experimental values and calculated values. The reference simulation performed using the method described in this article leads to a value for the viscosity: μ =0.481 ± mpas (46.3% 12
13 error) and therefore similar to the results obtained by others using different methods. In order to verify the sensitivity of several model parameters on the viscosity, the four other simulations are compared to this value. The first simulation compares the results of the reference simulation to the results obtained from a simulation where the distance of the walls is 6.4 nm which is 1.5 times larger than the reference case. This means the number of water molecules inside the channel is N = 3072 and the fluid is less confined, i.e. a larger part of the density profile does not show variations, compared to the reference case. In order to maintain the same accuracy in obtaining the electric interactions compared to the reference simulation, the PPPM grid size also needs to be increased. In order to do so, the method described by Deserno et al. [44] is used. The resulting PPPM grid size is [ ] with a splitting parameter β = The cut-off value for the Lennard-Jones interaction is kept at r c =1.0nm. As expected, the resulting density profile of this simulation showed a larger range where the value is the (expected) continuum value, while only near the walls large variations are visible, similar to those in Figure II A. However, the shape of the velocity profile did not change much. The viscosity is calculated from this velocity profile and the resulting value is: μ =0.508 ± mpas (43.2% error), which is the range of errors obtained by others previously. The next three simulations deal with the influence of the used cut-off radius on the velocity profile and the value of viscosity. The first two simulations check whether the PPPM size and therefore the accuracy of the electric interaction has an influence. In order to test this, the following grid sizes are compared: [ ] and [ ], with splitting parameter β =0.253 and β =0.350, respectively. This corresponds to a factor 10 decrease and increase in accuracy compared to the reference simulation, respectively. The results from these simulation showed that the computed values of viscosity are: μ =0.516 ± mpas (42.4% error) and μ =0.531 ± mpas (40.7% error), in the case of lower and higher accuracy, respectively. The third simulation tests whether the cut-off radius used for the Lennard-Jones interactions has an influence. This simulation uses a cut-off radius of: r c =1.2nm, while the accuracy of the electric interactions remains the same. The results from this simulation leads to a viscosity of: μ =0.455 ± (49.1% error). In conclusion, the results show that the calculated values of the viscosity are not highly sensitive to the change of simulation parameters like the PPPM size, cut-off radius, and size of the MD domain. The values of the viscosity that are obtained range from mpas to mpas, which means the error between the experimental and the calculated value is between 40.7% and 49.1%. This is within the range 13
14 Viscosity (mpa.s) Experiment TIP4P/2005 TIP4P/Ew SPC/E TIP4P Temperature (K) Figure 4: The values of the viscosity as a function of the temperature obtained from the curve fit of the velocity profile for the four different water models. The errors of the fits is also displayed. The lines in the figure are obtained from a fit of the type: μ =(T T 0 ) b, where the fit with experimental data from Bird et al. [43] is used for reference. Table III: The parameters obtained from the fit of the type μ =(T T 0 ) b from the data points as shown in figure 4. Experiment TIP4P/2005 TIP4P/Ew SPC/E TIP4P T o b of errors obtained by others using different methods. Further research is necessary in order to examine the full extend of the influence of the simulation parameters on the viscosity. Next the results of the simulations using the four different water models are discussed. Figure 4 shows the obtained values for the viscosity and the errors of the fits for the four different water models. The experimental values of the viscosity are displayed for reference. The curves are obtained from a fit of the type: μ =(T T 0 ) b, where temperatures are scaled by units Kelvin (K) and μ is in units of mpas, fitted to experimental data, e.g. from Bird et al. [43], where T 0 = K and b = Table II shows the obtained values of viscosity from the four different water models and the deviation from the experimental value of viscosity in percent. Table III shows the fit parameters obtained from the simulation results of the four water models. The results show that the TIP4P water model severely underpredicts the value of the viscosity at all liquid water temperatures, especially at the lower temperatures, where the deviation from the experi- 14
15 mental value is 45% to 60%, as found also by others [17, 18]. The SPC/E model and the TIP4P/Ew water model show comparable performance in predicting the value of the viscosity. The TIP4P/Ew water model is slightly better, but does so with more computational effort because of the extra interaction site involved. In general the deviations are about 15% to 30% for the SPC/E water model and 10% to 25% for the TIP4P/Ew water model at the lower temperatures. The error for the SPC/E water model at T = 293K and T = 303K are very similar to the errors reported by others [10, 12 15]. Both models predict the value of the viscosity within 10% to 15% for the higher temperatures. However, the TIP4P/2005 water model performed best. In general, the errors for the whole range of liquid water temperatures are below 8%, besides two outliers that are below 15%. Overall, the averaged data confirm the above conclusions; when the values are averaged over the whole temperature-range, the TIP4P model underpredicts the viscosity by as much as 39%, the SPC/E and TIP4P/Ew water models underpredict the viscosity by 19% and 15%, respectively, while the TIP4P/2005 water model underpredicts the value of viscosity by (only) 3%. IV. CONCLUSIONS The numerical results presented in this paper show how the viscosity for four different water models is calculated and how it compares to experimental values. This was accomplished by simulating Poiseuille flow in a MD nano channel. The value of the viscosity was determined by curve fitting the resulting velocity profile and comparison of the profile to a continuum Newtonian solution. The benefit of using this method is the fact that good statistics for the velocity profile can be obtained within only 1.2 ns of steady state flow, which is considerably faster than alternative methods for finding the viscosity from MD simulations. The results from the simulations showed that of the four models considered here the TIP4P/2005 water model gives the best prediction of the viscosity over a wide range of temperatures of liquid water, the TIP4P model performs worst, while the SPC/E and TIP4P/Ew water model performed similar and resulted in moderate accuracy for the value of the viscosity. Therefore, if a simulation is required where the viscosity plays an important role, the TIP4P/2005 water model is recommended. Finally, note that in this study, several parameters are varied and the effect of varying these for most of them is small. However, a more detailed parameter study is needed to understand the possibility of a systematic dependency of the viscosity on system and model parameters like, e.g. system size, cut-off radius or wall properties. Furthermore, the models were not evaluated concerning the phase transitions or other more advanced properties of water, neither was the polarisability of water taken into account, so 15
16 that enough options for future research remain. [1] H. Berendsen, J. Grigera, and T. Straatsma, J. Phys. Chem. 91, 6269 (1987). [2] W. Jorgensen and J. Madura, Mol. Phys. 56, 1381 (1985). [3] H. Horn, W. Swope, J. Pitera, J. Madura, T. Dick, G. Hura, and T. Head-Gordon, J. Chem. Phys. 120, 9665 (2004). [4] J. Abascal and C. Vega, J. Chem. Phys. 123, (2005). [5] M. Green, J. Chem. Phys. 22, 398 (1954). [6] R. Kubo, J. Phys. Soc. Japan 12, 570 (1957). [7] A. Einstein, Investigations on the Theory of the Brownian Movement (Dover, New York, 1956). [8] R. García-Rojo, S. Luding, and J. Brey, Phys. Rev. E 74 (2006). [9] V. Kumaran, Phys. Rev. Lett. 96 (2006). [10] B. Hess, J. Chem. Phys. 116, 209 (2002). [11] E. Gosling, I. McDonald, and K. Singer, Mol. Phys. 26, 1475 (1973). [12] S. Balasubramanian, C. Mundy, and M. Klein, J. Chem. Phys. 105, (1996). [13] G. Guo and Y. Zhang, Mol. Phys. 99, 283 (2001). [14] T. Chen, B. Smit, and A. Bell, J. Chem. Phys. 131, (2009). [15] Y. Wu, H. Tepper, and G. Voth, J. Chem. Phys. 124, (2006). [16] P. Bordat and F. Müller-Plathe, J. Chem. Phys. 116, 3362 (2002). [17] S. Yongli, S. Minhua, C. Weidong, M. Congxiao, and L. Fang, Comp. Mat. Science 38, 737 (2007). [18] E. Wensink, A. Hoffmann, P. van Maaren, and D. van der Spoel, J. Chem. Phys. 119, 7308 (2003). [19] Y. Song and L. Dai, Mol. Sim. 36, 560 (2010). [20] M. Gonzàlez and J. Abascal, J. Chem. Phys. 132, (2010). [21] G. Guevara-Carrion, J. Vrabec, and H. Hasse, J. Chem. Phys. 134, (2011). [22] J. Alejandre and G. Chapela, J. Chem. Phys. 132, (2010). [23] J. Abascal, E. Sanz, and C. Vega, Phys. Chem. Chem. Phys. 11, 556 (2009). [24] H. Pi, J. Aragones, C. Vega, E. Noya, J. Abascal, M. Gonzalez, and C. McBride, Mol. Phys. 107, 365 (2009). [25] J. Koplik, J. Banavar, and J. Willemsen, Phys. Rev. Lett. 60, 1282 (1988). [26] B. Todd and D. Evans, J. Chem. Phys. 103, 9804 (1995). 16
17 [27] I. Bitsanis, S. Somers, H. Davis, and M. Tirell, J. Chem. Phys. 93, 3427 (1990). [28] T. Li, J. Gao, R. Szoszkiewicz, U. Landman, and E. Riedo, Phys. Rev. B 75, (2007). [29] R. Hartkamp and S. Luding, in International Conference on Multiphase Flow. Tampa, Florida, May 2010 (2010). [30] K. Travis, B. Todd, and D. Evans, Phys. Rev. E 55, 4288 (1997). [31] J. Lyklema, Fundamentals of Interface and Colloid Science, Volume II (Academic Press Limited, 1995). [32] J. Ryckaert, G. Ciccotti, and H. Berendsen, J. Comp. Phys. 23, 327 (1977). [33] S. Nosé, J. Chem. Phys. 81, 511 (1984). [34] W. Hoover, Phys. Rev. A 31, 1695 (1985). [35] G. Karniadakis, A. Beskok, and N. Aluru, Microflows and nanoflows: fundamentals and simulation (Springer, 2005). [36] D. Frenkel and B. Smit, Understanding Molecular Simulations: From Algorithms to Applications, 2nd ed. (Academic Press, 2002). [37] L. Verlet, Phys. Rev. 159 (1967). [38] R. Hockney and J. Eastwood, Computer Simulation Using Particles (IOP, 1988). [39] E. Spohr, J. Chem. Phys. 107, 6342 (1997). [40] A. Arnold, J. de Joannis, and C. Holm, J. Chem. Phys. 117, 2496 (2002). [41] J. de Joannis, A. Arnold, and C. Holm, J. Chem. Phys. 117, 2503 (2002). [42] D. Chan and R. Horn, J. Chem. Phys. 83, 5311 (1985). [43] R. Bird, W. Stewart, and E. Lightfoot, Transport Phenomena, 2nd ed. (Wiley, 2002). [44] M. Deserno and C. Holm, J. Chem. Phys. 109, 7694 (1998). 17
Chemical Physics Letters
 Chemical Physics Letters 542 (2012) 37 41 Contents lists available at SciVerse ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Thermal conductivity, shear viscosity
Chemical Physics Letters 542 (2012) 37 41 Contents lists available at SciVerse ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Thermal conductivity, shear viscosity
Supporting Information for Solid-liquid Thermal Transport and its Relationship with Wettability and the Interfacial Liquid Structure
 Supporting Information for Solid-liquid Thermal Transport and its Relationship with Wettability and the Interfacial Liquid Structure Bladimir Ramos-Alvarado, Satish Kumar, and G. P. Peterson The George
Supporting Information for Solid-liquid Thermal Transport and its Relationship with Wettability and the Interfacial Liquid Structure Bladimir Ramos-Alvarado, Satish Kumar, and G. P. Peterson The George
Molecular Dynamics Simulation of a Nanoconfined Water Film
 Molecular Dynamics Simulation of a Nanoconfined Water Film Kyle Lindquist, Shu-Han Chao May 7, 2013 1 Introduction The behavior of water confined in nano-scale environment is of interest in many applications.
Molecular Dynamics Simulation of a Nanoconfined Water Film Kyle Lindquist, Shu-Han Chao May 7, 2013 1 Introduction The behavior of water confined in nano-scale environment is of interest in many applications.
Heat capacity of water: a signature of nuclear quantum effects. Abstract
 Heat capacity of water: a signature of nuclear quantum effects C. Vega a, M. M. Conde a, C. McBride a, J. L. F. Abascal a, E. G. Noya b, R. Ramirez c and L. M. Sesé d a Departamento de Química Física,
Heat capacity of water: a signature of nuclear quantum effects C. Vega a, M. M. Conde a, C. McBride a, J. L. F. Abascal a, E. G. Noya b, R. Ramirez c and L. M. Sesé d a Departamento de Química Física,
MD simulation of methane in nanochannels
 MD simulation of methane in nanochannels COCIM, Arica, Chile M. Horsch, M. Heitzig, and J. Vrabec University of Stuttgart November 6, 2008 Scope and structure Molecular model for graphite and the fluid-wall
MD simulation of methane in nanochannels COCIM, Arica, Chile M. Horsch, M. Heitzig, and J. Vrabec University of Stuttgart November 6, 2008 Scope and structure Molecular model for graphite and the fluid-wall
Atomistic simulation of electro-osmosis in a nanometer-scale channel
 Center for Turbulence Research Proceedings of the Summer Program Atomistic simulation of electro-osmosis in a nanometer-scale channel By J. B. Freund An atomistic simulation of an electro-osmostic flow
Center for Turbulence Research Proceedings of the Summer Program Atomistic simulation of electro-osmosis in a nanometer-scale channel By J. B. Freund An atomistic simulation of an electro-osmostic flow
Non-Equilibrium Molecular Dynamics. Investigation of Parameters Affecting Planar. Nanochannel Flows
 Contemporary Engineering Sciences, Vol., 009, no. 6, 83-98 Non-Equilibrium Molecular Dynamics Investigation of Parameters Affecting Planar Nanochannel Flows F. Sofos, T. E. Karakasidis and A. Liakopoulos
Contemporary Engineering Sciences, Vol., 009, no. 6, 83-98 Non-Equilibrium Molecular Dynamics Investigation of Parameters Affecting Planar Nanochannel Flows F. Sofos, T. E. Karakasidis and A. Liakopoulos
Stick and Slip Behaviour of Confined Oligomer Melts under Shear. A Molecular-Dynamics Study.
 EUROPHYSICS LETTERS Europhys. Lett., 24 (2), pp. 99-104 (1993) 10 October 1993 Stick and Slip Behaviour of Confined Oligomer Melts under Shear. A Molecular-Dynamics Study. E. MANIAS(*), G. HADZIIOANNOU(*),
EUROPHYSICS LETTERS Europhys. Lett., 24 (2), pp. 99-104 (1993) 10 October 1993 Stick and Slip Behaviour of Confined Oligomer Melts under Shear. A Molecular-Dynamics Study. E. MANIAS(*), G. HADZIIOANNOU(*),
Modeling the combined effect of surface roughness and shear rate on slip flow of simple fluids
 Modeling the combined effect of surface roughness and shear rate on slip flow of simple fluids Anoosheh Niavarani and Nikolai Priezjev www.egr.msu.edu/~niavaran November 2009 A. Niavarani and N.V. Priezjev,
Modeling the combined effect of surface roughness and shear rate on slip flow of simple fluids Anoosheh Niavarani and Nikolai Priezjev www.egr.msu.edu/~niavaran November 2009 A. Niavarani and N.V. Priezjev,
A comparative study between dissipative particle dynamics and molecular dynamics for simple- and complex-geometry flows
 THE JOURNAL OF CHEMICAL PHYSICS 123, 104107 2005 A comparative study between dissipative particle dynamics and molecular dynamics for simple- and complex-geometry flows Eric E. Keaveny, Igor V. Pivkin,
THE JOURNAL OF CHEMICAL PHYSICS 123, 104107 2005 A comparative study between dissipative particle dynamics and molecular dynamics for simple- and complex-geometry flows Eric E. Keaveny, Igor V. Pivkin,
Investigations of Freezing Pure Water
 Investigations of Freezing Pure Water David Meldgin Constanze Kalcher May 2, 2013 Abstract We use a the molecular simulation package LAAMPS to simulate the freezing of water. We analyze the SPC and TIP3P
Investigations of Freezing Pure Water David Meldgin Constanze Kalcher May 2, 2013 Abstract We use a the molecular simulation package LAAMPS to simulate the freezing of water. We analyze the SPC and TIP3P
Comparative Study of the Water Response to External Force at Nanoscale and Mesoscale
 Copyright 2013 Tech Science Press CMES, vol.95, no.4, pp.303-315, 2013 Comparative Study of the Water Response to External Force at Nanoscale and Mesoscale H.T. Liu 1,2, Z. Chen 2, S. Jiang 2, Y. Gan 3,
Copyright 2013 Tech Science Press CMES, vol.95, no.4, pp.303-315, 2013 Comparative Study of the Water Response to External Force at Nanoscale and Mesoscale H.T. Liu 1,2, Z. Chen 2, S. Jiang 2, Y. Gan 3,
Supporting Information
 Supporting Information Interface-Induced Affinity Sieving in Nanoporous Graphenes for Liquid-Phase Mixtures Yanan Hou, Zhijun Xu, Xiaoning Yang * State Key Laboratory of Material-Orientated Chemical Engineering,
Supporting Information Interface-Induced Affinity Sieving in Nanoporous Graphenes for Liquid-Phase Mixtures Yanan Hou, Zhijun Xu, Xiaoning Yang * State Key Laboratory of Material-Orientated Chemical Engineering,
Effects of molecular level surface roughness on electroosmotic flow
 Microfluid Nanofluid (7) 3: 33 38 DOI 1.17/s144-6-13-x RESEARCH PAPER R. Qiao Effects of molecular level surface roughness on electroosmotic flow Received: 4 March 6 / Accepted: 15 May 6 / Published online:
Microfluid Nanofluid (7) 3: 33 38 DOI 1.17/s144-6-13-x RESEARCH PAPER R. Qiao Effects of molecular level surface roughness on electroosmotic flow Received: 4 March 6 / Accepted: 15 May 6 / Published online:
Coupled continuum hydrodynamics and molecular dynamics method for multiscale simulation
 Coupled continuum hydrodynamics and molecular dynamics method for multiscale simulation Matthew K. BORG 1,, Duncan A. LOCKERBY 2, Jason M. REESE 1 * Corresponding author: Tel.: +44() 141 548 4386; Email:
Coupled continuum hydrodynamics and molecular dynamics method for multiscale simulation Matthew K. BORG 1,, Duncan A. LOCKERBY 2, Jason M. REESE 1 * Corresponding author: Tel.: +44() 141 548 4386; Email:
Introduction to Computer Simulations of Soft Matter Methodologies and Applications Boulder July, 19-20, 2012
 Introduction to Computer Simulations of Soft Matter Methodologies and Applications Boulder July, 19-20, 2012 K. Kremer Max Planck Institute for Polymer Research, Mainz Overview Simulations, general considerations
Introduction to Computer Simulations of Soft Matter Methodologies and Applications Boulder July, 19-20, 2012 K. Kremer Max Planck Institute for Polymer Research, Mainz Overview Simulations, general considerations
A Control Algorithm for Multiscale Simulations of Liquid Water
 A Control Algorithm for Multiscale Simulations of Liquid Water Evangelos M. Kotsalis and Petros Koumoutsakos Computational Science, ETH Zürich, CH-8092, Switzerland petros@ethz.ch Abstract. We present
A Control Algorithm for Multiscale Simulations of Liquid Water Evangelos M. Kotsalis and Petros Koumoutsakos Computational Science, ETH Zürich, CH-8092, Switzerland petros@ethz.ch Abstract. We present
STRUCTURE OF IONS AND WATER AROUND A POLYELECTROLYTE IN A POLARIZABLE NANOPORE
 International Journal of Modern Physics C Vol. 2, No. 9 (29) 1485 1492 c World Scientific Publishing Company STRUCTURE OF IONS AND WATER AROUND A POLYELECTROLYTE IN A POLARIZABLE NANOPORE LEI GUO and ERIK
International Journal of Modern Physics C Vol. 2, No. 9 (29) 1485 1492 c World Scientific Publishing Company STRUCTURE OF IONS AND WATER AROUND A POLYELECTROLYTE IN A POLARIZABLE NANOPORE LEI GUO and ERIK
International Journal of Heat and Mass Transfer
 International Journal of Heat and Mass Transfer 52 (2009) 735 743 Contents lists available at ScienceDirect International Journal of Heat and Mass Transfer journal homepage: www.elsevier.com/locate/ijhmt
International Journal of Heat and Mass Transfer 52 (2009) 735 743 Contents lists available at ScienceDirect International Journal of Heat and Mass Transfer journal homepage: www.elsevier.com/locate/ijhmt
Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
 Bull. Mater. Sci., Vol. 31, No. 3, June 2008, pp. 525 532. Indian Academy of Sciences. Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
Bull. Mater. Sci., Vol. 31, No. 3, June 2008, pp. 525 532. Indian Academy of Sciences. Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
Molecular Dynamics Simulation of Methanol-Water Mixture
 Molecular Dynamics Simulation of Methanol-Water Mixture Palazzo Mancini, Mara Cantoni University of Urbino Carlo Bo Abstract In this study some properties of the methanol-water mixture such as diffusivity,
Molecular Dynamics Simulation of Methanol-Water Mixture Palazzo Mancini, Mara Cantoni University of Urbino Carlo Bo Abstract In this study some properties of the methanol-water mixture such as diffusivity,
Equilibrium molecular dynamics studies on nanoscale-confined fluids
 DOI 10.1007/s10404-011-0794-5 RESEARCH PAPER Equilibrium molecular dynamics studies on nanoscale-confined fluids Murat Barisik Ali Beskok Received: 26 January 2011 / Accepted: 18 March 2011 Ó Springer-Verlag
DOI 10.1007/s10404-011-0794-5 RESEARCH PAPER Equilibrium molecular dynamics studies on nanoscale-confined fluids Murat Barisik Ali Beskok Received: 26 January 2011 / Accepted: 18 March 2011 Ó Springer-Verlag
Molecular Dynamics. Timothy O Sullivan Supervised by Naida Lacevic & John Sader The University of Melbourne
 Molecular Dynamics Timothy O Sullivan Supervised by Naida Lacevic & John Sader The University of Melbourne 1 1 Introduction Molecular Dynamics (MD) is a method of simulating systems of nanoscale volumes,
Molecular Dynamics Timothy O Sullivan Supervised by Naida Lacevic & John Sader The University of Melbourne 1 1 Introduction Molecular Dynamics (MD) is a method of simulating systems of nanoscale volumes,
Supporting information
 Electronic Supplementary Material ESI for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting information Understanding three-body contributions to coarse-grained force
Electronic Supplementary Material ESI for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting information Understanding three-body contributions to coarse-grained force
Scientific Computing II
 Scientific Computing II Molecular Dynamics Simulation Michael Bader SCCS Summer Term 2015 Molecular Dynamics Simulation, Summer Term 2015 1 Continuum Mechanics for Fluid Mechanics? Molecular Dynamics the
Scientific Computing II Molecular Dynamics Simulation Michael Bader SCCS Summer Term 2015 Molecular Dynamics Simulation, Summer Term 2015 1 Continuum Mechanics for Fluid Mechanics? Molecular Dynamics the
arxiv: v1 [cond-mat.soft] 26 Nov 2018
![arxiv: v1 [cond-mat.soft] 26 Nov 2018 arxiv: v1 [cond-mat.soft] 26 Nov 2018](/thumbs/90/104450801.jpg) Water diffusion in rough carbon nanotubes Bruno Henrique da Silva e Mendonça a,, Patricia Ternes a, Evy Salcedo b, Alan B. de Oliveira c, Marcia C. Barbosa a a Instituto de Física, Universidade Federal
Water diffusion in rough carbon nanotubes Bruno Henrique da Silva e Mendonça a,, Patricia Ternes a, Evy Salcedo b, Alan B. de Oliveira c, Marcia C. Barbosa a a Instituto de Física, Universidade Federal
Universal Repulsive Contribution to the. Solvent-Induced Interaction Between Sizable, Curved Hydrophobes: Supporting Information
 Universal Repulsive Contribution to the Solvent-Induced Interaction Between Sizable, Curved Hydrophobes: Supporting Information B. Shadrack Jabes, Dusan Bratko, and Alenka Luzar Department of Chemistry,
Universal Repulsive Contribution to the Solvent-Induced Interaction Between Sizable, Curved Hydrophobes: Supporting Information B. Shadrack Jabes, Dusan Bratko, and Alenka Luzar Department of Chemistry,
Introduction to molecular dynamics
 1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
Proceedings of the ASME 2009 International Mechanical Engineering Congress & Exposition
 Proceedings of the ASME 9 International Mechanical Engineering Congress & Exposition IMECE9 November 3-9, Lake Buena Vista, Florida, USA Proceedings of the ASME International Mechanical Engineering Congress
Proceedings of the ASME 9 International Mechanical Engineering Congress & Exposition IMECE9 November 3-9, Lake Buena Vista, Florida, USA Proceedings of the ASME International Mechanical Engineering Congress
Simulation of Nematic-Isotropic Phase Coexistence in Liquid Crystals under Shear
 Simulation of Nematic-Isotropic Phase Coexistence in Liquid Crystals under Shear Guido Germano 1,2 and Friederike Schmid 2 1 Physical Chemistry Philipps-University 3532 Marburg, Germany E-mail: germano@staff.uni-marburg.de
Simulation of Nematic-Isotropic Phase Coexistence in Liquid Crystals under Shear Guido Germano 1,2 and Friederike Schmid 2 1 Physical Chemistry Philipps-University 3532 Marburg, Germany E-mail: germano@staff.uni-marburg.de
On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water
 On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water Orsolya Gereben and László Pusztai Research Institute for
On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water Orsolya Gereben and László Pusztai Research Institute for
Interface Resistance and Thermal Transport in Nano-Confined Liquids
 1 Interface Resistance and Thermal Transport in Nano-Confined Liquids Murat Barisik and Ali Beskok CONTENTS 1.1 Introduction...1 1.2 Onset of Continuum Behavior...2 1.3 Boundary Treatment Effects on Interface
1 Interface Resistance and Thermal Transport in Nano-Confined Liquids Murat Barisik and Ali Beskok CONTENTS 1.1 Introduction...1 1.2 Onset of Continuum Behavior...2 1.3 Boundary Treatment Effects on Interface
Effects of wall roughness on flow in nanochannels
 PHYSICAL REVIEW E 79, 2635 29 Effects of wall roughness on flow in nanochannels Filippos D. Sofos, Theodoros E. Karakasidis,* and Antonios Liakopoulos Hydromechanics and Environmental Engineering Laboratory,
PHYSICAL REVIEW E 79, 2635 29 Effects of wall roughness on flow in nanochannels Filippos D. Sofos, Theodoros E. Karakasidis,* and Antonios Liakopoulos Hydromechanics and Environmental Engineering Laboratory,
Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases
 MD Simulation of Diatomic Gases Bull. Korean Chem. Soc. 14, Vol. 35, No. 1 357 http://dx.doi.org/1.51/bkcs.14.35.1.357 Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases Song
MD Simulation of Diatomic Gases Bull. Korean Chem. Soc. 14, Vol. 35, No. 1 357 http://dx.doi.org/1.51/bkcs.14.35.1.357 Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases Song
Oleg A. Mazyar, Guoai Pan and Clare McCabe*
 Molecular Physics Vol. 107, No. 14, 20 July 2009, 1423 1429 RESEARCH ARTICLE Transient time correlation function calculation of the viscosity of a molecular fluid at low shear rates: a comparison of stress
Molecular Physics Vol. 107, No. 14, 20 July 2009, 1423 1429 RESEARCH ARTICLE Transient time correlation function calculation of the viscosity of a molecular fluid at low shear rates: a comparison of stress
Dissipative Particle Dynamics: Foundation, Evolution and Applications
 Dissipative Particle Dynamics: Foundation, Evolution and Applications Lecture 4: DPD in soft matter and polymeric applications George Em Karniadakis Division of Applied Mathematics, Brown University &
Dissipative Particle Dynamics: Foundation, Evolution and Applications Lecture 4: DPD in soft matter and polymeric applications George Em Karniadakis Division of Applied Mathematics, Brown University &
The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity
 Draft: September 29, 1999 The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity N. G. Fuller 1 and R. L. Rowley 1,2 Abstract The influence of model flexibility upon simulated viscosity
Draft: September 29, 1999 The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity N. G. Fuller 1 and R. L. Rowley 1,2 Abstract The influence of model flexibility upon simulated viscosity
WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION
 WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION Taro Yamada 1 and Ryosuke Matsuzaki 2 1 Department of Mechanical Engineering, Tokyo University of Science, 2641 Yamazaki, Noda,
WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION Taro Yamada 1 and Ryosuke Matsuzaki 2 1 Department of Mechanical Engineering, Tokyo University of Science, 2641 Yamazaki, Noda,
Supporting Information for: Physics Behind the Water Transport through. Nanoporous Graphene and Boron Nitride
 Supporting Information for: Physics Behind the Water Transport through Nanoporous Graphene and Boron Nitride Ludovic Garnier, Anthony Szymczyk, Patrice Malfreyt, and Aziz Ghoufi, Institut de Physique de
Supporting Information for: Physics Behind the Water Transport through Nanoporous Graphene and Boron Nitride Ludovic Garnier, Anthony Szymczyk, Patrice Malfreyt, and Aziz Ghoufi, Institut de Physique de
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Ion-Gated Gas Separation through Porous Graphene
 Online Supporting Information for: Ion-Gated Gas Separation through Porous Graphene Ziqi Tian, Shannon M. Mahurin, Sheng Dai,*,, and De-en Jiang *, Department of Chemistry, University of California, Riverside,
Online Supporting Information for: Ion-Gated Gas Separation through Porous Graphene Ziqi Tian, Shannon M. Mahurin, Sheng Dai,*,, and De-en Jiang *, Department of Chemistry, University of California, Riverside,
Force Field for Water Based on Neural Network
 Force Field for Water Based on Neural Network Hao Wang Department of Chemistry, Duke University, Durham, NC 27708, USA Weitao Yang* Department of Chemistry, Duke University, Durham, NC 27708, USA Department
Force Field for Water Based on Neural Network Hao Wang Department of Chemistry, Duke University, Durham, NC 27708, USA Weitao Yang* Department of Chemistry, Duke University, Durham, NC 27708, USA Department
Effect of Nanostructure on Rapid Boiling of Water on a Hot Copper Plate: a Molecular Dynamics Study
 Effect of Nanostructure on Rapid Boiling of Water on a Hot Copper Plate: a Molecular Dynamics Study Ting Fu 1,2,3, Yijin Mao 3, Yong Tang 1 *,Yuwen Zhang 3 and Wei Yuan 1 1 Key Laboratory of Surface Functional
Effect of Nanostructure on Rapid Boiling of Water on a Hot Copper Plate: a Molecular Dynamics Study Ting Fu 1,2,3, Yijin Mao 3, Yong Tang 1 *,Yuwen Zhang 3 and Wei Yuan 1 1 Key Laboratory of Surface Functional
Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study
 Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study Julia Deitz, Yeneneh Yimer, and Mesfin Tsige Department of Polymer Science University of Akron
Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study Julia Deitz, Yeneneh Yimer, and Mesfin Tsige Department of Polymer Science University of Akron
Supplementary Information. Surface Microstructure Engenders Unusual Hydrophobicity in. Phyllosilicates
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Surface Microstructure Engenders Unusual Hydrophobicity in Phyllosilicates
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Surface Microstructure Engenders Unusual Hydrophobicity in Phyllosilicates
1.3 Molecular Level Presentation
 1.3.1 Introduction A molecule is the smallest chemical unit of a substance that is capable of stable, independent existence. Not all substances are composed of molecules. Some substances are composed of
1.3.1 Introduction A molecule is the smallest chemical unit of a substance that is capable of stable, independent existence. Not all substances are composed of molecules. Some substances are composed of
Surface tension of the most popular models of water by using the test-area simulation method
 THE JOURNAL OF CHEMICAL PHYSICS 126, 154707 2007 Surface tension of the most popular models of water by using the test-area simulation method C. Vega a Departamento de Química Física, Facultad de Ciencias
THE JOURNAL OF CHEMICAL PHYSICS 126, 154707 2007 Surface tension of the most popular models of water by using the test-area simulation method C. Vega a Departamento de Química Física, Facultad de Ciencias
Efficient viscosity estimation from molecular dynamics simulation via momentum impulse relaxation
 JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 6 8 AUGUST 2000 Efficient viscosity estimation from molecular dynamics simulation via momentum impulse relaxation Gaurav Arya, Edward J. Maginn, a) and Hsueh-Chia
JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 6 8 AUGUST 2000 Efficient viscosity estimation from molecular dynamics simulation via momentum impulse relaxation Gaurav Arya, Edward J. Maginn, a) and Hsueh-Chia
Supporting Information
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting Information Order in the chaos: The secret of the large negative entropy
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting Information Order in the chaos: The secret of the large negative entropy
PH 548 Atomistic Simulation Techniques
 PH 548 Atomistic Simulation Techniques Lectures: Lab: 2-0-2-6 Tuesday 12-1 PM Thursday 12-1 PM Monday 2-5 PM P. K. Padmanabhan PH548: Two Excellent Books How to do it? 1 2 F 12 F 23 3 F i = F ij j i F
PH 548 Atomistic Simulation Techniques Lectures: Lab: 2-0-2-6 Tuesday 12-1 PM Thursday 12-1 PM Monday 2-5 PM P. K. Padmanabhan PH548: Two Excellent Books How to do it? 1 2 F 12 F 23 3 F i = F ij j i F
Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation
 - 1 - Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation Tatjana Janzen, Gabriela Guevara-Carrión, Jadran Vrabec University of Paderborn, Germany
- 1 - Mutual diffusion in the ternary mixture of water + methanol + ethanol: Experiments and Molecular Simulation Tatjana Janzen, Gabriela Guevara-Carrión, Jadran Vrabec University of Paderborn, Germany
Self-Diffusion and Viscosity Coefficient of Fluids in Nanochannels
 Self-Diffusion and iscosity Coefficient of Fluids in Nanochannels alery Ya. RUDYAK 1,2*, Alexander A. BELKIN 1,2, Denis A. IANO 1,2, ladimir A. ANDRUSHENKO 1,2 *Corresponding author: Tel.: +7 383 266814;
Self-Diffusion and iscosity Coefficient of Fluids in Nanochannels alery Ya. RUDYAK 1,2*, Alexander A. BELKIN 1,2, Denis A. IANO 1,2, ladimir A. ANDRUSHENKO 1,2 *Corresponding author: Tel.: +7 383 266814;
Determining the shear viscosity of model liquids from molecular dynamics simulations Hess, Berk
 University of Groningen Determining the shear viscosity of model liquids from molecular dynamics simulations Hess, Berk Published in: Journal of Chemical Physics DOI: 1.163/1.1421362 IMPORA OE: You are
University of Groningen Determining the shear viscosity of model liquids from molecular dynamics simulations Hess, Berk Published in: Journal of Chemical Physics DOI: 1.163/1.1421362 IMPORA OE: You are
4. The Green Kubo Relations
 4. The Green Kubo Relations 4.1 The Langevin Equation In 1828 the botanist Robert Brown observed the motion of pollen grains suspended in a fluid. Although the system was allowed to come to equilibrium,
4. The Green Kubo Relations 4.1 The Langevin Equation In 1828 the botanist Robert Brown observed the motion of pollen grains suspended in a fluid. Although the system was allowed to come to equilibrium,
NON-EQUILIBRIUM MOLECULAR DYNAMICS CALCULATION OF THE
 FluidPhaseEquilibria, 53 (1989) 191-198 Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands 191 NON-EQUILIBRIUM MOLECULAR DYNAMICS CALCULATION OF THE TRANSPORT PROPERTIES OF CARBON
FluidPhaseEquilibria, 53 (1989) 191-198 Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands 191 NON-EQUILIBRIUM MOLECULAR DYNAMICS CALCULATION OF THE TRANSPORT PROPERTIES OF CARBON
Computer Simulation of Shock Waves in Condensed Matter. Matthew R. Farrow 2 November 2007
 Computer Simulation of Shock Waves in Condensed Matter Matthew R. Farrow 2 November 2007 Outline of talk Shock wave theory Results Conclusion Computer simulation of shock waves Shock Wave Theory Shock
Computer Simulation of Shock Waves in Condensed Matter Matthew R. Farrow 2 November 2007 Outline of talk Shock wave theory Results Conclusion Computer simulation of shock waves Shock Wave Theory Shock
UvA-DARE (Digital Academic Repository)
 UvA-DARE (Digital Academic Repository) Poiseuille flow to measure the viscosity of particle model fluids. Backer, J.A.; Lowe, C.P.; Hoefsloot, H.C.J.; Iedema, P.D. Published in: Journal of Chemical Physics
UvA-DARE (Digital Academic Repository) Poiseuille flow to measure the viscosity of particle model fluids. Backer, J.A.; Lowe, C.P.; Hoefsloot, H.C.J.; Iedema, P.D. Published in: Journal of Chemical Physics
Viscosity of confined inhomogeneous nonequilibrium fluids
 JOURNAL OF CHEMICAL PHYSICS VOLUME 121, NUMBER 21 1 DECEMBER 2004 Viscosity of confined inhomogeneous nonequilibrium fluids Junfang Zhang and B. D. Todd a) Centre for Molecular Simulation, Swinburne University
JOURNAL OF CHEMICAL PHYSICS VOLUME 121, NUMBER 21 1 DECEMBER 2004 Viscosity of confined inhomogeneous nonequilibrium fluids Junfang Zhang and B. D. Todd a) Centre for Molecular Simulation, Swinburne University
This Answer/Solution Example is taken from a student s actual homework report. I thank him for permission to use it here. JG
 This Answer/Solution Example is taken from a student s actual homework report. I thank him for permission to use it here. JG Chem 8021 Spring 2005 Project II Calculation of Self-Diffusion Coeffecient of
This Answer/Solution Example is taken from a student s actual homework report. I thank him for permission to use it here. JG Chem 8021 Spring 2005 Project II Calculation of Self-Diffusion Coeffecient of
An Introduction to Two Phase Molecular Dynamics Simulation
 An Introduction to Two Phase Molecular Dynamics Simulation David Keffer Department of Materials Science & Engineering University of Tennessee, Knoxville date begun: April 19, 2016 date last updated: April
An Introduction to Two Phase Molecular Dynamics Simulation David Keffer Department of Materials Science & Engineering University of Tennessee, Knoxville date begun: April 19, 2016 date last updated: April
Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems
 THE JOURNAL OF CHEMICAL PHYSICS 124, 184101 2006 Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems Igor V. Pivkin and George E. Karniadakis a Division of Applied Mathematics,
THE JOURNAL OF CHEMICAL PHYSICS 124, 184101 2006 Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems Igor V. Pivkin and George E. Karniadakis a Division of Applied Mathematics,
Flow of Fluids and Solids at the Nanoscale
 Proceedings of the 2 nd International Conference on Heat Transfer and Fluid Flow Barcelona, Spain July 20-21, 2015 Paper No. XXX (The number assigned by the OpenConf System) Flow of Fluids and Solids at
Proceedings of the 2 nd International Conference on Heat Transfer and Fluid Flow Barcelona, Spain July 20-21, 2015 Paper No. XXX (The number assigned by the OpenConf System) Flow of Fluids and Solids at
Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement
 Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement Xin Yin, Yeqi Shi, Yanbing Wei, Yongho Joo, Padma Gopalan, Izabela Szlufarska,
Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement Xin Yin, Yeqi Shi, Yanbing Wei, Yongho Joo, Padma Gopalan, Izabela Szlufarska,
MOLECULAR DYNAMICS SIMULATIONS OF CH4 CLATHRATE HYDRATE DISSOCIATION ADJACENT TO HYDRATED SILICA SURFACES
 Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, United Kingdom, July 17-21, 2011. MOLECULAR DYNAMICS SIMULATIONS OF CH4 CLATHRATE HYDRATE DISSOCIATION
Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, United Kingdom, July 17-21, 2011. MOLECULAR DYNAMICS SIMULATIONS OF CH4 CLATHRATE HYDRATE DISSOCIATION
Coupling Atomistic and Continuum Hydrodynamics
 Coupling Atomistic and Continuum Hydrodynamics Matej Praprotnik praprot@cmm.ki.si Laboratory for Molecular Modeling National Institute of Chemistry Ljubljana, Slovenia & Department of Physics Faculty of
Coupling Atomistic and Continuum Hydrodynamics Matej Praprotnik praprot@cmm.ki.si Laboratory for Molecular Modeling National Institute of Chemistry Ljubljana, Slovenia & Department of Physics Faculty of
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene
 Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
The microscopic aspects of solid-liquid-vapor interactions are usually crucial when we consider
 2.3 Microscopic Representation of Solid-Liquid-Vapor Interactions The microscopic aspects of solid-liquid-vapor interactions are usually crucial when we consider theories of phase change phenomena such
2.3 Microscopic Representation of Solid-Liquid-Vapor Interactions The microscopic aspects of solid-liquid-vapor interactions are usually crucial when we consider theories of phase change phenomena such
What is Classical Molecular Dynamics?
 What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential functions Newton s equations of motion are integrated
What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential functions Newton s equations of motion are integrated
arxiv: v1 [cond-mat.stat-mech] 25 May 2009
![arxiv: v1 [cond-mat.stat-mech] 25 May 2009 arxiv: v1 [cond-mat.stat-mech] 25 May 2009](/thumbs/85/92595076.jpg) Anomalies in water as obtained from computer simulations of the TIP4P/2005 model: density maxima, and density, isothermal compressibility and heat capacity minima arxiv:0905.4009v1 [cond-mat.stat-mech]
Anomalies in water as obtained from computer simulations of the TIP4P/2005 model: density maxima, and density, isothermal compressibility and heat capacity minima arxiv:0905.4009v1 [cond-mat.stat-mech]
Poiseuille flow of Lennard-Jones fluids in narrow slit pores
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 4 22 JANUARY 2000 Poiseuille flow of Lennard-Jones fluids in narrow slit pores Karl P. Travis a) and Keith E. Gubbins Department of Chemical Engineering,
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 4 22 JANUARY 2000 Poiseuille flow of Lennard-Jones fluids in narrow slit pores Karl P. Travis a) and Keith E. Gubbins Department of Chemical Engineering,
Chemical and Biomolecular Engineering 150A Transport Processes Spring Semester 2017
 Chemical and Biomolecular Engineering 150A Transport Processes Spring Semester 2017 Objective: Text: To introduce the basic concepts of fluid mechanics and heat transfer necessary for solution of engineering
Chemical and Biomolecular Engineering 150A Transport Processes Spring Semester 2017 Objective: Text: To introduce the basic concepts of fluid mechanics and heat transfer necessary for solution of engineering
Molecular Dynamics Simulations. Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia
 Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
c 2010 ANJAN V. RAGHUNATHAN
 c 2010 ANJAN V. RAGHUNATHAN MOLECULAR UNDERSTANDING OF OSMOSIS AND A MULTISCALE FRAMEWORK TO INVESTIGATE CONFINED FLUID PROPERTIES BY ANJAN V. RAGHUNATHAN DISSERTATION Submitted in partial fulfillment
c 2010 ANJAN V. RAGHUNATHAN MOLECULAR UNDERSTANDING OF OSMOSIS AND A MULTISCALE FRAMEWORK TO INVESTIGATE CONFINED FLUID PROPERTIES BY ANJAN V. RAGHUNATHAN DISSERTATION Submitted in partial fulfillment
A MOLECULAR DYNAMICS SIMULATION OF WATER DROPLET IN CONTACT WITH A PLATINUM SURFACE
 The 6th ASME-JSME Thermal Engineering Joint Conference March 16-2, 23 TED-AJ3-183 A MOLECULAR DYNAMICS SIMULATION OF WATER DROPLET IN CONTACT WITH A PLATINUM SURFACE Tatsuto KIMURA Department of Mechanical
The 6th ASME-JSME Thermal Engineering Joint Conference March 16-2, 23 TED-AJ3-183 A MOLECULAR DYNAMICS SIMULATION OF WATER DROPLET IN CONTACT WITH A PLATINUM SURFACE Tatsuto KIMURA Department of Mechanical
A potential model for the study of ices and amorphous water: TIP4P/Ice
 THE JOURNAL OF CHEMICAL PHYSICS 122, 234511 2005 A potential model for the study of ices and amorphous water: TIP4P/Ice J. L. F. Abascal, E. Sanz, R. García Fernández, and C. Vega Departamento de Química
THE JOURNAL OF CHEMICAL PHYSICS 122, 234511 2005 A potential model for the study of ices and amorphous water: TIP4P/Ice J. L. F. Abascal, E. Sanz, R. García Fernández, and C. Vega Departamento de Química
MOLECULAR DYNAMICS SIMULATIONS OF HEAT TRANSFER ISSUES IN CARBON NANOTUBES
 The st International Symposium on Micro & Nano Technology, 4-7 March, 4, Honolulu, Hawaii, USA MOLECULAR DYNAMICS SIMULATIONS OF HEAT TRANSFER ISSUES IN CARBON NANOTUBES S. Maruyama, Y. Igarashi, Y. Taniguchi
The st International Symposium on Micro & Nano Technology, 4-7 March, 4, Honolulu, Hawaii, USA MOLECULAR DYNAMICS SIMULATIONS OF HEAT TRANSFER ISSUES IN CARBON NANOTUBES S. Maruyama, Y. Igarashi, Y. Taniguchi
Mutual diffusion of binary liquid mixtures containing methanol, ethanol, acetone, benzene, cyclohexane, toluene and carbon tetrachloride
 - 1 - PLMMP 2016, Kyiv Mutual diffusion of binary liquid mixtures containing methanol, ethanol, acetone, benzene, cyclohexane, toluene and carbon tetrachloride Jadran Vrabec Tatjana Janzen, Gabriela Guevara-Carrión,
- 1 - PLMMP 2016, Kyiv Mutual diffusion of binary liquid mixtures containing methanol, ethanol, acetone, benzene, cyclohexane, toluene and carbon tetrachloride Jadran Vrabec Tatjana Janzen, Gabriela Guevara-Carrión,
Formation of water at a Pt(111) surface: A study using reactive force fields (ReaxFF)
 Formation of water at a Pt(111) surface: A study using reactive force fields (ReaxFF) Markus J. Buehler 1, Adri C.T. van Duin 2, Timo Jacob 3, Yunhee Jang 2, Boris Berinov 2, William A. Goddard III 2 1
Formation of water at a Pt(111) surface: A study using reactive force fields (ReaxFF) Markus J. Buehler 1, Adri C.T. van Duin 2, Timo Jacob 3, Yunhee Jang 2, Boris Berinov 2, William A. Goddard III 2 1
Supplementary Information for Atomistic Simulation of Spinodal Phase Separation Preceding Polymer Crystallization
 Supplementary Information for Atomistic Simulation of Spinodal Phase Separation Preceding Polymer Crystallization Richard H. Gee * Naida Lacevic and Laurence E. Fried University of California Lawrence
Supplementary Information for Atomistic Simulation of Spinodal Phase Separation Preceding Polymer Crystallization Richard H. Gee * Naida Lacevic and Laurence E. Fried University of California Lawrence
Supporting Information. for. Influence of Cononsolvency on the. Aggregation of Tertiary Butyl Alcohol in. Methanol-Water Mixtures
 Supporting Information for Influence of Cononsolvency on the Aggregation of Tertiary Butyl Alcohol in Methanol-Water Mixtures Kenji Mochizuki,, Shannon R. Pattenaude, and Dor Ben-Amotz Research Institute
Supporting Information for Influence of Cononsolvency on the Aggregation of Tertiary Butyl Alcohol in Methanol-Water Mixtures Kenji Mochizuki,, Shannon R. Pattenaude, and Dor Ben-Amotz Research Institute
Langevin Dynamics in Constant Pressure Extended Systems
 Langevin Dynamics in Constant Pressure Extended Systems D. Quigley and M.I.J. Probert CMMP 2004 1 Talk Outline Molecular dynamics and ensembles. Existing methods for sampling at NPT. Langevin dynamics
Langevin Dynamics in Constant Pressure Extended Systems D. Quigley and M.I.J. Probert CMMP 2004 1 Talk Outline Molecular dynamics and ensembles. Existing methods for sampling at NPT. Langevin dynamics
A Coupling Tool for Parallel Molecular Dynamics Continuum Simulations
 A Coupling Tool for Parallel Molecular Dynamics Continuum Simulations ISPDC 2012 Philipp Neumann and Nikola Tchipev 29.06.2012 ISPDC 2012, 29.06.2012 1 Contents Motivation The Macro Micro Coupling Tool
A Coupling Tool for Parallel Molecular Dynamics Continuum Simulations ISPDC 2012 Philipp Neumann and Nikola Tchipev 29.06.2012 ISPDC 2012, 29.06.2012 1 Contents Motivation The Macro Micro Coupling Tool
Surface property corrected modification of the classical nucleation theory
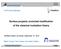 CCP5 Annual Meeting Surface property corrected modification of the classical nucleation theory Sheffield Hallam University, September 15, 2010 Martin Horsch, Hans Hasse, and Jadran Vrabec The critical
CCP5 Annual Meeting Surface property corrected modification of the classical nucleation theory Sheffield Hallam University, September 15, 2010 Martin Horsch, Hans Hasse, and Jadran Vrabec The critical
Atomistic molecular simulations for engineering applications: methods, tools and results. Jadran Vrabec
 Atomistic molecular simulations for engineering applications: methods, tools and results Jadran Vrabec Motivation Simulation methods vary in their level of detail The more detail, the more predictive power
Atomistic molecular simulations for engineering applications: methods, tools and results Jadran Vrabec Motivation Simulation methods vary in their level of detail The more detail, the more predictive power
Molecular Dynamics Investigation of Triethylene Glycol in Hydrated LTA Zeolite
 U N I V E R S I T E T E T I B E R G E N Institute of Physics and Technology Molecular Dynamics Investigation of Triethylene Glycol in Hydrated LTA Zeolite Emphasis on Evaluation of Potential Models Bjørnar
U N I V E R S I T E T E T I B E R G E N Institute of Physics and Technology Molecular Dynamics Investigation of Triethylene Glycol in Hydrated LTA Zeolite Emphasis on Evaluation of Potential Models Bjørnar
Flexible Bond and Angle, FBA/ǫ model of water
 Flexible Bond and Angle, FBA/ǫ model of water arxiv:1807.03460v1 [physics.comp-ph] 10 Jul 2018 Raúl Fuentes-Azcatl and Marcia C. Barbosa Instituto de Física, Universidade Federal do Rio Grande do Sul,
Flexible Bond and Angle, FBA/ǫ model of water arxiv:1807.03460v1 [physics.comp-ph] 10 Jul 2018 Raúl Fuentes-Azcatl and Marcia C. Barbosa Instituto de Física, Universidade Federal do Rio Grande do Sul,
 A Nobel Prize for Molecular Dynamics and QM/MM What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential
A Nobel Prize for Molecular Dynamics and QM/MM What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential
Water models in classical simulations
 Water models in classical simulations Maria Fyta Institut für Computerphysik, Universität Stuttgart Stuttgart, Germany Water transparent, odorless, tasteless and ubiquitous really simple: two H atoms attached
Water models in classical simulations Maria Fyta Institut für Computerphysik, Universität Stuttgart Stuttgart, Germany Water transparent, odorless, tasteless and ubiquitous really simple: two H atoms attached
Superhydrophobic surfaces: stability of the Cassie-Baxter state and its effect on liquid water slippage
 Superhydrophobic surfaces: stability of the Cassie-Baxter state and its effect on liquid water slippage Mauro Chinappi Center for Life Nano Science IIT@Sapienza Talk outlines Talk 1: Solid Molecular Dynamics
Superhydrophobic surfaces: stability of the Cassie-Baxter state and its effect on liquid water slippage Mauro Chinappi Center for Life Nano Science IIT@Sapienza Talk outlines Talk 1: Solid Molecular Dynamics
UB association bias algorithm applied to the simulation of hydrogen fluoride
 Fluid Phase Equilibria 194 197 (2002) 249 256 UB association bias algorithm applied to the simulation of hydrogen fluoride Scott Wierzchowski, David A. Kofke Department of Chemical Engineering, University
Fluid Phase Equilibria 194 197 (2002) 249 256 UB association bias algorithm applied to the simulation of hydrogen fluoride Scott Wierzchowski, David A. Kofke Department of Chemical Engineering, University
DSMC-Based Shear-Stress/Velocity-Slip Boundary Condition for Navier-Stokes Couette-Flow Simulations
 DSMC-Based Shear-Stress/Velocity-Slip Boundary Condition for Navier-Stokes Couette-Flow Simulations J. R. Torczynski and M. A. Gallis Engineering Sciences Center, Sandia National Laboratories, P. O. Box
DSMC-Based Shear-Stress/Velocity-Slip Boundary Condition for Navier-Stokes Couette-Flow Simulations J. R. Torczynski and M. A. Gallis Engineering Sciences Center, Sandia National Laboratories, P. O. Box
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE
 A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
Molecular dynamics simulation of nanofluidics and nanomachining
 Molecular dynamics simulation of nanofluidics and nanomachining M. T. Horsch,1, 4 S. Stephan,1 S. Becker,1 M. Heier,1 M. P. Lautenschläger,1 F. Diewald,2 R. Müller,2 H. M. Urbassek,3 and H. Hasse1 1 Engineering
Molecular dynamics simulation of nanofluidics and nanomachining M. T. Horsch,1, 4 S. Stephan,1 S. Becker,1 M. Heier,1 M. P. Lautenschläger,1 F. Diewald,2 R. Müller,2 H. M. Urbassek,3 and H. Hasse1 1 Engineering
ICONE COMPUTER SIMULATION OF DIFFUSION OF PB-BI EUTECTIC IN LIQUID SODIUM BY MOLECULAR DYNAMICS METHOD
 Proceedings of ICONE TH International Conference on Nuclear Engineering Arlington, VA, USA, April -, ICONE-3 COMPUTER SIMULATION OF DIFFUSION OF PB-BI EUTECTIC IN LIQUID SODIUM BY MOLECULAR DYNAMICS METHOD
Proceedings of ICONE TH International Conference on Nuclear Engineering Arlington, VA, USA, April -, ICONE-3 COMPUTER SIMULATION OF DIFFUSION OF PB-BI EUTECTIC IN LIQUID SODIUM BY MOLECULAR DYNAMICS METHOD
Nucleation rate (m -3 s -1 ) Radius of water nano droplet (Å) 1e+00 1e-64 1e-128 1e-192 1e-256
 Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
Supplemental Information: Ion-Specific Adsorption and Electroosmosis in Charged Amorphous Porous Silica
 Supplemental Information: Ion-Specific Adsorption and Electroosmosis in Charged Amorphous Porous Silica Remco Hartkamp,,, Bertrand Siboulet, Jean-François Dufrêche, and Benoit Coasne,, Institut Charles
Supplemental Information: Ion-Specific Adsorption and Electroosmosis in Charged Amorphous Porous Silica Remco Hartkamp,,, Bertrand Siboulet, Jean-François Dufrêche, and Benoit Coasne,, Institut Charles
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers
 Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation
 J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
Structural and Transport Properties of an SPC/E Electrolyte in a Nanopore
 Structural and Transport Properties of an SPC/E Electrolyte in a Nanopore Yuk Wai Tang, Kwong-Yu Chan,*, and István Szalai Department of Chemistry, The UniVersity of Hong Kong, Pokfulam Road, Hong Kong
Structural and Transport Properties of an SPC/E Electrolyte in a Nanopore Yuk Wai Tang, Kwong-Yu Chan,*, and István Szalai Department of Chemistry, The UniVersity of Hong Kong, Pokfulam Road, Hong Kong
