High temperature enthalpy and heat capacity of GaN
|
|
|
- Rosamond Merritt
- 5 years ago
- Views:
Transcription
1 Thermochimica Acta 401 (2003) High temperature enthalpy and heat capacity of GaN J. Leitner a,, A. Strejc b, D. Sedmidubský b,k.růžička c a Department of Solid State Engineering, Institute of Chemical Technology, Technická 5, Prague 6, Czech Republic b Department of Inorganic Chemistry, Institute of Chemical Technology, Technická 5, Prague 6, Czech Republic c Department of Physical Chemistry, Institute of Chemical Technology, Technická 5, Prague 6, Czech Republic Received 17 September 2002; received in revised form 30 September 2002; accepted 15 November 2002 Abstract The heat capacity and the heat content of gallium nitride were measured by calvet calorimetry ( K) and by drop calorimetry ( K), respectively. The temperature dependence of the heat capacity in the form C pm = T T T 3 was derived by the least squares method. Furthermore, thermodynamic functions calculated on the basis of our experimental results and literature data on the molar entropy and the heat of formation of GaN are given Elsevier Science B.V. All rights reserved. Keywords: Gallium nitride; Heat capacity; Heat content; Thermodynamic functions 1. Introduction Gallium nitride (GaN) and other nitrides of the group III elements are important materials for the fabrication of various semiconductor devices such as light emitting diodes, lasers, photodetectors or metal-semiconductor field effect transistors and high electron mobility transistors [1]. Various methods are utilized for the deposition of epitaxial layers and the heterostructures of these materials. Metalorganic vapor phase epitaxy (MOVPE) has some advantages over other techniques and it became the leading epitaxial technology in the last decade. A number of papers have recently been published dealing with the thermodynamic analysis of the deposition of GaN, (Al, Ga)N, (Ga, In)N as well as (Al, Ga, In)N epitaxial layers by metalorganic vapor phase epitaxy. Such theoretical calculations yield Corresponding author. Fax: address: jindrich.leitner@vscht.cz (J. Leitner). accurate results provided they are based on reliable thermodynamic data. Thermodynamic data for solid GaN are quoted in tables [2,3] and they have recently been assessed [4,5]. Unfortunately, the data show a large scatter and that is why new investigations should be performed. The heat capacity of the solid GaN was measured by Koshchenko et al. [6] in the temperature range of K using an adiabatic calorimeter. Chen et al. [7] have measured the heat capacity of GaN for temperatures from 113 to 1073 K. They obtained the temperature dependence of C pm in the form: = T T T 2 (1) Itagaki and Yamaguchi [8] have measured the heat content of GaN in the temperature range of K. The experimental data were fitted by the /03/$ see front matter 2002 Elsevier Science B.V. All rights reserved. PII: S (02)
2 170 J. Leitner et al. / Thermochimica Acta 401 (2003) following equation: H T H 298 (J mol 1 ) = 43.6T T T 1 15, 000 (2) from which heat capacity can be obtained by differentiating with respect to temperature: = T T 2 (3) The results of the calorimetric investigation on GaN are presented in this paper. Furthermore, thermodynamic functions calculated on the basis of our experimental results and literature data on the molar entropy [6] and the heat of formation [9] of GaN are given. 2. Experimental Powdered GaN (Aldrich, >99.99%) was used in all experiments. A heat conduction Setaram C-80 calorimeter was used for the heat capacity determination. The measurements were carried out in incremental temperature scanning mode with a number of 5 10 K steps (heating rate 0.2 K min 1 ) followed by isothermal delays of 9000 s. Three runs had to be performed with empty crucible (blank), with the reference material (synthetic sapphire, NIST Standard reference material no. 720) and with the sample. For the heat capacity of the sample the following relation holds: C pm,s (T i T i+1 ) = Q s Q blank m ref c p,ref M s (4) Q ref Q blank m s where Q is the relevant peak area for the sample (s), the reference material (ref) and blank, C p,ref the mean specific heat capacity of the reference material in the temperature range T i T i+1, m the mass and M the molar mass. The typical mass of samples was approximately 6 g. The accuracy of DSC measurements is estimated to be better than ±2%. Heat content determinations were carried out by the drop method using the high temperature calorimeter Setaram (Multi HTC 96). Isothermal measurements were made in the nitrogen atmosphere by alternate dropping of the reference material (small pieces of synthetic sapphire, NIST Standard reference material no. 720) and the sample (GaN pellets, 5 mm in diameter, thickness of 2 3 mm) from the feeding chamber held at room temperature (T 0 ) through a lock into the working cell of the preheated calorimeter. Endothermic effects are detected and the relevant peak area Q(T) is proportional to the heat content of the dropped specimen H(T): Q(T ) = ST m H (T ), M H T (T ) = C pm dt T 0 (5) where m and M are the mass and the molar mass of the specimen, respectively. The sensitivity of calorimeter S(T) at temperature T is determined from the known heat content of the standard. The measurements were performed at temperatures K on samples with the masses mg. The delays between two subsequent drops were min. In order to check the accuracy of the present measurement, the heat content of platinum was measured first. The values H = and kj mol 1 were observed at 970 K which differ approximately 0.8% from the recommended SGTE data [10]. 3. Results and discussion The molar heat capacity data are plotted in Fig. 1. The heat content data are listed in Table 1 and shown in Fig. 2. The raw data were treated simultaneously using the least square method with different weights for individual points. As the heat content data by Itagaki and Yamaguchi [8] are in good agreement with our values, they were taken into account during the optimization too. In order to smoothly link our temperature dependence to the low-temperature data of Koshchenko et al. [6] six points, namely C pm (200 K) = 26.8 J mol 1 K 1, C pm (210 K) = 27.8 J mol 1 K 1, C pm (230 K) = 29.5 J mol 1 K 1, C pm (250 K) = 31.1 J mol 1 K 1, C pm ( K) = 32.9 J mol 1 K 1 and C pm ( K) = 34.9 J mol 1 K 1 were included in the regression procedure. A four-parameters fitting equation with the T 3 term was used to reach higher flexibility around the room temperature. Thus, the temperature dependence of the molar heat capacity of solid
3 J. Leitner et al. / Thermochimica Acta 401 (2003) Fig. 1. Temperature dependence of molar heat capacity of solid GaN: (,, ) experimental points (calvet); ( ) [6]; (---) [7]; ( ) fitted curve (Eq. (6)). GaN can be expressed by the following equation (T = K): = ( ± 2.279)+(5.440 ± 2.936) 10 3 T (2.190 ± 0.288) 10 6 T 2 + (2.460 ± 0.459) 10 8 T 3 (6) Temperature dependence of the heat content is derived by integration of Eq. (6) with respect to temperature as follows: H T H 298 (J mol 1 ) = ( ± 2.279)T +(2.906 ± 1.468) 10 3 T 2 + (2.190 ± 0.288) 10 6 T 1 + (1.230 ± 0.459) 10 8 T 2 ( ± ) (7) The mean absolute deviation δ (δ = Σabs ( H T,calculated H T,experiment )/n) between the Table 1 Heat content of solid GaN T (K) H (kj mol 1 ) experiment H (kj mol 1 ) calculated Eq. (7) T (K) H (kj mol 1 ) experiment H (kj mol 1 ) calculated Eq. (7)
4 172 J. Leitner et al. / Thermochimica Acta 401 (2003) Fig. 2. Temperature dependence of heat content of solid GaN: ( ) experimental points (drop); ( ) [8]; (---) [7]; ( ) fitted curve (Eq. (7)). Table 2 Thermodynamic functions of solid GaN T (K) H m (kj mol 1 ) S m (J mol 1 K 1 ) G m (kj mol 1 ) calculated (Eq. (7)) and experimental values of H T = H T H 298 is 0.53 kj mol 1 with the maximum value of 1.89 J mol 1 at K. Our results are in good agreement with the high-temperature heat-content data of Itagaki and Yamaguchi [8]. On the other hand, there are relatively large differences (more then 10% in the temperature range K) between the temperature dependence of the heat capacity assessed in this work and published in literature [7]. Thermodynamic functions of solid GaN calculated on the basis of our temperature dependence of the heat capacity and literature data on the molar entropy [6] and the heat of formation [9] of GaN are given in Table 2. Acknowledgements This work was supported by the GACR (Grant no. 106/00/0568) and the Ministry of Education of the Czech Republic (Research Projects no. MSM and ).
5 J. Leitner et al. / Thermochimica Acta 401 (2003) References [1] P. Kung, M. Razeghi, Opto-Electron. Rev. 8 (2000) 201. [2] O. Knacke, O. Kubaschewski, K. Hesselmann, Thermochemical Properties of Inorganic Substances, 2nd ed., Springer, Berlin, [3] I. Barin, Thermochemical Data of Pure Substances, 2nd ed., VCH, Weinheim, [4] I.N. Przhevalskii, S.Yu. Karpov, Yu.N. Makarov, MRS Internet, J. Nitride Semicond. Res. 3 (1998) 30. [5] A.V. Davydov, T.J. Anderson, in: T.D. Moustakas, S. Mohney, S.J. Pearton (Eds.), Electrochemical Society Proceedings on III V Nitride Materials and Processes III, vol , Boston, 1998, p. 38. [6] V.I. Koshchenko, A.F. Demidenko, L.D. Sabanova, V.E. Yachmenev, Yu.M. Gran, A.F. Radchenko, Izv. Akad. Nauk SSSR, Neorg. Mater. 15 (1979) [7] X. Chen, Y. Lan, J. Liang, X. Cheng, Y. Xu, T. Xu, P. Jiang, K. Lu, Chin. Phys. Lett. 16 (1999) 107. [8] K. Itagaki, K. Yamaguchi, Thermochim. Acta 163 (1990) 1. [9] M.R. Ranade, F. Tessier, A. Navrotska, V.J. Leppert, S.H. Risbud, F.J. DiSalvo, C.M. Balkas, J. Phys. Chem. B 104 (2000) [10] A.T. Dinsdale, CALPHAD 15 (1991) 317.
Calorimetry. Chapter 2. Differential Scanning heat flux calorimetry
 Chapter 2 Calorimetry In this Chapter, the technique of differential scanning heat flux calorimetry is explained. We used a salt, of which the heat capacity is well-known, NaF, to test the equipment. After
Chapter 2 Calorimetry In this Chapter, the technique of differential scanning heat flux calorimetry is explained. We used a salt, of which the heat capacity is well-known, NaF, to test the equipment. After
Analysis of calorimetric measurement bismuth and tin system
 International Journal of Research in Engineering and Innovation Vol-1, Issue-1 (May-2017), 29-33 International Journal of Research in Engineering and Innovation (IJREI) journal home page: http://www.ijrei.com
International Journal of Research in Engineering and Innovation Vol-1, Issue-1 (May-2017), 29-33 International Journal of Research in Engineering and Innovation (IJREI) journal home page: http://www.ijrei.com
Calorimetric studies on urania thoria solid solutions
 Journal of Alloys and Compounds 299 (2000) 112 117 L www.elsevier.com/ locate/ jallcom Calorimetric studies on urania thoria solid solutions a b a, * S. Anthonysamy, Jose Joseph, P.R. Vasudeva Rao a Fuel
Journal of Alloys and Compounds 299 (2000) 112 117 L www.elsevier.com/ locate/ jallcom Calorimetric studies on urania thoria solid solutions a b a, * S. Anthonysamy, Jose Joseph, P.R. Vasudeva Rao a Fuel
VAPOUR PRESSURE OF DSC CALIBRATION SUBSTANCES
 Journal of Thermal Analysis and Calorimetry, Vol. 69 (2002) 333 338 VAPOUR PRESSURE OF DSC CALIBRATION SUBSTANCES G. Hakvoort 1,C.M.Hol 1 and P. J. van Ekeren 2 1 Delft University of Technology, c/o Wielengahof
Journal of Thermal Analysis and Calorimetry, Vol. 69 (2002) 333 338 VAPOUR PRESSURE OF DSC CALIBRATION SUBSTANCES G. Hakvoort 1,C.M.Hol 1 and P. J. van Ekeren 2 1 Delft University of Technology, c/o Wielengahof
A Calorimetric Investigation of the Liquid Bi-Ni Alloys
 A Calorimetric Investigation of the Liquid Bi-Ni Alloys M. El maniani 1*, M. Rechchach 1, A. El mahfoudi 1, M. El moudane 2, A. Sabbar 1 1 Equipe de Physico-chimie des matériaux et nanomatériaux: Dépollution,
A Calorimetric Investigation of the Liquid Bi-Ni Alloys M. El maniani 1*, M. Rechchach 1, A. El mahfoudi 1, M. El moudane 2, A. Sabbar 1 1 Equipe de Physico-chimie des matériaux et nanomatériaux: Dépollution,
Thermochimica Acta 531 (2012) Contents lists available at SciVerse ScienceDirect. Thermochimica Acta
 hermochimica Acta 531 (2012 6 11 Contents lists available at SciVerse ScienceDirect hermochimica Acta journa l h o me page: www.elsevier.com/locate/tca hermodynamic properties of potassium nitrate magnesium
hermochimica Acta 531 (2012 6 11 Contents lists available at SciVerse ScienceDirect hermochimica Acta journa l h o me page: www.elsevier.com/locate/tca hermodynamic properties of potassium nitrate magnesium
Finite element analysis of the temperature field in a vertical MOCVD reactor by induction heating
 Vol. 30, No. 11 Journal of Semiconductors November 2009 Finite element analysis of the temperature field in a vertical MOCVD reactor by induction heating Li Zhiming( ), Xu Shengrui( ), Zhang Jincheng(
Vol. 30, No. 11 Journal of Semiconductors November 2009 Finite element analysis of the temperature field in a vertical MOCVD reactor by induction heating Li Zhiming( ), Xu Shengrui( ), Zhang Jincheng(
M R S Internet Journal of Nitride Semiconductor Research
 Page 1 of 6 M R S Internet Journal of Nitride Semiconductor Research Volume 9, Article 7 The Ambient Temperature Effect on Current-Voltage Characteristics of Surface-Passivated GaN-Based Field-Effect Transistors
Page 1 of 6 M R S Internet Journal of Nitride Semiconductor Research Volume 9, Article 7 The Ambient Temperature Effect on Current-Voltage Characteristics of Surface-Passivated GaN-Based Field-Effect Transistors
High temperature heat capacity of Nd 2 Zr 2 O 7 and La 2 Zr 2 O 7 pyrochlores
 J. Chem. Thermodynamics 37 (2005) 1098 1103 www.elsevier.com/locate/jct High temperature heat capacity of Nd 2 Zr 2 O 7 and La 2 Zr 2 O 7 pyrochlores D. Sedmidubský a,b, *, O. Beneš a, R.J.M. Konings b
J. Chem. Thermodynamics 37 (2005) 1098 1103 www.elsevier.com/locate/jct High temperature heat capacity of Nd 2 Zr 2 O 7 and La 2 Zr 2 O 7 pyrochlores D. Sedmidubský a,b, *, O. Beneš a, R.J.M. Konings b
APPLICATION OF DIFFERENTIAL SCANNING CALORIMETRY TO CORE ANALYSIS
 SCA2013-055 1/7 APPLICATION OF DIFFERENTIAL SCANNING CALORIMETRY TO CORE ANALYSIS Evgeny Dyshlyuk, Schlumberger This paper was prepared for presentation at the International Symposium of the Society of
SCA2013-055 1/7 APPLICATION OF DIFFERENTIAL SCANNING CALORIMETRY TO CORE ANALYSIS Evgeny Dyshlyuk, Schlumberger This paper was prepared for presentation at the International Symposium of the Society of
Thermal Stress and Strain in a GaN Epitaxial Layer Grown on a Sapphire Substrate by the MOCVD Method
 CHINESE JOURNAL OF PHYSICS VOL. 48, NO. 3 June 2010 Thermal Stress and Strain in a GaN Epitaxial Layer Grown on a Sapphire Substrate by the MOCVD Method H. R. Alaei, 1 H. Eshghi, 2 R. Riedel, 3 and D.
CHINESE JOURNAL OF PHYSICS VOL. 48, NO. 3 June 2010 Thermal Stress and Strain in a GaN Epitaxial Layer Grown on a Sapphire Substrate by the MOCVD Method H. R. Alaei, 1 H. Eshghi, 2 R. Riedel, 3 and D.
10. Reaction Equations Module
 15005-ORC-J 1 (12) 10. Reaction Equations Module SUMMARY Clicking the Reaction Equations button in the main menu of HSC shows the Reaction Equations Window, see Fig. 1. With this module you can calculate
15005-ORC-J 1 (12) 10. Reaction Equations Module SUMMARY Clicking the Reaction Equations button in the main menu of HSC shows the Reaction Equations Window, see Fig. 1. With this module you can calculate
Traps in MOCVD n-gan Studied by Deep Level Transient Spectroscopy and Minority Carrier Transient Spectroscopy
 Traps in MOCVD n-gan Studied by Deep Level Transient Spectroscopy and Minority Carrier Transient Spectroscopy Yutaka Tokuda Department of Electrical and Electronics Engineering, Aichi Institute of Technology,
Traps in MOCVD n-gan Studied by Deep Level Transient Spectroscopy and Minority Carrier Transient Spectroscopy Yutaka Tokuda Department of Electrical and Electronics Engineering, Aichi Institute of Technology,
Measuring and Expressing Enthalpy Changes. Copyright Pearson Prentice Hall. Measuring and Expressing Enthalpy Changes. Calorimetry
 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
CH3511 EXPERIMENT: Determination of Thermal Properties Using a Differential Scanning Calorimeter
 CH3511 EXPERIMENT: Determination of Thermal Properties Using a Differential Scanning Calorimeter INTRODUCTION A Mettler Toledo 823E Differential Scanning Calorimeter (DSC) will be used to determine the
CH3511 EXPERIMENT: Determination of Thermal Properties Using a Differential Scanning Calorimeter INTRODUCTION A Mettler Toledo 823E Differential Scanning Calorimeter (DSC) will be used to determine the
APPLICATION NOTE. Investigation of Alkali Salts with the STA 449 F5 Jupiter. Dr. Alexander Schindler and Dr. Michael Schöneich.
 APPLICATION NOTE Investigation of Alkali Salts with the STA 449 F5 Jupiter Dr. Alexander Schindler and Dr. Michael Schöneich Introduction While analytical techniques such as EDX or ICP-MS provide a detailed
APPLICATION NOTE Investigation of Alkali Salts with the STA 449 F5 Jupiter Dr. Alexander Schindler and Dr. Michael Schöneich Introduction While analytical techniques such as EDX or ICP-MS provide a detailed
Lecture 7 Enthalpy. NC State University
 Chemistry 431 Lecture 7 Enthalpy NC State University Motivation The enthalpy change ΔH is the change in energy at constant pressure. When a change takes place in a system that is open to the atmosphere,
Chemistry 431 Lecture 7 Enthalpy NC State University Motivation The enthalpy change ΔH is the change in energy at constant pressure. When a change takes place in a system that is open to the atmosphere,
Major Concepts Calorimetry (from last time)
 Major Concepts Calorimetry (from last time) Heat capacity Molar heat capacity (per mole) Specific heat capacity (per mass) Standard state enthalpies: Hº Physical Changes Chemical Changes Hess's Law Balancing
Major Concepts Calorimetry (from last time) Heat capacity Molar heat capacity (per mole) Specific heat capacity (per mass) Standard state enthalpies: Hº Physical Changes Chemical Changes Hess's Law Balancing
Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods
 Int J Thermophys (2009) 30:1257 1269 DOI 10.1007/s10765-009-0641-z Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods Eva Günther Stefan
Int J Thermophys (2009) 30:1257 1269 DOI 10.1007/s10765-009-0641-z Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods Eva Günther Stefan
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction
 Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction Yang Bai 1,2, Guangping Zheng 1 and Sanqiang Shi 1 1 Department of Mechanical
Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction Yang Bai 1,2, Guangping Zheng 1 and Sanqiang Shi 1 1 Department of Mechanical
TAWN tests for quantitatively measuring the resolution and sensitivity of DSCs (version 2.1)
 TAWN tests for quantitatively measuring the resolution and sensitivity of DSCs (version 2.1) 1. Introduction There are many properties that characterise the performance of differential scanning calorimeters
TAWN tests for quantitatively measuring the resolution and sensitivity of DSCs (version 2.1) 1. Introduction There are many properties that characterise the performance of differential scanning calorimeters
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Thermodynamic investigation of ZrO 2 -BaO system
 Thermodynamic investigation of ZrO -BaO system ON Wei-ping( 龚伟平, CHEN Teng-fei( 陈腾飞, JIN Zhan-peng( 金展鹏 State Key Laboratory of Powder Metallurgy, Central South University, Changsha 4008, China Received
Thermodynamic investigation of ZrO -BaO system ON Wei-ping( 龚伟平, CHEN Teng-fei( 陈腾飞, JIN Zhan-peng( 金展鹏 State Key Laboratory of Powder Metallurgy, Central South University, Changsha 4008, China Received
Quiz I: Thermodynamics
 Quiz I: Thermodynamics SCH4U_2018-2019_V2 NAME: (Total Score: / 30) Multiple Choice (12) 1. What can be deduced from the following reaction profile? A. The reactants are less stable than the products and
Quiz I: Thermodynamics SCH4U_2018-2019_V2 NAME: (Total Score: / 30) Multiple Choice (12) 1. What can be deduced from the following reaction profile? A. The reactants are less stable than the products and
Chemistry Slide 1 of 33
 Chemistry 17.2 1 of 33 17.2 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will
Chemistry 17.2 1 of 33 17.2 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will
ISO INTERNATIONAL STANDARD. Plastics Differential scanning calorimetry (DSC) Part 4: Determination of specific heat capacity
 INTERNATIONAL STANDARD ISO 11357-4 First edition 2005-09-15 Plastics Differential scanning calorimetry (DSC) Part 4: Determination of specific heat capacity Plastiques Analyse calorimétrique différentielle
INTERNATIONAL STANDARD ISO 11357-4 First edition 2005-09-15 Plastics Differential scanning calorimetry (DSC) Part 4: Determination of specific heat capacity Plastiques Analyse calorimétrique différentielle
Thermochemistry. Mr.V
 Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Heat capacity and enthalpy of fusion of pyrimethanil laurate (C 24 H 37 N 3 O 2 )
 J. Chem. Thermodynamics 36 (24) 895 899 www.elsevier.com/locate/jct Heat capacity and enthalpy of fusion of pyrimethanil laurate (C 24 H 37 3 O 2 ) Xiao-Hong Sun a, *, Yuan-Fa Liu a, Zhi-Cheng Tan b, You-Ying
J. Chem. Thermodynamics 36 (24) 895 899 www.elsevier.com/locate/jct Heat capacity and enthalpy of fusion of pyrimethanil laurate (C 24 H 37 3 O 2 ) Xiao-Hong Sun a, *, Yuan-Fa Liu a, Zhi-Cheng Tan b, You-Ying
Chem 75 Winter, 2017 Practice Exam 1
 This was Exam 1 last year. It is presented here with, first, just the problems and then with the problems and their solutions. YOU WILL BENEFIT MOST if you attempt first just the problems as if you were
This was Exam 1 last year. It is presented here with, first, just the problems and then with the problems and their solutions. YOU WILL BENEFIT MOST if you attempt first just the problems as if you were
Thermal analysis of Li 2 O TeO 2 glass
 Journal of Non-Crystalline Solids 271 (2000) 12±17 www.elsevier.com/locate/jnoncrysol Thermal analysis of Li 2 O TeO 2 glass I. Avramov a, *, G. Guinev a, A.C.M. Rodrigues b a Institute of Physical Chemistry,
Journal of Non-Crystalline Solids 271 (2000) 12±17 www.elsevier.com/locate/jnoncrysol Thermal analysis of Li 2 O TeO 2 glass I. Avramov a, *, G. Guinev a, A.C.M. Rodrigues b a Institute of Physical Chemistry,
Kinetic evaluation of decabromodiphenil oxide as a ame retardant for unsaturated polyester
 Thermochimica Acta 388 (2002) 283±288 Kinetic evaluation of decabromodiphenil oxide as a ame retardant for unsaturated polyester V.J. Fernandes Jr. *, N.S. Fernandes, V.M. Fonseca, A.S. Araujo, D.R. Silva
Thermochimica Acta 388 (2002) 283±288 Kinetic evaluation of decabromodiphenil oxide as a ame retardant for unsaturated polyester V.J. Fernandes Jr. *, N.S. Fernandes, V.M. Fonseca, A.S. Araujo, D.R. Silva
Determination of the fictive temperature for a hyperquenched glass
 3 May 2002 Chemical Physics Letters 357 (2002) 20 24 www.elsevier.com/locate/cplett Determination of the fictive temperature for a hyperquenched glass Y.Z. Yue *, J.deC. Christiansen, S.L. Jensen Danish
3 May 2002 Chemical Physics Letters 357 (2002) 20 24 www.elsevier.com/locate/cplett Determination of the fictive temperature for a hyperquenched glass Y.Z. Yue *, J.deC. Christiansen, S.L. Jensen Danish
PCM specific heat capacity c p (T) measurements
 PCM specific heat capacity c p (T) measurements DSC on PCM Workshop AIT, Vienna, 04. - 05. April 2016 Daniel Lager Engineer Energy Department - Sustainable Thermal Energy Systems AIT Austrian Institute
PCM specific heat capacity c p (T) measurements DSC on PCM Workshop AIT, Vienna, 04. - 05. April 2016 Daniel Lager Engineer Energy Department - Sustainable Thermal Energy Systems AIT Austrian Institute
Electron leakage effects on GaN-based light-emitting diodes
 Opt Quant Electron (2010) 42:89 95 DOI 10.1007/s11082-011-9437-z Electron leakage effects on GaN-based light-emitting diodes Joachim Piprek Simon Li Received: 22 September 2010 / Accepted: 9 January 2011
Opt Quant Electron (2010) 42:89 95 DOI 10.1007/s11082-011-9437-z Electron leakage effects on GaN-based light-emitting diodes Joachim Piprek Simon Li Received: 22 September 2010 / Accepted: 9 January 2011
Entropy Changes & Processes
 Entropy Changes & Processes Chapter 4 of Atkins: he Second Law: he Concepts Section 4.3 Entropy of Phase ransition at the ransition emperature Expansion of the Perfect Gas Variation of Entropy with emperature
Entropy Changes & Processes Chapter 4 of Atkins: he Second Law: he Concepts Section 4.3 Entropy of Phase ransition at the ransition emperature Expansion of the Perfect Gas Variation of Entropy with emperature
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
Heat. Heat Terminology 04/12/2017. System Definitions. System Definitions
 System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
Energetics. Topic
 Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
 Class XI Chapter 6 Thermodynamics Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii)
Class XI Chapter 6 Thermodynamics Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii)
8.6 The Thermodynamic Standard State
 8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
Uncertainty Analysis for Heat-flux DSC Measurements
 Uncertainty Analysis for Heat-flux DSC Measurements metrology-focused coordinated R&D integration of national research programmes supported by the European Commission collaboration between National Measurement
Uncertainty Analysis for Heat-flux DSC Measurements metrology-focused coordinated R&D integration of national research programmes supported by the European Commission collaboration between National Measurement
APPLICATION OF THERMAL METHODS IN THE CHEMISTRY OF CEMENT: KINETIC ANALYSIS OF PORTLANDITE FROM NON- ISOTHERMAL THERMOGRAVIMETRC DATA
 The First International Proficiency Testing Conference Sinaia, România 11 th 13 th October, 2007 APPLICATION OF THERMAL METHODS IN THE CHEMISTRY OF CEMENT: KINETIC ANALYSIS OF PORTLANDITE FROM NON- ISOTHERMAL
The First International Proficiency Testing Conference Sinaia, România 11 th 13 th October, 2007 APPLICATION OF THERMAL METHODS IN THE CHEMISTRY OF CEMENT: KINETIC ANALYSIS OF PORTLANDITE FROM NON- ISOTHERMAL
NCORRECTED PROOF. High-temperature high-humidity and electrical static discharge stress effects on GaN p i n UV sensor
 Materials Science and Engineering B xxx (2005) xxx xxx 3 4 5 6 7 8 High-temperature high-humidity and electrical static discharge stress effects on GaN p i n UV sensor Su-Sir Liu a,, Pei-Wen Li a, W.H.
Materials Science and Engineering B xxx (2005) xxx xxx 3 4 5 6 7 8 High-temperature high-humidity and electrical static discharge stress effects on GaN p i n UV sensor Su-Sir Liu a,, Pei-Wen Li a, W.H.
Level 3 Chemistry, 2016
 91390 913900 3SUPERVISOR S Level 3 Chemistry, 2016 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 2.00 p.m. Monday 21 November 2016 Credits:
91390 913900 3SUPERVISOR S Level 3 Chemistry, 2016 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 2.00 p.m. Monday 21 November 2016 Credits:
A NEW MEASUREMENT AND EVALUATION METHOD FOR DSC OF PCM SAMPLES
 A NEW MEASUREMENT AND EVALUATION METHOD FOR DSC OF PCM SAMPLES H Mehling, E Günther, S Hiebler, Bavarian Center for Applied Energy Research (ZAE Bayern), Walther-Meißner-Str. 6, D-85748 Garching, Germany.
A NEW MEASUREMENT AND EVALUATION METHOD FOR DSC OF PCM SAMPLES H Mehling, E Günther, S Hiebler, Bavarian Center for Applied Energy Research (ZAE Bayern), Walther-Meißner-Str. 6, D-85748 Garching, Germany.
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Entropy Changes & Processes
 Entropy Changes & Processes Chapter 4 of Atkins: he Second Law: he Concepts Section 4.3, 7th edition; 3.3, 8th and 9th editions Entropy of Phase ransition at the ransition emperature Expansion of the Perfect
Entropy Changes & Processes Chapter 4 of Atkins: he Second Law: he Concepts Section 4.3, 7th edition; 3.3, 8th and 9th editions Entropy of Phase ransition at the ransition emperature Expansion of the Perfect
A pulse tube refrigerator below 2 K
 Cryogenics 39 (1999) 865±869 A pulse tube refrigerator below 2 K M.Y. Xu *, A.T.A.M. De Waele, Y.L. Ju Department of Physics, Eindhoven University of Technology, P.O. Box 513, NL-5600 MB Eindhoven, The
Cryogenics 39 (1999) 865±869 A pulse tube refrigerator below 2 K M.Y. Xu *, A.T.A.M. De Waele, Y.L. Ju Department of Physics, Eindhoven University of Technology, P.O. Box 513, NL-5600 MB Eindhoven, The
Level 3 Chemistry, 2018
 91390 913900 3SUPERVISOR S Level 3 Chemistry, 2018 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 2.00 p.m. Thursday 15 November 2018 Credits:
91390 913900 3SUPERVISOR S Level 3 Chemistry, 2018 91390 Demonstrate understanding of thermochemical principles and the properties of particles and substances 2.00 p.m. Thursday 15 November 2018 Credits:
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Estimation of vapour pressure and partial pressure of subliming compounds by low-pressure thermogravimetry
 Bull. Mater. Sci., Vol. 34, No. 7, December 2011, pp. 1633 1637. Indian Academy of Sciences. Estimation of vapour pressure and partial pressure of subliming compounds by low-pressure thermogravimetry G
Bull. Mater. Sci., Vol. 34, No. 7, December 2011, pp. 1633 1637. Indian Academy of Sciences. Estimation of vapour pressure and partial pressure of subliming compounds by low-pressure thermogravimetry G
molar surface area. The slope for this line gave the surface energy (J / m 2 ) for the hydrous
 Thermodynamics of manganese oxidzs: Effects of particle size and hydration on oxidation-reduction equilibria among hausmannite, bixbyite, and pyrolusite Nancy Birkner and Alexandra Navrotsky* SUPPORTING
Thermodynamics of manganese oxidzs: Effects of particle size and hydration on oxidation-reduction equilibria among hausmannite, bixbyite, and pyrolusite Nancy Birkner and Alexandra Navrotsky* SUPPORTING
Effects of Pressure and NH 3 Flow on the Two-Dimensional Electron Mobility in AlGaN/GaN Heterostructures
 Journal of the Korean Physical Society, Vol. 42, No. 5, May 2003, pp. 691 695 Effects of Pressure and NH 3 Flow on the Two-Dimensional Electron Mobility in AlGaN/GaN Heterostructures Dong-Joon Kim Optical
Journal of the Korean Physical Society, Vol. 42, No. 5, May 2003, pp. 691 695 Effects of Pressure and NH 3 Flow on the Two-Dimensional Electron Mobility in AlGaN/GaN Heterostructures Dong-Joon Kim Optical
Calorimetry &Thermochemistry
 Lecture 6 Calorimetry &Thermochemistry Suggested Reading Thermochemistry Chemistry 3, Chapter 14, Energy and Thermochemistry, pp.658-700. Elements of hysical Chemistry, 5 th edition, Atkins & de aula,
Lecture 6 Calorimetry &Thermochemistry Suggested Reading Thermochemistry Chemistry 3, Chapter 14, Energy and Thermochemistry, pp.658-700. Elements of hysical Chemistry, 5 th edition, Atkins & de aula,
Wavelength extension of GaInAs/GaIn(N)As quantum dot structures grown on GaAs
 PUBLICATION V Journal of Crystal Growth 248 (2003) 339 342 Wavelength extension of GaInAs/GaIn(N)As quantum dot structures grown on GaAs T. Hakkarainen*, J. Toivonen, M. Sopanen, H. Lipsanen Optoelectronics
PUBLICATION V Journal of Crystal Growth 248 (2003) 339 342 Wavelength extension of GaInAs/GaIn(N)As quantum dot structures grown on GaAs T. Hakkarainen*, J. Toivonen, M. Sopanen, H. Lipsanen Optoelectronics
Spin Transition and Structural Transformation in a
 Supporting Information for Spin Transition and Structural Transformation in a Mononuclear Cobalt(II) Complex Ying Guo, Xiu-Long Yang, Rong-Jia Wei, Lan-Sun Zheng, and Jun Tao* State Key Laboratory of Physical
Supporting Information for Spin Transition and Structural Transformation in a Mononuclear Cobalt(II) Complex Ying Guo, Xiu-Long Yang, Rong-Jia Wei, Lan-Sun Zheng, and Jun Tao* State Key Laboratory of Physical
Calorimetric investigations. in multi-component salt systems
 Calorimetric investigations in multi-component salt systems D. Sergeev 1, E. Yazhenskikh 1, N. Talukder 1, D. Kobertz 1, K. Hack 2, M. Müller 1 1 Forschungszentrum Jülich, IEK-2 2 - GTT-Technologies 1
Calorimetric investigations in multi-component salt systems D. Sergeev 1, E. Yazhenskikh 1, N. Talukder 1, D. Kobertz 1, K. Hack 2, M. Müller 1 1 Forschungszentrum Jülich, IEK-2 2 - GTT-Technologies 1
Low Temperature Heat Capacity and Magnetic Properties of UF 3
 pubs.acs.org/ic Low Temperature Heat Capacity and Magnetic Properties of UF 3 O. Benes,*, J.-C. Griveau, E. Colineau, D. Sedmidubsky, and R. J. M. Konings European Commission, Joint Research Centre, Institute
pubs.acs.org/ic Low Temperature Heat Capacity and Magnetic Properties of UF 3 O. Benes,*, J.-C. Griveau, E. Colineau, D. Sedmidubsky, and R. J. M. Konings European Commission, Joint Research Centre, Institute
U = 4.18 J if we heat 1.0 g of water through 1 C. U = 4.18 J if we cool 1.0 g of water through 1 C.
 CHAPER LECURE NOES he First Law of hermodynamics: he simplest statement of the First Law is as follows: U = q + w. Here U is the internal energy of the system, q is the heat and w is the work. CONVENIONS
CHAPER LECURE NOES he First Law of hermodynamics: he simplest statement of the First Law is as follows: U = q + w. Here U is the internal energy of the system, q is the heat and w is the work. CONVENIONS
Thermal Analysis measurements
 Thermal Analysis measurements R W McCallum Ames Laboratory And Materials Science and Engineering Phase vs Phase Field phase set of states of a macroscopic physical system that have relatively uniform chemical
Thermal Analysis measurements R W McCallum Ames Laboratory And Materials Science and Engineering Phase vs Phase Field phase set of states of a macroscopic physical system that have relatively uniform chemical
Heat capacity of α-gan: Isotope Effects
 Heat capacity of α-gan: Isotope Effects R. K. Kremer, M. Cardona, and E. Schmitt arxiv:cond-mat/0503522v1 [cond-mat.mtrl-sci] 21 Mar 2005 Max-Planck-Institut für Festkörperforschung, Heisenbergstr. 1,
Heat capacity of α-gan: Isotope Effects R. K. Kremer, M. Cardona, and E. Schmitt arxiv:cond-mat/0503522v1 [cond-mat.mtrl-sci] 21 Mar 2005 Max-Planck-Institut für Festkörperforschung, Heisenbergstr. 1,
Influence of excitation frequency on Raman modes of In 1-x Ga x N thin films
 Influence of excitation frequency on Raman modes of In 1-x Ga x N thin films A. Dixit 1,, J. S. Thakur 2, V. M. Naik 3, R. Naik 2 1 Center of Excellence in Energy & ICT, Indian Institute of Technology
Influence of excitation frequency on Raman modes of In 1-x Ga x N thin films A. Dixit 1,, J. S. Thakur 2, V. M. Naik 3, R. Naik 2 1 Center of Excellence in Energy & ICT, Indian Institute of Technology
Effects of Si doping on optical properties of GaN epitaxial layers
 (123) 31 Effects of Si doping on optical properties of GaN epitaxial layers Chiharu SASAKI (Department of Electrical and Electronic Engineering) Tatsuya YAMASHITA (Department of Electrical and Electronic
(123) 31 Effects of Si doping on optical properties of GaN epitaxial layers Chiharu SASAKI (Department of Electrical and Electronic Engineering) Tatsuya YAMASHITA (Department of Electrical and Electronic
STUDY OF LAYERS OF METAL NANOPARTICLES ON SEMICONDUCTOR WAFERS FOR HYDROGEN DETECTION
 STUDY OF LAYERS OF METAL NANOPARTICLES ON SEMICONDUCTOR WAFERS FOR HYDROGEN DETECTION Martin MULLER a, b, Karel ZDANSKY a, Jiri ZAVADIL a, Katerina PIKSOVA b a INSTITUTE OF PHOTONICS AND ELECTRONICS, CZECH
STUDY OF LAYERS OF METAL NANOPARTICLES ON SEMICONDUCTOR WAFERS FOR HYDROGEN DETECTION Martin MULLER a, b, Karel ZDANSKY a, Jiri ZAVADIL a, Katerina PIKSOVA b a INSTITUTE OF PHOTONICS AND ELECTRONICS, CZECH
2D MBE Activities in Sheffield. I. Farrer, J. Heffernan Electronic and Electrical Engineering The University of Sheffield
 2D MBE Activities in Sheffield I. Farrer, J. Heffernan Electronic and Electrical Engineering The University of Sheffield Outline Motivation Van der Waals crystals The Transition Metal Di-Chalcogenides
2D MBE Activities in Sheffield I. Farrer, J. Heffernan Electronic and Electrical Engineering The University of Sheffield Outline Motivation Van der Waals crystals The Transition Metal Di-Chalcogenides
Thermochemistry Enthalpy & Hess Law. Packet #35
 Thermochemistry Enthalpy & Hess Law Packet #35 Introduction I Thermochemistry, is the branch of chemistry that, investigates the amount of energy that is gained or lost during a chemical reaction. Introduction
Thermochemistry Enthalpy & Hess Law Packet #35 Introduction I Thermochemistry, is the branch of chemistry that, investigates the amount of energy that is gained or lost during a chemical reaction. Introduction
Energetics. These processes involve energy exchanges between the reacting system and its surroundings.
 Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
Energetics Chemical reactions involve: the breaking of bonds between atoms the making of new bonds between atoms These processes involve energy exchanges between the reacting system and its surroundings.
HSC Chemistry A. Roine June 28, ORC T
 HSC Chemistry 5.0 10 1 10. REACTION EQUATIONS Clicking the Reaction Equations button in the main menu shows the Reaction Equations Window, see Fig. 1. With this calculation option you can calculate the
HSC Chemistry 5.0 10 1 10. REACTION EQUATIONS Clicking the Reaction Equations button in the main menu shows the Reaction Equations Window, see Fig. 1. With this calculation option you can calculate the
Characterization of a NASICON based potentiometric CO 2 sensor
 Characterization of a NASICON based potentiometric CO 2 sensor S. Baliteau a, A-L. Sauvet b, C. Lopez a * and P. Fabry a a LEPMI, INPG-UJF-CNRS, 1130 rue de la piscine, 38 402 Saint Martin d Hères Cedex
Characterization of a NASICON based potentiometric CO 2 sensor S. Baliteau a, A-L. Sauvet b, C. Lopez a * and P. Fabry a a LEPMI, INPG-UJF-CNRS, 1130 rue de la piscine, 38 402 Saint Martin d Hères Cedex
Recent Developments in the Thermodynamics of Ionic liquids
 COST 1206 - EXIL workshop, Prague, April 21st-22 nd, 2015 Recent Developments in the Thermodynamics of Ionic liquids Luís M.N.B.F Santos Nanostructures and Self-Organization WG Universidade do Porto, Porto,
COST 1206 - EXIL workshop, Prague, April 21st-22 nd, 2015 Recent Developments in the Thermodynamics of Ionic liquids Luís M.N.B.F Santos Nanostructures and Self-Organization WG Universidade do Porto, Porto,
Ultrafast carrier dynamics in InGaN MQW laser diode
 Invited Paper Ultrafast carrier dynamics in InGaN MQW laser diode Kian-Giap Gan* a, Chi-Kuang Sun b, John E. Bowers a, and Steven P. DenBaars a a Department of Electrical and Computer Engineering, University
Invited Paper Ultrafast carrier dynamics in InGaN MQW laser diode Kian-Giap Gan* a, Chi-Kuang Sun b, John E. Bowers a, and Steven P. DenBaars a a Department of Electrical and Computer Engineering, University
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
Calorimetry: Heat of Solution
 Calorimetry: Heat of Solution When a substance undergoes a change in temperature, the quantity of heat lost or gained can be calculated using the following relationship: (heat) = m s T (1) The specific
Calorimetry: Heat of Solution When a substance undergoes a change in temperature, the quantity of heat lost or gained can be calculated using the following relationship: (heat) = m s T (1) The specific
Matter exchange - type of wall Yes - permeable - absence of wall. Energy exchange - type of wall. - diathermic - moving wall. Yes
 I. The concept of work, expansion and additional (useful) work. II. The concept of heat. III. Definition of internal energy and its molecular interpretation. I. Different forms of the first law of thermodynamics..
I. The concept of work, expansion and additional (useful) work. II. The concept of heat. III. Definition of internal energy and its molecular interpretation. I. Different forms of the first law of thermodynamics..
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water"
 "Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
Downloaded from
 THERMODYNAMICS Thermodynamics: is the branch of science which deals with deals with the study of different forms of energy and the quantitative relationship between them. Significance of Thermodynamics:
THERMODYNAMICS Thermodynamics: is the branch of science which deals with deals with the study of different forms of energy and the quantitative relationship between them. Significance of Thermodynamics:
Unit 7 Kinetics and Thermodynamics
 17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
PROPULSION LAB MANUAL
 PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
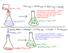 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Kinetic Analysis of the Oil Shale Pyrolysis using Thermogravimetry and Differential Scanning Calorimetry
 559 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 61, 2017 Guest Editors: Petar S Varbanov, Rongxin Su, Hon Loong Lam, Xia Liu, Jiří J Klemeš Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-51-8;
559 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 61, 2017 Guest Editors: Petar S Varbanov, Rongxin Su, Hon Loong Lam, Xia Liu, Jiří J Klemeš Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-51-8;
Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method
 International Journal of Thermophysics, Vol 24, No 2, March 23 ( 23) Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method T Ohmura, 1,2 M Tsuboi, 1 M Onodera, 1
International Journal of Thermophysics, Vol 24, No 2, March 23 ( 23) Specific Heat Measurement of High Temperature Thermal Insulations by Drop Calorimeter Method T Ohmura, 1,2 M Tsuboi, 1 M Onodera, 1
Thermodynamics of lithium ion batteries Hans J. Seifert
 Thermodynamics of lithium ion batteries Hans J. Seifert Institute for Applied Materials Applied Materials Physics (IAM-AWP) KIT University of the State of Baden-Wuerttemberg and National Research Center
Thermodynamics of lithium ion batteries Hans J. Seifert Institute for Applied Materials Applied Materials Physics (IAM-AWP) KIT University of the State of Baden-Wuerttemberg and National Research Center
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Implant isolation of AlGaAs multilayer DBR
 Nuclear Instruments and Methods in Physics Research B 218 (2004) 381 385 www.elsevier.com/locate/nimb Implant isolation of AlGaAs multilayer DBR A.V.P. Coelho a, *, H. Boudinov a, T. v. Lippen b, H.H.
Nuclear Instruments and Methods in Physics Research B 218 (2004) 381 385 www.elsevier.com/locate/nimb Implant isolation of AlGaAs multilayer DBR A.V.P. Coelho a, *, H. Boudinov a, T. v. Lippen b, H.H.
Analytical Evaluation of Energy and Electron Concentrations in Quantum Wells of the High Electron Mobility Transistors.
 Analytical Evaluation of Energy Electron Concentrations in Quantum Wells of the High Electron Mobility Transistors Salih SAYGI Department of Physics, Faculty of Arts Sciences, Gaziosmanpasa University,
Analytical Evaluation of Energy Electron Concentrations in Quantum Wells of the High Electron Mobility Transistors Salih SAYGI Department of Physics, Faculty of Arts Sciences, Gaziosmanpasa University,
FUNDAMENTALS OF THERMAL ANALYSIS AND DIFFERENTIAL SCANNING CALORIMETRY Application in Materials Science Investigations
 FUNDAMENTALS OF THERMAL ANALYSIS AND DIFFERENTIAL SCANNING CALORIMETRY Application in Materials Science Investigations Analiza cieplna i kalorymetria różnicowa w badaniach materiałów Tomasz Czeppe Lecture
FUNDAMENTALS OF THERMAL ANALYSIS AND DIFFERENTIAL SCANNING CALORIMETRY Application in Materials Science Investigations Analiza cieplna i kalorymetria różnicowa w badaniach materiałów Tomasz Czeppe Lecture
Measurement and correlation of solubility of xylitol in binary water+ethanol solvent mixtures between K and K
 Korean J. Chem. Eng., 3(4), 931-936 (213) DOI: 1.17/s11814-12-225-7 INVITED REVIEW PAPER Measurement and correlation of solubility of xylitol in binary water+ethanol solvent mixtures between 278. K and
Korean J. Chem. Eng., 3(4), 931-936 (213) DOI: 1.17/s11814-12-225-7 INVITED REVIEW PAPER Measurement and correlation of solubility of xylitol in binary water+ethanol solvent mixtures between 278. K and
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Effects of Current Spreading on the Performance of GaN-Based Light-Emitting Diodes
 IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. 48, NO. 6, JUNE 2001 1065 Effects of Current Spreading on the Performance of GaN-Based Light-Emitting Diodes Hyunsoo Kim, Seong-Ju Park, and Hyunsang Hwang Abstract
IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. 48, NO. 6, JUNE 2001 1065 Effects of Current Spreading on the Performance of GaN-Based Light-Emitting Diodes Hyunsoo Kim, Seong-Ju Park, and Hyunsang Hwang Abstract
S2004 Methods for characterization of biomolecular interactions - classical versus modern
 S2004 Methods for characterization of biomolecular interactions - classical versus modern Isothermal Titration Calorimetry (ITC) Eva Dubská email: eva.dubska@ceitec.cz Outline Calorimetry - history + a
S2004 Methods for characterization of biomolecular interactions - classical versus modern Isothermal Titration Calorimetry (ITC) Eva Dubská email: eva.dubska@ceitec.cz Outline Calorimetry - history + a
Chemical Energetics. First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another.
 Chemical Energetics First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another. All chemical reactions are accompanied by some form of energy
Chemical Energetics First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another. All chemical reactions are accompanied by some form of energy
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Thermochemistry Ch. 8
 De#initions I. Energy ( ): II. Heat ( ): A. Heat is not a substance. Objects do not contain heat, they B. Molecules with each other. III. Reaction perspectives: A. System: B. Surroundings: IV: Heat changes:
De#initions I. Energy ( ): II. Heat ( ): A. Heat is not a substance. Objects do not contain heat, they B. Molecules with each other. III. Reaction perspectives: A. System: B. Surroundings: IV: Heat changes:
Characterization of Solid State Drugs by Calorimetry
 Characterization of Solid State Drugs by Calorimetry Christin T. Choma TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA Drug product development and manufacture requires numerous studies to
Characterization of Solid State Drugs by Calorimetry Christin T. Choma TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA Drug product development and manufacture requires numerous studies to
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Thermal dehydration and degradation kinetics of chitosan Schiff bases of o- and m nitrobenzaldehyde Muraleedharan K.* & Viswalekshmi C.H.
 2017 St. Joseph s College (Autonomous), Devagiri www.devagirijournals.com ISSN 2454-2091 Thermal dehydration and degradation kinetics of chitosan Schiff bases of o- and m nitrobenzaldehyde Muraleedharan
2017 St. Joseph s College (Autonomous), Devagiri www.devagirijournals.com ISSN 2454-2091 Thermal dehydration and degradation kinetics of chitosan Schiff bases of o- and m nitrobenzaldehyde Muraleedharan
Standard Gibbs Energies of Formation of the Ferro and Paramagnetic Phases of AlNd 2
 Standard Gibbs Energies of Formation of the Ferro and Paramagnetic Phases of AlNd 2 M. Morishita*, H. Yamamoto*, M. Kodera**, N. Nishimura**, S. Miura** and Y. Yamada*** *Department of Materials Science
Standard Gibbs Energies of Formation of the Ferro and Paramagnetic Phases of AlNd 2 M. Morishita*, H. Yamamoto*, M. Kodera**, N. Nishimura**, S. Miura** and Y. Yamada*** *Department of Materials Science
