Astrochemistry and Molecular Astrophysics Paola Caselli
|
|
|
- Felicity Casey
- 6 years ago
- Views:
Transcription
1 School of Physics and Astronomy FACULTY OF MATHEMATICS & PHYSICAL SCIENCES Astrochemistry and Molecular Astrophysics Paola Caselli
2 Outline 1. The formation of H 2 2. The formation of H The chemistry initiated by H Formation and destruction of CO 5. The two-level system 6. Transfer of radiation in spectral lines 7. The rotational temperature diagram 8. The escape probability method
3 Interstellar Molecules Known Interstellar Molecules (Total: > 170) Amino acetonitrile in SgrB2(N) (Belloche et al. 2008) H C H O N C H H O H Glycine - the simplest amino acid
4 How do molecules form in the interstellar medium? The most elementary chemical reaction is the association of A and B to form a molecule AB with internal energy: A + B AB * The molecule AB * must lose the internal energy. In the Earth atmosphere, where the number of particles per cubic centimeter (cc) is very large (~10 19 ), the molecule looses its energy via three-body reactions: AB * + M AB But this is not an efficient process in interstellar clouds, where the number of particles per cc ranges between a few hundred and ~10 8.
5 1. The formation of H 2 The reaction that starts the chemistry in the interstellar medium is the one between two hydrogen atoms to form molecular hydrogen: H + H H 2 This reaction happens on the surface of dust grains.
6 1. The formation of H 2 The H 2 formation rate (cm -3 s -1 ) is given by (Gould & Salpeter 1963; Hollenbach & Salpeter 1970; Jura 1974; Pirronello et al. 1999; Cazaux & Tielens 2002; Bergin et al. 2004; Cuppen & Herbst 2005; Cazaux et al. 2008): R H 2 = 1 2 n Hv H A n g S H " # 10 $ cm -3 s -1 n H gas number density v H H atoms speed in gas-phase A grain cross sectional area n g dust grain number density S H sticking probability γ surface reaction probability PAHs
7 Once H2 is formed, the fun starts H 2 is the key to the whole of interstellar chemistry. Some important species that might react with H 2 are C, C+, O, N To decide whether a certain reaction is chemically favored, we need to examine internal energy changes. Molecule H 2 CH C + H 2 CH + H?? C + + H 2 CH + + H?? Dissociation energy (ev) OH 4.39 CH OH Question: Can the following reactions proceed in the cold interstellar medium? O + H 2 OH + H?? O + + H 2 OH + + H??
8 Once H2 is formed, the fun starts C + H 2 CH + H?? 4.48 ev 3.47 ev The bond strength of H 2 is larger than that of CH the reaction is not energetically favorable. The reaction is endothermic (by = 1.01 ev) and cannot proceed in cold clouds, where k b T < 0.01 ev! Dissociation energy or bond strength
9 Once H2 is formed, the fun starts Molecule H 2 CH Dissociation energy (ev) OH 4.39 CH OH C + H 2 CH + H C + + H 2 CH + + H O+ H 2 OH + H (endothermic by 1.01 ev) (endothermic by 0.39 ev) (endothermic by 0.09 ev) O + + H 2 OH + + H (exothermic by 0.62 ev!)
10 Rate coefficients and activation energies The rate coefficient k (cm 3 s -1 ) of a generic reaction A + B -> C + D is given by: k =< " v > σ total cross section of the reactants v relative velocity <The average is performed over the thermal distribution> Most reactions possess activation energies E a (~0.1-1 ev) even if exothermic and k is given by the Arrhenius formula (Herbst 1990): k = a(t)exp("e a /k B T)
11 Ion-Neutral reactions A + + B C + + D Exothermic ion-molecule reactions do not possess activation energy because of the strong long-range attractive force (Herbst & Klemperer 1973; Anicich & Huntress 1986): R V(R) = - α e 2 /2R 4 k LANGEVIN = 2 πe(α/µ) 1/ cm 3 s -1 independent of T
12 Neutral-Neutral reactions Energy to break the bond of the reactant. A + BC AB + C 1 ev for endothermic reactions E ev for exothermic reactions Energy released by the formation of the new bond. k b T < 0.01 ev in molecular clouds Example: O + H 2 OH + H (does not proceed in cold clouds) Duley & Williams 1984, Interstellar Chemistry; Bettens et al. 1995, ApJ
13 2. The formation of H 3 + After the formation of molecular hydrogen, cosmic rays ionize H 2 initiating fast routes towards the formation of complex molecules in dark clouds: H 2 + c.r. H e - + c.r. Once H 2 + is formed (in small percentages), it very quickly reacts with the abundant H 2 molecules to form H 3+, the most important molecular ion in interstellar chemistry: H H 2 H H H H H
14 3. The chemistry initiated by H 3 + Once H 3 + is formed, a cascade of reactions greatly enhance the chemical complexity of the ISM. In fact, H 3 + can easily donate a proton and allow larger molecules to form. Example OXYGEN CHEMISTRY (the formation of water in dark clouds) H 3 + O OH + H 2 H 2 O + H 2 H 3 O + e e e H 2 O OH O O O 2
15 3. The chemistry initiated by H 3 + CARBON CHEMISTRY (the formation of hydrocarbons) The formation of more complicated species from neutral atomic carbon begins with a sequence very similar to that which starts the oxygen chemistry: H 3 + H 2 H 2 e C CH+ CH 2 + CH 3 + e CH 2 CH A. Proton transfer from H 3 + to a neutral atom; B. Hydrogen abstraction reactions terminating in a molecular ion that does not react with H 2 ; C. Dissociative recombination with electrons.
16 4. Formation and destruction of CO [a] C + H 3 O + HCO + + H 2 [b] O + CH 3 + HCO + + H 2 [c] HCO + + e CO + H is the most important source of CO. CO is very stable and difficult to remove. It reacts with H 3+ : [d] H CO HCO + + H 2 but reaction (c) immediately reform CO. The main mechanisms for removing CO are: [e] He + + CO He + C + + O [f] hν + CO C + O Some of C + can react with OH and H 2 O (but not with H 2 ): [g] C + + OH CO + + H [h] CO + + H 2 HCO + + H [i] C + + H 2 O HCO + + H
17 The timescale to form CO Assume: dark region where all H is in H 2 and all atoms more massive than He are in neutral atomic form. The timescale on which almost all carbon becomes contained in CO (n O > n C ) is at least equal to the timescale for one H 2 to be ionized for every C: n C /[ζ n(h 2 )] = 2 n C /[ζ n H ] For ζ = s -1 and n C /n H = 10-4, the above expression gives a value of about 2x10 5 yr. O e - HCO + CO
18 5. The two-level system Collisional coefficient Einstein coefficient for absorption γ lu B lu n u ΔE n l γ ul B ul A ul Collisional deexcitation coefficient Einstein coefficient for stimulated emission Einstein coefficient for spontaneous emission Problem: find the level populations n u and n l as a function of the ambient kinetic temperature T kin and density n tot. In order for the populations of both levels to remain constant in time, we need: " lu n tot n l + B lu J n l = " ul n tot n u + B ul J n u + A ul n u Total rate of collisional excitations per unit time and per unit volume Probability per unit time of being excited radiatively J " % & 0 J # $(#)d#
19 In Local Thermodinamic Equilibrium (i.e. when radiative transitions are negligible): " lu " ul = n u n l = g $ u exp " #E ' & ) (T ex = T kin, in LTE) g l % k B T kin ( When n tot is so low that that radiative transitions dominate: J = A ul n u B lu n l B ul n u = A ul /B ul (g l B lu /g u B ul )exp(#e /k B T rad ) 1 Under the assumption of thermodynamic equilibrium (J ν =B ν ): J " B # 0 = 2h# 0 3 /c 2 exp($e /k B T rad ) %1 A ul = 2h" 3 0 B c 2 ul g l B lu = g u B ul
20 " lu n tot n l + B lu J n l = " ul n tot n u + B ul J n u + A ul n u $ exp &" #E % k B T ex ' $ ) = f coll exp " #E ' $ & ) + (1" f coll )exp&" #E ( % k B T kin ( % k B T rad ' ) ( f coll * n tot n tot + n crit (1+ c 2 J /2h+ 0 3 ) fraction of all downward transitions due to collisions n crit " A ul /# ul Excitation Temperature k B T ex /ΔE k B T kin /"E k B T rad /"E Ambient Density log(n tot /n crit )
21 Emission in the J=1 0 line of 12 C 16 O Increasing the density in a cloud can enhance the J=1-0 emission, but only for subcritical values of n tot. Observations in a given transition are most sensitive to gas with densities near the corresponding n crit.
22 Transfer of radiation in spectral lines The propagation of the specific intensity Iν is governed by the radiative transfer equation: di" = #$ " I" + j" ds Iν(0)! Iν(s) s=0 Iν(Δs) s=δs j" I" (#s) = I" (0)exp($% " #s) + [1$ exp($% " #s)] %" Stahler & Palla (2004)
23 We consider the case in which both absorption and emission are due to transitions between two discrete levels in an atom or molecule. The macroscopic emission and absorption coefficients can be written in terms of the microscopic Einstein coefficients: j " # " = j " = h" 0 4# n u A ul$(") % " = h" 0 4# (n lb lu & n u B ul )$(") n u A ul n l B lu $ n u B ul = 2h" 0 3 /c 2 exp(h" 0 /k B T ex ) $1 n u n l "(#) = 2 ln2 $ %# = g u exp ("#E /k T ex ) g l I " 0 (#$ 0 ) = I " 0 (0)e %#$ 0 + 2h" 0 3 /c 2 ( ) exp(h" 0 /k B T ex ) %1 1% e%#$ 0
24 The quantity of interest to the observer is not I ν itself, but rather the difference between I ν and the background intensity. Accordingly, we define a brightness temperature T B by: T B " c 2 2# 2 k B [I # ($% # ) & I # (0)] If we make the final assumption that the background radiation field is Planckian with an associated temperature T bg : T B0 = T 0 [ f (T ex ) " f (T bg )] [ 1" e "#$ ] 0 T B0 % T B (& = & 0 ), T 0 % h& 0 /k B f (T) % [exp(t 0 /T) "1] "1 T Bo is what we measure if we are observing the source with a perfect telescope above the atmosphere, and with an angular resolution much smaller than the source size.
25 Antenna and Brightness Temperature T A " # $ S $ A T B Brightness temperature Beam efficiency Ratio of solid angles subtended by the source and the antenna beams beam dilution factor The brightness temperature is the temperature which would result in the given brightness if inserted into the Rayleigh-Jeans law (T 0 / T<< 1 or hν<<kt, valid in radioastronomy) T B = c 2 2k 1 " 2 B "
26 Column Density " # = h# 0 4$ (n l B lu % n u B ul )&(#) "# 0 = 2 ln2 c 3 A ul (g u /g l ) 8$ 3 / 2 % 0 3 "v - ' / 1& exp )& "E. ( k B T ex * 0, 2 n l "s + 1 For the two-level system, n l ~n and n l Δs~N, the column density. Such an approximation is however not adequate for a system in which the two levels are closely spaced rungs in the ladder (e.g. CO). In these cases: Rotational partition function n = n l Q ) # Q = *(2J + 1)exp %" $ j= 0 BhJ(J + 1) k B T ex & ( '
27 The Rotational Temperature Diagram (RTD) The RTD is a customary technique to analyze the data of an individual molecule with many transitions. This method assumes LTE conditions so that a single excitation temperature (T rot ) characterizes all transitions. If the transitions are all optically thin, the RTD provides a good estimate of the column density of the molecule towards the source. Goldsmith & Langer (1999)
28 The Rotational Temperature Diagram (RTD) T A " $ # S T B T B = c 2 1 $ A 2k B " B 2 " T B0 = h" 0 /k B [ f (T rot ) # f (T bg )] [ 1# e # $% ] 0 T B0 & T B (" = " 0 ) f (T) & [exp(h" 0 /k B T) #1] #1 Now we assume that f (T rot ) >> f (T bg ). We can then write B " 0 as:
29 The Rotational Temperature Diagram (RTD) B " 0 1# e#$ = T 0 f (T rot ) $ $ (%$ 0 & $) The optical depth of the transition can be written as: Column density in the upper state " # h $v N u B ul (eh% / kt rot &1) Half-maximum line width in units of velocity Substituting these two expressions in: T B = c 2 2k B 1 " 2 B "
30 The Rotational Temperature Diagram (RTD) T B = hc 3 N u A ul 8"k B # 2 $v ' 1% e %& * ), ( & + We can now invert the above equation to yield an expression for N u. Considering the integrated line intensity, W = " T B dv ( " T B #v ) we obtain: N u = 8"k B# 2 W & $ ) ( + hc 3 A ul ' 1% e %$ *
31 The Rotational Temperature Diagram (RTD) If the molecule is in LTE: N u = N Q g u e" E u / kt Combining this with the previous expression of N u, assuming optically thin conditions, and taking the log $ log 8"k B# 2 W & % g u hc 3 A ul ' $ ) = log& ( % N ' ) * E u loge Q(T rot )( k B T rot
32 The Rotational Temperature Diagram (RTD) The previous expression shows that $ log 8"k B# 2 W ' & ) % g u hc 3 A ul ( function of E u /k B, with slope -(log e)/t rot and intercept log[n/q(t rot )] at E u = 0: is a linear $ log 8"k B# 2 W & % g u hc 3 A ul ' ) ( White et al. (2010)
33 Solving radiative transfer problems For the two level system, the statistical equilibrium equations are: dn l dt dn u dt = "n l (B lu J + # lu ) + n u (A ul + B ul J + # ul ) = n l (B lu J + # lu ) " n u (A ul + B ul J + # ul ) To solve this, we need to know the radiation field which was what we were after in the first place. This problem can be solved with some simplifying assumptions. van der Tak et al. (2007)
34 Solving radiative transfer problems: escape probability The problem is how to decouple the radiative transfer calculations from the calculations of the level populations. The escape probability β is a factor which allows us to determine the chance that a photon at some position in the cloud can escape the system (based on Sobolev 1960). We need to estimate to calculate the level population. is the amount of radiation inside the source. For a completely opaque source, = S, the source function. J J If β is the chance that a newly created photon can escape from the cloud, then J = S(1" #) J
35 Solving radiative transfer problems: escape probability Now the statistical equilibrium equations can be simplified: dn l dt dn u dt = "n l # lu + n u # ul + $n u A ul = n l # lu " n u # ul " $n u A ul And we can solve the level populations and the radiation field separately; they are decoupled. The contribution from background radiation can easily be added: - take the background intensity. The average chance that it penetrates into the source is (1 - β).
36 Solving radiative transfer problems: escape probability How to estimate β? Several forms have been proposed that depend on geometry (we have to find a form that estimates the average local escape probability over all directions). A very crude form of β in a one-dimensional case can be estimated as: " =< e #$ >= 1 $ $ & e # $ % d % 0 $ = 1# e#$ $ The form of β for a radially expanding sphere is equal to the above result. This is called the Sobolev or large velocity gradient (LVG) approximation (see also Elitzur 1992).
37 Solving radiative transfer problems: escape probability For a homogeneous slab: " = (1# e#3$ ) 3$ For a uniform sphere (Osterbrock 1974): " = # 1$ 2 # + % - ' 2 2 # + 2, & # 2 (. * e $# 0 ) / This last formula is used in RADEX (on-line code):
38
39 SUMMARY H 2 molecules are formed on the surface of dust grains with a rate R H 2 = 1 2 n H v H A n g S H" # 10 $ cm -3 s -1 H H H H 3 + is formed from cosmic-ray ionization of H 2, followed by H H 2. This starts interstellar chemistry. γ lu B lu n u ΔE γ ul B ul A ul The two level system and the brightness temperature of a molecular line at frequency ν = ν 0. T B0 = T 0 [ f (T ex ) " f (T bg )] [ 1" e " #$ ] 0 n l Log N u /g u E ul /k B (K) The rotational temperature diagram allows to derive the column density and gas temperature when multiple transitions of the same molecules are observed (assuming LTE and optically thin conditions).
Lecture 6: Molecular Transitions (1) Astrochemistry
 Lecture 6: Molecular Transitions (1) Astrochemistry Ehrenfreund & Charnley 2000, ARA&A, 38, 427 Outline Astrochemical processes: The formation of H2 H3 formation The chemistry initiated by H3 Formation
Lecture 6: Molecular Transitions (1) Astrochemistry Ehrenfreund & Charnley 2000, ARA&A, 38, 427 Outline Astrochemical processes: The formation of H2 H3 formation The chemistry initiated by H3 Formation
Lecture 7: Molecular Transitions (2) Line radiation from molecular clouds to derive physical parameters
 Lecture 7: Molecular Transitions (2) Line radiation from molecular clouds to derive physical parameters H 2 CO (NH 3 ) See sections 5.1-5.3.1 and 6.1 of Stahler & Palla Column density Volume density (Gas
Lecture 7: Molecular Transitions (2) Line radiation from molecular clouds to derive physical parameters H 2 CO (NH 3 ) See sections 5.1-5.3.1 and 6.1 of Stahler & Palla Column density Volume density (Gas
19. Interstellar Chemistry
 19. Interstellar Chemistry 1. Introduction to Interstellar Chemistry 2. Chemical Processes & Models 3. Formation & Destruction of H 2 4. Formation & Destruction of CO References Duley & Williams, "Interstellar
19. Interstellar Chemistry 1. Introduction to Interstellar Chemistry 2. Chemical Processes & Models 3. Formation & Destruction of H 2 4. Formation & Destruction of CO References Duley & Williams, "Interstellar
Spectroscopy and Molecular Emission. Fundamental Probes of Cold Gas
 Spectroscopy and Molecular Emission Fundamental Probes of Cold Gas Atomic Lines Few atoms have fine structure transitions at low enough energy levels to emit at radiofrequencies Important exceptions HI
Spectroscopy and Molecular Emission Fundamental Probes of Cold Gas Atomic Lines Few atoms have fine structure transitions at low enough energy levels to emit at radiofrequencies Important exceptions HI
The formation of stars and planets. Day 1, Topic 2: Radiation physics. Lecture by: C.P. Dullemond
 The formation of stars and planets Day 1, Topic 2: Radiation physics Lecture by: C.P. Dullemond Astronomical Constants CGS units used throughout lecture (cm,erg,s...) AU = Astronomical Unit = distance
The formation of stars and planets Day 1, Topic 2: Radiation physics Lecture by: C.P. Dullemond Astronomical Constants CGS units used throughout lecture (cm,erg,s...) AU = Astronomical Unit = distance
First studies for cold stars under the hyphotesis of TE : Russell (1934) Fujita (1939, 1940, 1941)
 First studies for cold stars under the hyphotesis of TE : Russell (1934) Fujita (1939, 1940, 1941) These models were able to predict the abundances of the most conspicous diatomic molecules detected in
First studies for cold stars under the hyphotesis of TE : Russell (1934) Fujita (1939, 1940, 1941) These models were able to predict the abundances of the most conspicous diatomic molecules detected in
Astrochemistry with SOFIA. Paola Caselli Center for Astrochemical Studies Max-Planck-Ins:tute for Extraterrestrial Physics
 1 Astrochemistry with SOFIA Paola Caselli Center for Astrochemical Studies Max-Planck-Ins:tute for Extraterrestrial Physics 2 Molecular clouds in the Milky Way ~ 100,000 light years Taurus-Auriga 3 Molecular
1 Astrochemistry with SOFIA Paola Caselli Center for Astrochemical Studies Max-Planck-Ins:tute for Extraterrestrial Physics 2 Molecular clouds in the Milky Way ~ 100,000 light years Taurus-Auriga 3 Molecular
21. Introduction to Interstellar Chemistry
 21. Introduction to Interstellar Chemistry 1. Background 2. Gas Phase Chemistry 3. Formation and Destruction of H 2 4. Formation and Destruction of CO 5. Other Simple Molecules References Tielens, Physics
21. Introduction to Interstellar Chemistry 1. Background 2. Gas Phase Chemistry 3. Formation and Destruction of H 2 4. Formation and Destruction of CO 5. Other Simple Molecules References Tielens, Physics
Astro 201 Radiative Processes Problem Set 6. Due in class.
 Astro 201 Radiative Processes Problem Set 6 Due in class. Readings: Hand-outs from Osterbrock; Rybicki & Lightman 9.5; however much you like of Mihalas 108 114, 119 127, 128 137 (even skimming Mihalas
Astro 201 Radiative Processes Problem Set 6 Due in class. Readings: Hand-outs from Osterbrock; Rybicki & Lightman 9.5; however much you like of Mihalas 108 114, 119 127, 128 137 (even skimming Mihalas
Photodissociation Regions Radiative Transfer. Dr. Thomas G. Bisbas
 Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
Some recent work I. Cosmic microwave background, seeds of large scale structure (Planck) Formation and evolution of galaxies (Figure: Simpson et al.
 Radio astronomy Radio astronomy studies celestial objects at wavelengths longward of λ 100 µm (frequencies below ν 3 THz) A radio telecope can see cold gas and dust (Wien s displacement law of BB emision,
Radio astronomy Radio astronomy studies celestial objects at wavelengths longward of λ 100 µm (frequencies below ν 3 THz) A radio telecope can see cold gas and dust (Wien s displacement law of BB emision,
Spontaneous Emission, Stimulated Emission, and Absorption
 Chapter Six Spontaneous Emission, Stimulated Emission, and Absorption In this chapter, we review the general principles governing absorption and emission of radiation by absorbers with quantized energy
Chapter Six Spontaneous Emission, Stimulated Emission, and Absorption In this chapter, we review the general principles governing absorption and emission of radiation by absorbers with quantized energy
CHAPTER 22. Astrophysical Gases
 CHAPTER 22 Astrophysical Gases Most of the baryonic matter in the Universe is in a gaseous state, made up of 75% Hydrogen (H), 25% Helium (He) and only small amounts of other elements (called metals ).
CHAPTER 22 Astrophysical Gases Most of the baryonic matter in the Universe is in a gaseous state, made up of 75% Hydrogen (H), 25% Helium (He) and only small amounts of other elements (called metals ).
Some fundamentals. Statistical mechanics. The non-equilibrium ISM. = g u
 Some fundamentals Statistical mechanics We have seen that the collision timescale for gas in this room is very small relative to radiative timesscales such as spontaneous emission. The frequent collisions
Some fundamentals Statistical mechanics We have seen that the collision timescale for gas in this room is very small relative to radiative timesscales such as spontaneous emission. The frequent collisions
Components of Galaxies Gas The Importance of Gas
 Components of Galaxies Gas The Importance of Gas Fuel for star formation (H 2 ) Tracer of galaxy kinematics/mass (HI) Tracer of dynamical history of interaction between galaxies (HI) The Two-Level Atom
Components of Galaxies Gas The Importance of Gas Fuel for star formation (H 2 ) Tracer of galaxy kinematics/mass (HI) Tracer of dynamical history of interaction between galaxies (HI) The Two-Level Atom
Thermal Equilibrium in Nebulae 1. For an ionized nebula under steady conditions, heating and cooling processes that in
 Thermal Equilibrium in Nebulae 1 For an ionized nebula under steady conditions, heating and cooling processes that in isolation would change the thermal energy content of the gas are in balance, such that
Thermal Equilibrium in Nebulae 1 For an ionized nebula under steady conditions, heating and cooling processes that in isolation would change the thermal energy content of the gas are in balance, such that
Astrochemistry the summary
 Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Theory of Interstellar Phases
 Theory of Interstellar Phases 1. Relevant Observations 2. Linear Stability Theory 3. FGH Model 4. Update and Summary References Tielens, Secs. 8.1-5 Field ApJ 142 531 1965 (basic stability theory) Field,
Theory of Interstellar Phases 1. Relevant Observations 2. Linear Stability Theory 3. FGH Model 4. Update and Summary References Tielens, Secs. 8.1-5 Field ApJ 142 531 1965 (basic stability theory) Field,
The Gas Grain Chemistry of Translucent Molecular Clouds
 The Gas Grain Chemistry of Translucent Molecular Clouds Dominique Maffucci Department of Chemistry University of Virginia people.virginia.edu/ dmm2br/tools.html 17 July 2017 / Current and Future Perspectives
The Gas Grain Chemistry of Translucent Molecular Clouds Dominique Maffucci Department of Chemistry University of Virginia people.virginia.edu/ dmm2br/tools.html 17 July 2017 / Current and Future Perspectives
Relations between the Einstein coefficients
 Relations between the Einstein coefficients Additional reading: Böhm-Vitense Ch 13.1, 13.2 In thermodynamic equilibrium, transition rate (per unit time per unit volume) from level 1 to level 2 must equal
Relations between the Einstein coefficients Additional reading: Böhm-Vitense Ch 13.1, 13.2 In thermodynamic equilibrium, transition rate (per unit time per unit volume) from level 1 to level 2 must equal
Astrochimistry Spring 2013
 Astrochimistry Spring 2013 Lecture 3: H2 Formation NGC 7023 - HST Julien Montillaud 1st February 2013 Outline I. The most abundant molecule in the Universe (6 p.) I.1 Relative abundances I.2 Historical
Astrochimistry Spring 2013 Lecture 3: H2 Formation NGC 7023 - HST Julien Montillaud 1st February 2013 Outline I. The most abundant molecule in the Universe (6 p.) I.1 Relative abundances I.2 Historical
2. NOTES ON RADIATIVE TRANSFER The specific intensity I ν
 1 2. NOTES ON RADIATIVE TRANSFER 2.1. The specific intensity I ν Let f(x, p) be the photon distribution function in phase space, summed over the two polarization states. Then fdxdp is the number of photons
1 2. NOTES ON RADIATIVE TRANSFER 2.1. The specific intensity I ν Let f(x, p) be the photon distribution function in phase space, summed over the two polarization states. Then fdxdp is the number of photons
6. Interstellar Medium. Emission nebulae are diffuse patches of emission surrounding hot O and
 6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
2- The chemistry in the. The formation of water : gas phase and grain surface formation. The present models. Observations of molecules in the ISM.
 2- The chemistry in the ISM. The formation of water : gas phase and grain surface formation. The present models. Observations of molecules in the ISM. 1 Why studying the ISM chemistry? 1- The thermal balance,
2- The chemistry in the ISM. The formation of water : gas phase and grain surface formation. The present models. Observations of molecules in the ISM. 1 Why studying the ISM chemistry? 1- The thermal balance,
Plasma Spectroscopy Inferences from Line Emission
 Plasma Spectroscopy Inferences from Line Emission Ø From line λ, can determine element, ionization state, and energy levels involved Ø From line shape, can determine bulk and thermal velocity and often
Plasma Spectroscopy Inferences from Line Emission Ø From line λ, can determine element, ionization state, and energy levels involved Ø From line shape, can determine bulk and thermal velocity and often
The Interstellar Medium
 The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
Photoionization Modelling of H II Region for Oxygen Ions
 Journal of Materials Science and Chemical Engineering, 2015, 3, 7-16 Published Online April 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.34002 Photoionization Modelling
Journal of Materials Science and Chemical Engineering, 2015, 3, 7-16 Published Online April 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.34002 Photoionization Modelling
1 Radiative transfer etc
 Radiative transfer etc Last time we derived the transfer equation dτ ν = S ν I v where I ν is the intensity, S ν = j ν /α ν is the source function and τ ν = R α ν dl is the optical depth. The formal solution
Radiative transfer etc Last time we derived the transfer equation dτ ν = S ν I v where I ν is the intensity, S ν = j ν /α ν is the source function and τ ν = R α ν dl is the optical depth. The formal solution
Cosmic Evolution, Part II. Heavy Elements to Molecules
 Cosmic Evolution, Part II Heavy Elements to Molecules First a review of terminology: Element Atom Electro- magnetic Electrons Nucleus Electromagnetic Strong Nuclear Compound Molecule Protons Neutrons Neutral
Cosmic Evolution, Part II Heavy Elements to Molecules First a review of terminology: Element Atom Electro- magnetic Electrons Nucleus Electromagnetic Strong Nuclear Compound Molecule Protons Neutrons Neutral
Lecture 10: "Chemistry in Dense Molecular Clouds"
 Lecture 10: "Chemistry in Dense Molecular Clouds" Outline 1. Observations of molecular clouds 2. Physics of dense clouds 3. Chemistry of dense clouds: C-, O-, N-chemistry Types of molecular clouds Diffuse
Lecture 10: "Chemistry in Dense Molecular Clouds" Outline 1. Observations of molecular clouds 2. Physics of dense clouds 3. Chemistry of dense clouds: C-, O-, N-chemistry Types of molecular clouds Diffuse
Photoionized Gas Ionization Equilibrium
 Photoionized Gas Ionization Equilibrium Ionization Recombination H nebulae - case A and B Strömgren spheres H + He nebulae Heavy elements, dielectronic recombination Ionization structure 1 Ionization Equilibrium
Photoionized Gas Ionization Equilibrium Ionization Recombination H nebulae - case A and B Strömgren spheres H + He nebulae Heavy elements, dielectronic recombination Ionization structure 1 Ionization Equilibrium
Quantum Electronics/Laser Physics Chapter 4 Line Shapes and Line Widths
 Quantum Electronics/Laser Physics Chapter 4 Line Shapes and Line Widths 4.1 The Natural Line Shape 4.2 Collisional Broadening 4.3 Doppler Broadening 4.4 Einstein Treatment of Stimulated Processes Width
Quantum Electronics/Laser Physics Chapter 4 Line Shapes and Line Widths 4.1 The Natural Line Shape 4.2 Collisional Broadening 4.3 Doppler Broadening 4.4 Einstein Treatment of Stimulated Processes Width
Cosmic Evolution, Part II. Heavy Elements to Molecules
 Cosmic Evolution, Part II Heavy Elements to Molecules Heavy elements molecules First a review of terminology: Electromagnetic Electrons Element Atom Nucleus Compound Molecule Electromagnetic Strong Nuclear
Cosmic Evolution, Part II Heavy Elements to Molecules Heavy elements molecules First a review of terminology: Electromagnetic Electrons Element Atom Nucleus Compound Molecule Electromagnetic Strong Nuclear
Spectral Line Intensities - Boltzmann, Saha Eqs.
 Spectral Line Intensities - Boltzmann, Saha Eqs. Absorption in a line depends on: - number of absorbers along the line-of-sight, and -their cross section(s). Absorp. n a σl, where n a is the number of
Spectral Line Intensities - Boltzmann, Saha Eqs. Absorption in a line depends on: - number of absorbers along the line-of-sight, and -their cross section(s). Absorp. n a σl, where n a is the number of
Monte Carlo methods for molecular line observations at (sub)mm wavelengths. Michiel Hogerheijde Leiden Observatory Allegro ALMA ARC node
 Monte Carlo methods for molecular line observations at (sub)mm wavelengths Michiel Hogerheijde Leiden Observatory Allegro ALMA ARC node Outline Lecture 1: How does molecular line transfer and non- LTE
Monte Carlo methods for molecular line observations at (sub)mm wavelengths Michiel Hogerheijde Leiden Observatory Allegro ALMA ARC node Outline Lecture 1: How does molecular line transfer and non- LTE
3: Interstellar Absorption Lines: Radiative Transfer in the Interstellar Medium. James R. Graham University of California, Berkeley
 3: Interstellar Absorption Lines: Radiative Transfer in the Interstellar Medium James R. Graham University of California, Berkeley Interstellar Absorption Lines Example of atomic absorption lines Structure
3: Interstellar Absorption Lines: Radiative Transfer in the Interstellar Medium James R. Graham University of California, Berkeley Interstellar Absorption Lines Example of atomic absorption lines Structure
OUTLINE OF A GAS-GRAIN CHEMICAL CODE. Dima Semenov MPIA Heidelberg
 OUTLINE OF A GAS-GRAIN CHEMICAL CODE Dima Semenov MPIA Heidelberg MOTTO OF ASTROCHEMISTRY CODE DEVELOPMENT We had 5 ODE solvers, 9 Jacobi matrix inversion routines, several approaches to calculate reaction
OUTLINE OF A GAS-GRAIN CHEMICAL CODE Dima Semenov MPIA Heidelberg MOTTO OF ASTROCHEMISTRY CODE DEVELOPMENT We had 5 ODE solvers, 9 Jacobi matrix inversion routines, several approaches to calculate reaction
FORMATION OF PRIMORDIAL STARS
 Talk@INT, UW, July 5, 2006 FORMATION OF PRIMORDIAL STARS Naoki Yoshida Department of Physics Nagoya University Outline Thermal evolution of a primordial gas - Physics at high densities (cooling, chem.
Talk@INT, UW, July 5, 2006 FORMATION OF PRIMORDIAL STARS Naoki Yoshida Department of Physics Nagoya University Outline Thermal evolution of a primordial gas - Physics at high densities (cooling, chem.
ASTR240: Radio Astronomy
 ASTR240: Radio Astronomy HW#3 Due Feb 27, 2013 Problem 1 (4 points) (Courtesy J. J. Condon & S. M. Ransom) The GBT (Green Bank Telescope, a steerable radio telescope roughly the size of a football field
ASTR240: Radio Astronomy HW#3 Due Feb 27, 2013 Problem 1 (4 points) (Courtesy J. J. Condon & S. M. Ransom) The GBT (Green Bank Telescope, a steerable radio telescope roughly the size of a football field
Gas 1: Molecular clouds
 Gas 1: Molecular clouds > 4000 known with masses ~ 10 3 to 10 5 M T ~ 10 to 25 K (cold!); number density n > 10 9 gas particles m 3 Emission bands in IR, mm, radio regions from molecules comprising H,
Gas 1: Molecular clouds > 4000 known with masses ~ 10 3 to 10 5 M T ~ 10 to 25 K (cold!); number density n > 10 9 gas particles m 3 Emission bands in IR, mm, radio regions from molecules comprising H,
Understanding the chemistry of AGB circumstellar envelopes through the study of IRC
 Understanding the chemistry of AGB circumstellar envelopes through the study of IRC +10216 Marcelino Agúndez LUTH, Observatoire de Paris 28 janvier 2010 1 1.65 m 2MASS PART I. INTRODUCTION: - Interest
Understanding the chemistry of AGB circumstellar envelopes through the study of IRC +10216 Marcelino Agúndez LUTH, Observatoire de Paris 28 janvier 2010 1 1.65 m 2MASS PART I. INTRODUCTION: - Interest
PHYS 172: Modern Mechanics Fall 2009
 PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
Ay Fall 2004 Lecture 6 (given by Tony Travouillon)
 Ay 122 - Fall 2004 Lecture 6 (given by Tony Travouillon) Stellar atmospheres, classification of stellar spectra (Many slides c/o Phil Armitage) Formation of spectral lines: 1.excitation Two key questions:
Ay 122 - Fall 2004 Lecture 6 (given by Tony Travouillon) Stellar atmospheres, classification of stellar spectra (Many slides c/o Phil Armitage) Formation of spectral lines: 1.excitation Two key questions:
The Interstellar Medium
 http://www.strw.leidenuniv.nl/~pvdwerf/teaching/ The Interstellar Medium Lecturer: Dr. Paul van der Werf Fall 2014 Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium
http://www.strw.leidenuniv.nl/~pvdwerf/teaching/ The Interstellar Medium Lecturer: Dr. Paul van der Werf Fall 2014 Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium
Notes: Most of the material presented in this chapter is taken from Stahler and Palla (2004), Chap. 3. v r c, (3.1) ! obs
 Chapter 3. Molecular Clouds Notes: Most of the material presented in this chapter is taken from Stahler and Palla 2004), Chap. 3. 3.1 Definitions and Preliminaries We mainly covered in Chapter 2 the Galactic
Chapter 3. Molecular Clouds Notes: Most of the material presented in this chapter is taken from Stahler and Palla 2004), Chap. 3. 3.1 Definitions and Preliminaries We mainly covered in Chapter 2 the Galactic
a few more introductory subjects : equilib. vs non-equil. ISM sources and sinks : matter replenishment, and exhaustion Galactic Energetics
 Today : a few more introductory subjects : equilib. vs non-equil. ISM sources and sinks : matter replenishment, and exhaustion Galactic Energetics photo-ionization of HII assoc. w/ OB stars ionization
Today : a few more introductory subjects : equilib. vs non-equil. ISM sources and sinks : matter replenishment, and exhaustion Galactic Energetics photo-ionization of HII assoc. w/ OB stars ionization
Model of Hydrogen Deficient Nebulae in H II Regions at High Temperature
 Journal of Materials Science and Chemical Engineering, 2015, 3, 21-29 Published Online August 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.38004 Model of Hydrogen
Journal of Materials Science and Chemical Engineering, 2015, 3, 21-29 Published Online August 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.38004 Model of Hydrogen
Spectroscopy Lecture 2
 Spectroscopy Lecture 2 I. Atomic excitation and ionization II. Radiation Terms III. Absorption and emission coefficients IV. Einstein coefficients V. Black Body radiation I. Atomic excitation and ionization
Spectroscopy Lecture 2 I. Atomic excitation and ionization II. Radiation Terms III. Absorption and emission coefficients IV. Einstein coefficients V. Black Body radiation I. Atomic excitation and ionization
Models of molecular line emission from circumstellar disks
 Chapter 2 Models of molecular line emission from circumstellar disks Abstract High-resolution observations of molecular line emission allow the determination of the chemical composition at each radius
Chapter 2 Models of molecular line emission from circumstellar disks Abstract High-resolution observations of molecular line emission allow the determination of the chemical composition at each radius
RADIO SPECTRAL LINES. Nissim Kanekar National Centre for Radio Astrophysics, Pune
 RADIO SPECTRAL LINES Nissim Kanekar National Centre for Radio Astrophysics, Pune OUTLINE The importance of radio spectral lines. Equilibrium issues: kinetic, excitation, brightness temperatures. The equation
RADIO SPECTRAL LINES Nissim Kanekar National Centre for Radio Astrophysics, Pune OUTLINE The importance of radio spectral lines. Equilibrium issues: kinetic, excitation, brightness temperatures. The equation
Radiation processes and mechanisms in astrophysics I. R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 2009
 Radiation processes and mechanisms in astrophysics I R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 009 Light of the night sky We learn of the universe around us from EM radiation, neutrinos,
Radiation processes and mechanisms in astrophysics I R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 009 Light of the night sky We learn of the universe around us from EM radiation, neutrinos,
Lecture 2 Interstellar Absorption Lines: Line Radiative Transfer
 Lecture 2 Interstellar Absorption Lines: Line Radiative Transfer 1. Atomic absorption lines 2. Application of radiative transfer to absorption & emission 3. Line broadening & curve of growth 4. Optical/UV
Lecture 2 Interstellar Absorption Lines: Line Radiative Transfer 1. Atomic absorption lines 2. Application of radiative transfer to absorption & emission 3. Line broadening & curve of growth 4. Optical/UV
Diffuse Interstellar Medium
 Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
7. Non-LTE basic concepts
 7. Non-LTE basic concepts LTE vs NLTE occupation numbers rate equation transition probabilities: collisional and radiative examples: hot stars, A supergiants 10/13/2003 Spring 2016 LTE LTE vs NLTE each
7. Non-LTE basic concepts LTE vs NLTE occupation numbers rate equation transition probabilities: collisional and radiative examples: hot stars, A supergiants 10/13/2003 Spring 2016 LTE LTE vs NLTE each
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
VI. 21cm Radiation. 1 AY230-21cm. A. History
 1 AY230-21cm VI. 21cm Radiation A. History B. Physics van der Hulst 1941 Collq in a bunker! Ground state of HI: l = 0, s = 1/2, j = 1/2 Degeneracy of the G.S. is removed by the interaction of the magnetic
1 AY230-21cm VI. 21cm Radiation A. History B. Physics van der Hulst 1941 Collq in a bunker! Ground state of HI: l = 0, s = 1/2, j = 1/2 Degeneracy of the G.S. is removed by the interaction of the magnetic
Electron temperature is the temperature that describes, through Maxwell's law, the kinetic energy distribution of the free electrons.
 10.3.1.1 Excitation and radiation of spectra 10.3.1.1.1 Plasmas A plasma of the type occurring in spectrochemical radiation sources may be described as a gas which is at least partly ionized and contains
10.3.1.1 Excitation and radiation of spectra 10.3.1.1.1 Plasmas A plasma of the type occurring in spectrochemical radiation sources may be described as a gas which is at least partly ionized and contains
1/30/11. Astro 300B: Jan. 26, Thermal radia+on and Thermal Equilibrium. Thermal Radia0on, and Thermodynamic Equilibrium
 Astro 300B: Jan. 26, 2011 Thermal radia+on and Thermal Equilibrium Thermal Radia0on, and Thermodynamic Equilibrium 1 Thermal radiation is radiation emitted by matter in thermodynamic equilibrium. When
Astro 300B: Jan. 26, 2011 Thermal radia+on and Thermal Equilibrium Thermal Radia0on, and Thermodynamic Equilibrium 1 Thermal radiation is radiation emitted by matter in thermodynamic equilibrium. When
Equilibrium Properties of Matter and Radiation
 Equilibrium Properties of Matter and Radiation Temperature What is it? A measure of internal energy in a system. Measure from (1) velocities of atoms/molecules () population of excited/ionized states (3)
Equilibrium Properties of Matter and Radiation Temperature What is it? A measure of internal energy in a system. Measure from (1) velocities of atoms/molecules () population of excited/ionized states (3)
X-ray Radiation, Absorption, and Scattering
 X-ray Radiation, Absorption, and Scattering What we can learn from data depend on our understanding of various X-ray emission, scattering, and absorption processes. We will discuss some basic processes:
X-ray Radiation, Absorption, and Scattering What we can learn from data depend on our understanding of various X-ray emission, scattering, and absorption processes. We will discuss some basic processes:
Collisionally Excited Spectral Lines (Cont d) Diffuse Universe -- C. L. Martin
 Collisionally Excited Spectral Lines (Cont d) Please Note: Contrast the collisionally excited lines with the H and He lines in the Orion Nebula spectrum. Preview: Pure Recombination Lines Recombination
Collisionally Excited Spectral Lines (Cont d) Please Note: Contrast the collisionally excited lines with the H and He lines in the Orion Nebula spectrum. Preview: Pure Recombination Lines Recombination
Lecture 2 Line Radiative Transfer for the ISM
 Lecture 2 Line Radiative Transfer for the ISM Absorption lines in the optical & UV Equation of transfer Absorption & emission coefficients Line broadening Equivalent width and curve of growth Observations
Lecture 2 Line Radiative Transfer for the ISM Absorption lines in the optical & UV Equation of transfer Absorption & emission coefficients Line broadening Equivalent width and curve of growth Observations
Chemical Diagnostics of Star Forming Regions
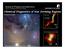 School of Physics and Astronomy FACULTY OF MATHEMATICS & PHYSICAL SCIENCES Chemical Diagnostics of Star Forming Regions Paola Caselli N 2 H + (1-0) Di Francesco et al. 2004 N 2 H + (3-2) Bourke et al.
School of Physics and Astronomy FACULTY OF MATHEMATICS & PHYSICAL SCIENCES Chemical Diagnostics of Star Forming Regions Paola Caselli N 2 H + (1-0) Di Francesco et al. 2004 N 2 H + (3-2) Bourke et al.
Interstellar Medium Physics
 Physics of gas in galaxies. Two main parts: atomic processes & hydrodynamic processes. Atomic processes deal mainly with radiation Hydrodynamics is large scale dynamics of gas. Start small Radiative transfer
Physics of gas in galaxies. Two main parts: atomic processes & hydrodynamic processes. Atomic processes deal mainly with radiation Hydrodynamics is large scale dynamics of gas. Start small Radiative transfer
Lec. 4 Thermal Properties & Line Diagnostics for HII Regions
 Lec. 4 Thermal Properties & Line Diagnostics for HII Regions 1. General Introduction* 2. Temperature of Photoionized Gas: Heating & Cooling of HII Regions 3. Thermal Balance 4. Line Emission 5. Diagnostics
Lec. 4 Thermal Properties & Line Diagnostics for HII Regions 1. General Introduction* 2. Temperature of Photoionized Gas: Heating & Cooling of HII Regions 3. Thermal Balance 4. Line Emission 5. Diagnostics
Lecture 4: Absorption and emission lines
 Lecture 4: Absorption and emission lines Senior Astrophysics 2018-03-13 Senior Astrophysics () Lecture 4: Absorption and emission lines 2018-03-13 1 / 35 Outline 1 Absorption and emission line spectra
Lecture 4: Absorption and emission lines Senior Astrophysics 2018-03-13 Senior Astrophysics () Lecture 4: Absorption and emission lines 2018-03-13 1 / 35 Outline 1 Absorption and emission line spectra
Lecture 19 CO Observations of Molecular Clouds
 Lecture 9 CO Observations of Molecular Clouds. CO Surveys 2. Nearby molecular clouds 3. Antenna temperature and radiative transfer 4. Determining cloud conditions from CO References Tielens, Ch. 0 Myers,
Lecture 9 CO Observations of Molecular Clouds. CO Surveys 2. Nearby molecular clouds 3. Antenna temperature and radiative transfer 4. Determining cloud conditions from CO References Tielens, Ch. 0 Myers,
Collisional radiative model
 Lenka Dosoudilová Lenka Dosoudilová 1 / 14 Motivation Equations Approximative models Emission coefficient Particles J ij = 1 4π n j A ij hν ij, atoms in ground state atoms in excited states resonance metastable
Lenka Dosoudilová Lenka Dosoudilová 1 / 14 Motivation Equations Approximative models Emission coefficient Particles J ij = 1 4π n j A ij hν ij, atoms in ground state atoms in excited states resonance metastable
Published in: LOW-METALLICITY STAR FORMATION: FROM THE FIRST STARS TO DWARF GALAXIES
 University of Groningen Interstellar Chemistry Spaans, Marco Published in: LOW-METALLICITY STAR FORMATION: FROM THE FIRST STARS TO DWARF GALAXIES DOI: 10.1017/S1743921308024885 IMPORTANT NOTE: You are
University of Groningen Interstellar Chemistry Spaans, Marco Published in: LOW-METALLICITY STAR FORMATION: FROM THE FIRST STARS TO DWARF GALAXIES DOI: 10.1017/S1743921308024885 IMPORTANT NOTE: You are
Lecture 3: Emission and absorption
 Lecture 3: Emission and absorption Senior Astrophysics 2017-03-10 Senior Astrophysics Lecture 3: Emission and absorption 2017-03-10 1 / 35 Outline 1 Optical depth 2 Sources of radiation 3 Blackbody radiation
Lecture 3: Emission and absorption Senior Astrophysics 2017-03-10 Senior Astrophysics Lecture 3: Emission and absorption 2017-03-10 1 / 35 Outline 1 Optical depth 2 Sources of radiation 3 Blackbody radiation
Cosmic Rays in the Interstellar Medium. Nick Indriolo University of Illinois at Urbana- Champaign
 Cosmic Rays in the Interstellar Medium Nick Indriolo University of Illinois at Urbana- Champaign November 3, 2010 Stormy Cosmos Cosmic Ray Basics Energetic charged particles and nuclei protons, alpha particles,
Cosmic Rays in the Interstellar Medium Nick Indriolo University of Illinois at Urbana- Champaign November 3, 2010 Stormy Cosmos Cosmic Ray Basics Energetic charged particles and nuclei protons, alpha particles,
Theory of optically thin emission line spectroscopy
 Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Class #4 11 September 2008
 Class #4 11 September 2008 Review Stellar evolution/nucleosynthesis/h-r diagrams Phases of the Interstellar Medium The Hydrogen Atom H-R diagram for 47 Tuc Evolution+nucleosynt hesis each box is a different
Class #4 11 September 2008 Review Stellar evolution/nucleosynthesis/h-r diagrams Phases of the Interstellar Medium The Hydrogen Atom H-R diagram for 47 Tuc Evolution+nucleosynt hesis each box is a different
II. HII Regions (Ionization State)
 1 AY230-HIIReg II. HII Regions (Ionization State) A. Motivations Theoretical: HII regions are intamitely linked with past, current and future starforming regions in galaxies. To build theories of star-formation
1 AY230-HIIReg II. HII Regions (Ionization State) A. Motivations Theoretical: HII regions are intamitely linked with past, current and future starforming regions in galaxies. To build theories of star-formation
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines!
 Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
Protostars 1. Early growth and collapse. First core and main accretion phase
 Protostars 1. First core and main accretion phase Stahler & Palla: Chapter 11.1 & 8.4.1 & Appendices F & G Early growth and collapse In a magnetized cloud undergoing contraction, the density gradually
Protostars 1. First core and main accretion phase Stahler & Palla: Chapter 11.1 & 8.4.1 & Appendices F & G Early growth and collapse In a magnetized cloud undergoing contraction, the density gradually
Astrochemistry. Lecture 10, Primordial chemistry. Jorma Harju. Department of Physics. Friday, April 5, 2013, 12:15-13:45, Lecture room D117
 Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
Chapter V: Interactions of neutrons with matter
 Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Lecture 3: Specific Intensity, Flux and Optical Depth
 Lecture 3: Specific Intensity, Flux and Optical Depth We begin a more detailed look at stellar atmospheres by defining the fundamental variable, which is called the Specific Intensity. It may be specified
Lecture 3: Specific Intensity, Flux and Optical Depth We begin a more detailed look at stellar atmospheres by defining the fundamental variable, which is called the Specific Intensity. It may be specified
M.Phys., M.Math.Phys., M.Sc. MTP Radiative Processes in Astrophysics and High-Energy Astrophysics
 M.Phys., M.Math.Phys., M.Sc. MTP Radiative Processes in Astrophysics and High-Energy Astrophysics Professor Garret Cotter garret.cotter@physics.ox.ac.uk Office 756 in the DWB & Exeter College Radiative
M.Phys., M.Math.Phys., M.Sc. MTP Radiative Processes in Astrophysics and High-Energy Astrophysics Professor Garret Cotter garret.cotter@physics.ox.ac.uk Office 756 in the DWB & Exeter College Radiative
CHM 5423 Atmospheric Chemistry Notes on kinetics (Chapter 4)
 CHM 5423 Atmospheric Chemistry Notes on kinetics (Chapter 4) Introduction A mechanism is one or a series of elementary reactions that convert reactants into products or otherwise model the chemistry of
CHM 5423 Atmospheric Chemistry Notes on kinetics (Chapter 4) Introduction A mechanism is one or a series of elementary reactions that convert reactants into products or otherwise model the chemistry of
The chemistry and thermodynamics of Pop III star formation
 The chemistry and thermodynamics of Pop III star formation Where do the first stars form Form in dark matter minihalos with mass Mhalo 5 10 5 M Redshift z = 16-20 Tvir ~ 1000 K Gas density is around 1
The chemistry and thermodynamics of Pop III star formation Where do the first stars form Form in dark matter minihalos with mass Mhalo 5 10 5 M Redshift z = 16-20 Tvir ~ 1000 K Gas density is around 1
Physics and Chemistry of the Interstellar Medium
 Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
Chapter 10 The Interstellar Medium
 Chapter 10 The Interstellar Medium Guidepost You have begun your study of the sun and other stars, but now it is time to study the thin gas and dust that drifts through space between the stars. This chapter
Chapter 10 The Interstellar Medium Guidepost You have begun your study of the sun and other stars, but now it is time to study the thin gas and dust that drifts through space between the stars. This chapter
Lec 3. Radiative Processes and HII Regions
 Lec 3. Radiative Processes and HII Regions 1. Photoionization 2. Recombination 3. Photoionization-Recombination Equilibrium 4. Heating & Cooling of HII Regions 5. Strömgren Theory (for Hydrogen) 6. The
Lec 3. Radiative Processes and HII Regions 1. Photoionization 2. Recombination 3. Photoionization-Recombination Equilibrium 4. Heating & Cooling of HII Regions 5. Strömgren Theory (for Hydrogen) 6. The
Notes on Photoionized Regions Wednesday, January 12, 2011
 Notes on Photoionized Regions Wednesday, January 12, 2011 CONTENTS: 1. Introduction 2. Hydrogen Nebulae A. Ionization equations B. Recombination coefficients and cross sections C. Structure of the hydrogen
Notes on Photoionized Regions Wednesday, January 12, 2011 CONTENTS: 1. Introduction 2. Hydrogen Nebulae A. Ionization equations B. Recombination coefficients and cross sections C. Structure of the hydrogen
Giant Star-Forming Regions
 University of Heidelberg, Center for Astronomy Dimitrios A. Gouliermis & Ralf S. Klessen Lecture #7 Physical Processes in Ionized Hydrogen Regions Part II (tentative) Schedule of the Course Lect. 1 Lect.
University of Heidelberg, Center for Astronomy Dimitrios A. Gouliermis & Ralf S. Klessen Lecture #7 Physical Processes in Ionized Hydrogen Regions Part II (tentative) Schedule of the Course Lect. 1 Lect.
Giant Star-Forming Regions
 University of Heidelberg, Center for Astronomy Dimitrios A. Gouliermis & Ralf S. Klessen Lecture #1 Introduction & Overview Introduction to HII Regions In this Lecture Motivation for this Course Schedule
University of Heidelberg, Center for Astronomy Dimitrios A. Gouliermis & Ralf S. Klessen Lecture #1 Introduction & Overview Introduction to HII Regions In this Lecture Motivation for this Course Schedule
Possible Extra Credit Option
 Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
The Birth Of Stars. How do stars form from the interstellar medium Where does star formation take place How do we induce star formation
 Goals: The Birth Of Stars How do stars form from the interstellar medium Where does star formation take place How do we induce star formation Interstellar Medium Gas and dust between stars is the interstellar
Goals: The Birth Of Stars How do stars form from the interstellar medium Where does star formation take place How do we induce star formation Interstellar Medium Gas and dust between stars is the interstellar
The KInetic Database for Astrochemistry
 The KInetic Database for Astrochemistry Valentine Wakelam and the KIDA team Laboratory astrophysics of Bordeaux France ICE Interstellar chemical models Compute species abundances as a function of time
The KInetic Database for Astrochemistry Valentine Wakelam and the KIDA team Laboratory astrophysics of Bordeaux France ICE Interstellar chemical models Compute species abundances as a function of time
University of Groningen. Molecular data and radiative transfer tools for ALMA Tak, F F S van der;; Hogerheijde, M. Published in: Default journal
 University of Groningen Molecular data and radiative transfer tools for ALMA Tak, F F S van der;; Hogerheijde, M Published in: Default journal IMPORTANT NOTE: You are advised to consult the publisher's
University of Groningen Molecular data and radiative transfer tools for ALMA Tak, F F S van der;; Hogerheijde, M Published in: Default journal IMPORTANT NOTE: You are advised to consult the publisher's
arxiv: v2 [astro-ph.ga] 15 Apr 2015
![arxiv: v2 [astro-ph.ga] 15 Apr 2015 arxiv: v2 [astro-ph.ga] 15 Apr 2015](/thumbs/74/71382419.jpg) APJ LETTERS ACCEPTED Preprint typeset using L A TEX style emulateapj v. 05/1/1 WATER FORMATION DURING THE EPOCH OF FIRST METAL ENRICHMENT SHMUEL BIALY 1, AMIEL STERNBERG 1 AND ABRAHAM LOEB 1, ApJ Letters
APJ LETTERS ACCEPTED Preprint typeset using L A TEX style emulateapj v. 05/1/1 WATER FORMATION DURING THE EPOCH OF FIRST METAL ENRICHMENT SHMUEL BIALY 1, AMIEL STERNBERG 1 AND ABRAHAM LOEB 1, ApJ Letters
Physics of Photon Dominated Regions. PDR Models SS 2007 M. Röllig
 Physics of Photon Dominated Regions PDR Models SS 2007 M. Röllig The standard view from planeparallel models So far: all necessary components of a PDR model introduced: energy balance: important heating
Physics of Photon Dominated Regions PDR Models SS 2007 M. Röllig The standard view from planeparallel models So far: all necessary components of a PDR model introduced: energy balance: important heating
Lecture 26 Clouds, Clumps and Cores. Review of Molecular Clouds
 Lecture 26 Clouds, Clumps and Cores 1. Review of Dense Gas Observations 2. Atomic Hydrogen and GMCs 3. Formation of Molecular Clouds 4. Internal Structure 5. Observing Cores 6. Preliminary Comments on
Lecture 26 Clouds, Clumps and Cores 1. Review of Dense Gas Observations 2. Atomic Hydrogen and GMCs 3. Formation of Molecular Clouds 4. Internal Structure 5. Observing Cores 6. Preliminary Comments on
Outline. Today we will learn what is thermal radiation
 Thermal Radiation & Outline Today we will learn what is thermal radiation Laws Laws of of themodynamics themodynamics Radiative Radiative Diffusion Diffusion Equation Equation Thermal Thermal Equilibrium
Thermal Radiation & Outline Today we will learn what is thermal radiation Laws Laws of of themodynamics themodynamics Radiative Radiative Diffusion Diffusion Equation Equation Thermal Thermal Equilibrium
Effects of Massive Stars
 Effects of Massive Stars Classical HII Regions Ultracompact HII Regions Stahler Palla: Sections 15.1, 15. HII Regions The salient characteristic of any massive star is its extreme energy output, much of
Effects of Massive Stars Classical HII Regions Ultracompact HII Regions Stahler Palla: Sections 15.1, 15. HII Regions The salient characteristic of any massive star is its extreme energy output, much of
Astrophysics of Gaseous Nebulae and Active Galactic Nuclei
 SECOND EDITION Astrophysics of Gaseous Nebulae and Active Galactic Nuclei Donald E. Osterbrock Lick Observatory, University of California, Santa Cruz Gary J. Ferland Department of Physics and Astronomy,
SECOND EDITION Astrophysics of Gaseous Nebulae and Active Galactic Nuclei Donald E. Osterbrock Lick Observatory, University of California, Santa Cruz Gary J. Ferland Department of Physics and Astronomy,
PDR Modelling with KOSMA-τ
 PDR Modelling with KOSMA-τ M. Röllig, V. Ossenkopf-Okada, C. Bruckmann; Y. Okada, N. Schneider, U. Graf, J. Stutzki I. Physikalisches Institut, Universität zu Köln The KOSMA-τ PDR Code 1-D, spherical geometry
PDR Modelling with KOSMA-τ M. Röllig, V. Ossenkopf-Okada, C. Bruckmann; Y. Okada, N. Schneider, U. Graf, J. Stutzki I. Physikalisches Institut, Universität zu Köln The KOSMA-τ PDR Code 1-D, spherical geometry
Physics of Primordial Star Formation
 Physics of Primordial Star Formation Naoki Yoshida University of Tokyo SFDE17 Bootcamp, Quy Nhon, August 6th So far, we learned 1. The standard cosmological model 2. Growth of density fluctuations 3. Assembly
Physics of Primordial Star Formation Naoki Yoshida University of Tokyo SFDE17 Bootcamp, Quy Nhon, August 6th So far, we learned 1. The standard cosmological model 2. Growth of density fluctuations 3. Assembly
