Electrochemistry Part 2: Getting quantitative and looking at electrochemical cells in action
|
|
|
- Samson Cox
- 6 years ago
- Views:
Transcription
1 Electrochemistry Part 2: Getting quantitative and looking at electrochemical cells in action Everything we want to learn in the way of quantitative information about electrochemistry can be found in the familiar diagram below: The e- e- e- e- is the amount of charge, q, and can be related to mass through a simple stoichiometry problem The potential hill, E, is the value on your battery it depends on the half cell potentials and the concentrations Thermodynamic values like w and DG are realized from q times E. E(V) e e e e e (this is the voltage (V) difference measured by a voltmeter) This is the number of charges, as per unit time, the current. 1. For electrochemistry to do much work, the size of the hill (V) and the amount of charge (q) both need to be large. 2. Oh, this must mean work= w= qv These three general categories of calculations are examined in order on the following pages:
2 Everything you wanted to know about measuring charge: basic stuff they should have told you in your high school physics class Electrical Charge. The fundamental unit of charge, q, is measured in Coulombs. One electron has a charge of x Coulombs (C). Therefore, one mole of electrons has a special amount of charge F= Faraday (Coulombs/mole) = q (Coulombs) / n (moles) F = x 10 4 C/mole So q = nf So electrons are our currency for electrochemical measurements in the same way that protons were our currency for acid/base reactions. A Faraday is simply an amount of charge in the same way a dozen is an amount of eggs and we can do routine stoichiometry calculations with this charge to mole conversion factor. Example: In a redox reaction involving the reduction of Fe +++ to Fe ++, a total of x 10 3 C are used up. How much Fe +++ was reduced? 1mole Fe +3 1mole Fe g (9.649 x 10 3 C)( ) ( ) ( ) = 5.585g Fe x 10 4 C/mol 1mole Fe +3 1mole Fe +2 Note that for this reaction, a single electron is transferred from the Fe +2 to the Fe +3. This is analogous to a single H + transfer in a monoprotic acid.
3 Adding time to the equation and get CURRENT. The measure of current as a function of time is the ampere: One Ampere = one Coulomb/second Having current measured as a function of time means we can talk about a rate of reaction (kinetics stuff, something we haven t considered since we started equilibrium.) Example: What is the current measured in a circuit involving the half cell reaction Sn e - Sn +2 which is occurring at a rate of 4.2 x 10-3 mole/hr? 1hr 2 mole e x 10 4 C i = (4.24 x 10-3 mole/hr) ( )( )( ) = 0.227A 3600s 1 mole Sn +4 mole e - By the way, what do you think about this magnitude of current? Will it kill you? Will it power a small city or a small toy? I d say, that s a pretty nasty shock right at the edge of where a DC voltage can kill you.
4 Standard Potentials. You will recall from our brief detour into physics that you need the difference between TWO potentials to do work. A single half cell reaction is only a figment of your imagination (or an incorrect answer on an exam.) The only value of interest to interest to us is this difference, this CHANGE IN POTENTIAL. Now here is the problem, do you know how many half cell reactions there are in the world? Well a lot, since every chemical reaction can be described as an oxidation or reduction. So what do we do? We create a STANDARD REFERENCE POTENTIAL (E o ) to which we arbitrarily assign a number. Then all the other half cell reactions can be compared to the reference and through that reference, to each other. Let s let our STANDARD REFERENCE POTENTIAL for a half cell reaction be equal to 0 V, so we can do the math in our head. Now, let s choose a half cell to be our reference AGAINST WHICH ALL OTHERS THINGS ARE MEASURED!!! Let s choose THE STANDARD HYDROGEN ELECTRODE (SHE). E 1/2 o = 0 assigned to 1/2 H 2(gas, PH2 = 1) H + (1M, ie.ph = 0) + e - Now looking at this half cell, it doesn t seem to have a lot going for it. To build one you need to have a gas bulb filled with an explosive gas, AND a solution that is at ph = 0. But we don t get to pick, it was already chosen for us. Fortunately we don t have to use this half cell in the lab, we just use it to make the table of standard half cell reactions. In the lab we will use something else as a reference cell, like a STANDARD CALOMEL ELECTRODE (SCE) and we will simply note the difference between the SCE and the SHE when we do our calculations.
5 TABLES OF STANDARD HALF CELL REACTIONS. Let s go in the lab, pull out a standard hydrogen electrode, and hook it up in a cell like the one below. Now let s hook up all the half cell reactions in the world and obtain standard reference potentials ( E o ) in the case above we started with a zinc solution that was lying around. But a couple of things to consider before we get too far along. First, electrochemistry is just thermodynamics, and you may recall from thermodynamics that things like concentration and temperature matter, so we need some standard concentrations and a standard temperature so that our electrochemistry friends can pitch in and help. How about we choose the following standard conditions: all reactants and products must be at 1 atm or 1M
6 a standard temperature like 298K. Reduction potentials: One more thing. We need to either write our half reactions as either oxidations or reductions. (We can t switch back and forth or we get our signs mixed up.) Now this may seem no big deal to you, but it sure was worth fighting over among electrochemists. For example, famous UT electrochemists like Al Bard and former Presidernt Larry Faulkner were big on writing everything as an oxidation half reaction. But they lost the This means the electrons will appear on the left side of the equation. Anyway, here it is, our very abbreviated table: Notice in my small table that the SHE sits pretty much in the middle of things. Half of the reactions when written as reductions are spontaneous (E o is positive) and half are not spontaneous (E o is negative).
7 There is a much bigger collection of standard reduction potentials half reactions in the appendix of your text. Spend some time looking at them and making sense out of what you see from everyday life. Also, look at the bounds for this table. Does it make sense that Li and F 2 half reactions exhibit the largest absolute magnitudes? Using the Tables of Standard Reduction Potentials. The whole purpose of the reduction potential tables was to avoid writing an exponentially increasing number of reactions between every new half cell reaction and every half cell reaction we already knew. So how do we put two half cell reactions together using the SHE? By convention: E o (net) = E o (cathode) - E o (anode) Use this equation exactly as written, inserting values directly from the table of standard reductions. DON T switch signs in the half cell reaction and then use this equation or you will mess up. Example: Given below is an electrochemical cell reaction. Is the reaction spontaneous as written? Cu s + Ni ++ Cu ++ + Ni s Break it down into the half cell reactions: Anode Cu s Cu e - E o (reduction) = 0.339V Cathode Ni e - Ni s E o (reduction) = V Now use the equation E o = (-0.236V) - (0.339V) = Cathode - anode = net Note that AS WRITTEN, this reaction is not spontaneous. However we can reverse the reaction and E o = and the reaction is spontaneous. Cu ++ + Ni s Cu s + Ni ++ (spontaneous )
8 COMPLICATION: THE NERNST EQUATION If electrochemistry was simply a matter of calculating E o values, life would be easy. But it is not. Do you really think that if we put together a nickel and copper cell and measured the potential, it would be 0.575? If you do, you are probably the same sort of person who believes that the ph of a 0.01M HCl solution is 2 (Go measure it, it is not.) In fact, there are a lot of complications to solution chemistry that need to be addressed if we are going to get a reasonable quantitative handle on the actual potential of an electrochemical cell. We need to deal with CONCENTRATION and how it affects E. We will borrow what we learned back in thermodynamics about relating ΔG to ΔG o through Q ΔG = ΔG o + RT ln Q Deriving the Nernst Equation. You may have noticed that batteries go dead. This means that even when they read 1.5V on the label, the battery s potential to do work is no longer 1.5V. Why is this? The problem is, we aren t accounting for concentration changes in the electrochemical cell. Remember that electrochemistry is just thermodynamics, and thermodynamics always considers relative concentrations. So clearly we will have to add corrections for concentrations to our equation for E. This is where the Nernst equation comes in. The Nernst equation accounts for the concentration of electroactive species in an electrochemical cell; i.e., the Nernst equation turns E o into E, the actual cell potential. It is easily obtained by combining the above equation with the one on p.131 that relates E to ΔG. [B] b For a reaction: aa + ne - bb Q = [A] a RT [B] b R = ideal gas constant n = # of e - E = E o - ln T = temperature F = Faraday nf [A] a Here, Q has the same form as an equilibrium constant in that it is equal to products over reactants, but the difference is that Q is used when NOT AT EQUILIBRIUM. (In other words, Q applied back when you added NaOH to your titration flask and things were kind of pink just before the solution went clear.) It is important to note from the Nernst equation that when Q = 1, then ln Q = 0 and E = E o. This makes sense, because remember that E o is the standard potential and by definition occurs when all our concentrations are 1 M. If you stick the number 1 into every concentration in Q, obviously you end up with the ln Q term going away.
9 A more practical form of the Nernst assumes that T = 25 o C and uses log base 10. In this case a Nernst equation half cell reaction is E 1/2 = E 1/2 o - log Q n and for the cell reaction E cell = E cell o - log Q n E cell o = E o cathode - E o anode where Q is the reaction quotient for the balanced redox reaction. Here n is the # of electrons in the balanced reaction. Example of a Nernst Equation Calculation Consider a cell formed with the half reactions below and a CdCl 2 solution that is M. The two half cell reactions, Cd e - Cd(s) E o 1/2 = AgCl(s) + 2e - 2Ag(s) + 2Cl - E o 1/2 = yield a standard voltaic cell reaction potential of Cd(s) + 2AgCl(s) Cd Ag(s) + 2Cl - E o = (-0.412) = 0.624v the Nernst equation is E cell = log [Cl - ] 2 [Cd ++ ] 2 and for the non-standard concentrations of 0.167M Cd ++ and Cl E cell = 0.624V - log [0.0334] 2 [0.0167] = E cell = 0.764V 2 Note that as written, by lowering the CdCl 2 concentration from 1M, we are able to make a stronger battery.
10 Another example of the Nernst equation in action. This one is fun because you get to apply some of what you ve learned about acid base chemistry!! For the two half cell reactions, 2H + + 2e - H 2(g) E o 1/2 = 0V Cd e - Cd (s) E o 1/2 = V which give a cell reaction Cd ++ + H 2(g) Cd (s) + 2H + E o cell = V the Nernst equation is [H + ] 2 E cell = V - log 2 [Cd ++ ]P H2 Now according to the Nernst equation, if all the concentrations were unity ( in this case it would mean the ph = 0), then the reaction is not spontaneous. But is it possible to make this reaction spontaneous? According to LeChatelier we can do so by altering the concentrations. What if we left P H2 = [Cd ++ ] = 1, what would the ph have to be to make the reaction spontaneous? Mathematically, this means we want to find the ph when E goes from negative to positive: [H + ] 2 0 = V - log 2 (1)(1) Now solve for H + and you get [H + ] = 1.6 x 10-7 M or ph = 6.8 So this is an example of a reaction that does not occur in acidic solution but is spontaneous at physiological ph and has a large driving force in base!! This is a startling example of how easily we can manipulate the balance of materials in a chemical system to observe very different types of chemistry. It is also why body ph is so important to all you medical types.
11 The Dead Battery: What happens at equilibrium. Galvanic cells (batteries) work when a reaction is NOT at equilibrium. But in a battery, reactants decrease, products increase and then we reach a point where nothing happens. This place is of course, EQUILIBRIUM. While it is the case that EQUILIBRIUM is just one special case for an electrochemical cell, it is an important case at a boundary. when Q------> K E cell > 0 At that point we can substitute K into the Nernst equation and do a little rearranging = E o - log K 25 n E o = log K 25 n K eq = 10 ne/ WOW!!! An equation that allows us to use an electrochemical cell to measure equilibrium constants. I m sure many of you were wondering how we made all those tables of K values, for the most part they were developed from electrochemical measurements of dead batteries!!
12 Example of a dead battery as an equilibrium problem. Given the following cell reaction: Cu (s) +2Fe +3 2Fe +2 + Cu +2 E o = 0.433V What is the equilibrium constant for the reaction? Keq = 10 (2)(0.433)/ = 4 x Note that when Eo is positive, K is greater than 1. Another example: Combine the two half cell reactions: FeCO 3(s) + 2e - Fe (s) + CO 3-2 E o = Fe (s) Fe e - E o = FeCO 2(s) Fe CO 3 E o = what we have is a K sp equilibrium for FeCO 3. Keq = Ksp = 10 2(-0.316)/ = 2 x Note that as expected, our K sp is a very small number. This occurs when E o is negative.
13 Tying Electrochemistry to Thermodynamics putting q and E together to do electrical work What is the WORK necessary to move electrons? In electrochemistry the electric POTENTIAL, E, measured in Volts (V), is the measure of the potential difference between two points. Work is the physical measure of what happens when an amount of charge passes between two points with a potential difference. Work has the units of Joules, and with V and C also in mks units, we have then Work (J) = q E = (volts)(coulombs) So one Joule of work is done when one Coulomb moves across a potential gradient of one Volt. Now don't blow this off, w = qe is an extremely important concept in any kind of science associated with moving charged particles. Example. How much work does it take to move over 2.36 x 10-3 moles of electrons across a 1.05 V potential? Work(J) = (1.05V)q = (1.05V)(2.36 x 10-3 moles e - )(9.65 x 10 4 C/mol) Work = 239 J By the way, is 239 J a lot or a little? Will it power a city, or a flashlight? We just did a problem in which we used a 1.05 V potential difference. If we had wanted to do more work, we could have used a bigger potential difference; this is the idea behind purchasing a bigger battery, (a 12 V rather than a 1.5 V battery.) So where did that in the Nernst equation come from? Without going into the reasons why, when dealing with electrochemical cells, we can relate this idea of work done in electrochemistry to the thermodynamic concept of free energy, through the equation: w = free energy = ΔG = -q E = - nfe
14 You will also remember that free energy = ΔG = -RT ln K so and RTln K = -nfe E = (RT/nF) ln K (this is where in the Nernst comes from) And looking just at the transitions from spontaneous to non-spontaneous reactions, the following must be true: type of reaction thermodynamics electrochemistry equilibria spontaneous reaction ΔG is negative E is positive K is > 1 non-spontaneous reaction ΔG is positive E is negative K is < 1 As we leave thermodynamics and head into kinetics, spend a moment reflecting back on the very first day in CH301 when you were told about calorimetry. We haven t look at a subject since then, from enthalpy to entropy to free energy to physical equilibria to chemical equilibria to acids and bases and solubility to batteries and the Nernst equation. Every one of these subjects asked: how has the energy of a system changed from start to finish. Thermodynamics, equilibria and electrochemistry are the tools we have used to answer that question. And now, the last piece of the puzzle how the speed with which a reaction happens matters in defining the natural world.
Chapter 21: ELECTROCHEMISTRY TYING IT ALL TOGETHER
 Chapter 21: ELECTROCHEMISTRY TYING IT ALL TOGETHER -RT ln K = G = -nfe o CH 17-20 = CH15 = CH 21 1 Important errata to fix. F = 9.649 x 10 4 C/mole in problem on current measurement. Beginning of section
Chapter 21: ELECTROCHEMISTRY TYING IT ALL TOGETHER -RT ln K = G = -nfe o CH 17-20 = CH15 = CH 21 1 Important errata to fix. F = 9.649 x 10 4 C/mole in problem on current measurement. Beginning of section
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Electrochemical System
 Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
 Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Galvanic Cells Spontaneous Electrochemistry. Electrolytic Cells Backwards Electrochemistry
 Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Electrochemistry objectives
 Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Part One: Introduction. a. Chemical reactions produced by electric current. (electrolysis)
 CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
Ch. 13 Fundamentals of Electrochemistry
 Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Copyright 2018 Dan Dill 1
 when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
Redox and Electrochemistry (BLB chapter 20, p.723)
 Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq)
 The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS
 AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
Chapter Nineteen. Electrochemistry
 Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Section Electrochemistry represents the interconversion of chemical energy and electrical energy.
 Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
Types of Cells Chemical transformations to produce electricity- Galvanic cell or Voltaic cell (battery)
 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
AP Chemistry Readiness Thermodynamics and Electrochemistry Review Page 1 of 15. AP Chemistry Review Session UCLA April 23, 2016
 AP Chemistry Readiness Thermodynamics and Electrochemistry Review Page 1 of 15 AP Chemistry Review Session UCLA April 23, 2016 Michael A. Morgan Richard Erdman Larry Walker mmorgan@lausd.net xchemteach@yahoo.com
AP Chemistry Readiness Thermodynamics and Electrochemistry Review Page 1 of 15 AP Chemistry Review Session UCLA April 23, 2016 Michael A. Morgan Richard Erdman Larry Walker mmorgan@lausd.net xchemteach@yahoo.com
Zn+2 (aq) + Cu (s) Oxidation: An atom, ion, or molecule releases electrons and is oxidized. The oxidation number of the atom oxidized increases.
 Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Electrochemical Cells
 Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Techniques: Constructing Cells Measuring Potential
 Nernst Electrochemistry Faraday Cells, Half-cells, and the Nernst Equation RT E = E 0 - -------ln Q - nf Chemical reactions with electrons 1 Objectives: Examine electrical consequences of oxidation reduction
Nernst Electrochemistry Faraday Cells, Half-cells, and the Nernst Equation RT E = E 0 - -------ln Q - nf Chemical reactions with electrons 1 Objectives: Examine electrical consequences of oxidation reduction
Electrochemical Properties of Materials for Electrical Energy Storage Applications
 Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 2 March 4, 2015 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se 78.96
Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 2 March 4, 2015 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se 78.96
CHAPTER 17: ELECTROCHEMISTRY. Big Idea 3
 CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website:
 General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website: www.austincc.edu/samorde Email: samorde@austincc.edu Lecture Notes Chapter 21 (21.1-21.25) Suggested Problems () Outline 1. Introduction
General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website: www.austincc.edu/samorde Email: samorde@austincc.edu Lecture Notes Chapter 21 (21.1-21.25) Suggested Problems () Outline 1. Introduction
17.1 Redox Chemistry Revisited
 Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Electrochem: It s Got Potential!
 Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
We can use chemistry to generate electricity... this is termed a Voltaic (or sometimes) Galvanic Cell
 Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
Answer Key, Problem Set 9
 Chemistry 122 Mines, Spring 2018 Answer Key, Problem Set 9 1. 19.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 19.46 (do this for all cells in 19.44);
Chemistry 122 Mines, Spring 2018 Answer Key, Problem Set 9 1. 19.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 19.46 (do this for all cells in 19.44);
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
A + B C +D ΔG = ΔG + RTlnKp. Me n+ + ne - Me. Me n n
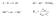 A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
Electrochemical Cells: Virtual Lab
 Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemistry. The study of the interchange of chemical and electrical energy.
 Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Oxidation-Reduction Review. Electrochemistry. Oxidation-Reduction Reactions. Oxidation-Reduction Reactions. Sample Problem.
 1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
Chapter 19 ElectroChemistry
 Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
ΔG = -nfe cell. Electrode Potentials. The cell potential E cell is related to the free energy of the reaction ΔG by:
 Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Lab #14: Electrochemical Cells
 Lab #14: Electrochemical Cells Objectives: 1. To understand the nature of electrochemical cells. 2. To construct a table listing the reduction potentials of a series of metal ions, in order of ease of
Lab #14: Electrochemical Cells Objectives: 1. To understand the nature of electrochemical cells. 2. To construct a table listing the reduction potentials of a series of metal ions, in order of ease of
Chapter 18 problems (with solutions)
 Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Electrochemistry 1 1
 Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells
 Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells Oxidation loss of electrons (oxidation number increases) OIL RIG Reduction gain of electrons (oxidation number decreases)
Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells Oxidation loss of electrons (oxidation number increases) OIL RIG Reduction gain of electrons (oxidation number decreases)
Electrochemistry. 1. For example, the reduction of cerium(iv) by iron(ii): Ce 4+ + Fe 2+ Ce 3+ + Fe 3+ a. The reduction half-reaction is given by...
 Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
2. Using Half Cell Potentials and Latimer Diagrams. 100 measured half cell potentials generate 10,000 full reactions
 Electrochemistry 1. Balancing Redox Reactions 2. Using Half Cell Potentials and Latimer Diagrams 100 measured half cell potentials generate 10,000 full reactions 3. E as a Thermodynamic state function
Electrochemistry 1. Balancing Redox Reactions 2. Using Half Cell Potentials and Latimer Diagrams 100 measured half cell potentials generate 10,000 full reactions 3. E as a Thermodynamic state function
Electrochemistry. Remember from CHM151 G E R L E O 6/24/2014. A redox reaction in one in which electrons are transferred.
 Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Lecture 14. Thermodynamics of Galvanic (Voltaic) Cells.
 Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
ELECTROCHEMISTRY Chapter 14
 ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
#13 Electrochemical Cells
 #13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
#13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
Chemistry 1011 TOPIC TEXT REFERENCE. Electrochemistry. Masterton and Hurley Chapter 18. Chemistry 1011 Slot 5 1
 Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Electrochemistry. Galvanic Cell. Page 1. Applications of Redox
 Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Chapter 21 Electrochemistry
 Chapter 21 Electrochemistry - electrochemistry and electrochemical processes are some of the most important sources of power that we have - batteries - much publicized hydrogen fuel cells - photosynthesis
Chapter 21 Electrochemistry - electrochemistry and electrochemical processes are some of the most important sources of power that we have - batteries - much publicized hydrogen fuel cells - photosynthesis
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Oxidation number. The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
 Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Electrochemical Reactions
 1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Oxidation-reduction (redox) reactions
 Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
CHEM 112 Final Exam (New Material) Practice Test Solutions
 CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials. Compiled by. Dr. A.O. Oladebeye
 CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
Advanced Chemistry: Chemical Reactions
 ILLINOIS MATHEMATICS & SCIENCE ACADEMY Teacher: Dave DeVol Advanced Chemistry: Chemical Reactions January 2014 Unit 1: Equilibrium Theme: Equilibrium is a dynamic process that involves change at the molecular
ILLINOIS MATHEMATICS & SCIENCE ACADEMY Teacher: Dave DeVol Advanced Chemistry: Chemical Reactions January 2014 Unit 1: Equilibrium Theme: Equilibrium is a dynamic process that involves change at the molecular
Oxidation (oxidized): the loss of one or more electrons. Reduction (reduced): the gain of one or more electrons
 1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
Oxidation refers to any process in which the oxidation number of an atom becomes more positive
 Lecture Notes 3 rd Series: Electrochemistry Oxidation number or states When atoms gain or lose electrons they are said to change their oxidation number or oxidation state. If an element has gained electrons
Lecture Notes 3 rd Series: Electrochemistry Oxidation number or states When atoms gain or lose electrons they are said to change their oxidation number or oxidation state. If an element has gained electrons
CHEM 116 Electrochemical Cells
 CHEM 116 Electrochemical Cells Lecture 22 Prof. Sevian Today s agenda Big picture of electrochemistry Redox reactions and oxidation numbers (last lecture) Charge flow in electrochemical cells and diagramming
CHEM 116 Electrochemical Cells Lecture 22 Prof. Sevian Today s agenda Big picture of electrochemistry Redox reactions and oxidation numbers (last lecture) Charge flow in electrochemical cells and diagramming
CHEM Pharmacy Week 9: Nernst Equation. Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone:
 CHEM1612 - Pharmacy Week 9: Nernst Equation Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone: 9351 4196 E-mail: siegbert.schmid@sydney.edu.au Unless otherwise stated, all images in this file have
CHEM1612 - Pharmacy Week 9: Nernst Equation Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone: 9351 4196 E-mail: siegbert.schmid@sydney.edu.au Unless otherwise stated, all images in this file have
Electrochemistry. Chapter 19. Concept Check Concept Check Solution. Solution
 Chapter 19 Electrochemistry Concept Check 19.1 If you were to construct a wet cell and decided to replace the salt bridge with a piece of copper wire, would the cell produce sustainable current? Explain
Chapter 19 Electrochemistry Concept Check 19.1 If you were to construct a wet cell and decided to replace the salt bridge with a piece of copper wire, would the cell produce sustainable current? Explain
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook
 Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
SHOCK TO THE SYSTEM! ELECTROCHEMISTRY
 SHOCK TO THE SYSTEM! ELECTROCHEMISTRY REVIEW I. Re: Balancing Redox Reactions. A. Every redox reaction requires a substance to be... 1. oxidized (loses electrons). a.k.a. reducing agent 2. reduced (gains
SHOCK TO THE SYSTEM! ELECTROCHEMISTRY REVIEW I. Re: Balancing Redox Reactions. A. Every redox reaction requires a substance to be... 1. oxidized (loses electrons). a.k.a. reducing agent 2. reduced (gains
5) do sample calculations 1) In electrogravimetry, analyte deposited as a solid ("plated") onto one of the electrodes.
 Page 1 of 1 Chem 201 Lecture 8b Summer 09 Return tests Last time: 0) Intro to Electrochemistry 1) E, Galvanic cells Today: Potentiometry Lecture: GALVANIC CELLS: -spontaneous reaction is utilized. ; voltaic
Page 1 of 1 Chem 201 Lecture 8b Summer 09 Return tests Last time: 0) Intro to Electrochemistry 1) E, Galvanic cells Today: Potentiometry Lecture: GALVANIC CELLS: -spontaneous reaction is utilized. ; voltaic
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Redox reactions & electrochemistry
 Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
ELEMENTS OF ELEC TROCHEMIS TRY. A. A number of analytical techniques are based upon oxidation-reduction reactions.
 Page 1 of 8 Chem 201 Winter 2006 I. Introduction ELEMENTS OF ELEC TROCHEMIS TRY A. A number of analytical techniques are based upon oxidationreduction reactions. B. Examples of these techniques would include:
Page 1 of 8 Chem 201 Winter 2006 I. Introduction ELEMENTS OF ELEC TROCHEMIS TRY A. A number of analytical techniques are based upon oxidationreduction reactions. B. Examples of these techniques would include:
CHEM J-14 June 2014
 CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
ELECTROCHEMISTRY I. The science concerned with the study of electron transfer across phase boundary
 ELECTROCHEMISTRY I The science concerned with the study of electron transfer across phase boundary Electrode: Is a conducting material immersed in a media. Electrode potential: Is the potential difference
ELECTROCHEMISTRY I The science concerned with the study of electron transfer across phase boundary Electrode: Is a conducting material immersed in a media. Electrode potential: Is the potential difference
Acid/Base Reactions & Electrochemistry
 Adult Basic Education Science Acid/Base Reactions & Electrochemistry Prerequisite: Chemistry 3102B Credit Value: 1 Chemistry Concentration Chemistry 1102 Chemistry 2102A Chemistry 2102B Chemistry 2102C
Adult Basic Education Science Acid/Base Reactions & Electrochemistry Prerequisite: Chemistry 3102B Credit Value: 1 Chemistry Concentration Chemistry 1102 Chemistry 2102A Chemistry 2102B Chemistry 2102C
Electrochemistry. Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions).
 Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Chapter 17 Electrochemistry
 Chapter 17 Electrochemistry 17.1 Galvanic Cells A. Oxidation-Reduction Reactions (Redox Rxns) 1. Oxidation = loss of electrons a. the substance oxidized is the reducing agent 2. Reduction = gain of electrons
Chapter 17 Electrochemistry 17.1 Galvanic Cells A. Oxidation-Reduction Reactions (Redox Rxns) 1. Oxidation = loss of electrons a. the substance oxidized is the reducing agent 2. Reduction = gain of electrons
REDOX EQUILIBRIA AND FEASIBILITY OF A REACTION
 REDOX EQUILIBRIA AND FEASIBILITY OF A REACTION Oxidizing agent Reducing agent Oxidation-Reduction Reactions Electron transfer reactions Electrons transferred from one substance to another Change in oxidation
REDOX EQUILIBRIA AND FEASIBILITY OF A REACTION Oxidizing agent Reducing agent Oxidation-Reduction Reactions Electron transfer reactions Electrons transferred from one substance to another Change in oxidation
Chemistry 2000 Lecture 15: Electrochemistry
 Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chpt 20: Electrochemistry
 Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. + A
 20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
Electrochemistry C020. Electrochemistry is the study of the interconversion of electrical and chemical energy
 Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Electrochemistry. Goal: Understand basic electrochemical reactions. Half Cell Reactions Nernst Equation Pourbaix Diagrams.
 Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Chapter 18 Electrochemistry
 Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Electrode Potentials and Their Measurement
 Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
electron mass per mole 1/2000 g/mole 1 g/mole 1 g/mole 23 g/mole
 Lecture 16: Electrochemistry-- The Big Picture Electrochemistry follows the adventures of the electron e electron Recall that we first discussed the electron when it came up as a fundamental particle back
Lecture 16: Electrochemistry-- The Big Picture Electrochemistry follows the adventures of the electron e electron Recall that we first discussed the electron when it came up as a fundamental particle back
Electrochemistry and Concentration Effects on Electrode Potentials Prelab
 Electrochemistry and Concentration Effects on Electrode Potentials Prelab 1. What is the purpose of this experiment? 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and
Electrochemistry and Concentration Effects on Electrode Potentials Prelab 1. What is the purpose of this experiment? 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and
RedOx Chemistry. with. Dr. Nick
 RedOx Chemistry with Dr. Nick What is RedOx Chemistry? The defining characteristic of a RedOx reaction is that electron(s) have completely moved from one atom / molecule to another. The molecule receiving
RedOx Chemistry with Dr. Nick What is RedOx Chemistry? The defining characteristic of a RedOx reaction is that electron(s) have completely moved from one atom / molecule to another. The molecule receiving
Chemistry 222 Exam 4: Chapters 11, 13, 14 Spring Points
 Chemistry 222 Name Exam 4: Chapters 11, 13, 14 Spring 2014 80 Points Complete five (5) of the following problems. Each problem is worth 16 points. CLEARLY mark the problems you do not want graded. You
Chemistry 222 Name Exam 4: Chapters 11, 13, 14 Spring 2014 80 Points Complete five (5) of the following problems. Each problem is worth 16 points. CLEARLY mark the problems you do not want graded. You
Topic 19 Redox 19.1 Standard Electrode Potentials. IB Chemistry T09D04
 Topic 19 Redox 19.1 Standard Electrode Potentials IB Chemistry T09D04 19.1 Standard Electrode Potentials 19.1.1 Describe the standard hydrogen electrode. (2) 19.1.2 Define the term standard electrode potential,
Topic 19 Redox 19.1 Standard Electrode Potentials IB Chemistry T09D04 19.1 Standard Electrode Potentials 19.1.1 Describe the standard hydrogen electrode. (2) 19.1.2 Define the term standard electrode potential,
If you're told the reaction is in basic solution: Exam 3 moved to Wednesday. More of Ch 18 today. Add OH to both sides to cancel H +
 Announcements Monday, May 03, 2010 Exam 3 moved to Wednesday. Balancing in basic solution 5. If you're told the reaction is in basic solution: More of Ch 18 today. Answer keys for Quiz 3 and the titration
Announcements Monday, May 03, 2010 Exam 3 moved to Wednesday. Balancing in basic solution 5. If you're told the reaction is in basic solution: More of Ch 18 today. Answer keys for Quiz 3 and the titration
Guide to Chapter 18. Electrochemistry
 Guide to Chapter 18. Electrochemistry We will spend three lecture days on this chapter. During the first class meeting we will review oxidation and reduction. We will introduce balancing redox equations
Guide to Chapter 18. Electrochemistry We will spend three lecture days on this chapter. During the first class meeting we will review oxidation and reduction. We will introduce balancing redox equations
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
