Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq)
|
|
|
- Magnus Perry
- 6 years ago
- Views:
Transcription
1 The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to spontaneous processes having negative values for energy terms. However, since batteries were historically measured looking at the change in energy of the surroundings rather than for the reaction, the opposite sign convention applies. Work We can now write an expression for the maximum work attainable by a voltaic cell. Let n be the number of mole electrons transferred in the overall balanced cell reaction. The maximum work for molar amounts of reactants is: LP#5. The EMF of a voltaic cell with the following reaction is V. Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq) Calculate the max work that can be done by this cell when 0.500g H2 is consumed. Step 1: Identify the number of electrons transferred during the overall reaction. Oxid ½ Rxn: Red. ½ Rxn: Step 2: Compute the maximum work per mole. Wmax = -(n)(f)(ecell) = Step 3: Determine the total energy based on the total moles of e - transferred
2 Standard Cell EMF s and Standard Electrode Potentials A cell emf is a measure of the driving force of the cell reaction. A reduction potential is a measure of the in the reduction half-reaction. You can look at the oxidation half-reaction as the reverse of a corresponding reduction half-reaction. The oxidation potential for an oxidation half-reaction (the reverse reaction) is the of the reduction potential. By convention, the Table of Standard Electrode Potentials are tabulated as reduction potentials. Standard Reduction Potentials E 0 cell = standard cell potential under standard conditions, Voltage listed at standard conditions are at temperature: concentration: 19-14
3 The actual cell voltage will depend upon a) b) c) Individual potentials cannot be measured, only differences in potential. By convention, for values in the table, all others must be compared to a standard reference reaction The reference reaction is the reduction of H + (aq) ions to produce H2(g): 2H + (aq, 1M) + 2e - H2(g, 1 atm) Eºred = This is known as the: Standard electrode potentials are measured relative to this hydrogen reference. A cell will always include one oxidation reaction (at the anode) and one reduction reaction (at the cathode). When we reverse a reaction to get an oxidation half reaction, the sign of the potential must be changed. We can then sum the energy potentials the same way we have treated other energy variable (e.g. G & H) this semester. E cell = Note: The book uses the convention E cell = E cathode - E anode Where all potentials are reduction potentials! Both processes will give the same correct answer
4 Lets look at a section of an Activity Series for metals and hydrogen ion: Standard Potential, (Eºred) in Volts Reduction Half- Reaction Li + (aq) + e - Li(s) Zn 2+ (aq) + 2e - Zn(s) 0 2H + (aq) + 2e - H2(g) 0.34 Cu 2+ (aq) + 2e - Cu(s) 0.80 Ag + (aq) + e - Ag(s) The more positive the value of Eºred the greater the driving force for reduction Reactions with positive E values - want to undergo:. - are good: Reactions with negative E values - prefer to undergo: - are good: The net difference between the standard reduction potentials of the two reactions, is the excess potential that can be used to drive electrons through the cell, Eºcell Active metals are. The more active they are, the greater the oxidation potential for the metal. Since tables usually list reduction potentials, the more active they are, the more negative the reduction potential for the ion
5 When comparing Zinc with Copper in the activity series, which reaction would we expect to happen? Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) OR Zn 2+ (aq) + Cu(s) Zn(s) + Cu 2+ (aq) Zinc is higher on the activity series. Zinc is more easily oxidized than copper. We would expect the reaction to happen. Example: Consider the zinc-copper cell described earlier. Zn( s) Zn 2 ( aq) Cu The two half-reactions are: ( aq) Cu( s) Oxidation ½ Rxn: Reduction ½ Rxn: Find the oxidation potential for this half reaction. Zn(s) Zn 2+ (aq) + 2e - E o ox = Find the reduction potential for this half reaction. Cu 2+ (aq) + 2e - Cu(s) E o red = Find the overall cell potential. 2 Eº cell = The electrode potential is an intensive property whose value is independent of the amount of species in the reaction. Thus, the electrode potential for the half-reaction would be: 2Cu 2+ (aq) + 4e - 2Cu(s) E o = 19-17
6 LP#6. Consider a cell constructed of the following two half-reactions. What would the overall reaction be that would create a voltaic cell and what would the voltage of that cell be at standard conditions? 2 o Cd ( aq ) 2e Cd ( s); E 0.40 V Ag ( aq) 1e Ag( s); E o 0.80 V Which reaction should be reversed? Half reactions and associated voltages therefore are: Cd(s) Cd 2+ (aq) + 2e - ; E = Ag + (aq) + 1e - Ag(s) ; E = 0.80V We must double the silver half-reaction so that the electrons cancel. Cd(s) Cd 2+ (aq) + 2e - ; E = 0.40 V 2Ag + (aq) + 2e - 2Ag(s) ; E = Now we can add the two half-reactions. The corresponding cell notation would be: Spontaneity of Redox Reactions Eºcell = Eºred + Eºoxid Eº will be positive for the case where the reaction is: Eº will be zero for a redox reaction that is: Eº will be negative for the case where the reaction is: LP#7. Can copper be dissolved (oxidized) by acid (i.e., H + )? Really asking if the following reaction is spontaneous: Cu(s) + 2H + (aq) Cu 2+ (aq) + H2(g) This reaction can be broken down into two half-reactions: Oxidation: Cu(s) Cu 2+ (aq) + 2e - E 0 ox = Reduction: 2H + (aq) + 2e - H2(g) E 0 red = 19-18
7 The standard cell potential for this reaction is: Eº cell = Since this value is, this redox reaction is and. What if we only had the activity series without any numerical voltages? (Remember the most active at the top is most easily Since H is higher than Cu, it is more easily oxidized. Cu cannot replace it as the oxidized species. Li Zn H Cu Ag EMF, Free Energy Changes, and Equilibrium Free energy change, Gº, Kc, and Eºcell, all measure spontaneity of a reaction. What is the relationship between these variables? Gº and Eºcell Previously, we saw that G is the free energy available which equals the maximum useful work of a reaction. Remember, for a voltaic cell, work = -nfecell, so when reactants are in their standard states The Gibbs Free Energy (G) can be related to the EMF of the cell. Where n= number of moles of e- transferred F = Faraday s constant = 96,485 C/mole e - C = Coulombs Our typical unit of energy, Joules can be related to the cell EMF: Since 1 V = 1J/C 19-19
8 º and Kc The measurement of cell EMF s gives you yet another way of calculating equilibrium constants. Combining the previous equation, G o = -nfe o cell, with the equation G o = -RT lnk, (from our previous chapter) we get Or, rearranging, we get LP#8. The standard EMF for the following cell is 1.10 V. Zn( s) Zn 2 ( aq) Cu ( aq) Cu( s) Calculate the equilibrium constant Kc at 25 o C for the reaction: Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) Note that n=. Substituting into the equation relating E o cell and K gives 2 Solving for log Kc, you find: logk= Now take the antilog of both sides: K c = Effect of Concentration on Cell EMF The EMF of a voltaic cell is determined by a) the identity of the redox reaction and b) the concentrations of the reactants and products. The EMF of the cell will fall as the reactants are used up and products increase in concentration 19-20
9 The Nernst Equation This is an equation that related the EMF of a redox reaction on the concentration of reactants and products. Developed by Walther Hermann Nernst ( ), a German chemist. E cell o RT Ecell lnq nf At equilibrium concentrations of reactants and products, the EMF =. Electrons flow spontaneously in a redox reaction because the system is attempting to achieve equilibrium. When equilibrium is achieved, net electron flow is zero. 0 E o cell RT nf ln K E cell 0 RT ln K at equilibrium nf A common form of the equation does away with the natural log and puts the equation in the form of log10: E cell o 2.303RT Ecell logq Nernst Equation nf At 25 C and using base 10 logs this becomes: E = E log Q n The Nernst Equation. Allows us to determine cell potentials at 19-21
10 LP#9. A voltaic cell that utilizes the oxidation of zinc by copper ion is set up with an initial concentration of 5.0M copper ion and 0.050M zinc ion. Calculate the cell potential at 20C. Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) E 0 cell = 1.10V n= Determination of ph A ph electrode works by relating cell EMF (i.e., potential) to concentration. As the concentration of H + in the solution changes, the amount of voltage that can be measured changes. We can then calibrate the voltage to actual ph values
11 CONCENTRATION CELLS Since cell potentials cepend not only on the half reactions but on the concentrations, it is possible to create a cell where the two half reactions are the same, but only th e concentrations are different. The standard cell potential would be zero: Oxidation: Cu(s) Cu 2+ (aq) + 2e - E 0 ox = Reduction: Cu 2+ (aq) + 2e - Cu(s) E 0 red = Using the Nernst Equation the actual cell potential is: 19-23
12 LP#10. Consider a cell with the shorthand notation: Al(s) Al 3+ (aq) (5.0M) Cu 2+ (aq)(0.020m) Cu(s) A) What is the cell voltage at 20C given: Al 3+ (aq) + 3e Al(s) E = 1.66V Cu 2+ (aq) + 2e Cu(s) E = V Label each part: a) anode & cathode: b) signs of electrodes; c) oxidation and reduction cell; d) electron flow; e) ion flows in beakers; f) ion flows in salt bridge, g) cathode and anode processes, h) salt bridge, i) solutions in beakers; j) electrode materials
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Zn+2 (aq) + Cu (s) Oxidation: An atom, ion, or molecule releases electrons and is oxidized. The oxidation number of the atom oxidized increases.
 Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Electrochemical System
 Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemistry objectives
 Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
CHEM J-8 June /01(a)
 CHEM1001 2012-J-8 June 2012 22/01(a) A galvanic cell has the following cell reaction: D(s) + 2Zn 2+ (aq) 2Zn(s) + D 4+ (aq) Write the overall cell reaction in shorthand cell notation. E = 0.18 V 8 D(s)
CHEM1001 2012-J-8 June 2012 22/01(a) A galvanic cell has the following cell reaction: D(s) + 2Zn 2+ (aq) 2Zn(s) + D 4+ (aq) Write the overall cell reaction in shorthand cell notation. E = 0.18 V 8 D(s)
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
17.1 Redox Chemistry Revisited
 Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Electrochemical Cells: Virtual Lab
 Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Electrochemistry. Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions).
 Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
A + B C +D ΔG = ΔG + RTlnKp. Me n+ + ne - Me. Me n n
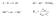 A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
Electrochemistry. Remember from CHM151 G E R L E O 6/24/2014. A redox reaction in one in which electrons are transferred.
 Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
Electrochemistry 1 1
 Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry. The study of the interchange of chemical and electrical energy.
 Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Chemistry 132 NT. Electrochemistry. Oxidation-Reduction Reactions
 Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
 Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Chapter 19 - Electrochemistry. the branch of chemistry that examines the transformations between chemical and electrical energy
 Chapter 19 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy 19.1 Redox Chemistry Revisited A Spontaneous Redox Reaction Znº(s) + Cu 2+
Chapter 19 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy 19.1 Redox Chemistry Revisited A Spontaneous Redox Reaction Znº(s) + Cu 2+
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture 14. Thermodynamics of Galvanic (Voltaic) Cells.
 Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
Spontaneous Redox Between Zinc Metal and Copper(II) Ions. Zn 2+ Zn + 2e- Cu 2+ NO 3
 Spontaneous Redox Between Zinc Metal and Copper(II) Ions Zn 2+ Cu 2+ NO 3 _ Zn + 2e- Cu Zn 0 + Cu 2+ º Zn 2+ + Cu 0 spontaneous red 1 ox 2 ox 1 red 2 Spontaneous Redox Between Copper Metal and Silver Ions
Spontaneous Redox Between Zinc Metal and Copper(II) Ions Zn 2+ Cu 2+ NO 3 _ Zn + 2e- Cu Zn 0 + Cu 2+ º Zn 2+ + Cu 0 spontaneous red 1 ox 2 ox 1 red 2 Spontaneous Redox Between Copper Metal and Silver Ions
Oxidation-Reduction Review. Electrochemistry. Oxidation-Reduction Reactions. Oxidation-Reduction Reactions. Sample Problem.
 1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
Chapter 20. Electrochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Electrochemical Reactions
 1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Electrochemistry. Galvanic Cell. Page 1. Applications of Redox
 Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
Types of Cells Chemical transformations to produce electricity- Galvanic cell or Voltaic cell (battery)
 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
CHEM J-12 June 2013
 CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
CHEM J-14 June 2014
 CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
REVIEW QUESTIONS Chapter 19
 Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
Chemistry 2000 Lecture 15: Electrochemistry
 Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
18.2 Voltaic Cell. Generating Voltage (Potential) Dr. Fred Omega Garces. Chemistry 201. Miramar College. 1 Voltaic Cell.
 18.2 Voltaic Cell Generating Voltage (Potential) Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Voltaic Cell Redox Between If Zn (s) and Cu 2+ (aq) is in the same solution, then the electrons transfer
18.2 Voltaic Cell Generating Voltage (Potential) Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Voltaic Cell Redox Between If Zn (s) and Cu 2+ (aq) is in the same solution, then the electrons transfer
Announcements. Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87
 Announcements Exam 3 March 17 Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 Problems Chapter 21: 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87 Up to but not including
Announcements Exam 3 March 17 Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 Problems Chapter 21: 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87 Up to but not including
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
CHAPTER 17: ELECTROCHEMISTRY. Big Idea 3
 CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
Galvanic Cells Spontaneous Electrochemistry. Electrolytic Cells Backwards Electrochemistry
 Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Electrochemical Cells at Non-Standard Conditions
 Electrochemical Cells at Non-Standard Conditions Oxidation-reduction reactions in the real world rarely occur under standard conditions. Even if the cell started out with all dissolved species at 1M concentration,
Electrochemical Cells at Non-Standard Conditions Oxidation-reduction reactions in the real world rarely occur under standard conditions. Even if the cell started out with all dissolved species at 1M concentration,
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
Redox reactions & electrochemistry
 Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Answer Key, Problem Set 9
 Chemistry 122 Mines, Spring 2018 Answer Key, Problem Set 9 1. 19.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 19.46 (do this for all cells in 19.44);
Chemistry 122 Mines, Spring 2018 Answer Key, Problem Set 9 1. 19.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 19.46 (do this for all cells in 19.44);
Section Electrochemistry represents the interconversion of chemical energy and electrical energy.
 Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example OK, then here
Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example OK, then here
CHEMISTRY - CLUTCH CH.18 - ELECTROCHEMISTRY.
 !! www.clutchprep.com CONCEPT: OXIDATION-REDUCTION REACTIONS Chemists use some important terminology to describe the movement of electrons. In reactions we have the movement of electrons from one reactant
!! www.clutchprep.com CONCEPT: OXIDATION-REDUCTION REACTIONS Chemists use some important terminology to describe the movement of electrons. In reactions we have the movement of electrons from one reactant
Electrochem: It s Got Potential!
 Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Oxidation (oxidized): the loss of one or more electrons. Reduction (reduced): the gain of one or more electrons
 1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
Electrochemical Cells
 Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
CHEM Pharmacy Week 9: Nernst Equation. Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone:
 CHEM1612 - Pharmacy Week 9: Nernst Equation Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone: 9351 4196 E-mail: siegbert.schmid@sydney.edu.au Unless otherwise stated, all images in this file have
CHEM1612 - Pharmacy Week 9: Nernst Equation Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone: 9351 4196 E-mail: siegbert.schmid@sydney.edu.au Unless otherwise stated, all images in this file have
EXPERIMENT C4: ELECTROCHEMISTRY. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT C4: ELECTROCHEMISTRY Upon completion of this lab, the student will be able to: 1) Construct an electrochemical cell. 2) Measure the cell potential for an electrochemical
1 Learning Outcomes EXPERIMENT C4: ELECTROCHEMISTRY Upon completion of this lab, the student will be able to: 1) Construct an electrochemical cell. 2) Measure the cell potential for an electrochemical
Copyright 2018 Dan Dill 1
 when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
Electrochemistry. 1. For example, the reduction of cerium(iv) by iron(ii): Ce 4+ + Fe 2+ Ce 3+ + Fe 3+ a. The reduction half-reaction is given by...
 Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
Assigning Oxidation Numbers:
 Assigning Oxidation Numbers: 1. Oxidation number of a free element or diatomic molecule is zero. Ex: Na(s), Cu(s), H 2 (g), F 2 (g) 2. In most cases the oxidation number of hydrogen is +1, oxygen is -2,
Assigning Oxidation Numbers: 1. Oxidation number of a free element or diatomic molecule is zero. Ex: Na(s), Cu(s), H 2 (g), F 2 (g) 2. In most cases the oxidation number of hydrogen is +1, oxygen is -2,
Chapter 18 problems (with solutions)
 Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Chapter 19: Redox & Electrochemistry
 Chapter 19: Redox & Electrochemistry 1. Oxidation-Reduction Reactions Definitions Oxidation - refers to the of electrons by a molecule, atom or ion Reduction - refers to the of electrons by an molecule,
Chapter 19: Redox & Electrochemistry 1. Oxidation-Reduction Reactions Definitions Oxidation - refers to the of electrons by a molecule, atom or ion Reduction - refers to the of electrons by an molecule,
RedOx Chemistry. with. Dr. Nick
 RedOx Chemistry with Dr. Nick What is RedOx Chemistry? The defining characteristic of a RedOx reaction is that electron(s) have completely moved from one atom / molecule to another. The molecule receiving
RedOx Chemistry with Dr. Nick What is RedOx Chemistry? The defining characteristic of a RedOx reaction is that electron(s) have completely moved from one atom / molecule to another. The molecule receiving
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
The relevant half cell reactions and potentials are: Calculate the equilibrium constant, K, for the reaction at 25 C. lnk
 CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
Ch. 13 Fundamentals of Electrochemistry
 Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Chapter 19 ElectroChemistry
 Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Electrode Potentials and Their Measurement
 Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
Chapter Nineteen. Electrochemistry
 Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Introduction to Electrochemical reactions. Schweitzer
 Introduction to Electrochemical reactions Schweitzer Electrochemistry Create and or store electricity chemically. Use electricity to drive a reaction that normally would not run. Plating metal onto a metal
Introduction to Electrochemical reactions Schweitzer Electrochemistry Create and or store electricity chemically. Use electricity to drive a reaction that normally would not run. Plating metal onto a metal
CH 223 Friday Sept. 08, 2017 L14B
 CH 223 Friday Sept. 08, 2017 L14B Previously: Relationships between E cell, K, and ΔG Concentration and cell potential Nernst equation for non-standard conditions: E cell = E 0 cell - 0.0592 n log Q at
CH 223 Friday Sept. 08, 2017 L14B Previously: Relationships between E cell, K, and ΔG Concentration and cell potential Nernst equation for non-standard conditions: E cell = E 0 cell - 0.0592 n log Q at
Chpt 20: Electrochemistry
 Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Lecture #15. Chapter 18 - Electrochemistry
 Lecture #15 Chapter 18 - Electrochemistry Chapter 18 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy Redox Chemistry Revisited A Spontaneous
Lecture #15 Chapter 18 - Electrochemistry Chapter 18 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy Redox Chemistry Revisited A Spontaneous
We can use chemistry to generate electricity... this is termed a Voltaic (or sometimes) Galvanic Cell
 Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Part One: Introduction. a. Chemical reactions produced by electric current. (electrolysis)
 CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Oxidation-Reduction Reactions Review of Terms Oxidation-reduction (redox) reactions always involve a transfer of electrons from one species to another. Oxidation number - the
Chapter 18 Electrochemistry Oxidation-Reduction Reactions Review of Terms Oxidation-reduction (redox) reactions always involve a transfer of electrons from one species to another. Oxidation number - the
Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells
 Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells Oxidation loss of electrons (oxidation number increases) OIL RIG Reduction gain of electrons (oxidation number decreases)
Electrochemistry (Galvanic and Electrolytic Cells) Exchange of energy in chemical cells Oxidation loss of electrons (oxidation number increases) OIL RIG Reduction gain of electrons (oxidation number decreases)
AP* Electrochemistry Free Response Questions page 1
 Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Review. Chapter 17 Electrochemistry. Outline. Voltaic Cells. Electrochemistry. Mnemonic
 Review William L Masterton Cecile N. Hurley Edward J. Neth cengage.com/chemistry/masterton Chapter 17 Electrochemistry Oxidation Loss of electrons Occurs at electrode called the anode Reduction Gain of
Review William L Masterton Cecile N. Hurley Edward J. Neth cengage.com/chemistry/masterton Chapter 17 Electrochemistry Oxidation Loss of electrons Occurs at electrode called the anode Reduction Gain of
ELECTROCHEMICAL CELLS
 ELECTROCHEMICAL CELLS Electrochemistry 1. Redox reactions involve the transfer of electrons from one reactant to another 2. Electric current is a flow of electrons in a circuit Many reduction-oxidation
ELECTROCHEMICAL CELLS Electrochemistry 1. Redox reactions involve the transfer of electrons from one reactant to another 2. Electric current is a flow of electrons in a circuit Many reduction-oxidation
Electrochemistry C020. Electrochemistry is the study of the interconversion of electrical and chemical energy
 Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Lab.12. Electrochemistry
 Key words: oxidation, reduction, anode, cathode, potential, galvanic cell, Nernst equation, electromotive force Literature: D.A. Skoog, F.J. Holler, T.A. Nieman: Principles of Instrumental Analysis J.
Key words: oxidation, reduction, anode, cathode, potential, galvanic cell, Nernst equation, electromotive force Literature: D.A. Skoog, F.J. Holler, T.A. Nieman: Principles of Instrumental Analysis J.
Electrochemistry. (Hebden Unit 5 ) Electrochemistry Hebden Unit 5
 (Hebden Unit 5 ) is the study of the interchange of chemical energy and electrical energy. 2 1 We will cover the following topics: Review oxidation states and assigning oxidation numbers Redox Half-reactions
(Hebden Unit 5 ) is the study of the interchange of chemical energy and electrical energy. 2 1 We will cover the following topics: Review oxidation states and assigning oxidation numbers Redox Half-reactions
Electrochemistry. Chapter 18. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
CHEM 112 Final Exam (New Material) Practice Test Solutions
 CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook
 Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. + A
 20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
Chapter 18 Electrochemistry
 Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Redox Reactions and Electrochemistry
 Redox Reactions and Electrochemistry Redox Reactions and Electrochemistry Redox Reactions (19.1) Galvanic Cells (19.2) Standard Reduction Potentials (19.3) Thermodynamics of Redox Reactions (19.4) The
Redox Reactions and Electrochemistry Redox Reactions and Electrochemistry Redox Reactions (19.1) Galvanic Cells (19.2) Standard Reduction Potentials (19.3) Thermodynamics of Redox Reactions (19.4) The
Electrochemical Cells II: Stoichiometry and Nernst Equation
 CH302 LaBrake and Vanden Bout Electrochemical Cells II: Stoichiometry and Nernst Equation All the electrochemical cells on this worksheet are the same ones on the first Electrochemical Cells worksheet.
CH302 LaBrake and Vanden Bout Electrochemical Cells II: Stoichiometry and Nernst Equation All the electrochemical cells on this worksheet are the same ones on the first Electrochemical Cells worksheet.
ELECTROCHEMICAL CELLS NAME ROW PD
 4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
Module-1: Electrode Potential And Cells 2015
 Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Electrolysis. Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element.
 Electrolysis Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element. Electrolysis is done in an electrolytic cell. Electrolytic cells
Electrolysis Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element. Electrolysis is done in an electrolytic cell. Electrolytic cells
CHEM 116 Electrochemical Cells
 CHEM 116 Electrochemical Cells Lecture 22 Prof. Sevian Today s agenda Big picture of electrochemistry Redox reactions and oxidation numbers (last lecture) Charge flow in electrochemical cells and diagramming
CHEM 116 Electrochemical Cells Lecture 22 Prof. Sevian Today s agenda Big picture of electrochemistry Redox reactions and oxidation numbers (last lecture) Charge flow in electrochemical cells and diagramming
Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon
 Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon Electrolytic cells the use of electrical energy to drive thermodynamically
Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon Electrolytic cells the use of electrical energy to drive thermodynamically
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials. Compiled by. Dr. A.O. Oladebeye
 CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
Dr. Anand Gupta
 By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
ii. Gains Oxygen and/or loses hydrogen (hydrocarbons): Example:
 Identify element being oxidized and element being reduced 1. Oxidation: Increasing oxidation number 2. Reduction: Decreasing oxidation number 3. Hierarchical guidelines 4. OXIDIZED i. Free elements have
Identify element being oxidized and element being reduced 1. Oxidation: Increasing oxidation number 2. Reduction: Decreasing oxidation number 3. Hierarchical guidelines 4. OXIDIZED i. Free elements have
Topic 4 Electrochem. The study of interchange energy chemical electrical
 Topic 4 lectrochem The study of interchange energy chemical electrical Review of Terms Oxidation reduction (redox) reaction involves a transfer of electrons from the reducing agent to the oxidizing agent
Topic 4 lectrochem The study of interchange energy chemical electrical Review of Terms Oxidation reduction (redox) reaction involves a transfer of electrons from the reducing agent to the oxidizing agent
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Electrochemical Cell Consists of electrodes which dip into an electrolyte & in which a chem. rxn. uses or generates an electric current Voltaic (Galvanic) Cell Spont. rxn. -
Chapter 20 Electrochemistry Electrochemical Cell Consists of electrodes which dip into an electrolyte & in which a chem. rxn. uses or generates an electric current Voltaic (Galvanic) Cell Spont. rxn. -
Redox and Electrochemistry (BLB chapter 20, p.723)
 Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
