l μ M Right hand Screw rule
|
|
|
- Trevor Carroll
- 5 years ago
- Views:
Transcription
1 Magnetic materials
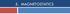
2 Magnetic property The response of the materials to external magnetic field All the materials are magnetic, only the degree of response varies, which is measured in terms of their magnetization (strong or weak) The parameters used to study the magnetic behaviors of the materials are as follows: 1.Magnetic dipoles & magnetic moment: Magnetic dipoles are analogous to electric dipoles ; consists of a north pole and a south pole of strength m each separated by a small distance l Magneic moment = mxl For a circular current loop equivalent to a magnetic dipole, magnetic moment μ M = NIxA ( amp. m 2 ) & torque on the dipole τ = μ M XB Where I is the current in the loop and A is the area of the loop,n is no. of turns N l μ M S I Right hand Screw rule
3 2. Magnetisation = dipole moment / volume M = μ / V ( amp. / met.) 3.Magnetic susceptibility = magnetization / mag. field strength χ = M / H (no unit) 4.Magnetic permeability = magnetic induction / mag. field strength μ =μ 0 μ r μ = B / H (Wb / amp. met. = H/met) 5.Relative permeability μ r = μ / μ 0, μ 0 = absolute permeability = 4πX 10-7 H/m 6.Relation between H,B & M is B = μ H = μ 0 μ r H so 7. μ r =(1 + χ) B = μ 0 (M+H) = μ 0 (χh + H) = μ 0 (1 + χ) H
4 Classification of magnetic materials Diamagnetism Paramagnetism Ferromagnetism 1.Normally referred as nonmagnetic as the response is very weak 2.In ext. magnetic field magnetic moment induced in a direction opposite to applied field repelled by the field H=0, M=0 H=H M= - M 3. Permeability μ<1 4. susceptibility χ <0 5. Susceptibility does not depend on temperature 5. Ex; Cu, Ag, Hg, Au, Zn,SC 1.Normally referred as nonmagnetic as the response is very weak 2. They posses permanent magnetic moments, which are randomly oriented in the absence of ext. magnetic field., hence net magnetization is zero. When a field is applied moments get aligned in the field direction, giving positive magnetization H=0, M=0 H=H M= M 3. Permeability μ>1 4. susceptibility χ is positive, small and temp. dependant χ = C/ T Curie law 1.Referred as magnetic as response is strong(exchange coupling) 2. Posses permanent dipoles 3. Show spontaneous magnetization even in the absence of ext. field, magnetization shown is high, when field is applied, M increases 4.Permeability μ>1 5.susceptibility χ is positive, large and temp. dependant χ = C/ T-θ Curie Weiss law Ex; Fe, Co, Ni ferromagnetic domains
5 Origin of magnetic moment The three sources of magnetic moment in an atom are 1. orbital motion of the electron 2.spin motion of the electron 3. nuclear spin If the vector sum of all the contribution is zero then net magnetization is zero 1. orbital motion of the electron Motion of the electron (charged particle)around the nucleus in a circular orbit (orbital motion) is equivalent to a circular current and behaves as a magnetic dipole. Associated magnetic moment is μ M = IxA ( amp. m 2 ) I= - q/t, where T is the time period for one rotation = 2 π r / v v is the velocity of the electron in the orbit μ M = (- q v / 2 π r ) (π r 2 ) = - qvr/2 = - q/ 2m ( mvr) = - (q/ 2m ) L Orbital magnetic moment μ orb = - (q/ 2m ) L
6 2.spin motion of the electron Similarly, the spin motion of the electron around their own axis give rise to spin magnetic moment μ spin = - (q/ 2m ) S Total magnetic moment due to electron motion inside the atom is μ M = - (q/ 2m ) (L+S) = - (q/ 2m ) J, J is the total angular momentum ( from L+S to L- S Quantum no. associated with L is * l(l+1) ħ+, l is the orbital quantum no. l=0---s shell, l=1---p shell, l=2-----d shell, l= f shell Quantum no. associated with S ± ħ/2 Total magnetic moment = μ M = - g(q/ 2m ) J If magnetic field is applied along z-direction, the component of the total magnetic moment in that direction is, μ M = -g(qħ/2m)m j
7 Where mj is the magnetic quantum no. Varying from (J to -J) & g is called Lande s g-factor g = Calculation rules 1.if electrons are in the s-orbit, orbital magnetic moment is zero (l=0) 2. for completely filled shell, orbital magnetic moment is zero (l=0) As ml = l to l (s shell,l=0, p shell, l=1, d shell, l=2 and f shell, l=3..) 3. partially filled p, d and f shells contribute to orbital magnetic moment 4.If all electrons are paired, spin magnetic momentum is zero 3. Nuclear spin 1 J ( J 1) S( S 2J ( J 1) 1) Due to the spin of nucleus, a magnetic moment is associated which is very small as compared to the electronic contribution as heavy mass is involved (10-3 times) is masked by electronic mag. Mom. L( L 1)
8 Bohr magnetron If there is only a single electron it will have only spin magnetic moment μ s = -2 (qħ / 2m) ±1/2, as g=2 and m j = ±1/2 This is the fundamental magnetic moment called Bohr magnetron μ B All the magnetic moments are expressed in terms of μ B μ B = q = 9.27x Am 2 2m Ex;-1. hydrogen atom One electron in s shell. So l=0 and s= +1/2 or -1/2 implies that orbital magnetic mom. Is zero and spin mag. Mom. Is same as the Bohr magnetron ( μ B is the magnetic moment possesed by hydrogen electron) 2. Helium atom?...calculate
9 For other atoms apply the rules to calculate Hund s rule: 1. spins of electrons remain parallel to each other to the max. Extent 2. max. Value of L is consistent with the spin S 3. if shell is less than half filled J= L- S, if more than half filled J= L+ S & if exactly half filled then L=0 and J=S
10 Langevin s theory of diamagnetism motion of the electron in the orbit around the nucleus is equivalent to a current in the closed circuit. When magnetic field is applied electric field is induced in the circuit. The induced emf is opposite to the applied field and electrons are accelerated in the opposite direction. Acceleration a =- ee i /m, E i = V i /d, where V i is induced emf and d is the path covered by the electron in the orbit, d=2πρ ρ is the radius of projection of the orbit in the X-Y plane. V i = induced emf = - (rate of change of magnetic flux.) If MF is applied along Z-direction, flux= B(πρ 2 ) Putting all these, we get acceleration a= - e m(2 ) d dt ( B 2 Or, dv dt e m(2 2 ) db dt
11 dv e 2m Or, v 2 -v 1 = v= (eρ/ 2m) B B 0 db indicates that the velocity of the electron in the orbit changes, so angular momentum also changes the magnetic moment. μ M = IxA = -ea/t = -ea v / 2π ρ = - eρ/2 ( eρ/2m)b = - (e 2 ρ 2 /4m ) B As the plane of the orbit varies continuously due to applied MF we take average value of ρ av 2 = x av2 +y av 2 if r is the radius of the atom, then r av2 = x av2 +y av2 +z av 2 For spherical symmetric atom x av2 =y av2 = z av2 = r av2 / 3 So, ρ av2 = (2/3) r av 2
12 Hence, μ M = - (e 2 r av 2 /6m ) B If there are Z no. of electrons, then, μ M = - Z(e 2 r av 2 /6m ) B If N is no. of atoms per vol. Then magnetization M= - NZ(e 2 r av 2 /4m ) B Negative sign indicates that Magnetization is opposite to applied field So the diamagnetic susceptibility χ = μ 0 M/B or, χ = -NZ(μ 0 e 2 r av 2 /6m) It indicates that Susceptibility depends on average mean square radius and independent of temperature
13 Theories of Paramagnetism Classical theory (Langevin's): Let n = no. of dipoles in a system = n 0 exp ( - E/ k β T) (Boltzmann s distribution formula) B = magnetic field applied τ = torque experienced by the dipoles in the magnetic field = μ M X B = μ M B sinθ E = energy of the dipoles = τ dθ = μ M B sinθ dθ = - μ M B cosθ = - μ M.B Now we have n = n 0 exp (μ M B cosθ / k β T ) Total dipole moment for all these dipoles = M cos dn < μ M > = ; μ M cosθ is the component of μ M dn along B n 0 M dn μ M θ B
14 but dn= n0 k M B T exp M B cos k T sin d So, < μ M > = If we put cosθ = x, dx= - sinθdθ & μ M B / k β T = a then the above expression becomes < μ M > = 1 1 M 0 0 M x 1 1 cos exp( exp( M exp( ax) dx = μ M [ coth a- 1/a ] M exp( ax) dx B cos / k T )sin d B cos / k T )sin d
15 < μ M > = μ M L (a) ; L(a) is called Langevin s function Now magnetisation M= N μ M L(a) Case-I :- a is large when T is small or B is large L(a) 1 hence M = N μ M = M s = saturation magnetisation Case-II :- a is small when T is large or B is small coth = 1/a + a/3 a2/ /a + a/3 L(a) = a/3 magnetisation M = Nμ m μ m B/ 3k β T = N μ m2 B/ 3k β T Susceptibility or, χ = M/ H = N μ m2 μ 0 / 3k β T χ = C/T Curie law
16 Quantum Theory : The energy of the system in the magnetic field applied in Z- direction is E = - μ z.b μ z = -gm j μ B so, E = 2m j μ B B For a system of one electron (l=0), m j =+ ½ or ½ E = - μ B B when m j =- ½ & E = μ B B when m j = + ½ One corresponding to parallel and other to antiparallel spin magnetic moment orientation w.r.t. Magnetic field Difference in energy between the two E= 2 μ B B Let out of N total atoms per volume In the system N1 are parallel and N2 are antiparallel E B=0 E2 E1 B 0 m j =- ½ Z- dir m j =+ ½
17 Distribution of atoms at thermal equilibrium in the two corresponding states are given by Maxwell Boltzmann distribution function N1/ N = e E 1 / k β T / N & N2/ N = e E 2 / k β T /N Net magnetization M = (N1-N2) μ z Or M = N B e e k k B B B T B T e e BB k T k B B T M = N μ B tanh ( μ B B/ k β T) In case μ B B < < k β T M= N μ B ( μ B B/ k β T) = N μ 2 BB/ k β T χ = M / H or χ = C/T Curie law χ = N μ 0 μ 2 B/ k β T
18 Ferromagnetic theory ( Weiss ) As per Weiss in ferromagnetic materials spontaneous magnetization is observed, which is due to a strong internal field arising from an exchange interaction between the magnetic moments in the neighborhood domains exchange interaction between two atoms I and j = U= -2 J S i S j J is called the exchange integral Internal field H is proportional to the magnetisation H int α M Or H int = λm H tot = H appl + H int H tot = H appl + λm As, χ = M / H = M / H appl + λm = C / T by Curie law
19 C/ T = M/ H appl. +λ M C H appl. = MT - C λ M or, M = C H appl. / T C λ or, M = C H / T-Tc Susceptibility Curie-Weiss law χ = M / H = C / T-Tc Ferromagnetic domains: B
20 Domain wall 10-2 μm Ms Ms(0) Tc T Ferromagnetic hysteresis M s = saturation magnetisation M r = remanent magnetisation H c = coercive field
21 Soft ferromagnetic 1. Can be easily magnetized or demagnetized 2. Thin and long hysteresis loop 3. High permeability and low coercive field 4. Large susceptibility & low remanent mag. 5. As area of the loop is small, magnetic energy loss per volume is less during magnetisation and demagnetisation 6. Application: electromagnet, in motors, generators, dynamos and switching circuits 7. Ex: Fe-Si alloy, Fe-Co-Mn alloy and Fe-Ni alloy Soft and hard FM materials Hard ferromagnetic 1. Can not be magnetised or demagnetised easily 2. Wide hysteresis loop 3. Low permeability and high coercive field 4. small susceptibility & high remanent mag. 5. Large area of the loop indicates, magnetic energy loss per volume is high during magnetisation and demagnetisation 6. For permanent magnet in speakers, clocks 7. Rare earth alloys with Mn, Fe, Co, Ni
22 SOFT & HARD FERROMAGNETIC HYSTERESIS LOOP
23 Ferrimagnetic & Antiferromagnetic materials Ferrimagnetic material are special class of ferromagnetic material called ferrites with high permeability, saturation magnetisation and show hysteresis (square loop) Suitable for high frequency application and magnetic devices Some spin magnetic moments are in opposite direction in the magnetised state, but are of different magnitudes, giving rise to net finite magnetic moment. Molecular formula: Me 2+ Fe 2 3+ O 3 ; Me is a divalent atom like, Fe, Mn, Zn,Cd,Cu,Ni,Co,Mg Crystal structure: Inverse spinel In the cubic cell 8 atoms per unit cell. In the unit cell, 32 O -2 ions, 16 Fe 3+ ions, and 8 Me 2+ ions 8- Fe 3+ ions, 8- Me 2+ ions are surrounded by 6 oxygen ion octahedral site and spins are parallel
24 Rest 8- Fe 3+ ions are surrounded by 4 oxygen ions tetrahedral site and spins antiparallel Hence net spin moment of Fe 3+ ions cancel ( 8 up spin and 8 down spin) Only, 8 Me 2+ ions contribute to magnetic moment. As spin magnetic moment is μ = g μ B S since g=2, magnetic moment of one divalent atom μ di = 2μ B S in a unit cell 8 such atoms are present ( for Fe2+, s= 2, for Co =3/2,Ni=1, Cu=1/2, Mn=5/2 ) total moment in unit cell= 8 x μ di Magnetisation M = total moment per volume = 8 x μ di / a 3 Where a is lattice parameter Applications: resistivity of ferrites are very high so applied for high frequency application (eddy current energy loss less) Mixed ferrites are produced by combining two suitable divalent atoms
25 Ferrites have square hysteresis loop. So used for digital storage device ( two values of magnetisation +Ms & - Ms; so 1 or 0 ) Soft ferrites are used for high freq. Transformer core, computer memory, hard disc, floppy disk audio video cassette, recorder head Hard ferrites are used for permanent magnets in generator, motor, loud speaker, telephone Non-volatile memory called magnetic bubbles Antiferromagnetism: when exchange interaction between adjacent or neighboring domains give rise to ordered antiparallel spin arrangement, below a temp. called Neel temp. ex.- MnO,MnS,FeCl2, CoO. Net moment or magnetisation is Zero χ= C / (T +θ ) χ T N T
26 Fe 3+ octahedral Fe 3+ tetrahedral Me 2+ S= 2 S= 5/2 S= 5/2 Mn 2+ = 3d 5 μ = g μ B S = 2 x 5/2 x μ B = 5μ B Fe 2+ = 3d 6, μ = 4 μ B Co 2+ = 3d 7, μ = 3 μ B Ni 2+ = 3d 8, μ = 2μ B Cu 2+ = 3d 9 μ = 1μ B
27 Ferrites = Me 2+ O Fe 3+ 2O 3 Me= Fe, Mn, Co, Ni, Cu, Mg, Zn, Cd Magnetite has the empirical formula Fe 3 O 4, or Fe 2+ (Fe 3+ O 2 ) 2, ferrous ferrite. Its formula as a spinel would be Fe 3+ tetfe 2+ octfe 3+ octo 4, where "tet" and "oct" stand for tetrahedral and octahedral coordinations by the oxide anions. In the above model, the blue spheres represent the tetrahedral iron(iii) cations, and the red spheres are the octahedrally coordinated iron(ii) and (III) cations. The oxide anions are shown as the green spheres. Because of the fortuitous inverse nature of the magnetite structure, ferrous and ferric cations are both in the similar octahedral coordination by oxides. In "normal" spinels, such as the mineral spinel itself (magnesium aluminate), the A cation is tetrahedral and the M cations are both octahedral
CHAPTER 2 MAGNETISM. 2.1 Magnetic materials
 CHAPTER 2 MAGNETISM Magnetism plays a crucial role in the development of memories for mass storage, and in sensors to name a few. Spintronics is an integration of the magnetic material with semiconductor
CHAPTER 2 MAGNETISM Magnetism plays a crucial role in the development of memories for mass storage, and in sensors to name a few. Spintronics is an integration of the magnetic material with semiconductor
Chapter 14. Optical and Magnetic Materials. 경상대학교 Ceramic Design Lab.
 Chapter 14 Optical and Magnetic Materials Magnetic field strength = H H = Ni/l (amp-turns/m) N = # turns i = current, amps l = conductor length B = Magnetic Induction or Magnetic flux density (Wb/m 2 )
Chapter 14 Optical and Magnetic Materials Magnetic field strength = H H = Ni/l (amp-turns/m) N = # turns i = current, amps l = conductor length B = Magnetic Induction or Magnetic flux density (Wb/m 2 )
Lecture contents. Magnetic properties Diamagnetism Band paramagnetism Atomic paramagnetism Ferromagnetism. Molecular field theory Exchange interaction
 1 Lecture contents Magnetic properties Diamagnetism and paramagnetism Atomic paramagnetism Ferromagnetism Molecular field theory Exchange interaction NNSE 58 EM Lecture #1 [SI] M magnetization or magnetic
1 Lecture contents Magnetic properties Diamagnetism and paramagnetism Atomic paramagnetism Ferromagnetism Molecular field theory Exchange interaction NNSE 58 EM Lecture #1 [SI] M magnetization or magnetic
Magnetism. Ram Seshadri MRL 2031, x6129, Some basics:
 Magnetism Ram Seshadri MRL 2031, x6129, seshadri@mrl.ucsb.edu Some basics: A magnet is associated with magnetic lines of force, and a north pole and a south pole. he lines of force come out of the north
Magnetism Ram Seshadri MRL 2031, x6129, seshadri@mrl.ucsb.edu Some basics: A magnet is associated with magnetic lines of force, and a north pole and a south pole. he lines of force come out of the north
Lecture 5. Chapters 3 & 4. Induced magnetization: that which is induced in the presence of an applied magnetic field. diamagnetic.
 Lecture 5 Induced magnetization: that which is induced in the presence of an applied magnetic field diamagnetic paramagnetic Remanent magnetization: that which remains in the absence of an external field
Lecture 5 Induced magnetization: that which is induced in the presence of an applied magnetic field diamagnetic paramagnetic Remanent magnetization: that which remains in the absence of an external field
PHYSICS 4750 Physics of Modern Materials Chapter 8: Magnetic Materials
 PHYSICS 475 Physics of Modern Materials Chapter 8: Magnetic Materials 1. Atomic Magnetic Dipole Moments A magnetic solid is one in which at least some of the atoms have a permanent magnetic dipole moment
PHYSICS 475 Physics of Modern Materials Chapter 8: Magnetic Materials 1. Atomic Magnetic Dipole Moments A magnetic solid is one in which at least some of the atoms have a permanent magnetic dipole moment
Physics of Magnetism. Chapter references are to Essentials of Paleomagnetism, UC Press, 2010
 Physics of Magnetism Chapter references are to Essentials of Paleomagnetism, UC Press, 2010 http://magician.ucsd.edu/essentials 1 Magnetic units (sorry!) SI cgs Magnetic fields as the gradient of a scalar
Physics of Magnetism Chapter references are to Essentials of Paleomagnetism, UC Press, 2010 http://magician.ucsd.edu/essentials 1 Magnetic units (sorry!) SI cgs Magnetic fields as the gradient of a scalar
3 MAGNETIC MATERIALS 3.1 INTRODUCTION
 3 MAGNETIC MATERIALS 3.1 INTRODUCTION Magnetic force is one of the oldest physical phenomena that human knows. The story of magnetism and magnetic materials begins with minerals called Magnetite (Fe 3
3 MAGNETIC MATERIALS 3.1 INTRODUCTION Magnetic force is one of the oldest physical phenomena that human knows. The story of magnetism and magnetic materials begins with minerals called Magnetite (Fe 3
Paramagnetism and Diamagnetism. Paramagnets (How do paramagnets differ fundamentally from ferromagnets?)
 Paramagnetism and Diamagnetism Paramagnets (How do paramagnets differ fundamentally from ferromagnets?) The study of paramagnetism allows us to investigate the atomic magnetic moments of atoms almost in
Paramagnetism and Diamagnetism Paramagnets (How do paramagnets differ fundamentally from ferromagnets?) The study of paramagnetism allows us to investigate the atomic magnetic moments of atoms almost in
Transition Elements. pranjoto utomo
 Transition Elements pranjoto utomo Definition What is transition metal? One of which forms one or more stable ions which have incompletely filled d orbitals. 30Zn? Definition Zink is not transition elements
Transition Elements pranjoto utomo Definition What is transition metal? One of which forms one or more stable ions which have incompletely filled d orbitals. 30Zn? Definition Zink is not transition elements
Electromagnetism II. Instructor: Andrei Sirenko Spring 2013 Thursdays 1 pm 4 pm. Spring 2013, NJIT 1
 Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
Magnetic materials, & inductance & Torque. P.Ravindran, PHY041: Electricity & Magnetism 8 February 2013: Magnetic materials, inductance, and torque
 Magnetic materials, & inductance & Torque Magnetic Properties of Materials Magnetic behavior of a material is due to the interaction of magnetic dipole moments of its atoms with an external magnetic field.
Magnetic materials, & inductance & Torque Magnetic Properties of Materials Magnetic behavior of a material is due to the interaction of magnetic dipole moments of its atoms with an external magnetic field.
Ferromagnetism. In free space, the flux density and magnetizing field strength are related by the expression
 1 Ferromagnetism B In free space, the flux density and magnetizing field strength are related by the expression H B =µ 0 H µ 0 =4π x 10-7 H.m -1, the permeability of free space. 2 Ferromagnetism B H For
1 Ferromagnetism B In free space, the flux density and magnetizing field strength are related by the expression H B =µ 0 H µ 0 =4π x 10-7 H.m -1, the permeability of free space. 2 Ferromagnetism B H For
Displacement Current. Ampere s law in the original form is valid only if any electric fields present are constant in time
 Displacement Current Ampere s law in the original form is valid only if any electric fields present are constant in time Maxwell modified the law to include timesaving electric fields Maxwell added an
Displacement Current Ampere s law in the original form is valid only if any electric fields present are constant in time Maxwell modified the law to include timesaving electric fields Maxwell added an
Ferromagnetism. Iron, nickel, and cobalt are ferromagnetic.
 Ferromagnetism Technische Universität Graz Institute of Solid State Physics Ferromagnetism elow a critical temperature (called the Curie temperature) a magnetization spontaneously appears in a ferromagnet
Ferromagnetism Technische Universität Graz Institute of Solid State Physics Ferromagnetism elow a critical temperature (called the Curie temperature) a magnetization spontaneously appears in a ferromagnet
Magnetic Materials. The inductor Φ B = LI (Q = CV) = L I = N Φ. Power = VI = LI. Energy = Power dt = LIdI = 1 LI 2 = 1 NΦ B capacitor CV 2
 Magnetic Materials The inductor Φ B = LI (Q = CV) Φ B 1 B = L I E = (CGS) t t c t EdS = 1 ( BdS )= 1 Φ V EMF = N Φ B = L I t t c t B c t I V Φ B magnetic flux density V = L (recall I = C for the capacitor)
Magnetic Materials The inductor Φ B = LI (Q = CV) Φ B 1 B = L I E = (CGS) t t c t EdS = 1 ( BdS )= 1 Φ V EMF = N Φ B = L I t t c t B c t I V Φ B magnetic flux density V = L (recall I = C for the capacitor)
PHY331 Magnetism. Lecture 3
 PHY331 Magnetism Lecture 3 Last week Derived magnetic dipole moment of a circulating electron. Discussed motion of a magnetic dipole in a constant magnetic field. Showed that it precesses with a frequency
PHY331 Magnetism Lecture 3 Last week Derived magnetic dipole moment of a circulating electron. Discussed motion of a magnetic dipole in a constant magnetic field. Showed that it precesses with a frequency
μ (vector) = magnetic dipole moment (not to be confused with the permeability μ). Magnetism Electromagnetic Fields in a Solid
 Magnetism Electromagnetic Fields in a Solid SI units cgs (Gaussian) units Total magnetic field: B = μ 0 (H + M) = μ μ 0 H B = H + 4π M = μ H Total electric field: E = 1/ε 0 (D P) = 1/εε 0 D E = D 4π P
Magnetism Electromagnetic Fields in a Solid SI units cgs (Gaussian) units Total magnetic field: B = μ 0 (H + M) = μ μ 0 H B = H + 4π M = μ H Total electric field: E = 1/ε 0 (D P) = 1/εε 0 D E = D 4π P
MAGNETIC MATERIALS. Fundamentals and device applications CAMBRIDGE UNIVERSITY PRESS NICOLA A. SPALDIN
 MAGNETIC MATERIALS Fundamentals and device applications NICOLA A. SPALDIN CAMBRIDGE UNIVERSITY PRESS Acknowledgements 1 Review of basic magnetostatics 1.1 Magnetic field 1.1.1 Magnetic poles 1.1.2 Magnetic
MAGNETIC MATERIALS Fundamentals and device applications NICOLA A. SPALDIN CAMBRIDGE UNIVERSITY PRESS Acknowledgements 1 Review of basic magnetostatics 1.1 Magnetic field 1.1.1 Magnetic poles 1.1.2 Magnetic
Lecture 24 - Magnetism
 Lecture 24: Magnetism (Kittel Ch. 1112) Quantum Mechanics Magnetism ElectronElectron Interactions Physics 460 F 2006 Lect 24 1 Outline Magnetism is a purely quantum phenomenon! Totally at variance with
Lecture 24: Magnetism (Kittel Ch. 1112) Quantum Mechanics Magnetism ElectronElectron Interactions Physics 460 F 2006 Lect 24 1 Outline Magnetism is a purely quantum phenomenon! Totally at variance with
PHY331 Magnetism. Lecture 6
 PHY331 Magnetism Lecture 6 Last week Learned how to calculate the magnetic dipole moment of an atom. Introduced the Landé g-factor. Saw that it compensates for the different contributions from the orbital
PHY331 Magnetism Lecture 6 Last week Learned how to calculate the magnetic dipole moment of an atom. Introduced the Landé g-factor. Saw that it compensates for the different contributions from the orbital
Interaction of matter with magnetic fields
 LN07-1 Interaction of matter with magnetic fields All substances have magnetic properties, which can be determined by examining their behaviour in the presence of an external magnetic field, H. N S When
LN07-1 Interaction of matter with magnetic fields All substances have magnetic properties, which can be determined by examining their behaviour in the presence of an external magnetic field, H. N S When
An introduction to magnetism in three parts
 An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
复习题. 2 Calculate the intensity of magnetic field in the air gap of the magnetic circuit shown in the figure. Use the values N=200,
 复习题 1 Calculate the magnetic moment of a sphere of radius R made from a magnetic material with magnetic susceptibility, when it is magnetized by an external magnetic field H. How is the value of the moment
复习题 1 Calculate the magnetic moment of a sphere of radius R made from a magnetic material with magnetic susceptibility, when it is magnetized by an external magnetic field H. How is the value of the moment
Material Science. Chapter 16. Magnetic properties
 Material Science Chapter 16. Magnetic properties Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
Material Science Chapter 16. Magnetic properties Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
Electromagnetic fields Learning outcome
 Electromagnetic fields Learning outcome At the end of this lecture you will be able to: List the most important electromagnetic field quantities Explain what these quantities describe Calculate some of
Electromagnetic fields Learning outcome At the end of this lecture you will be able to: List the most important electromagnetic field quantities Explain what these quantities describe Calculate some of
Chapter 23. Transition Metals and Coordination Chemistry
 Chapter 23 Transition Metals and Coordination Chemistry The Transition Metals: Exact Definition Transition metal: An element whose atom has an incomplete d subshell or which can give rise to cations with
Chapter 23 Transition Metals and Coordination Chemistry The Transition Metals: Exact Definition Transition metal: An element whose atom has an incomplete d subshell or which can give rise to cations with
Module-16. Magnetic properties
 Module-16 Magnetic properties Contents 1) Dia-, Para-, and Ferro-magnetism (Antiferro-magnetism and ferri-magnetism) 2) Influence of temperature on magnetic behavior 3) Domains and Hysteresis Introduction
Module-16 Magnetic properties Contents 1) Dia-, Para-, and Ferro-magnetism (Antiferro-magnetism and ferri-magnetism) 2) Influence of temperature on magnetic behavior 3) Domains and Hysteresis Introduction
PHY331 Magnetism. Lecture 4
 PHY331 Magnetism Lecture 4 Last week Discussed Langevin s theory of diamagnetism. Use angular momentum of precessing electron in magnetic field to derive the magnetization of a sample and thus diamagnetic
PHY331 Magnetism Lecture 4 Last week Discussed Langevin s theory of diamagnetism. Use angular momentum of precessing electron in magnetic field to derive the magnetization of a sample and thus diamagnetic
Contents. Acknowledgments
 MAGNETIC MATERIALS Fundamentals and Applications Second edition NICOLA A. SPALDIN University of California, Santa Barbara CAMBRIDGE UNIVERSITY PRESS Contents Acknowledgments page xiii I Basics 1 Review
MAGNETIC MATERIALS Fundamentals and Applications Second edition NICOLA A. SPALDIN University of California, Santa Barbara CAMBRIDGE UNIVERSITY PRESS Contents Acknowledgments page xiii I Basics 1 Review
Solid State Physics MAGNETISM I. Lecture 27. A.H. Harker. Physics and Astronomy UCL
 Solid State Physics MAGNETISM I Lecture 27 A.H. Harker Physics and Astronomy UCL 10 Magnetic Materials Magnet technology has made enormous advances in recent years without the reductions in size that have
Solid State Physics MAGNETISM I Lecture 27 A.H. Harker Physics and Astronomy UCL 10 Magnetic Materials Magnet technology has made enormous advances in recent years without the reductions in size that have
THE INFLUENCE OF A SURFACE ON HYSTERESIS LOOPS FOR SINGLE-DOMAIN FERROMAGNETIC NANOPARTICLES
 THE INFLUENCE OF A SURFACE ON HYSTERESIS LOOPS FOR SINGLE-DOMAIN FERROMAGNETIC NANOPARTICLES A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Saad Alsari
THE INFLUENCE OF A SURFACE ON HYSTERESIS LOOPS FOR SINGLE-DOMAIN FERROMAGNETIC NANOPARTICLES A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Saad Alsari
2. When the current flowing through a wire loop is halved, its magnetic moment will become a. half. b. one-fourth. c. double. d. quadruple.
 13 1. When a magnetic needle is kept in a uniform magnetic field, it experiences a. neither a force nor a torque. b. a force and not a torque. c. a torque and a force. d. only a torque.. Magnetic lines
13 1. When a magnetic needle is kept in a uniform magnetic field, it experiences a. neither a force nor a torque. b. a force and not a torque. c. a torque and a force. d. only a torque.. Magnetic lines
PHY331 Magnetism. Lecture 1
 PHY331 Magnetism Lecture 1 Overview Course syllabus / general information Quick revision of basic concepts Magnetization and susceptibility Using susceptibility to define magnetic materials Diamagnetic
PHY331 Magnetism Lecture 1 Overview Course syllabus / general information Quick revision of basic concepts Magnetization and susceptibility Using susceptibility to define magnetic materials Diamagnetic
Material Property. Dr. Cherdsak Bootjomchai (Dr. Per)
 Material Property By Dr. Cherdsak Bootjomchai (Dr. Per) Chapter IV Magnetic Properties Objectives - Magnetic Properties I 1. Equations describing magnetic field strength, induction (several versions),
Material Property By Dr. Cherdsak Bootjomchai (Dr. Per) Chapter IV Magnetic Properties Objectives - Magnetic Properties I 1. Equations describing magnetic field strength, induction (several versions),
Chapter 2 Magnetic Properties
 Chapter 2 Magnetic Properties Abstract The magnetic properties of a material are the basis of their applications. Specifically, the contrast agents that will be developed in Chaps. 4 and 5 use their magnetic
Chapter 2 Magnetic Properties Abstract The magnetic properties of a material are the basis of their applications. Specifically, the contrast agents that will be developed in Chaps. 4 and 5 use their magnetic
Luigi Paolasini
 Luigi Paolasini paolasini@esrf.fr LECTURE 5: MAGNETIC STRUCTURES - Mean field theory and magnetic order - Classification of magnetic structures - Collinear and non-collinear magnetic structures. - Magnetic
Luigi Paolasini paolasini@esrf.fr LECTURE 5: MAGNETIC STRUCTURES - Mean field theory and magnetic order - Classification of magnetic structures - Collinear and non-collinear magnetic structures. - Magnetic
Magnetism in low dimensions from first principles. Atomic magnetism. Gustav Bihlmayer. Gustav Bihlmayer
 IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
IFF 10 p. 1 Magnetism in low dimensions from first principles Atomic magnetism Gustav Bihlmayer Institut für Festkörperforschung, Quantum Theory of Materials Gustav Bihlmayer Institut für Festkörperforschung
B for a Long, Straight Conductor, Special Case. If the conductor is an infinitely long, straight wire, θ 1 = 0 and θ 2 = π The field becomes
 B for a Long, Straight Conductor, Special Case If the conductor is an infinitely long, straight wire, θ 1 = 0 and θ 2 = π The field becomes μ I B = o 2πa B for a Curved Wire Segment Find the field at point
B for a Long, Straight Conductor, Special Case If the conductor is an infinitely long, straight wire, θ 1 = 0 and θ 2 = π The field becomes μ I B = o 2πa B for a Curved Wire Segment Find the field at point
MSE 7025 Magnetic Materials (and Spintronics)
 MSE 7025 Magnetic Materials (and Spintronics) Lecture 4: Category of Magnetism Chi-Feng Pai cfpai@ntu.edu.tw Course Outline Time Table Week Date Lecture 1 Feb 24 Introduction 2 March 2 Magnetic units and
MSE 7025 Magnetic Materials (and Spintronics) Lecture 4: Category of Magnetism Chi-Feng Pai cfpai@ntu.edu.tw Course Outline Time Table Week Date Lecture 1 Feb 24 Introduction 2 March 2 Magnetic units and
( (Chapter 5)(Magnetism and Matter)
 Additional Exercises Question 5.16: Answer the following questions: (a) Why does a paramagnetic sample display greater magnetisation (for the same magnetising field) when cooled? (b) Why is diamagnetism,
Additional Exercises Question 5.16: Answer the following questions: (a) Why does a paramagnetic sample display greater magnetisation (for the same magnetising field) when cooled? (b) Why is diamagnetism,
Solid state physics. Lecture 9: Magnetism. Prof. Dr. U. Pietsch
 Solid state physics Lecture 9: Magnetism Prof. Dr. U. Pietsch Diamagnetism and Paramagnetsim Materie in magnetic field m 0 0 H M H(1 H 0 0M m M magnetiszation magnetic susceptibility - magnetic permeability
Solid state physics Lecture 9: Magnetism Prof. Dr. U. Pietsch Diamagnetism and Paramagnetsim Materie in magnetic field m 0 0 H M H(1 H 0 0M m M magnetiszation magnetic susceptibility - magnetic permeability
Ch. 28: Sources of Magnetic Fields
 Ch. 28: Sources of Magnetic Fields Electric Currents Create Magnetic Fields A long, straight wire A current loop A solenoid Slide 24-14 Biot-Savart Law Current produces a magnetic field The Biot-Savart
Ch. 28: Sources of Magnetic Fields Electric Currents Create Magnetic Fields A long, straight wire A current loop A solenoid Slide 24-14 Biot-Savart Law Current produces a magnetic field The Biot-Savart
UNIT - IV SEMICONDUCTORS AND MAGNETIC MATERIALS
 1. What is intrinsic If a semiconductor is sufficiently pure, then it is known as intrinsic semiconductor. ex:: pure Ge, pure Si 2. Mention the expression for intrinsic carrier concentration of intrinsic
1. What is intrinsic If a semiconductor is sufficiently pure, then it is known as intrinsic semiconductor. ex:: pure Ge, pure Si 2. Mention the expression for intrinsic carrier concentration of intrinsic
Internal Fields in Solids: (Lorentz Method)
 Internal Fields in Solids: (Lorentz Method) Let a dielectric be placed between the plates of a parallel plate capacitor and let there be an imaginary spherical cavity around the atom A inside the dielectric.
Internal Fields in Solids: (Lorentz Method) Let a dielectric be placed between the plates of a parallel plate capacitor and let there be an imaginary spherical cavity around the atom A inside the dielectric.
Magnetic Force on a Moving Charge
 Magnetic Force on a Moving Charge Electric charges moving in a magnetic field experience a force due to the magnetic field. Given a charge Q moving with velocity u in a magnetic flux density B, the vector
Magnetic Force on a Moving Charge Electric charges moving in a magnetic field experience a force due to the magnetic field. Given a charge Q moving with velocity u in a magnetic flux density B, the vector
Physics 202, Lecture 14
 Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch. 30) Review: iot-savart Law, Ampere s Law Displacement Current: Ampere-Maxwell Law Magnetism in Matter Maxwell s Equations (prelude)
Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch. 30) Review: iot-savart Law, Ampere s Law Displacement Current: Ampere-Maxwell Law Magnetism in Matter Maxwell s Equations (prelude)
Electromagnetism - Lecture 10. Magnetic Materials
 Electromagnetism - Lecture 10 Magnetic Materials Magnetization Vector M Magnetic Field Vectors B and H Magnetic Susceptibility & Relative Permeability Diamagnetism Paramagnetism Effects of Magnetic Materials
Electromagnetism - Lecture 10 Magnetic Materials Magnetization Vector M Magnetic Field Vectors B and H Magnetic Susceptibility & Relative Permeability Diamagnetism Paramagnetism Effects of Magnetic Materials
Electromagnetism II. Cristina Lazzeroni Lecture 5
 Electromagnetism II Cristina Lazzeroni c.lazzeroni@bham.ac.uk Lecture 5 Maxwell s equations for free space They apply simultaneously at any given point in free space. How do they change in presence of
Electromagnetism II Cristina Lazzeroni c.lazzeroni@bham.ac.uk Lecture 5 Maxwell s equations for free space They apply simultaneously at any given point in free space. How do they change in presence of
J 12 J 23 J 34. Driving forces in the nano-magnetism world. Intra-atomic exchange, electron correlation effects: Inter-atomic exchange: MAGNETIC ORDER
 Driving forces in the nano-magnetism world Intra-atomic exchange, electron correlation effects: LOCAL (ATOMIC) MAGNETIC MOMENTS m d or f electrons Inter-atomic exchange: MAGNETIC ORDER H exc J S S i j
Driving forces in the nano-magnetism world Intra-atomic exchange, electron correlation effects: LOCAL (ATOMIC) MAGNETIC MOMENTS m d or f electrons Inter-atomic exchange: MAGNETIC ORDER H exc J S S i j
Magnetic Oxides. Gerald F. Dionne. Department of Materials Science and Engineering Massachusetts Institute of Technology
 Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
Current Loop as a Magnetic Dipole & Dipole Moment:
 MAGNETISM 1. Bar Magnet and its properties 2. Current Loop as a Magnetic Dipole and Dipole Moment 3. Current Solenoid equivalent to Bar Magnet 4. Bar Magnet and it Dipole Moment 5. Coulomb s Law in Magnetism
MAGNETISM 1. Bar Magnet and its properties 2. Current Loop as a Magnetic Dipole and Dipole Moment 3. Current Solenoid equivalent to Bar Magnet 4. Bar Magnet and it Dipole Moment 5. Coulomb s Law in Magnetism
Follow this and additional works at: Part of the Physics Commons
 Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2016 Surface Effect Of Ferromagnetic Nanoparticles On Transition Between Single- And Multi-Domain Structure
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2016 Surface Effect Of Ferromagnetic Nanoparticles On Transition Between Single- And Multi-Domain Structure
Coaxial cable. Coaxial cable. Magnetic field inside a solenoid
 Divergence and circulation Surface S Ampere s Law A vector field is generally characterized by 1) how field lines possibly diverge away from or converge upon (point) sources plus 2) how field lines circulate,
Divergence and circulation Surface S Ampere s Law A vector field is generally characterized by 1) how field lines possibly diverge away from or converge upon (point) sources plus 2) how field lines circulate,
Physics 12. Unit 8 Magnetic Field and Electromagnetism Part I
 Physics 12 Unit 8 Magnetic Field and Electromagnetism Part I 1. Basics about magnets Magnets have been known by ancient people since long time ago, referring to the iron-rich rocks, called magnetite or
Physics 12 Unit 8 Magnetic Field and Electromagnetism Part I 1. Basics about magnets Magnets have been known by ancient people since long time ago, referring to the iron-rich rocks, called magnetite or
physics 590 ruslan prozorov magnetic measurements Nov 9,
 physics 590 ruslan prozorov magnetic measurements Nov 9, 2009 - magnetic moment of free currents Magnetic moment of a closed loop carrying current I: Magnetic field on the axis of a loop of radius R at
physics 590 ruslan prozorov magnetic measurements Nov 9, 2009 - magnetic moment of free currents Magnetic moment of a closed loop carrying current I: Magnetic field on the axis of a loop of radius R at
Chapter 5. Magnetism and Matter
 Chapter 5 Magnetism and Matter TABLE 5.1 THE DIPOLE ANALO Diamagnetic materials, when placed in a magnetic field, are magnetized in the direction opposite to the magnetic field; whereas paramagnetic and
Chapter 5 Magnetism and Matter TABLE 5.1 THE DIPOLE ANALO Diamagnetic materials, when placed in a magnetic field, are magnetized in the direction opposite to the magnetic field; whereas paramagnetic and
Geophysics 223 January Geophysics 223 C1: Basics of Geomagnetism. C1.1 Introduction
 Geophysics 223 C1: Basics of Geomagnetism C1.1 Introduction Lodestone was known to the Greeks (800 BC) and Chinese (300 BC) First compass (200 BC) made by Chinese, but not clear why it worked Europeans
Geophysics 223 C1: Basics of Geomagnetism C1.1 Introduction Lodestone was known to the Greeks (800 BC) and Chinese (300 BC) First compass (200 BC) made by Chinese, but not clear why it worked Europeans
~~r ~o~/, ' , I. l: z: n.-b -z 01. ?;Cl. 60) Pro CD'fCJ7 '; ftu-0j~
 i -1- ~~r ~o~/, ------', I l: z: n.-b -z 01?;Cl 60) 1---.-- Pro CD'fCJ7 '; ftu-0j~ APPLICATIONS 1/ What features of atomic structure determine whether an element is diamagnetic or paramagnetic? Explain.
i -1- ~~r ~o~/, ------', I l: z: n.-b -z 01?;Cl 60) 1---.-- Pro CD'fCJ7 '; ftu-0j~ APPLICATIONS 1/ What features of atomic structure determine whether an element is diamagnetic or paramagnetic? Explain.
Electricity & Optics
 Physics 24100 Electricity & Optics Lecture 15 Chapter 27 sec. 3-5 Fall 2016 Semester Professor Koltick Magnetic Fields B = μ 0 4π I dl r r 2 = μ 0 4π I dl r r 3 B = μ 0 2I 4π R B = μ 0 2 IR 2 R 2 + z 2
Physics 24100 Electricity & Optics Lecture 15 Chapter 27 sec. 3-5 Fall 2016 Semester Professor Koltick Magnetic Fields B = μ 0 4π I dl r r 2 = μ 0 4π I dl r r 3 B = μ 0 2I 4π R B = μ 0 2 IR 2 R 2 + z 2
Ferromagnetism and antiferromagnetism ferromagnetism (FM) antiferromagnetism (AFM) ferromagnetic domains nanomagnetic particles
 Dept of Phys Ferromagnetism and antiferromagnetism ferromagnetism (FM) exchange interaction, Heisenberg model spin wave, magnon antiferromagnetism (AFM) ferromagnetic domains nanomagnetic particles M.C.
Dept of Phys Ferromagnetism and antiferromagnetism ferromagnetism (FM) exchange interaction, Heisenberg model spin wave, magnon antiferromagnetism (AFM) ferromagnetic domains nanomagnetic particles M.C.
Luigi Paolasini
 Luigi Paolasini paolasini@esrf.fr LECTURE 2: LONELY ATOMS - Systems of electrons - Spin-orbit interaction and LS coupling - Fine structure - Hund s rules - Magnetic susceptibilities Reference books: -
Luigi Paolasini paolasini@esrf.fr LECTURE 2: LONELY ATOMS - Systems of electrons - Spin-orbit interaction and LS coupling - Fine structure - Hund s rules - Magnetic susceptibilities Reference books: -
Fundamentals of Magnetism
 Fundamentals of Magnetism Part II Albrecht Jander Oregon State University Real Magnetic Materials, Bulk Properties M-H Loop M M s M R B-H Loop B B s B R H ci H H c H M S - Saturation magnetization H ci
Fundamentals of Magnetism Part II Albrecht Jander Oregon State University Real Magnetic Materials, Bulk Properties M-H Loop M M s M R B-H Loop B B s B R H ci H H c H M S - Saturation magnetization H ci
Torque on a Current Loop
 Today Chapter 19 Magnetism Torque on a current loop, electrical motor Magnetic field around a current carrying wire. Ampere s law Solenoid Material magnetism Clicker 1 Which of the following is wrong?
Today Chapter 19 Magnetism Torque on a current loop, electrical motor Magnetic field around a current carrying wire. Ampere s law Solenoid Material magnetism Clicker 1 Which of the following is wrong?
Electromagnetism - Lecture 12. Ferromagnetism & Superconductivity
 Electromagnetism - Lecture 12 Ferromagnetism & Superconductivity Ferromagnetism Hysteresis & Permanent Magnets Ferromagnetic Surfaces Toroid with Ferromagnetic Core Superconductivity The Meissner Effect
Electromagnetism - Lecture 12 Ferromagnetism & Superconductivity Ferromagnetism Hysteresis & Permanent Magnets Ferromagnetic Surfaces Toroid with Ferromagnetic Core Superconductivity The Meissner Effect
S j H o = gµ o H o. j=1
 LECTURE 17 Ferromagnetism (Refs.: Sections 10.6-10.7 of Reif; Book by J. S. Smart, Effective Field Theories of Magnetism) Consider a solid consisting of N identical atoms arranged in a regular lattice.
LECTURE 17 Ferromagnetism (Refs.: Sections 10.6-10.7 of Reif; Book by J. S. Smart, Effective Field Theories of Magnetism) Consider a solid consisting of N identical atoms arranged in a regular lattice.
Magnetism and Levitation
 Magnetism and Levitation Brent Hobbs Dan Stark Timothy Wofford Junior Lab I Wednesday, December 11, 2002 Types of Magnetism Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism Superparamagnetism
Magnetism and Levitation Brent Hobbs Dan Stark Timothy Wofford Junior Lab I Wednesday, December 11, 2002 Types of Magnetism Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism Superparamagnetism
CHAPTER 20 MAGNETIC PROPERTIES PROBLEM SOLUTIONS
 CHAPTER 20 MAGNETIC PROPERTIES PROBLEM SOLUTIONS Basic Concepts 20.1 A coil of wire 0.20 m long and having 200 turns carries a current of 10 A. (a) What is the magnitude of the magnetic field strength
CHAPTER 20 MAGNETIC PROPERTIES PROBLEM SOLUTIONS Basic Concepts 20.1 A coil of wire 0.20 m long and having 200 turns carries a current of 10 A. (a) What is the magnitude of the magnetic field strength
Faraday s Law of Induction I
 Faraday s Law of Induction I Physics 2415 Lecture 19 Michael Fowler, UVa Today s Topics Magnetic Permeability Faraday s Law of Induction Lenz s Law Paramagnets and Diamagnets Electromagnets Electromagnets
Faraday s Law of Induction I Physics 2415 Lecture 19 Michael Fowler, UVa Today s Topics Magnetic Permeability Faraday s Law of Induction Lenz s Law Paramagnets and Diamagnets Electromagnets Electromagnets
Basic Magnetism (I. Fundamentals)
 Paolo Allia DISAT Politecnico di Torino Associate, INRiM - Torino Basic Magnetism (I. Fundamentals) P. Allia - Italian School of Magnetism 018 1 A journey through Magnetism or, From atoms to macroscopic
Paolo Allia DISAT Politecnico di Torino Associate, INRiM - Torino Basic Magnetism (I. Fundamentals) P. Allia - Italian School of Magnetism 018 1 A journey through Magnetism or, From atoms to macroscopic
EWING S MOLECULAR THEORY OF MAGNETISM AND ITS FAILURES
 8. MAGNETISM Salient features Ewing s molecular theory and its failures Domain theory Inverse square law of magnetism Magnetic induction Magnetic field due to a bar magnet Magnetic lines of force Magnetic
8. MAGNETISM Salient features Ewing s molecular theory and its failures Domain theory Inverse square law of magnetism Magnetic induction Magnetic field due to a bar magnet Magnetic lines of force Magnetic
Kirchhoff s rules, example
 Kirchhoff s rules, example Magnets and Magnetism Poles of a magnet are the ends where objects are most strongly attracted. Two poles, called north and south Like poles repel each other and unlike poles
Kirchhoff s rules, example Magnets and Magnetism Poles of a magnet are the ends where objects are most strongly attracted. Two poles, called north and south Like poles repel each other and unlike poles
1. Aims. 2. Apparatus. 3. Background
 1. Aims The aims of this experiment are to measure the magnetic susceptibility of a solution of manganese sulphate and to determine the magnetic dipole moment of a Mn + ion in units of the Bohr magneton,
1. Aims The aims of this experiment are to measure the magnetic susceptibility of a solution of manganese sulphate and to determine the magnetic dipole moment of a Mn + ion in units of the Bohr magneton,
Lecture 24 Origins of Magnetization (A number of illustrations in this lecture were generously provided by Prof. Geoffrey Beach)
 Lecture 4 Origins of Magnetization (A number of illustrations in this lecture were generously provided by Prof. Geoffrey Beach) Today 1. Magnetic dipoles.. Orbital and spin angular momenta. 3. Non-interacting
Lecture 4 Origins of Magnetization (A number of illustrations in this lecture were generously provided by Prof. Geoffrey Beach) Today 1. Magnetic dipoles.. Orbital and spin angular momenta. 3. Non-interacting
Physics 202, Lecture 14
 Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch 30) Review: The Biot-Savart Law The Ampere s Law Applications And Exercises of ampere s Law Straight line, Loop, Solenoid, Toroid
Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch 30) Review: The Biot-Savart Law The Ampere s Law Applications And Exercises of ampere s Law Straight line, Loop, Solenoid, Toroid
Chapter 8: Magnetic and Electrical Properties
 Chapter 8: Magnetic and Electrical Properties 1 In solids, properties of individual atoms can interact cooperatively to produce effects not found in fluids. Magnetic Susceptibility: magnetic field produces
Chapter 8: Magnetic and Electrical Properties 1 In solids, properties of individual atoms can interact cooperatively to produce effects not found in fluids. Magnetic Susceptibility: magnetic field produces
Magnetic systems for refrigeration & thermometry
 Magnetic systems for refrigeration & thermometry * Paramagnetic salts * * Hyperfine enhanced systems * * Nuclear spins * Magnetic moments can be aligned by external magnetic field Thermal disorder counteracts
Magnetic systems for refrigeration & thermometry * Paramagnetic salts * * Hyperfine enhanced systems * * Nuclear spins * Magnetic moments can be aligned by external magnetic field Thermal disorder counteracts
MAGNETIC PARTICLE INSPECTION (MPI)
 MAGNETIC PARTICLE INSPECTION (MPI) Magnetic particle inspection (MPI) is a method that can be used to detect surface and near surface defects or flaws in ferromagnetic materials such as steel and iron.
MAGNETIC PARTICLE INSPECTION (MPI) Magnetic particle inspection (MPI) is a method that can be used to detect surface and near surface defects or flaws in ferromagnetic materials such as steel and iron.
Lecture 19: Magnetic properties and the Nephelauxetic effect
 Lecture 19: Magnetic properties and the Nephelauxetic effect sample balance thermometer connection to balance left: the Gouy balance for Gouy Tube determining the magnetic susceptibility of materials north
Lecture 19: Magnetic properties and the Nephelauxetic effect sample balance thermometer connection to balance left: the Gouy balance for Gouy Tube determining the magnetic susceptibility of materials north
The magnetic circuits and fields in materials
 The magnetic circuits and fields in materials Lecture 14 1 Linear current above a magnetic block In this example, assume a current density J above an infinite slab of linear magnetic material, with permeability,
The magnetic circuits and fields in materials Lecture 14 1 Linear current above a magnetic block In this example, assume a current density J above an infinite slab of linear magnetic material, with permeability,
1 CHAPTER 12 PROPERTIES OF MAGNETIC MATERIALS
 1 CHAPTER 12 PROPERTIES OF MAGNETIC MATERIALS 12.1 Introduction This chapter is likely to be a short one, not least because it is a subject in which my own knowledge is, to put it charitably, a little
1 CHAPTER 12 PROPERTIES OF MAGNETIC MATERIALS 12.1 Introduction This chapter is likely to be a short one, not least because it is a subject in which my own knowledge is, to put it charitably, a little
Electric vs Magnetic Comparison
 5. MAGNETOSTATICS Electric vs Magnetic Comparison J=σE Most dielectrics µ = µo excluding ferromagnetic materials Gauss s Law E field is conservative Gauss s law (integral) Conservative E field Electric
5. MAGNETOSTATICS Electric vs Magnetic Comparison J=σE Most dielectrics µ = µo excluding ferromagnetic materials Gauss s Law E field is conservative Gauss s law (integral) Conservative E field Electric
The Physics of Ferromagnetism
 Terunobu Miyazaki Hanmin Jin The Physics of Ferromagnetism Springer Contents Part I Foundation of Magnetism 1 Basis of Magnetism 3 1.1 Basic Magnetic Laws and Magnetic Quantities 3 1.1.1 Basic Laws of
Terunobu Miyazaki Hanmin Jin The Physics of Ferromagnetism Springer Contents Part I Foundation of Magnetism 1 Basis of Magnetism 3 1.1 Basic Magnetic Laws and Magnetic Quantities 3 1.1.1 Basic Laws of
Physics 202, Lecture 14
 Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch. 27) Review of Biot-Savart Law Ampere s Law Magnetism in Matter Magnetic Fields (Biot-Savart): Summary Current loop, distance on
Physics 202, Lecture 14 Today s Topics Sources of the Magnetic Field (Ch. 27) Review of Biot-Savart Law Ampere s Law Magnetism in Matter Magnetic Fields (Biot-Savart): Summary Current loop, distance on
2 B B D (E) Paramagnetic Susceptibility. m s probability. A) Bound Electrons in Atoms
 Paramagnetic Susceptibility A) Bound Electrons in Atoms m s probability B +½ p ½e x Curie Law: 1/T s=½ + B ½ p + ½e +x With increasing temperature T the alignment of the magnetic moments in a B field is
Paramagnetic Susceptibility A) Bound Electrons in Atoms m s probability B +½ p ½e x Curie Law: 1/T s=½ + B ½ p + ½e +x With increasing temperature T the alignment of the magnetic moments in a B field is
Magnetic Materials. 2. Diamagnetism. Numan Akdoğan.
 Magnetic Materials. Diamagnetism Numan Akdoğan akdogan@gyte.edu.tr Gebze Institute of Technology Department of Physics Nanomagnetism and Spintronic Research Center (NASAM) Magnetic moments of electrons
Magnetic Materials. Diamagnetism Numan Akdoğan akdogan@gyte.edu.tr Gebze Institute of Technology Department of Physics Nanomagnetism and Spintronic Research Center (NASAM) Magnetic moments of electrons
Winmeen Tnpsc Group 1 & 2 Self Preparation Course Physics UNIT 10. Magnetism
 Physics UNIT 10 Magnetism The word magnetism is derived from iron ore magnetite (Fe3O4), which was found in the island of magnesia in Greece. It was Gilbert who laid the foundation for magnetism and had
Physics UNIT 10 Magnetism The word magnetism is derived from iron ore magnetite (Fe3O4), which was found in the island of magnesia in Greece. It was Gilbert who laid the foundation for magnetism and had
MAGNETIC DIPOLES, HYSTERESIS AND CORE LOSES
 Power Quality For The Digital Age MAGNETIC DIPOLES, HYSTERESIS AND CORE LOSES A N E N V I R O N M E N T A L P O T E N T I A L S W H I T E P A P E R By Professor Edward Price Director of Research and Development
Power Quality For The Digital Age MAGNETIC DIPOLES, HYSTERESIS AND CORE LOSES A N E N V I R O N M E N T A L P O T E N T I A L S W H I T E P A P E R By Professor Edward Price Director of Research and Development
The goal of this chapter is to understand
 A photograph of a commercial highspeed maglev train that connects the Shanghai, China airport to the city center. Maglev trains do not run on wheels; instead, they are lifted from the track and propelled
A photograph of a commercial highspeed maglev train that connects the Shanghai, China airport to the city center. Maglev trains do not run on wheels; instead, they are lifted from the track and propelled
Magnetic field and magnetic poles
 Magnetic field and magnetic poles Magnetic Field B is analogically similar to Electric Field E Electric charges (+ and -)are in analogy to magnetic poles(north:n and South:S). Paramagnetism, Diamagnetism,
Magnetic field and magnetic poles Magnetic Field B is analogically similar to Electric Field E Electric charges (+ and -)are in analogy to magnetic poles(north:n and South:S). Paramagnetism, Diamagnetism,
Chapter 13 Principles of Electromechanics
 Chapter 13 Principles of Electromechanics Jaesung Jang Electrostatics B-H Magnetization Curves & Magnetic Hysteresis 1 Electrostatics & Magnetic Flux The force on a stationary charge q in an electric field
Chapter 13 Principles of Electromechanics Jaesung Jang Electrostatics B-H Magnetization Curves & Magnetic Hysteresis 1 Electrostatics & Magnetic Flux The force on a stationary charge q in an electric field
Competing Ferroic Orders The magnetoelectric effect
 Competing Ferroic Orders The magnetoelectric effect Cornell University I would found an institution where any person can find instruction in any study. Ezra Cornell, 1868 Craig J. Fennie School of Applied
Competing Ferroic Orders The magnetoelectric effect Cornell University I would found an institution where any person can find instruction in any study. Ezra Cornell, 1868 Craig J. Fennie School of Applied
Lecture 11: Transition metals (1) Basics and magnetism
 Lecture 11: Transition metals (1) Basics and magnetism Oxidation states in transition metal compounds Ligand field theory Magnetism Susceptibility Temperature dependence Magnetic moments Figure: Wikipedia
Lecture 11: Transition metals (1) Basics and magnetism Oxidation states in transition metal compounds Ligand field theory Magnetism Susceptibility Temperature dependence Magnetic moments Figure: Wikipedia
Metropolis Monte Carlo simulation of the Ising Model
 Metropolis Monte Carlo simulation of the Ising Model Krishna Shrinivas (CH10B026) Swaroop Ramaswamy (CH10B068) May 10, 2013 Modelling and Simulation of Particulate Processes (CH5012) Introduction The Ising
Metropolis Monte Carlo simulation of the Ising Model Krishna Shrinivas (CH10B026) Swaroop Ramaswamy (CH10B068) May 10, 2013 Modelling and Simulation of Particulate Processes (CH5012) Introduction The Ising
Problems in Magnetic Properties of Materials
 Problems in Magnetic Properties of Materials Notations used: H: Magnetic field stregth B: Magnetic flux density I: Intensity of Magentization (Please note that, in text book, notation, M, is used for Intensity
Problems in Magnetic Properties of Materials Notations used: H: Magnetic field stregth B: Magnetic flux density I: Intensity of Magentization (Please note that, in text book, notation, M, is used for Intensity
1.1 Units, definitions and fundamental equations. How should we deal with B and H which are usually used for magnetic fields?
 Advance Organizer: Chapter 1: Introduction to single magnetic moments: Magnetic dipoles Spin and orbital angular momenta Spin-orbit coupling Magnetic susceptibility, Magnetic dipoles in a magnetic field:
Advance Organizer: Chapter 1: Introduction to single magnetic moments: Magnetic dipoles Spin and orbital angular momenta Spin-orbit coupling Magnetic susceptibility, Magnetic dipoles in a magnetic field:
Outside the solenoid, the field lines are spread apart, and at any given distance from the axis, the field is weak.
 Applications of Ampere s Law continued. 2. Field of a solenoid. A solenoid can have many (thousands) of turns, and perhaps many layers of windings. The figure shows a simple solenoid with just a few windings
Applications of Ampere s Law continued. 2. Field of a solenoid. A solenoid can have many (thousands) of turns, and perhaps many layers of windings. The figure shows a simple solenoid with just a few windings
Class XII Chapter 5 Magnetism And Matter Physics
 Question 5.1: the following questions regarding earth s magnetism: (a) A vector needs three quantities for its specification. Name the three independent quantities conventionally used to specify the earth
Question 5.1: the following questions regarding earth s magnetism: (a) A vector needs three quantities for its specification. Name the three independent quantities conventionally used to specify the earth
Chapter 8 Magnetic Resonance
 Chapter 8 Magnetic Resonance 9.1 Electron paramagnetic resonance 9.2 Ferromagnetic resonance 9.3 Nuclear magnetic resonance 9.4 Other resonance methods TCD March 2007 1 A resonance experiment involves
Chapter 8 Magnetic Resonance 9.1 Electron paramagnetic resonance 9.2 Ferromagnetic resonance 9.3 Nuclear magnetic resonance 9.4 Other resonance methods TCD March 2007 1 A resonance experiment involves
Properties of Materials. Chapter Two Magnetic Properties of Materials
 1896 1920 1987 2006 Properties of Materials Chapter Two Magnetic Properties of Materials Key Magnetic Parameters How does M respond to H? Ferromagnetic Fe, Co, Ni Ferrimagnetic Fe 3 O 4 Antiferromagnetic
1896 1920 1987 2006 Properties of Materials Chapter Two Magnetic Properties of Materials Key Magnetic Parameters How does M respond to H? Ferromagnetic Fe, Co, Ni Ferrimagnetic Fe 3 O 4 Antiferromagnetic
