The orbital-specific-virtual local coupled cluster singles and doubles method
|
|
|
- Holly Gardner
- 5 years ago
- Views:
Transcription
1 The orbital-specific-virtual local coupled cluster singles and doubles method Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, Martin Schütz, and Hans-Joachim Werner Citation: J. Chem. Phys. 136, (2012); doi: / View online: View Tle of Contents: Published by the American Institute of Physics. Additional information on J. Chem. Phys. Journal Homepage: Journal Information: Top downloads: Information for Authors:
2 THE JOURNAL OF CHEMICAL PHYSICS 136, (2012) The orbital-specific-virtual local coupled cluster singles and doubles method Jun Yang, 1,a) Garnet Kin-Lic Chan, 1,b) Frederick R. Manby, 2,c) Martin Schütz, 3,d) and Hans-Joachim Werner 4,e) 1 Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, USA 2 Center for Computational Chemistry, School of Chemistry, University of Bristol, Bristol BS8 1TS, United Kingdom 3 Institut für Physikalische und Theoretische Chemie, Universität Regensburg, Regensburg, D-93040, Germany 4 Institut für Theoretische Chemie, Universität Stuttgart, Stuttgart D-70569, Germany (Received 7 January 2012; accepted 6 March 2012; published online 10 April 2012) We extend the orbital-specific-virtual tensor factorization, introduced for local Møller-Plesset perturbation theory in Ref. [J. Yang, Y. Kurashige, F. R. Manby and G. K. L. Chan, J. Chem. Phys. 134, (2011)], to local coupled cluster singles and doubles theory (OSV-LCCSD). The method is implemented by modifying an efficient projected-atomic-orbital local coupled cluster program (PAO- LCCSD) described recently, [H.-J. Werner and M. Schütz, J. Chem. Phys. 135, (2011)]. By comparison of both methods we find that the compact representation of the amplitudes in the OSV approach affords various advantages, including smaller computational time requirements (for comparle accuracy), as well as a more systematic control of the error through a single energy threshold. Overall, the OSV-LCCSD approach together with an MP2 correction yields small domain errors in practical calculations. The applicility of the OSV-LCCSD is demonstrated for molecules with up to 73 atoms and realistic basis sets (up to 2334 basis functions) American Institute of Physics. [ I. INTRODUCTION Ab initio quantum chemistry defines hierarchies of correlation theories, such as perturbation theory (PT), coupled cluster (CC), and configuration interaction. Despite much progress, conventional correlation treatments are still too expensive to apply to large systems, due to high scaling of computational effort with system size. For example, conventional second-order Møller-Plesset perturbation theory (MP2) requires N 5 cost for the energy (where N measures system size); coupled cluster singles and doubles (CCSD) theory requires N 6 cost; and the gold standard, CC with perturbative triples CCSD(T), requires N 7 cost. The steep computational scalings stem from the high tensor rank of the mathematical objects in the theories, and the delocalized nature of the underlying orbital basis. By tensor, we mean an array of numbers, written T n1 n 2, where n i are the tensor indices, and the number of indices is the tensor rank. These objects include wavefunction amplitudes as well as integral intermediates. To reduce the computational complexity, we can impose special structures on the tensors. Such a structure can be interpreted as defining a tensor factorization, where a high rank tensor is written as products of tensors, 1, 2 with possible contractions over auxiliary indices. Matrix factorizations, such as the Cholesky decomposition, and density fitting (DF) [somea) Electronic mail: jy459@cornell.edu. b) Electronic mail: gc238@cornell.edu. c) Electronic mail: fred.manby@bris.ac.uk. d) Electronic mail: martin.schuetz@chemie.uni-regensburg.de. e) Electronic mail: werner@theochem.uni-stuttgart.de. times also called resolution of the identity] (Refs. 3 15) are obvious examples, but methods which define new occupied and virtual orbital sets, such as the projected atomic orbital (PAO), frozen natural orbital, and pair natural orbital (PNO) (Refs ) methods, can also be understood in this mathematical language. PAO methods define a single global set of occupied and virtual orbitals for correlation, while PNO methods define an adapted set of virtual orbitals for every pair of correlated occupied orbitals. We recently described a tensor factorization that lies between the PAO and PNO methods, the orbitalspecific virtual (OSV) method. 1, 34 The OSV factorization associates a set of virtual orbitals with each occupied orbital ( orbital specific ) rather than to each orbital pair ij as in the PNO method. This leads to simplifications relative to the PNO method, in particular in the integral transformation. The adaption of the virtual orbitals to the occupied space provides a more compact description of correlation space with OSVs than with PAOs, which in most cases leads to computational savings if one aims at the same accuracy. More importantly, the OSV scheme allows to improve the accuracy systematically based on a single parameter, and at least in principle the canonical result can be approached smoothly. Other theoretical improvements offered by the OSV method include the recovery of smooth potential energy surfaces (without the need for unphysical smoothing schemes ) even for modest numbers of OSVs, particularly when fully optimized OSVs are used. 34 It should be noted, however, that there are also disadvantages relative to PAOs, such as higher memory requirements and less straightforward generalization to openshell cases /2012/136(14)/144105/16/$ , American Institute of Physics
3 Yang et al. J. Chem. Phys. 136, (2012) The orbital-specific-virtual local coupled cluster singles and doubles (OSV-LCCSD) ansatz improves the treatment of the virtual space only, and without further approximations still exhibits a relatively steep computational scaling [mostly O(m 3 ), some terms scale even as O(m 4 )] with respect to the number m of occupied orbitals. This problem is exactly as in other local correlation methods. 16, 17, 38, 39 One way to avoid this is to use local pair approximations, as first introduced by Pulay and used in many later PAO methods. 12, 15, Local pair approximations provide a simple way to reach linear scaling and to extend OSV-LCCSD to large systems. However, as pointed out in previous work, 15, 46, 47 the application of local approximations requires a careful balancing of errors (e.g., local pair error versus domain error) and in the current work we reconsider these issues in the context of the OSV method. The work presented in this paper can be separated into three parts. First, we will outline the theory. We will then investigate the performance of the OSV method without pair approximations. Here, we primarily contrast the compactness and cost of OSV-LCCSD with that of PAO-LCCSD, and study the accuracy and computational cost requirements as a function of the number of OSVs. In the third part, pair approximations are introduced and their impact on the accuracy and efficiency is demonstrated. Finally, we present some applications that demonstrate the applicility of the method to real problems. II. THEORY In this section, we first briefly introduce the coupled cluster equations in order to define the relevant quantities and notation. Subsequently, we will discuss various choices of virtual orbitals and introduce the OSV-LCCSD method. In the following indices i, j, k, l will denote localized occupied molecular orbitals (LMOs), and a, b, c, d canonical virtual orbitals (VMOs). It will be assumed that the occupied orbitals are orthonormal and that the occupied and virtual orbital spaces are mutually orthogonal, i.e., i a = 0 a, i. Other choices of virtual orbitals will be denoted by indices r, s, t, u. A. Definition of the CCSD wavefunction The CCSD wavefunction in an orthonormal orbital basis is defined as = e ˆT 0, (1) where 0 is the closed-shell Hartree-Fock Slater determinant, and ˆT = ˆT 1 + ˆT 2 is the singles and doubles cluster operator ˆT 1 = t aêa i i, (2) i a Ê a i ˆT 2 = 1 2 T ij Êa i Êb j. (3) i,j are spin-summed one-electron excitation operators, and ta i, T ij are the singles and doubles amplitudes, respectively. These quantities can be considered as second- and fourthorder tensors, respectively. However, since in local treatments with pair approximations the list of pairs ij is very sparse, we prefer to denote them as vectors and matrices, respectively, where the superscripts denote different matrices (upper case quantities) or vectors (lower case quantities), and the subscripts their elements. Such vectors and matrices will be written in bold face if reference to the individual elements is not needed, e.g., T ij = [Tij ]. The elements of such matrices always correspond to virtual orbitals. Since Êi a and Êj b commute, T ij = T ji ba. The amplitudes are determined by solving the CC amplitude equations ra i = a i e ˆT HeˆT ˆ 0 = 0 a,i, (4) = ij e ˆT HeˆT ˆ 0 = 0 i j,a,b. (5) R ij The quantities ra i and Rij are called residual vectors and matrices, respectively. They vanish for the optimized amplitudes. The (contravariant) configurations a i and ij are defined as a 1 i = 2Êai 0, (6) 1( ij = 2Ê i a 6 Êb j + ) Êa j Êb i 0. (7) They have the property that ta i = a i, (8) where = ij, (9) C ij C ij = T ij + t a i t j b. (10) The choice of projection functions in Eqs. (6) and (7) leads to the most compact form of the CCSD equations For convenience in later expressions we also define the corresponding contravariant doubles amplitudes T ij ij = 2T T ji. (11) The two-electron integrals can be represented by matrices and vectors as well, for example J ij = ( ij ), (12) K ij = (ai bj ), (13) L ij = 2Kij Kij ba. (14) In terms of these quantities, the CCSD correlation energy is E corr = ij C ij Lij = (2 δ ij )tr[c ij L ji ]. (15) i j
4 Yang et al. J. Chem. Phys. 136, (2012) B. General transformations for the virtual orbitals We now consider transformations of the CCSD equations to different virtual orbital representations. The new orbitals, which may in general be non-orthonormal, will be leled by indices r, s. In general, different orbital sets can be defined for each pair ij, and this will then be indicated by superscripts, r ij = a The amplitudes transform as t i a = r T ij = rs a Q ij ar. (16) Q ii ar t i r, (17) Q ij ar T ij rs Qij bs. (18) Single excitations from an LMO i are made into the same virtual orbitals as for the diagonal double excitation (i = j) from the same LMO. Inserting these expressions into the CCSD residual equations and transforming the residuals to the new basis, i.e., r i r = a R ij rs = r i a Qii ar, (19) Q ij ar Rij Qij bs, (20) yields equations in which all integrals and amplitudes are in the new virtual basis. They differ formally from the equations in an orthonormal virtual basis only by the multiplications with the overlap matrix, r ij s kl =[S ij,kl ] rs = [Q ij Q kl ] rs, (21) in all places where an amplitude index is not matched by an integral lel. The explicit form of the resulting equations can be found in Appendix B of Ref. 15. So far, there is no advantage of these transformations. However, if the virtual orbitals are suitly chosen, the number of amplitudes and residual equations can be strongly reduced by introducing domain approximations, t i a r [i] T ij rs [ij ] Q ii ar t i r, (22) Q ij ar T ij rs Qij bs. (23) The subset of orbitals r ij used to approximate the amplitude matrix T ij is denoted as pair domain [ij]. The domains [i] for single excitations correspond to the pair domains of the diagonal pairs, i.e., [i] = [ii]. The residual equations have then to be solved only for the same domains r i r = 0 r [i], (24) Rrs ij = 0 r, s [ij ], (25) and the correlation energy is given by E corr = (2 δ ij ) i j rs [ij ] C ij rs Lij rs. (26) For large molecules, the domain approximation leads to a strong reduction of the computational effort and its scaling with molecular size. Note that the domain approximation involves only the virtual lels r, s in the tensor quantities. Pair approximations allow us to use different levels of theory based on the occupied lels i, j. For example, only strong pairs, which contribute most to the correlation energy, are included in the LCCSD; the remaining weak or distant pairs are either approximated by LMP2 or neglected. It is then possible to achieve linear scaling of the computational effort with molecular size. 15, 40, 43, 51 We discuss pair approximations further in Sec. II H. The important point is that the convergence of the correlation energy and other molecular properties as a function of domain sizes depends crucially on the choice of the transformation matrices Q ij. In Subsections II C II E, we will discuss three different choices and their implications on the computational efficiency. C. Projected atomic orbitals (PAOs) Pulay 16 suggested spanning the virtual orbital space by projected atomic orbitals, r = a a Q ar, (27) Q ar = a χr AO, (28) where χr AO are atomic orbitals (AOs). Usually, the AOs are taken to be the contracted Gaussian type orbitals (CGTOs), and then each PAO is associated to a CGTO. For example, the local correlation methods of Pulay and Saebø and of Werner, Schütz and co-workers 12, 15, 40 46, are based on PAOs. The PAOs are local by construction, pair-independent and nonorthogonal r s =[S] rs = [Q Q] rs. (29) The standard way to select domains in the PAO-LCCSD method is either to use the method of Boughton and Pulay 56 (BP) or natural population analysis (NPA). 53, 57 Both methods can be used with any localization scheme, e.g., Pipek- Mezey 58 53, 59 (PM) or natural localized orbitals (NLMOs). Unless otherwise noted, we will use the NLMO/NPA method 53 in the current paper. The domain selection with the BP or NPA methods depends on thresholds l bp and l npa, respectively. l bp is a completeness criterion, and with l bp = 1 domains that span the full virtual space are obtained. l npa refers to the natural charge of a center in a given orbital, and all centers are included which have charges larger then l npa. In the current work we use l npa = Smaller values yield larger domains, and in this case l npa = 0 gives full domains. However, when these thresholds are close to 1 or 0, respectively, the domains may become unphysical, and therefore a variation of these thresholds is not very suitle to approach the canonical limit systematically.
5 Yang et al. J. Chem. Phys. 136, (2012) One way to overcome this problem is to use the BP or NPA methods just to determine standard (or primary ) domains, which include the most important atoms for each orbital and usually correspond to chemical intuition. The accuracy can be improved by extending the domains by adding all PAOs at shells of neighboring centers. 47 The fraction of correlation energy then converges quickly towards 100%. The disadvantage of this method is, however, that it is quite coarse grained and the domains grow rapidly. In order to achieve a more fine-grained variation of the domain sizes we have adopted an approach that is based on contributions of individual centers to the correlation energies of the diagonal pairs ii (similar to the OSV case, see later). Initially, an LMP2 calculation is carried out, in which the domains of the diagonal pairs are extended by several shells of neighboring atoms. The energy contributions of individual centers A to the pair energy ɛ ii are then evaluated as ɛii A = r [A] s T ii rs Kii rs, (30) where the sum over r is restricted to PAOs at center A. Equivalent to this would be to partition the pair energy to contributions ɛii AB, and to assign half of these to the centers A and B. The orbital domains [i] include all centers that yield energy contributions larger than an energy threshold l pao. Unfortunately, due to the non-orthogonality of the PAOs, this selection procedure slightly depends on the domains used in the initial LMP2. In the current work, we have used complete domains for the diagonal pairs, and standard domains for the remaining pairs. Pair domains [ij] are then taken to be the union of the orbital domains [i] and [j]. As will be shown in Sec. III, on the average typically ( ) PAOs per pair are needed to recover around 99% (99.8%) of the canonical correlation energy for an augmented triple-ζ basis set. These domain sizes grow linearly with the size of the basis set per atom. However, the domain sizes are (asymptotically) independent of the molecular size. D. Pair natural orbitals (PNOs) Much better convergence of the correlation energy as a function of the domain sizes can be achieved with pairspecific virtual orbitals. An excellent choice is to use MP2 pair natural orbitals, where Q ij is defined by diagonalizing the MP2-like density matrix D ij = 1 ( T ij T ij + T ij T ij ), (31) 1 + δ ij [ Q ij D ij Q ij ] rs = nij r δ rs, (32) for pair ij. The amplitudes in Eq. (31) are computed as T ij = K ij ɛ a + ɛ b f ii f jj. (33) Here the virtual orbitals are assumed to be canonical, i.e., f = ɛ a δ, and f ii are the diagonal elements of the Fock matrix in the LMO basis. The domain [ij] can then be determined by neglecting orbitals that have natural occupation numbers n ij r below a certain threshold. 60 This is the approach used by Neese and co-workers The PNOs for a given pair ij are orthonormal, but PNOs of different pairs are non-orthogonal. Using this ansatz one typically needs only PNOs per pair in order to recover 99.8% of the canonical CCSD correlation energy (again for a triple-ζ basis set and independent of the molecular size). However, a severe disadvantage of the PNO method is that the total number of virtual orbitals may become very large; for example, if 1000 pairs ij are correlated, one needs out virtual orbitals. This leads to difficulties in the integral transformations and storage of the integral matrices unless drastic approximations are used, as described in Sec. II F. E. Orbital specific virtuals (OSVs) Recently, Yang et al. 1 have proposed orbital-specific virtual orbitals as a compromise between the pair-specific PNOs and the pair-independent PAOs. In this case, a set of virtual orbitals is associated with each LMO. An excellent choice is to generate the OSVs by singular value decomposition (SVD) of the diagonal MP2 pair amplitudes, [Q i T ii Q i ] rs = tr ii δ rs, (34) r i = a Q i ar. (35) a The amplitudes T ii are approximated according to Eq. (33). Since the diagonal amplitude matrices are symmetrical, the left and right singular vectors are identical, and SVD is equivalent to diagonalization of T ii. The OSVs r i are also identical to the PNOs r ii, and n ii r = (tr i)2. Based on the magnitude of the eigenvalues tr ii or of the occupation numbers n ii r, a domain [i] of OSVs can be selected for each LMO i. Alternatively, here we will use an energy criterion. The diagonal MP2 pair correlation energies are written as ɛ ii = k ii r T ii Kii = r t ii r kii r, (36) = Q i ar Kii Qi br, (37) and as many orbitals r i are included in the domain [i] as needed to make the error of r [i] t r iikii r relative to the exact pair energy smaller than a threshold l osv (the orbitals are ordered according to decreasing tr ii). Note that l pao and l osv are not directly comparle, as the former is a threshold on the contribution of a center and its set of PAOs to the diagonal pair energy, while the latter is a threshold on the contribution of a single orbital to the diagonal pair energy. Consequently, for the same error in ɛ ii, l pao will typically be larger than l osv. As in PAO methods, pair domains [ij] are then formed as the union of the orbital domains [i] and [j]. Thus, the transformation matrix Q ij ar that generates the pair domain from the canonical orbitals can be written in block form as ( Q ij ) = ( Q i Q j ), (38)
6 Yang et al. J. Chem. Phys. 136, (2012) which indicates that the columns of Q i are collated with those of Q j. The canonical amplitudes can then be approximated as in Eqs. (22) and (23). The OSVs for a given LMO are orthonormal, but OSVs for different LMOs are non-orthogonal. Thus, the overlap matrix S ij, ij for a pair domain is block diagonal. However, this sparsity is not exploited in our current implementation. It should be noted that the orbitals r ij in a pair domain [ij] may become (nearly) linear dependent. Such linear dependencies are removed by diagonalizing S ij, ij and removing eigenvectors that correspond to very small eigenvalues. This is exactly as in the PAO case and technical details can be found in Ref. 15. As will be demonstrated in Sec. III, typically 100 OSVs per pair are needed to recover 99.8% of the canonical correlation energy. This is one third to one half of the number of PAOs required for the same accuracy, but out twice as many PNOs. The advantage of using OSVs rather than PNOs is that the total number of virtual orbitals is very much smaller. Finally, it should be noted that the generation of the OSVs scales as O(N 4 ), where N is a measure of the molecular size (e.g., the number of correlated electrons). This scaling is steeper than of all other terms in an OSV-LCCSD calculation, but since efficient density fitting methods are used to generate the necessary integrals K ii this step did not present a bottleneck in any of the calculations presented in this paper. F. The OSV-LCCSD residuals In this section, we will discuss the solution of the coupled-cluster equations and the required integral transformations when using OSVs. Our implementation of the OSV- LCCSD method is based on the DF-LCCSD method that has recently been described by two of us, 15 and that is part of the MOLPRO program package. 61, 62 Formally, the OSV-LCCSD equations are exactly the same as given in Appendix B of Ref. 15 for PAO-LCCSD. However, larger integral and overlap matrices are needed. In order to illustrate this, we consider a typical contribution in the doubles residual, R ij [ij,ij ] =... k Y kj [ k, j] = Kkj [ k, j] S [ij,ik] T ik [ik,ik] Ykj [ik,ij], (39) [ ] L kl lj T [ k,lj] [lj,lj ] S [ j, j] +... l (40) The first and second lels in square brackets indicate the domains of the rows and columns of the matrices, respectively, and obviously these must match in the matrix multiplications. This makes it necessary to use gather operations to extract the appropriate blocks from the overlap and integral matrices. For example, in order to evaluate Eq. (39), the block [ij, ik] isextracted from the full overlap matrix S, and the block [ik, ij] is extracted from the intermediate matrix Y kj. Since Y kj can be used to compute all residuals R ij for a fixed j, it is computed in the united domains [ k, j]. The united domain [ k] is the union of all pair domains [ik] that share the same k, and the united domain [ j] is the union of all [ij] for fixed j. Thus, the blocks Y kj [ik,ij] can be extracted from the larger matrix Ykj for all [ k, j] i. Note that for large molecules the united domains are independent of the molecular size (if pair approximations are applied), and therefore linear scaling is automatically achieved for these terms. In Eq. (40) each term in the summation over l involves a different domain [lj]. Therefore, the block L kl must be [ k,lj] extracted from the integral matrix L kl, and the matrix product must be added to the appropriate blocks in Y kj. The result in [ k, j] square brackets has then dimension [ k, j] and is finally multiplied with S [ j, j]. The matrix multiplications in the residual equations can be carried out in various possible orders. For example, one could also evaluate the second term of Y kj as [ ] + 1 L kl lj T 4 [ k,lj] [lj,lj ] S [lj, j], (41) l i.e., the multiplication with S is now done within the loop over l. However, the number of operations is in this case larger than Eq. (40) since the union of all [lj](forfixedj) equals the union of all [l], while the sum of all dimensions of [lj] is larger (since it contains [j] repeatedly). Therefore Eq. (40) is used. Similar considerations apply for other contributions to the residuals. In our program, the whole overlap matrix S of all OSVs is kept in memory. The required blocks are obtained when needed by gather operations as described ove. The total dimension of the overlap matrix is in i, where n i is the number of OSVs for LMO i. On the average, n i 50 in order to recover 99.8 % of the correlation energy. Thus, in a calculation with 100 correlated LMOs the dimension of S is out Note that this dimension can be larger than the number of virtual orbitals and depends on the OSV selection threshold l osv. For example, in the polyglycine (Gly) 8 calculation that will be presented in Sec. III there are 92 correlated LMOs, 1757 VMOs, and in total 2819 (l osv = ) or 3855 (l osv = ) OSVs. In contrast, in a PAO calculation the dimension of the overlap matrix can never be larger than the number of basis functions (in our example this is 1882). Similar considerations hold for the integral matrices J kl and K kl. If all pairs are included in the LCCSD, one needs all m(m + 1)/2 matrices of each type in the full OSV basis, where m is the number of correlated LMOs. The number of matrices, as well as their dimensions, are reduced if pair approximations are introduced, i.e., if weak pairs are approximated by MP2 (cf. Sec. II H). Then i, j, k, l must all be within a finite distance, since i is close to j through pair ij; i close to k though pair ik; and j close to l through pair jl. Using such considerations one can form operator lists and operator domains, as discussed previously for PAO-LCCSD. 43 Despite the fact that more PAOs than OSVs are needed to reach a certain accuracy, one usually needs more integrals J kl and K kl for OSV-LCCSD than for PAO- LCCSD (in particular for small values of the threshold l osv ). This means that in most cases the reduced CPU-time of the OSV-LCCSD iterations comes at the expense of greater memory requirements.
7 Yang et al. J. Chem. Phys. 136, (2012) This situation is even much more pronounced in the PNO-LCCSD method. Even though one needs only out 40 PNOs per pair to recover 99.8% of the canonical correlation energy, the overlap and integral matrices in the basis of all PNOs would be huge. If no pairs were neglected the total dimension of the overlap matrix would for the ove example be ( )/2 = In practice, one can approximate weak pairs by LMP2, and then only out 1000 pairs need to be included in the LCCSD. But the total dimension of S would still be (out 6 GB if stored in triangular form). Again, similar considerations hold for the integral matrices J kl, K kl, and it would obviously be quite impossible to store them. In order to overcome this problem, Neese et al. 32 have introduced some rather drastic approximations: in some terms the operators K kl [ik,lj] are projected onto the domain [kl, kl], i.e., K kl [ik,lj] S [ik,kl]k kl [kl,kl] S [kl,lj]. (42) These approximations introduce errors which eliminate some of the advantages of the systematic convergence achievle using PNOs. Alternatively, the integrals are stored in the canonical molecular orbital (MO) basis and transformed on the fly into the PNO basis when needed. As the canonical MO basis is involved, this loses the local scaling. 63 G. OSV-LCCSD integrals The computation of two-electron integrals in the OSV representations forms a large part of the cost of the OSV- LCCSD method. All required integrals are computed by DF approximations as described in Ref. 15. However, somewhat different restrictions to the virtual orbital lels apply. In the PAO-LCCSD method the necessary integrals are defined 14, 15, 45 by quadruplets of centers (for PAOs) and/or LMOs. Since the OSVs are not related to centers but only to LMOs, center lels are now replaced by LMO lels. Local density fitting approximations as described previously for PAO methods 12, 15, 64 should be possible for OSVs as well, but have not yet been implemented in our program. We first consider the contributions of integrals over four OSVs (in the following denoted 4-ext integrals): R ij rs += t,u [ij ] (rt su)c ij tu r, s [ij ]. (43) All four lels r, s, t, u are associated to the same pair domain [ij]. Consequently, it is sufficient to generate the 4-ext integrals where r, s, t, u are OSVs for LMOs i or j. Thus, there are only four integral classes, namely (r i t i s i u i ), (r i t j s i u j ), (r i t i s j u j ), and (r i t i s i u j ). The total number of unique 4-external integrals is approximately (7N p /4 13m/8)L 4, where L is the average number of OSVs per LMO, m is the number of correlated LMOs, and N p is the number of pairs included in the LCCSD. Since both m and N p are proportional to the molecular size and L is independent of the molecular size the total number of integrals scales linearly with molecular size. Similar considerations apply to the 3-external integrals (rs tk). In this case r, s, t must be OSVs for the LMOs i, j,ork. In addition, ij and ik or jk must be strong pairs (see Appendix BofRef.15 for more details). It follows that the number of 3-external integrals scales linearly as well (provided distant pairs are neglected). Lastly, 0-ext, 1-ext, and 2-ext integrals appear in a number of different contractions with the singles and doubles amplitudes. In most terms the LMO lels are related by strong pair conditions, as, e.g., discussed in Sec. II F. There are a few terms, however, where ij and kl are not directly related. For example, this is the case for the contribution with R ij rs += kl α ij,kl = K ij kl + r,s [ij ] α ij,kl [SC kl S] rs, (44) Crs ij Kkl rs + tr i (rk lj ) + r [i] r [j] tr j (rl ki). (45) However, the sum over kl can be restricted since the integrals (ik jl), (rk lj), and (rl ki) decay exponentially with the distance of i and k or j and l. Furthermore, the integrals (rk ls) become small if the r, s [ij] are far from k, l. Finally, the overlap integrals in Eq. (44) become also small if ij is far from kl. Similar considerations apply to some other terms. In the current work, we use exactly the same restrictions as described in detail in Ref. 43. Overall, the generation and storage of the 3-ext and 4-ext integrals is the major bottleneck of OSV-LCCSD. As will be shown in Sec. III, the total number of these integrals depends crucially on the threshold l osv. Depending on this threshold, the number of integrals and the computational effort may be smaller or larger than for PAO-LCCSD. H. Local pair approximations If all pairs are included in the LCCSD, the CPU time as well as the disk space scale formally as O(N 4 ). However, both can be reduced to linear scaling by introducing pair approximations. In principle, it would be sufficient to neglect very distant pairs which have negligible contributions to the correlation energy. However, the cross-over point to low-order scaling then occurs only for quite large molecular sizes and will not be reached in most applications involving mediumsize molecules ( atoms). In the past, additional approximations were therefore introduced for weak pairs, 15 17, 40, 43 which have small but non-negligible contributions to the correlation energy. The current work follows the earlier developments. We classify the orbital pairs according to the distance of the atoms that contribute to the primary domains. This can be done either by distance or connectivity criteria. Here we use the latter, in which the pair classes depend on the minimum number of bonds between any atom in domain [i] and any atom in domain [j]. We distinguish strong, close, weak, and very distant pairs. The latter are entirely neglected. The amplitudes of strong pairs are fully optimized by LCCSD, while the remaining amplitudes are determined by LMP2. The weak pair approximation usually leads to an overestimation of the correlation energy. 15, 47 In most cases this is not caused by an overshooting of the LMP2 weak pair
8 Yang et al. J. Chem. Phys. 136, (2012) correlation energies, but due to the neglect of the weak pair amplitudes in the LCCSD equations for the strong pairs. The overshooting due to the pair approximation partly compensates the error caused by the domain approximation (domain error), since the latter reduces the correlation energies. This error compensation is favorle if one uses standard PAO domains and medium size basis sets. However, if the accuracy of the domain approximation is improved by extending the domains, using OSVs, or by including explicitly correlated terms in the wavefunction, 46 the error compensation is lost and the error of the pair approximation dominates. It is then necessary to include more pairs in the LCCSD. Furthermore, the error can be significantly reduced with small additional cost by including the LMP2 close pair amplitudes in the 15, 46 LCCSD residual equations for the strong pairs. If connectivity criteria are used, the pair approximation can be specified by three integers w, c, and k. w and c specify the minimum number of bonds between pairs of orbitals that form weak and close pairs, respectively. k = 1 means that close pairs are included in the LCCSD residuals for the strong pairs. For k = 0 this is not done, and in the sence of triple excitations there is then no difference between close and weak pairs. The default for standard LCCSD calculations with triple-ζ basis sets is wck = 210. This means that in strong pairs the two orbital domains must share at least one atom, close pairs are separated by 1 bond, and weak pairs are separated by at least 2 bonds. Very distant pairs are neglected if the distance between the two orbitals exceeds 15 a 0.IfMP2 corrections are applied or explicitly correlated wavefunctions are used, the lists of strong and close pairs must be increased, and wck = 321 has been recommended as a good compromise 15, 46 between accuracy and cost. One may very well argue that the use of distance criteria is incompatible with the goal of avoiding physically motivated ad hoc approximations in the OSV-LCCSD method. The main reason for still employing the same distance criteria as in the previous work was to be le to directly compare the OSV and PAO values to previous results, using exactly the same pair approximations. One could equally well determine the pair classes solely on the basis of LMP2 pair energies, so that no definitions of distances or bonds between LMOs would be necessary any more. It would then be possible to control the whole calculation by a single energy threshold. III. BENCHMARK CALCULATIONS In this section, we will investigate the dependence of the LCCSD correlation energy and the computational cost as a function of the domain sizes, using PAOs or OSVs. In order to isolate the effect of the domains, we begin with calculations in which all pairs are treated at the LCCSD level (Sec. III A). Additional pair approximations will be considered in Sec. III B. A benchmark for reaction energies will be presented in Sec. III C, and applications to larger molecular systems in Sec. IV. In the following, we will first investigate the convergence of the correlation energies as a function of the thresholds l pao and l osv towards the canonical CCSD limit obtained with the same basis set. We will denote basis sets consisting of ccpvxz for hydrogen atoms and aug-cc-pvxz for other atoms as avxz. It will be shown that this convergence can be much 15, 47 improved by adding an MP2 domain correction E CCSD E LCCSD + E MP2 E LMP2, (46) where all energies are computed with the same basis set. In the following, we denote this as MP2 correction. One might argue that the calculation of the canonical MP2 energy E MP2 leads to O(N 5 ) scaling and might therefore dominate the computational cost in large molecules. However, the DF- MP2 method is very efficient and well parallelized. For example, the DF-MP2 calculation for (Gly) 10 (2334 basis functions, 228 correlated electrons) took 429 minutes elapsed time on a single core. Using 12 cores and 2 Nvidia C2070 graphics processor units on the same machine, this can be reduced to just 11.5 min elapsed time (without HF). With increasing domain sizes, the ove procedure should converge to the canonical CCSD limit for a given basis set. However, the basis set error is often larger than the domain error, and the goal should be to approach the CCSD complete basis set (CBS) limit. This can be achieved by replacing, say a triple-zeta MP2 energy by the CBS limit, i.e., E CCSD/CBS E LCCSD/aVTZ + E MP2/CBS E LMP2/aVTZ. (47) This corrects both for domain and basis set incompleteness errors. The performance of this approximation for reaction energies will be investigated in Sec. III C. We note that the expensive MP2 extrapolations could be avoided by using the explicitly correlated LMP2-F12 method, 64 which scales much lower with molecular size and should be at least as accurate as avqz/av5z extrapolated MP2 values. A. Dependence of the correlation energy and computational cost on the domain sizes As test examples we have chosen a subset of the molecules used by Neese et al. 32 in their benchmarks of the PNO-LQCISD and PNO-LCCSD methods, namely pyrazole, 2-hydroxypyridine, cyclooctatetraene, neopentane, vinyl acetate, and vinylcyclopropane. All the geometries were obtained based on MP2/VTZ optimizations. 65 In all cases the avtz basis set was used. The corresponding avtz/mp2fit basis sets of Weigend et al. 66 were used in the density fitting for all integrals except for the 4-external ones. As shown in Ref. 15, the cardinal number should be increased by one for the latter integral class in order to keep the fitting error on the solute correlation energies small. For example, in the case of pyrazine the correlation energies are overestimated by out 0.05% when the avtz/mp2fit fitting sets are used for the 4-external integrals. Thus, we have used the avqz/mp2fit sets for these integrals, and then the fitting errors are negligible. The reference CCSD calculations did not involve any density fitting approximations, but in the MP2, LMP2, and LCCSD calculations all integrals were obtained by density fitting.
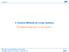
9 Yang et al. J. Chem. Phys. 136, (2012) TABLE I. Average pair domain sizes AVD (including redundant functions), correlation energies (in E h ), computation times (in min), and file sizes (in GB) for various molecules and domain selection thresholds. The percentage of correlation energy relative to the canonical CCSD value is given in parenthesis. All pairs are included in the LCCSD. Basis set: hydrogen atoms cc-pvtz, other atoms aug-cc-pvtz. Calculations were carried out on a single core Xeon 3.47 GHz. Timings for the complete LCCSD calculations (including integral evaluation and transformations, 10 iterations). Correlation energies Timings File sizes Molecule AVD LMP2 LCCSD LCCSD+ MP2 CPU WALL 3-ext. 4-ext. OSV, l osv = Vinylcyclopropane (98.90) (100.31) Pyrazole (98.88) (100.32) Neopentane (98.73) (100.37) Vinylacetate (98.95) (100.20) hydroxypyridine (98.75) (100.32) Cyclooctatetraene (98.72) (100.38) OSV, l osv = Vinylcyclopropane (99.53) (100.11) Pyrazole (99.53) (100.10) Neopentane (99.45) (100.14) Vinylacetate (99.53) (100.05) hydroxypyridine (99.49) (100.09) Cyclooctatetraene (99.44) (100.13) OSV, l osv = Vinylcyclopropane (99.79) (100.02) Pyrazole (99.80) (100.01) Neopentane (99.76) (100.04) Vinylacetate (99.78) (99.99) hydroxypyridine (99.76) (100.01) Cyclooctatetraene (99.74) (100.02) PAO,l pao = Vinylcyclopropane (98.83) (100.14) Pyrazole (99.05) (100.11) Neopentane (98.66) (100.16) Vinylacetate (99.21) (100.06) hydroxypyridine (98.94) (100.11) Cyclooctatetraene (98.63) (100.20) PAO,l pao = Vinylcyclopropane (99.14) (100.10) Pyrazole (99.75) (100.02) Neopentane (99.23) (100.09) Vinylacetate (99.61) (100.03) hydroxypyridine (99.56) (100.04) Cyclooctatetraene (99.06) (100.09) PAO,l pao = Vinylcyclopropane (99.69) (100.03) Pyrazole (99.85) (100.01) Neopentane (99.47) (100.07) Vinylacetate (99.81) (100.01) hydroxypyridine (99.84) (100.01) Cyclooctatetraene (99.72) (100.03) The results for the six test molecules are presented in Tle I, which shows the convergence of the correlation energy and of the computational resources (CPU and elapsed times, disk space) as a function of the domain selection thresholds l osv and l pao for OSV-LCCSD and PAO-LCCSD, respectively. As an example, the convergence of the OSV- LCCSD and OSV-LCCSD+ MP2 correlation energies as a function of the domain sizes are shown for pyrazole in Fig. 1. For all molecules one can observe that much smaller domains are sufficient with OSVs than with PAOs to achieve a certain accuracy of the correlation energy. Typically, with OSVs 99.5% of the correlation energy is recovered with average pair domain sizes of (l osv = ). For the same accuracy, the PAO domains need to be 2 3 times larger (l pao ). If the MP2 domain correction is added, the total correlation energies overestimate the canonical limit, indicating that the domain error is somewhat larger for LMP2 than for LCCSD. This overshooting is more pronounced with OSVs
10 Yang et al. J. Chem. Phys. 136, (2012) Percent of canonical correlation energy OSV-LCCSD+ΔMP2 OSV-LCCSD Average domain size FIG. 1. Fraction of the OSV-LCCSD and OSV-LCCSD + MP2 correlation energies relative to the CCSD value as a function of the average domain size for pyrazole (basis avtz, see text). No pair approximations are applied. than with PAOs for small domains. Already with the smallest PAO domains considered here (l pao = ), the MP2- corrected error is below 0.2% of the canonical correlation energy (typical domain sizes 140). For a comparle accuracy an OSV threshold of l osv = is required (average domain size 85). It is very satisfying that the average OSV domain sizes as well as the percentage of correlation energy for a given threshold are very similar for all molecules. In the PAO case the fraction of correlation energy varies considerly more, since the domain selection is less fine grained. We now consider the computation times and the required disk space. Because of the smaller pair domain sizes, the OSV calculations can be faster than the PAO calculations for a similar accuracy. For example, for an accuracy of 99.5% in the correlation energy (l pao = and l osv = ), the OSV calculations are up to out a factor of two faster. However, it is obvious that there must be a cross-over point between PAOs and OSVs at some accuracy. This is due to the fact as we approach higher accuracies the total space of OSVs (for all LMOs) eventually becomes larger than the number of virtual orbitals, and therefore the number of required transformed integrals strongly increases with decreasing OSV threshold. With PAOs the number of integrals becomes independent of the threshold much earlier (in fact, the number of 3-external integrals is independent of the threshold if all pairs are included). This is because in the PAO case different pairs share the PAOs at certain centers, and the minimum number of 4-external integrals is determined by center quadruplets (AB CD), where the PAOs at all four centers A, B, C, D must belong to the same pair domain. 15, 43 In the OSV case this is replaced by the condition that the four OSVs for a pair ij belong either to orbital i or to orbital j, i.e., the smallest number of OSVs that can be shared by different pairs is determined by the number of OSVs per orbital, cf. Sec. II G. The ove also means that with decreasing energy thresholds the I/O times grow more rapidly for OSV-LCCSD than for PAO-LCCSD. This can be observed, e.g., for cyclooctatetraene or 2-hydroxypyridine, where for the lowest thresholds the difference between the elapsed and CPU times is much larger in the OSV calculation. It should be noted that the calculations were carried out on a machine with rather large memory (96 GB) and fast file systems (two RAID0 file systems with a bandwidth of out 300 MB/s each). If a machine with less memory and slower I/O would be used, the elapsed times would be more dominated by the I/O overhead, and this would change the relative timings for small thresholds in favor of the PAO method. B. Dependence of the correlation energy and computational cost on pair approximations The ove calculations included all pairs. A very significant speedup can be achieved by pair approximations. Tle II shows the results for the same molecules and domain selection thresholds as used in Tle I, but now including pair approximations (wck = 321). As expected, the savings by the pair approximations increase with molecular size. In the case of cyclooctatetraene both the timings as well as the file sizes are reduced by out a factor of two. Clearly, the savings would be more pronounced for larger molecules. As for the calculation with all pairs, the OSV results for a given threshold yield more consistent fractions of the canonical correlation energies than the PAO ones. It can also be seen that the pair approximations lead to a slight overestimation of the correlation energies, as discussed in Sec. II H. Note that the LMP2 correlation energies are smaller than the LCCSD ones for all six test molecules, and therefore the overestimation is not due to an overestimation of the LMP2 weak pair energies. Tle III shows the percentage of correlation energy recovered by OSV-LCCSD and PAO-LCCSD relative to canonical CCSD calculations for glycine polypeptides (Gly) n, n = 1 4 (with our computational resources, larger calculations are not possible with canonical CCSD). The basis set and other computational details are the same as in Sec. III A. The results for different choices of the weak pair parameters wck demonstrate again the overestimation of the correlation energies by the pair approximations. Comparison of the calculations for wck = 210 and wck = 211 shows that this is significantly reduced by setting k = 1 (cf. Section II H). It is further reduced if the close and weak pair parameters are increased. For the larger peptides, the percentage of correlation energy that is recovered for each choice of wck is nearly independent of the molecular size. This means that the solute error is proportional to the molecular size. However, in most situations this error will cancel to a large extent when energy differences are considered. This is similar to the basis set error on the correlation energies, which also increases with molecular size. The dependence of the computational resources on the molecular size is demonstrated in Tle IV. As before, the avtz basis set was used and the weak pair parameters were varied. The scaling of the OSV-LCCSD and PAO-LCCSD calculation as a function of the molecular size is very similar, and therefore the larger calculations have only been done with OSV-LCCSD. Since wall-clock (elapsed) times depend strongly on the machine configuration (memory, disk), we only report pure user-cpu times. The disk space given is the maximum used at any stage of the calculation. The
11 Yang et al. J. Chem. Phys. 136, (2012) TABLE II. Average pair domain sizes AVD (including redundant functions), correlation energies (in E h ), computation times (in min), and file sizes (in GB) for various molecules. The percentage of correlation energy relative to the canonical CCSD value is given in parenthesis. Pairs are classified using wck=321 (see text). Basis set: hydrogen atoms cc-pvtz, other atoms aug-cc-pvtz. Calculations were carried out on a single core Xeon 3.47 GHz. Timings for the complete LCCSD calculations (including integral evaluation and transformations, 10 iterations). Correlation energies Timings File sizes Molecule AVD LMP2 LCCSD LCCSD+ MP2 CPU WALL 3-ext. 4-ext. OSV, l osv = Vinylcyclopropane (98.94) (100.35) Pyrazole (98.90) (100.34) Neopentane (98.78) (100.41) Vinylacetate (99.01) (100.26) hydroxypyridine (99.80) (100.38) Cyclooctatetraene (98.95) (100.61) OSV, l osv = Vinylcyclopropane (99.58) (100.15) Pyrazole (99.56) (100.12) Neopentane (99.50) (100.19) Vinylacetate (99.59) (100.11) hydroxypyridine (99.55) (100.16) Cyclooctatetraene (99.69) (100.38) PAO,l pao = Vinylcyclopropane (98.88) (100.19) Pyrazole (99.08) (100.14) Neopentane (98.71) (100.22) Vinylacetate (99.28) (100.13) hydroxypyridine (99.01) (100.18) Cyclooctatetraene (98.88) (100.46) PAO,l pao = Vinylcyclopropane (99.19) (100.15) Pyrazole (99.78) (100.05) Neopentane (99.29) (100.15) Vinylacetate (99.67) (100.10) hydroxypyridine (99.62) (100.10) Cyclooctatetraene (99.33) (100.36) number of correlated electrons (N el ) is taken as a measure for the molecular size, but virtually the same scalings would be obtained by using the number of basis functions, since in the current case N el 10 N AO. We find that for all choices of wck the scaling behaviour is nearly the same. The overall OSV- LCCSD CPU-times [evaluated from (Gly) 6 and (Gly) 8 ] scale cubically [O(Nel 3.1 )too(nel 3.2 )], while the disk space scales quadratically [O(Nel 2.0 )too(nel 2.1 )]. These are the expected values, since no local density fitting approximations are applied. TABLE III. Comparison of OSV and PAO correlation energies relative to the canonical CCSD correlation energy for linear polyglycine chains (in percent). The pair selection thresholds wck (see text) are varied. The avtz basis set has been used. Average domain size (AVD) includes redundant functions. LCCSD LCCSD+ MP2 Molecule AVD wck = 210 wck = 211 wck = 311 wck = 321 wck = 210 wck = 211 wck = 311 wck = 321 OSV (l osv = ) (Gly) (Gly) (Gly) (Gly) PAO (l pao = ) (Gly) (Gly) (Gly) (Gly)
The orbital-specific virtual local triples correction: OSV-L(T)
 The orbital-specific virtual local triples correction: OSV-L(T) Martin Schütz, Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, and Hans-Joachim Werner Citation: The Journal of Chemical Physics 138,
The orbital-specific virtual local triples correction: OSV-L(T) Martin Schütz, Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, and Hans-Joachim Werner Citation: The Journal of Chemical Physics 138,
On the selection of domains and orbital pairs in local correlation treatments
 On the selection of domains and orbital pairs in local correlation treatments Hans-Joachim Werner and Klaus Pflüger Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569
On the selection of domains and orbital pairs in local correlation treatments Hans-Joachim Werner and Klaus Pflüger Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569
Linear Scaling Local Coupled Cluster Theory with Density Fitting. I: 4-External Integrals
 Linear Scaling Local Coupled Cluster Theory with Density Fitting. I: 4-External Integrals Martin Schütz Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
Linear Scaling Local Coupled Cluster Theory with Density Fitting. I: 4-External Integrals Martin Schütz Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
OVERVIEW OF QUANTUM CHEMISTRY METHODS
 OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism
 Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism Stéphanie Essafi,*,1 Simone Tomasi, 2 Varinder K. Aggarwal, 1 Jeremy Harvey*,1 1 School of Chemistry,
Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism Stéphanie Essafi,*,1 Simone Tomasi, 2 Varinder K. Aggarwal, 1 Jeremy Harvey*,1 1 School of Chemistry,
Methods for Treating Electron Correlation CHEM 430
 Methods for Treating Electron Correlation CHEM 430 Electron Correlation Energy in the Hartree-Fock approximation, each electron sees the average density of all of the other electrons two electrons cannot
Methods for Treating Electron Correlation CHEM 430 Electron Correlation Energy in the Hartree-Fock approximation, each electron sees the average density of all of the other electrons two electrons cannot
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Exercise 1: Structure and dipole moment of a small molecule
 Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Performance of Hartree-Fock and Correlated Methods
 Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule. Vesa Hänninen
 Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the electronic energy
Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the electronic energy
Introduction to Computational Chemistry
 Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems
 John von Neumann Institute for Computing Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems Christof Hättig published in Computational Nanoscience: Do It Yourself, J. Grotendorst, S.
John von Neumann Institute for Computing Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems Christof Hättig published in Computational Nanoscience: Do It Yourself, J. Grotendorst, S.
We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include,
 1 Common Notation We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include, µ, ν, λ, σ: Primary basis functions, i.e., atomic orbitals (AOs) {φ µ ( r)}. The size
1 Common Notation We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include, µ, ν, λ, σ: Primary basis functions, i.e., atomic orbitals (AOs) {φ µ ( r)}. The size
Lecture 5: More about one- Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory.
 Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lecture 4: methods and terminology, part II
 So theory guys have got it made in rooms free of pollution. Instead of problems with the reflux, they have only solutions... In other words, experimentalists will likely die of cancer From working hard,
So theory guys have got it made in rooms free of pollution. Instead of problems with the reflux, they have only solutions... In other words, experimentalists will likely die of cancer From working hard,
( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r)
 Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
4 Post-Hartree Fock Methods: MPn and Configuration Interaction
 4 Post-Hartree Fock Methods: MPn and Configuration Interaction In the limit of a complete basis, the Hartree-Fock (HF) energy in the complete basis set limit (ECBS HF ) yields an upper boundary to the
4 Post-Hartree Fock Methods: MPn and Configuration Interaction In the limit of a complete basis, the Hartree-Fock (HF) energy in the complete basis set limit (ECBS HF ) yields an upper boundary to the
Fast and accurate Coulomb calculation with Gaussian functions
 Fast and accurate Coulomb calculation with Gaussian functions László Füsti-Molnár and Jing Kong Q-CHEM Inc., Pittsburgh, Pennysylvania 15213 THE JOURNAL OF CHEMICAL PHYSICS 122, 074108 2005 Received 8
Fast and accurate Coulomb calculation with Gaussian functions László Füsti-Molnár and Jing Kong Q-CHEM Inc., Pittsburgh, Pennysylvania 15213 THE JOURNAL OF CHEMICAL PHYSICS 122, 074108 2005 Received 8
6. Iterative Methods for Linear Systems. The stepwise approach to the solution...
 6 Iterative Methods for Linear Systems The stepwise approach to the solution Miriam Mehl: 6 Iterative Methods for Linear Systems The stepwise approach to the solution, January 18, 2013 1 61 Large Sparse
6 Iterative Methods for Linear Systems The stepwise approach to the solution Miriam Mehl: 6 Iterative Methods for Linear Systems The stepwise approach to the solution, January 18, 2013 1 61 Large Sparse
Beyond the Hartree-Fock Approximation: Configuration Interaction
 Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Density functional calculation of nuclear magnetic resonance chemical shifts
 Density functional calculation of nuclear magnetic resonance chemical shifts Christoph van Wüllen Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, D-44780 Bochum, Germany Received 22 August
Density functional calculation of nuclear magnetic resonance chemical shifts Christoph van Wüllen Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, D-44780 Bochum, Germany Received 22 August
Coupled-Cluster Theory. Nuclear Structure
 Coupled-Cluster Theory! for Nuclear Structure!!!! Sven Binder INSTITUT FÜR KERNPHYSIK! 1 Nuclear Interactions from Chiral EFT NN 3N 4N NLO LO N 2 LO +... N 3 LO +... +... +... 2 Nuclear Interactions from
Coupled-Cluster Theory! for Nuclear Structure!!!! Sven Binder INSTITUT FÜR KERNPHYSIK! 1 Nuclear Interactions from Chiral EFT NN 3N 4N NLO LO N 2 LO +... N 3 LO +... +... +... 2 Nuclear Interactions from
Local correlation in the virtual space in multireference singles and doubles configuration interaction
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 18 8 MAY 2003 ARTICLES Local correlation in the virtual space in multireference singles and doubles configuration interaction Derek Walter, Arun Venkatnathan,
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 18 8 MAY 2003 ARTICLES Local correlation in the virtual space in multireference singles and doubles configuration interaction Derek Walter, Arun Venkatnathan,
Basis sets for electron correlation
 Basis sets for electron correlation Trygve Helgaker Centre for Theoretical and Computational Chemistry Department of Chemistry, University of Oslo, Norway The 12th Sostrup Summer School Quantum Chemistry
Basis sets for electron correlation Trygve Helgaker Centre for Theoretical and Computational Chemistry Department of Chemistry, University of Oslo, Norway The 12th Sostrup Summer School Quantum Chemistry
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory
 Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Highly accurate quantum-chemical calculations
 1 Highly accurate quantum-chemical calculations T. Helgaker Centre for Theoretical and Computational Chemistry, Department of Chemistry, University of Oslo, Norway A. C. Hennum and T. Ruden, University
1 Highly accurate quantum-chemical calculations T. Helgaker Centre for Theoretical and Computational Chemistry, Department of Chemistry, University of Oslo, Norway A. C. Hennum and T. Ruden, University
v(r i r j ) = h(r i )+ 1 N
 Chapter 1 Hartree-Fock Theory 1.1 Formalism For N electrons in an external potential V ext (r), the many-electron Hamiltonian can be written as follows: N H = [ p i i=1 m +V ext(r i )]+ 1 N N v(r i r j
Chapter 1 Hartree-Fock Theory 1.1 Formalism For N electrons in an external potential V ext (r), the many-electron Hamiltonian can be written as follows: N H = [ p i i=1 m +V ext(r i )]+ 1 N N v(r i r j
Calculations of band structures
 Chemistry and Physics at Albany Planning for the Future Calculations of band structures using wave-function based correlation methods Elke Pahl Centre of Theoretical Chemistry and Physics Institute of
Chemistry and Physics at Albany Planning for the Future Calculations of band structures using wave-function based correlation methods Elke Pahl Centre of Theoretical Chemistry and Physics Institute of
Using BLIS for tensor computations in Q-Chem
 Using BLIS for tensor computations in Q-Chem Evgeny Epifanovsky Q-Chem BLIS Retreat, September 19 20, 2016 Q-Chem is an integrated software suite for modeling the properties of molecular systems from first
Using BLIS for tensor computations in Q-Chem Evgeny Epifanovsky Q-Chem BLIS Retreat, September 19 20, 2016 Q-Chem is an integrated software suite for modeling the properties of molecular systems from first
Wave function methods for the electronic Schrödinger equation
 Wave function methods for the electronic Schrödinger equation Zürich 2008 DFG Reseach Center Matheon: Mathematics in Key Technologies A7: Numerical Discretization Methods in Quantum Chemistry DFG Priority
Wave function methods for the electronic Schrödinger equation Zürich 2008 DFG Reseach Center Matheon: Mathematics in Key Technologies A7: Numerical Discretization Methods in Quantum Chemistry DFG Priority
Local Approaches to the Simulation of Electron Correlation in complex systems
 Local Approaches to the Simulation of Electron Correlation in complex systems Martin Schütz Institut für Physikalische und Theoretische Chemie, Universität Regensburg Universitätsstraße 31, D-93040 Regensburg
Local Approaches to the Simulation of Electron Correlation in complex systems Martin Schütz Institut für Physikalische und Theoretische Chemie, Universität Regensburg Universitätsstraße 31, D-93040 Regensburg
Divergence in Møller Plesset theory: A simple explanation based on a two-state model
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 22 8 JUNE 2000 Divergence in Møller Plesset theory: A simple explanation based on a two-state model Jeppe Olsen and Poul Jørgensen a) Department of Chemistry,
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 22 8 JUNE 2000 Divergence in Møller Plesset theory: A simple explanation based on a two-state model Jeppe Olsen and Poul Jørgensen a) Department of Chemistry,
Linear Algebra and Eigenproblems
 Appendix A A Linear Algebra and Eigenproblems A working knowledge of linear algebra is key to understanding many of the issues raised in this work. In particular, many of the discussions of the details
Appendix A A Linear Algebra and Eigenproblems A working knowledge of linear algebra is key to understanding many of the issues raised in this work. In particular, many of the discussions of the details
EE731 Lecture Notes: Matrix Computations for Signal Processing
 EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University September 22, 2005 0 Preface This collection of ten
EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University September 22, 2005 0 Preface This collection of ten
Building a wavefunction within the Complete-Active. Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy
 Building a wavefunction within the Complete-Active Active-Space Coupled-Cluster Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy Dmitry I. Lyakh (Karazin Kharkiv
Building a wavefunction within the Complete-Active Active-Space Coupled-Cluster Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy Dmitry I. Lyakh (Karazin Kharkiv
Computational Linear Algebra
 Computational Linear Algebra PD Dr. rer. nat. habil. Ralf Peter Mundani Computation in Engineering / BGU Scientific Computing in Computer Science / INF Winter Term 2017/18 Part 2: Direct Methods PD Dr.
Computational Linear Algebra PD Dr. rer. nat. habil. Ralf Peter Mundani Computation in Engineering / BGU Scientific Computing in Computer Science / INF Winter Term 2017/18 Part 2: Direct Methods PD Dr.
1 Rayleigh-Schrödinger Perturbation Theory
 1 Rayleigh-Schrödinger Perturbation Theory All perturbative techniques depend upon a few simple assumptions. The first of these is that we have a mathematical expression for a physical quantity for which
1 Rayleigh-Schrödinger Perturbation Theory All perturbative techniques depend upon a few simple assumptions. The first of these is that we have a mathematical expression for a physical quantity for which
Density-Fitting Approximations to the Electron Repulsion Integrals
 Density-Fitting Approximations to the Electron Repulsion Integrals C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Created on 19 July 2010 Here we will follow some
Density-Fitting Approximations to the Electron Repulsion Integrals C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Created on 19 July 2010 Here we will follow some
Lecture 4: Linear Algebra 1
 Lecture 4: Linear Algebra 1 Sourendu Gupta TIFR Graduate School Computational Physics 1 February 12, 2010 c : Sourendu Gupta (TIFR) Lecture 4: Linear Algebra 1 CP 1 1 / 26 Outline 1 Linear problems Motivation
Lecture 4: Linear Algebra 1 Sourendu Gupta TIFR Graduate School Computational Physics 1 February 12, 2010 c : Sourendu Gupta (TIFR) Lecture 4: Linear Algebra 1 CP 1 1 / 26 Outline 1 Linear problems Motivation
NPA/NBO-Analysis. Examples POP =
 NPA/NBO-Analysis Examples POP = NBO Requests a full Natural Bond Orbital analysis, using NBO version 3 NPA Requests just the Natural Population Analysis phase of NBO. NBORead Requests a full NBO analysis,
NPA/NBO-Analysis Examples POP = NBO Requests a full Natural Bond Orbital analysis, using NBO version 3 NPA Requests just the Natural Population Analysis phase of NBO. NBORead Requests a full NBO analysis,
Self-consistent Field
 Chapter 6 Self-consistent Field A way to solve a system of many electrons is to consider each electron under the electrostatic field generated by all other electrons. The many-body problem is thus reduced
Chapter 6 Self-consistent Field A way to solve a system of many electrons is to consider each electron under the electrostatic field generated by all other electrons. The many-body problem is thus reduced
Chemistry 334 Part 2: Computational Quantum Chemistry
 Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Electric properties of molecules
 Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Numerical Methods. Elena loli Piccolomini. Civil Engeneering. piccolom. Metodi Numerici M p. 1/??
 Metodi Numerici M p. 1/?? Numerical Methods Elena loli Piccolomini Civil Engeneering http://www.dm.unibo.it/ piccolom elena.loli@unibo.it Metodi Numerici M p. 2/?? Least Squares Data Fitting Measurement
Metodi Numerici M p. 1/?? Numerical Methods Elena loli Piccolomini Civil Engeneering http://www.dm.unibo.it/ piccolom elena.loli@unibo.it Metodi Numerici M p. 2/?? Least Squares Data Fitting Measurement
Domain decomposition on different levels of the Jacobi-Davidson method
 hapter 5 Domain decomposition on different levels of the Jacobi-Davidson method Abstract Most computational work of Jacobi-Davidson [46], an iterative method suitable for computing solutions of large dimensional
hapter 5 Domain decomposition on different levels of the Jacobi-Davidson method Abstract Most computational work of Jacobi-Davidson [46], an iterative method suitable for computing solutions of large dimensional
Electron Correlation Methods
 Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
Math 471 (Numerical methods) Chapter 3 (second half). System of equations
 Math 47 (Numerical methods) Chapter 3 (second half). System of equations Overlap 3.5 3.8 of Bradie 3.5 LU factorization w/o pivoting. Motivation: ( ) A I Gaussian Elimination (U L ) where U is upper triangular
Math 47 (Numerical methods) Chapter 3 (second half). System of equations Overlap 3.5 3.8 of Bradie 3.5 LU factorization w/o pivoting. Motivation: ( ) A I Gaussian Elimination (U L ) where U is upper triangular
Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little
 Vinay Ramasesh Getting Involved with Research Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little experience with actual scientific research. As a freshman, I d
Vinay Ramasesh Getting Involved with Research Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little experience with actual scientific research. As a freshman, I d
Jack Simons, Henry Eyring Scientist and Professor Chemistry Department University of Utah
 1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
Lecture 4: Hartree-Fock Theory
 Lecture 4: Hartree-Fock Theory One determinant to rule them all, One determinant to find them, One determinant to bring them all and in the darkness bind them Second quantization rehearsal The formalism
Lecture 4: Hartree-Fock Theory One determinant to rule them all, One determinant to find them, One determinant to bring them all and in the darkness bind them Second quantization rehearsal The formalism
Linear Solvers. Andrew Hazel
 Linear Solvers Andrew Hazel Introduction Thus far we have talked about the formulation and discretisation of physical problems...... and stopped when we got to a discrete linear system of equations. Introduction
Linear Solvers Andrew Hazel Introduction Thus far we have talked about the formulation and discretisation of physical problems...... and stopped when we got to a discrete linear system of equations. Introduction
Density Functional Theory. Martin Lüders Daresbury Laboratory
 Density Functional Theory Martin Lüders Daresbury Laboratory Ab initio Calculations Hamiltonian: (without external fields, non-relativistic) impossible to solve exactly!! Electrons Nuclei Electron-Nuclei
Density Functional Theory Martin Lüders Daresbury Laboratory Ab initio Calculations Hamiltonian: (without external fields, non-relativistic) impossible to solve exactly!! Electrons Nuclei Electron-Nuclei
Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices
 Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices Vahid Dehdari and Clayton V. Deutsch Geostatistical modeling involves many variables and many locations.
Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices Vahid Dehdari and Clayton V. Deutsch Geostatistical modeling involves many variables and many locations.
This is called a singlet or spin singlet, because the so called multiplicity, or number of possible orientations of the total spin, which is
 9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Notes on basis changes and matrix diagonalization
 Notes on basis changes and matrix diagonalization Howard E Haber Santa Cruz Institute for Particle Physics, University of California, Santa Cruz, CA 95064 April 17, 2017 1 Coordinates of vectors and matrix
Notes on basis changes and matrix diagonalization Howard E Haber Santa Cruz Institute for Particle Physics, University of California, Santa Cruz, CA 95064 April 17, 2017 1 Coordinates of vectors and matrix
arxiv:quant-ph/ v1 20 Apr 1995
 Combinatorial Computation of Clebsch-Gordan Coefficients Klaus Schertler and Markus H. Thoma Institut für Theoretische Physik, Universität Giessen, 3539 Giessen, Germany (February, 008 The addition of
Combinatorial Computation of Clebsch-Gordan Coefficients Klaus Schertler and Markus H. Thoma Institut für Theoretische Physik, Universität Giessen, 3539 Giessen, Germany (February, 008 The addition of
Ab initio treatment of electron correlations in polymers: Lithium hydride
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 10 8 MARCH 2000 Ab initio treatment of electron correlations in polymers: Lithium hydride chain and beryllium hydride polymer Ayjamal Abdurahman a) Max-Planck-Institut
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 10 8 MARCH 2000 Ab initio treatment of electron correlations in polymers: Lithium hydride chain and beryllium hydride polymer Ayjamal Abdurahman a) Max-Planck-Institut
Introduction to Electronic Structure Theory
 Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Coupled-cluster and perturbation methods for macromolecules
 Coupled-cluster and perturbation methods for macromolecules So Hirata Quantum Theory Project and MacroCenter Departments of Chemistry & Physics, University of Florida Contents Accurate electronic structure
Coupled-cluster and perturbation methods for macromolecules So Hirata Quantum Theory Project and MacroCenter Departments of Chemistry & Physics, University of Florida Contents Accurate electronic structure
Course Notes: Week 1
 Course Notes: Week 1 Math 270C: Applied Numerical Linear Algebra 1 Lecture 1: Introduction (3/28/11) We will focus on iterative methods for solving linear systems of equations (and some discussion of eigenvalues
Course Notes: Week 1 Math 270C: Applied Numerical Linear Algebra 1 Lecture 1: Introduction (3/28/11) We will focus on iterative methods for solving linear systems of equations (and some discussion of eigenvalues
Parallel Singular Value Decomposition. Jiaxing Tan
 Parallel Singular Value Decomposition Jiaxing Tan Outline What is SVD? How to calculate SVD? How to parallelize SVD? Future Work What is SVD? Matrix Decomposition Eigen Decomposition A (non-zero) vector
Parallel Singular Value Decomposition Jiaxing Tan Outline What is SVD? How to calculate SVD? How to parallelize SVD? Future Work What is SVD? Matrix Decomposition Eigen Decomposition A (non-zero) vector
Math 123, Week 2: Matrix Operations, Inverses
 Math 23, Week 2: Matrix Operations, Inverses Section : Matrices We have introduced ourselves to the grid-like coefficient matrix when performing Gaussian elimination We now formally define general matrices
Math 23, Week 2: Matrix Operations, Inverses Section : Matrices We have introduced ourselves to the grid-like coefficient matrix when performing Gaussian elimination We now formally define general matrices
QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
The Matrix Representation of a Three-Dimensional Rotation Revisited
 Physics 116A Winter 2010 The Matrix Representation of a Three-Dimensional Rotation Revisited In a handout entitled The Matrix Representation of a Three-Dimensional Rotation, I provided a derivation of
Physics 116A Winter 2010 The Matrix Representation of a Three-Dimensional Rotation Revisited In a handout entitled The Matrix Representation of a Three-Dimensional Rotation, I provided a derivation of
Computational Chemistry I
 Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
CS 246 Review of Linear Algebra 01/17/19
 1 Linear algebra In this section we will discuss vectors and matrices. We denote the (i, j)th entry of a matrix A as A ij, and the ith entry of a vector as v i. 1.1 Vectors and vector operations A vector
1 Linear algebra In this section we will discuss vectors and matrices. We denote the (i, j)th entry of a matrix A as A ij, and the ith entry of a vector as v i. 1.1 Vectors and vector operations A vector
Numerical Linear and Multilinear Algebra in Quantum Tensor Networks
 Numerical Linear and Multilinear Algebra in Quantum Tensor Networks Konrad Waldherr October 20, 2013 Joint work with Thomas Huckle QCCC 2013, Prien, October 20, 2013 1 Outline Numerical (Multi-) Linear
Numerical Linear and Multilinear Algebra in Quantum Tensor Networks Konrad Waldherr October 20, 2013 Joint work with Thomas Huckle QCCC 2013, Prien, October 20, 2013 1 Outline Numerical (Multi-) Linear
Strassen-like algorithms for symmetric tensor contractions
 Strassen-like algorithms for symmetric tensor contractions Edgar Solomonik Theory Seminar University of Illinois at Urbana-Champaign September 18, 2017 1 / 28 Fast symmetric tensor contractions Outline
Strassen-like algorithms for symmetric tensor contractions Edgar Solomonik Theory Seminar University of Illinois at Urbana-Champaign September 18, 2017 1 / 28 Fast symmetric tensor contractions Outline
Coupled Cluster Theory and Tensor Decomposition Techniques
 Coupled Cluster Theory and Tensor Decomposition Techniques Alexander A. Auer Atomistic Modelling Group Interface Chemistry and Surface Engineering Hierarchy of quantum chemical methods Analysis of orbitals
Coupled Cluster Theory and Tensor Decomposition Techniques Alexander A. Auer Atomistic Modelling Group Interface Chemistry and Surface Engineering Hierarchy of quantum chemical methods Analysis of orbitals
DS-GA 1002 Lecture notes 0 Fall Linear Algebra. These notes provide a review of basic concepts in linear algebra.
 DS-GA 1002 Lecture notes 0 Fall 2016 Linear Algebra These notes provide a review of basic concepts in linear algebra. 1 Vector spaces You are no doubt familiar with vectors in R 2 or R 3, i.e. [ ] 1.1
DS-GA 1002 Lecture notes 0 Fall 2016 Linear Algebra These notes provide a review of basic concepts in linear algebra. 1 Vector spaces You are no doubt familiar with vectors in R 2 or R 3, i.e. [ ] 1.1
Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner
 JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 21 1 DECEMBER 2001 Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner Helena Larsen,
JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 21 1 DECEMBER 2001 Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner Helena Larsen,
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014
 Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Answers Quantum Chemistry NWI-MOL406 G. C. Groenenboom and G. A. de Wijs, HG00.307, 8:30-11:30, 21 jan 2014 Question 1: Basis sets Consider the split valence SV3-21G one electron basis set for formaldehyde
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 28, December 08, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Study of Molecular Clusters with Explicitly Correlated Wave Function Methods
 Study of Molecular Clusters with Explicitly Correlated Wave Function Methods Von der Fakultät Chemie der Universität Stuttgart zur Erlangung der Würde eines Doktors der Naturwissenschaften (Dr. rer. nat.)
Study of Molecular Clusters with Explicitly Correlated Wave Function Methods Von der Fakultät Chemie der Universität Stuttgart zur Erlangung der Würde eines Doktors der Naturwissenschaften (Dr. rer. nat.)
LU Factorization. LU factorization is the most common way of solving linear systems! Ax = b LUx = b
 AM 205: lecture 7 Last time: LU factorization Today s lecture: Cholesky factorization, timing, QR factorization Reminder: assignment 1 due at 5 PM on Friday September 22 LU Factorization LU factorization
AM 205: lecture 7 Last time: LU factorization Today s lecture: Cholesky factorization, timing, QR factorization Reminder: assignment 1 due at 5 PM on Friday September 22 LU Factorization LU factorization
BLAS: Basic Linear Algebra Subroutines Analysis of the Matrix-Vector-Product Analysis of Matrix-Matrix Product
 Level-1 BLAS: SAXPY BLAS-Notation: S single precision (D for double, C for complex) A α scalar X vector P plus operation Y vector SAXPY: y = αx + y Vectorization of SAXPY (αx + y) by pipelining: page 8
Level-1 BLAS: SAXPY BLAS-Notation: S single precision (D for double, C for complex) A α scalar X vector P plus operation Y vector SAXPY: y = αx + y Vectorization of SAXPY (αx + y) by pipelining: page 8
Linear Algebra March 16, 2019
 Linear Algebra March 16, 2019 2 Contents 0.1 Notation................................ 4 1 Systems of linear equations, and matrices 5 1.1 Systems of linear equations..................... 5 1.2 Augmented
Linear Algebra March 16, 2019 2 Contents 0.1 Notation................................ 4 1 Systems of linear equations, and matrices 5 1.1 Systems of linear equations..................... 5 1.2 Augmented
I. CSFs Are Used to Express the Full N-Electron Wavefunction
 Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
QUANTUM CHEMISTRY FOR TRANSITION METALS
 QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
B553 Lecture 5: Matrix Algebra Review
 B553 Lecture 5: Matrix Algebra Review Kris Hauser January 19, 2012 We have seen in prior lectures how vectors represent points in R n and gradients of functions. Matrices represent linear transformations
B553 Lecture 5: Matrix Algebra Review Kris Hauser January 19, 2012 We have seen in prior lectures how vectors represent points in R n and gradients of functions. Matrices represent linear transformations
4 Matrix product states
 Physics 3b Lecture 5 Caltech, 05//7 4 Matrix product states Matrix product state (MPS) is a highly useful tool in the study of interacting quantum systems in one dimension, both analytically and numerically.
Physics 3b Lecture 5 Caltech, 05//7 4 Matrix product states Matrix product state (MPS) is a highly useful tool in the study of interacting quantum systems in one dimension, both analytically and numerically.
Fully Automated Implementation of the Incremental Scheme: Application. to CCSD Energies for Hydrocarbons and Transition Metal Compounds
 Fully Automated Implementation of the Incremental Scheme: Application to CCSD Energies for Hydrocarbons and Transition Metal Compounds Joachim Friedrich 1, Michael Hanrath 1, Michael Dolg 1 1 Institute
Fully Automated Implementation of the Incremental Scheme: Application to CCSD Energies for Hydrocarbons and Transition Metal Compounds Joachim Friedrich 1, Michael Hanrath 1, Michael Dolg 1 1 Institute
V(t) = Total Power = Calculating the Power Spectral Density (PSD) in IDL. Thomas Ferree, Ph.D. August 23, 1999
 Calculating the Power Spectral Density (PSD) in IDL Thomas Ferree, Ph.D. August 23, 1999 This note outlines the calculation of power spectra via the fast Fourier transform (FFT) algorithm. There are several
Calculating the Power Spectral Density (PSD) in IDL Thomas Ferree, Ph.D. August 23, 1999 This note outlines the calculation of power spectra via the fast Fourier transform (FFT) algorithm. There are several
Chapter 2. Linear Algebra. rather simple and learning them will eventually allow us to explain the strange results of
 Chapter 2 Linear Algebra In this chapter, we study the formal structure that provides the background for quantum mechanics. The basic ideas of the mathematical machinery, linear algebra, are rather simple
Chapter 2 Linear Algebra In this chapter, we study the formal structure that provides the background for quantum mechanics. The basic ideas of the mathematical machinery, linear algebra, are rather simple
SPARSE signal representations have gained popularity in recent
 6958 IEEE TRANSACTIONS ON INFORMATION THEORY, VOL. 57, NO. 10, OCTOBER 2011 Blind Compressed Sensing Sivan Gleichman and Yonina C. Eldar, Senior Member, IEEE Abstract The fundamental principle underlying
6958 IEEE TRANSACTIONS ON INFORMATION THEORY, VOL. 57, NO. 10, OCTOBER 2011 Blind Compressed Sensing Sivan Gleichman and Yonina C. Eldar, Senior Member, IEEE Abstract The fundamental principle underlying
Chapter 3. The (L)APW+lo Method. 3.1 Choosing A Basis Set
 Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
Consequently, the exact eigenfunctions of the Hamiltonian are also eigenfunctions of the two spin operators
 VI. SPIN-ADAPTED CONFIGURATIONS A. Preliminary Considerations We have described the spin of a single electron by the two spin functions α(ω) α and β(ω) β. In this Sect. we will discuss spin in more detail
VI. SPIN-ADAPTED CONFIGURATIONS A. Preliminary Considerations We have described the spin of a single electron by the two spin functions α(ω) α and β(ω) β. In this Sect. we will discuss spin in more detail
2.6 Complexity Theory for Map-Reduce. Star Joins 2.6. COMPLEXITY THEORY FOR MAP-REDUCE 51
 2.6. COMPLEXITY THEORY FOR MAP-REDUCE 51 Star Joins A common structure for data mining of commercial data is the star join. For example, a chain store like Walmart keeps a fact table whose tuples each
2.6. COMPLEXITY THEORY FOR MAP-REDUCE 51 Star Joins A common structure for data mining of commercial data is the star join. For example, a chain store like Walmart keeps a fact table whose tuples each
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found.
 Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
A practical view on linear algebra tools
 A practical view on linear algebra tools Evgeny Epifanovsky University of Southern California University of California, Berkeley Q-Chem September 25, 2014 What is Q-Chem? Established in 1993, first release
A practical view on linear algebra tools Evgeny Epifanovsky University of Southern California University of California, Berkeley Q-Chem September 25, 2014 What is Q-Chem? Established in 1993, first release
High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules
 High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules Markus Schneider Fritz Haber Institute of the MPS, Berlin, Germany École Polytechnique Fédérale de Lausanne, Switzerland دانشگاه
High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules Markus Schneider Fritz Haber Institute of the MPS, Berlin, Germany École Polytechnique Fédérale de Lausanne, Switzerland دانشگاه
Session 1. Introduction to Computational Chemistry. Computational (chemistry education) and/or (Computational chemistry) education
 Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.1677 Entangled quantum electronic wavefunctions of the Mn 4 CaO 5 cluster in photosystem II Yuki Kurashige 1 *, Garnet Kin-Lic Chan 2, Takeshi Yanai 1 1 Department of Theoretical and
DOI: 10.1038/NCHEM.1677 Entangled quantum electronic wavefunctions of the Mn 4 CaO 5 cluster in photosystem II Yuki Kurashige 1 *, Garnet Kin-Lic Chan 2, Takeshi Yanai 1 1 Department of Theoretical and
Introduction to Electronic Structure Theory
 CSC/PRACE Spring School in Computational Chemistry 2017 Introduction to Electronic Structure Theory Mikael Johansson http://www.iki.fi/~mpjohans Objective: To get familiarised with the, subjectively chosen,
CSC/PRACE Spring School in Computational Chemistry 2017 Introduction to Electronic Structure Theory Mikael Johansson http://www.iki.fi/~mpjohans Objective: To get familiarised with the, subjectively chosen,
Introduction to DFTB. Marcus Elstner. July 28, 2006
 Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
MTH 309 Supplemental Lecture Notes Based on Robert Messer, Linear Algebra Gateway to Mathematics
 MTH 309 Supplemental Lecture Notes Based on Robert Messer, Linear Algebra Gateway to Mathematics Ulrich Meierfrankenfeld Department of Mathematics Michigan State University East Lansing MI 48824 meier@math.msu.edu
MTH 309 Supplemental Lecture Notes Based on Robert Messer, Linear Algebra Gateway to Mathematics Ulrich Meierfrankenfeld Department of Mathematics Michigan State University East Lansing MI 48824 meier@math.msu.edu
Renormalization Group for the Two-Dimensional Ising Model
 Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Boolean Inner-Product Spaces and Boolean Matrices
 Boolean Inner-Product Spaces and Boolean Matrices Stan Gudder Department of Mathematics, University of Denver, Denver CO 80208 Frédéric Latrémolière Department of Mathematics, University of Denver, Denver
Boolean Inner-Product Spaces and Boolean Matrices Stan Gudder Department of Mathematics, University of Denver, Denver CO 80208 Frédéric Latrémolière Department of Mathematics, University of Denver, Denver
