Linear Scaling Local Coupled Cluster Theory with Density Fitting. I: 4-External Integrals
|
|
|
- Prosper Gilmore
- 5 years ago
- Views:
Transcription
1 Linear Scaling Local Coupled Cluster Theory with Density Fitting. I: 4-External Integrals Martin Schütz Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D Stuttgart, Germany Frederick R. Manby School of Chemistry, University of Bristol, Cantocks Close, Bristol BS8 1TS, UK (Dated: April 24, 2003, submitted to Phys. Chem. Chem. Phys.) The density fitting approximation is applied to the most expensive class of 2-electron integrals in local CCSD, i.e., to those integrals that involve four virtual orbitals (or projected AOs). The fitting error in the correlation energy is systematic and considerably smaller than the deviation between the local and the canonical CCSD energy. In order to restore O(N ) scaling locality must be exploited for the fitting functions as well as for orbitals. Local fitting domains specified for individual centre pairs provide an adequate basis for such a local description, however, Dunlap s robust formula for the approximate integrals then no longer simplifies to the usual expression known as the V approximation. A symmetric formula is proposed as an alternative, which, although formally nonrobust, yields virtually the same results as the robust formalism. The additional fitting error due to the introduction of local fitting domains is considerably smaller than the original fitting error itself (by at least an order of magnitude). Test calculations demonstrate O(N ) scaling for the new LDF-LCCSD method. The approximate calculation of the 4-external integrals via density fitting in LDF-LCCSD is times faster than the exact calculation via the O(N ) 4-index transformation in LCCSD. I. INTRODUCTION Coupled-cluster (CC) theory is one of the most successful models to treat many-electron molecular wavefunctions at high accuracy. By virtue of the underlying exponential ansatz for the substitution operator CC theory is size-extensive, i.e., the CC wavefunction is multiplicatively separable, which is a prerequisite for the treatment of large molecules. Furthermore, the exponential ansatz yields a very compact description for the wavefunction, much more compact than linear configuration interaction (CI). The CCSD wavefunction (obtained by truncating the cluster expansion beyond double substitutions) depends on the same number of amplitudes as a corresponding CISD wavefunction, but carries triply substituted and higher configurations in the form of disconnected clusters, which are not included in CISD. Finally, CC wavefunctions that include single substitutions are inherently stable with respect to orbital relaxation effects. According to the Thouless theorem [1, 2] most of the orbital relaxation effects are implicitly taken care of by solving the CC equations. One severe disadvantage of CC theory, which it shares with most other correlation methods, is the unfavourable scaling of the computational cost with molecular size N : canonical CCSD scales as O(N 6 ), and if triple substitutions are included even perturbatively the scaling rises to O(N 7 ). The implications of this steep scaling are Electronic address: schuetz@theochem.uni-stuttgart.de severe: an increase of computational capacity by three orders of magnitude leads to a modest increase of 2.7 in tractable molecular size, thus parallel supercomputers do not offer a practical solution to the scaling problem. During the last few years considerable effort has been invested in the development of low-order scaling electron correlation methods.these new methods are based on the short-ranged nature of dynamic electron correlation. Between remote parts of a molecule the correlation energy is dominated by dispersion, decaying as R 6 with respect to the distance R between fragments, i.e., much more quickly than the electrostatic energy components that are treated at the Hartree-Fock (HF) level. Conventional correlation methods rely on the convenient properties of the canonical molecular orbitals (MOs) supplied by HF theory. However these MOs are generally delocalized, and the short-range nature of correlation can only be exploited by abandoning the canonical orbitals. Instead one has to use localized orbitals and generalize the new local theories for non-canonical orbitals. In Stuttgart, using the local correlation approach of Pulay and Saebø [3 8], O(N ) scaling was achieved for local Møller-Plesset perturbation theory of second-order (LMP2) [9, 10], local CCSD theory (LCCSD) [11 13], and the treatment of triple substitutions in the context of local CC theory (LCCSD(T), LCCSDT-1b) [14, 15]. O(N ) scaling was also achieved by Ayala and Scuseria at the level of MP2 [16], who employed the Laplacetransform idea of Almlöf and Häser [17 19]. Recently, this O(N ) Laplace-transform method was extended for periodic systems [20]. Other approaches to low-order scaling electron correlation methods include the local tri-
2 2 atomics in molecules model for MP2 by Lee, Maslen, and Head-Gordon [21]; a recent LMP2 implementation by Saebø and Pulay [22]; the pseudo-spectral LMP2 (and multiconfigurational LMP2) methods by Murphy, Dunietz, and Friesner [23, 24]; and the pseudo-spectral local multi-reference CI by Walter and Carter [25]. For the LCCSD method developed in Stuttgart the number of amplitudes (the coefficients to be determined) is O(N ) by construction.yet in order to arrive at a method with linear scaling the number of 2-electron repulsion integrals (4-index objects) in the local orbital basis, must also be O(N ). In LCCSD three different classes of transformed integrals are needed for the computation of the residual vectors, characterized by having four, three, or two orbitals in the virtual space. In what follows we refer to these integral classes as 4-ext, 3-ext, and 2-ext. Integrals with zero or one virtual orbital, which are needed as well, are produced on-the-fly during the construction of the 2-ext integral set. By introducing the concept of operator lists and operator domains [12, 13] integral distributions of size O(N ) can be specified a priori for all three classes, which are sufficient to compute all relevant couplings in the local CC equations. In LCCSD the calculation of these integral distributions constitutes the dominant part of the computational cost, while the subsequent local CCSD iterations proceed rapidly [13]. This is in strong contrast to canonical CCSD theory, where the dominant computational cost resides in the iterative construction of the residual vectors from integrals and amplitudes. Hence, in the local case the computational cost is moved outside the iterations to the initial phase of integral evaluation and transformation, where the distributions for the three integral classes are generated and stored. Of these the 4-ext set is particularly expensive, especially for larger basis sets, since it scales worst with the number of basis functions per atom N atom (in spite of the O(N ) scaling with respect to molecular size). Obviously, in order to improve the efficiency of local CC further, the calculation of the integral distribution (in particular that of the 4-ext class) has to be accelerated. A possible route is the combination of local CC with density fitting. Density fitting (DF) has a long history in electron correlation [26 28], although it is frequently cited in the context of the Coulomb problem in HF and Kohn-Sham theory [29 31]. By now, it is well established and has been used for Coulomb fitting in HF and DFT [32, 33], exchange fitting in HF [34], as well as for approximating transformed 2-electron integrals in MP2 [35, 36], MP2 gradients [37], and CCSD(T) [38]. It has been demonstrated that the fitting errors introduced by the DF approximation are substantially smaller than other typical errors of the calculation (like basis set truncation) and systematic, provided that suitable fitting basis sets are employed. In the context of correlated methods the name resolution of the identity (RI) is often used as a synonym for DF (e.g. RI-MP2), yet we prefer the name density fitting for reasons stated elsewhere [39]. Very recently, Werner et al. implemented an O(N ) method for LMP2 using the DF approximation of the transformed 2-electron integrals (DF-LMP2) [39]. The resulting method is so much faster than the preceeding HF calculation that those authors are currently implementing a local density fitted exchange engine to increase efficiency. In this work we use density fitting to approximate the 4-ext integrals in local CC, removing the most severe bottleneck in our current LCCSD implementation. By virtue of the DF approximation the 4-index 2-electron integrals can be written in terms of 2- and 3-index integrals, which are much easier to handle. In particular the computational complexity with respect to the number of basis functions per atom N atom is only O(Natom) 4 for the generation of the 3-index integrals, compared to O(Natom) 5 of the 4-index transformation generating the exact 4-ext integrals, which allows for the use of bigger basis sets in local CC calculations. In the next section we will briefly review the basic concepts of local correlation methods, and how DF is used to approximate the 4-ext integrals in local CC theory. The O(N ) scaling is lost when density fitting is used, but we will discuss the way in which it can be restored by introducing the concept of fitting domains. In section 3 we analyse the accuracy of using fitted 4-ext integrals in LCCSD, and of introducing fitting domains. Furthermore, the gain in efficiency due to DF will be demonstrated for a number of test molecules. II. THEORY AND IMPLEMENTATION A. Local correlation methods The O(N ) LCCSD method was described in detail previously [12, 13]. It is based on the local correlation ansatz of Pulay and Saebø [3 8], and the local formalism for CCSD by Hampel and Werner [11]. It uses mutually orthogonal localized MOs (LMOs) to span the occupied space, generated from the occupied canonical Hartree- Fock orbitals by unitary transformation [40, 41]. The virtual space, on the other hand, is spanned by nonorthogonal orbitals, obtained by projecting the atomic orbital (AO) basis onto it (to give projected AOs, PAOs). The PAOs are inherently local and can still be assigned to individual atoms. In order to truncate the virtual space excitation domains are introduced, which consist of all PAOs on atoms spatially close to the LMOs out of which the excitation takes place. For example, double excitations (or substitutions) out of the LMO pair φ i, φ j = ij to a PAO pair φ r, φ s = rs are restricted to PAOs in the pair domain [ij], i.e., to PAOs spatially close either to φ i or to φ j. The motivation for this truncation is the exponential decay of the matrix element between such a doubly excited configuration and the ground state, with respect to the distance between φ i and φ r (or φ j and φ s ) [42]. The excitation domains can be determined automatically just
3 3 by specifying a single parameter [43]. The virtual space for an individual LMO pair ij is pair specific, but its size becomes independent of molecular size N. In order to truncate the occupied space, i.e., the number of LMO pairs (or triples) that have to be treated at a certain level of theory a hierarchy of pairs based on the distance R between the two nearest atoms of the two LMOs of the pair was introduced. The physical motivation behind this is the above mentioned R 6 decay of the pair energy with respect to R. Only the small subset of strong pairs, where the two LMOs typically have a common atom, is treated at the CC level (usually, about 98 % of the correlation energy is covered by these strong pairs), the rest being treated at a lower level of theory. Together with the truncation of the virtual space outlined above the number of amplitudes in the local CC becomes O(N ). In order to arrive at a method with O(N ) scaling the number of 2-electron repulsion integrals (4-index objects) required for the computation of the residuals must also be O(N ). Since in CC amplitudes are coupled via 2- electron integrals this is not as trivially fulfilled as in LMP2. It was already mentioned before (and described in detail elsewhere [12, 13]), that for all required integral classes (2-ext, 3-ext, 4-ext) subsets of size O(N ) can be specified a priori, which comprise all integrals needed for the computation of the LCCSD residuals. All these integral subsets are generated before the CC iteration loop is entered. The 4-ext integrals, which contribute to the doubles residual exclusively via the external exchange matrices [44], require some special attention. These quantities are computed as 2-index contractions of the 4-ext integrals with the doubles amplitudes, i.e., K(C ij ) rs = tu [ij] C ij tu(rt su) (1) with Crs ij = Trs ij + t i rt j s. This contraction must be carried out in each CCSD iteration since the K(C ij ) depend on the amplitudes. At this point it is timely to define the notation used in this paper: i, j, k,... and r, s, t,... denote (occupied) LMO and (virtual) PAO indices, respectively. Capital letters P, Q,..., on the other hand, are used as indices for the fitting functions and t i r, Trs ij represent the CC singles and doubles amplitudes in eq. (1). Note that the summation is restricted to PAOs t, u within the pair domain [ij] of pair ij. Since there is a one-to-one correspondence between the doubles residual matrices R ij and the external exchange matrices K(C ij ), all four PAO indices of the 4-ext integrals (rs tu) contributing to K(C ij ) for fixed pair ij must belong to the same pair domain [ij]. As a consequence, all four PAOs must be spatially close, and the 4-ext integral set becomes very compact and naturally O(N ). The optimum specification of this integral set is based on a decomposition into blocks related to quadruples of centres [13]. Since individual atoms are the natural building blocks of all domains, any redundancy in the integrals is avoided that way. In strong contrast to canonical CCSD the computational cost for the construction of the K(C ij ) according to eq. (1) is entirely negligible in LCCSD. On the other hand, the formation of the 4-ext integrals (outside the CC iteration loop) is expensive in spite of its O(N ) scaling behaviour, and presently dominates an LCCSD calculation. As already mentioned in the Introduction, the 4-ext integral transformation scales worst with the number of basis functions per atom, i.e., with Natom, 5 while the remaining transformations scale with Natom. 4 It thus constitutes the main obstacle thwarting the use of bigger basis sets in LCCSD. A possible fix for this problem is to employ density fitting to calculate approximate 4-ext integrals (rs tu) directly instead of obtaining the exact ones by transformation. In the context of canonical CCSD this has been tried before by Rendell and Lee [45], but with the intention to reduce the I/O and storage bottlenecks in conventional (non integral-direct) CCSD algorithms and not the CPU cost. Since in canonical CCSD most of the CPU time is spent for the calculation of the residuals during the iterations, e.g., for the construction of the K(C ij ), employing the density fitting approach to get the integrals quicker has little effect on the total CPU time. In fact, the use of the density fitting approach in canonical CCSD actually leads to a somewhat higher CPU cost for the iterations [45], caused by the additional assembly of the approximated integrals (rs tu), carried out on-the-fly in each iteration. The situation for LCCSD is entirely different, of course, and we will show that density fitting has a tremendous impact on the overall CPU cost. B. Density fitting for local coupled-cluster By using the Density Fitting approach in the context of LCC for the 4-ext integrals (rs tu) = = dr 1 dr 1 dr 2 φr (r 1 ) φ s (r 1 )r 1 12 φ t (r 2 ) φ u (r 2 ) dr 2 ρ rs (r 1 )r 1 12 ρ tu(r 2 ) (2) the one-particle orbital product densities ρ rs (r) = φ r (r) φ s (r) appearing in the integrals are substituted by approximated densities ρ rs (r). The latter are expanded in an auxiliary or fitting basis {Ξ P (r)}, i.e., ρ rs (r) = φ r (r) φ s (r) ρ rs (r) = P P Ξ P (r). (3) The optimal fitting coefficients P are determined by minimizing an appropriate measure for the fitting error,
4 4 generally written as w = dr 1 dr 2 [ρ rs ρ rs ](r 1 ) ŵ 12 [ρ rs ρ rs ](r 2 ) = (ρ rs ŵ 12 ρ rs ) 2 P + P Q P (P ŵ 12 ρ rs ) P (P ŵ 12 Q) Q. (4) Here, ŵ 12 is an appropriate, positive definite weight operator. Minimization of w with respect to the fitting coefficients yields the linear equation system (P ŵ 12 Q) Q = (P ŵ 12 rs), (5) Q which has to be solved to determine the fitting coefficients. For the calculation of the approximate 4-ext integrals (rs tu) the robust formula (rs tu) = ( ρ rs ρ tu ) + (ρ rs ρ tu ) ( ρ rs ρ tu ) = P P Q P (P tu) + (rs Q)d tu Q Q P (P Q)d tu Q (6) of Dunlap [46 48] is employed, which leads to a fitting error in the integrals that is second order with respect to the fitting error in the densities, regardless of the choice of ŵ 12 : (rs tu) (rs tu) = (ρ rs ρ tu ) ( ρ rs ρ tu ) (ρ rs ρ tu ) + ( ρ rs ρ tu ) = (ρ rs ρ rs ρ tu ρ tu ). (7) Choosing ŵ 12 = r12 1 however is advantageous [28, 49 52], as eq. (5) then reads (P Q) Q = (P rs) (8) Q or (P ρ rs ) = (P ρ rs ), with (P Q) = dr 1 dr 2 Ξ P (r 1 )r12 1 Ξ Q(r 2 ) = J P Q, (9) (P rs) = dr 1 dr 2 Ξ P (r 1 )r12 1 φ r (r 2 ) φ s (r 2 ). (10) Using eq. (8) the robust formula (6) simplifies to (Dunlap, [46]) (rs tu) = ( ρ rs ρ tu ) = ( ρ rs ρ tu ) = (ρ rs ρ tu ) = P P (P tu) = P Q(rs P )[J 1 ] P Q (Q tu), (11) provided that the same fitting basis is employed for ρ rs and ρ tu. Eq. (11) corresponds to the V approximation of Vahtras et al. [32] and is commonly used in canonical correlation methods based on density fitting [37, 38, 53 55]. The calculation of the 3-index Coulomb integrals (P rs) (cf. eq. (10)) requires a 2-index transformation from AO to PAO basis, i.e., (P rs) = P µr P νs (P µν), (12) µ ν where P µr is an element of the PAO projection matrix. The number of relevant integrals (P rs) and the CPU cost for this 2-index transformation scale as O(N 2 ) with molecular size, because the two PAOs φ r, φ s must be spatially close (cf. eq. (1), Ref. [13]), whereas for the fitting function Ξ P there is no immediate spatial connection to φ r, φ s. Furthermore, the number of 2-index Coulomb integrals J P Q as defined in eq. (9) scales also as O(N 2 ), since Ξ P and Ξ Q are coupled by the long-range Coulomb operator r12 1 and there is again no immediate spatial connection (like a sparse test density) relating Ξ P and Ξ Q. Assembling the 4-index object (rs tu) according to eq. (11) also scales as O(N 2 ), whereas solving the equation system (8) (or inverting the dense J matrix) scales even as O(N 3 ). This has to be compared with the O(N ) scaling behaviour of the original local CC method without density fitting. It should be mentioned at this point, that there is a neat trick based on the Poisson equation, which can be exploited to replace most of the slowly decaying 2- and 3- index Coulomb 2-electron integrals by rapidly decaying 2- and 3-index one-electron integrals [56 58]. This restores O(N ) scaling for the transformation, yet the assembly and solve step still scale as O(N 2 ) and O(N 3 ), respectively, since even though the J matrix becomes sparse, its inverse remains dense. The use of the Poisson trick for local coupled-clusters is the subject of a forthcoming paper. With respect to the number of basis functions per atom N atom the situation is different. Here the conventional 4-index transformation scales as O(Natom), 5 while the 2-index transformation according to eq. (10) scales as O(Natom). 4 The computational complexity of the assembly step in eq. (11) however is NPAO 4 N FIT, thus remaining O(Natom). 5 It is possible to get O(Natom) 4 scaling by direct contraction of the 3-index integrals with the amplitudes in each iteration, which avoids the explicit construction of the (rs tu) integrals altogether. Such a scheme has a computational complexity N pair NPAO 3 N FIT. Yet since the number of electron pairs is much larger than the number of PAOs (which are spatially restricted) even for large AO basis sets, explicit assembly of the (rs tu) integrals appears to be preferable. This implies though, that in contrast to the MP2 case there is no reduction achieved in the overall scaling with respect to N atom by employing density fitting
5 5 for the 4-ext integrals in coupled-cluster theory. Nevertheless, the computational savings due to density fitting in local coupled-cluster theory turn out to be even more dramatic than in local MP2, as will be demonstrated in section III. C. Local fitting basis and O(N ) scaling It has been discussed above that in the density fitting approximation the O(N ) scaling behaviour of the original local CC method is lost, which is due to the absence of a spatial connection (like a sparse test density) between the PAOs and the fitting functions. The Poisson trick offers a partial remedy to this problem, restoring O(N ) scaling for the 2-index transformation, but not for the assembly and the solve step, with the latter still scaling as O(N 3 ). Another possibility is to introduce the concept of locality also for the fitting functions, i.e., to restrict the range of fitting functions Ξ P for a local orbital density ρ rs to the spatial vicinity of ρ rs. These fitting domains are specific for individual groups of densities ρ rs, or even groups of pairs of densities (ρ rs ρ tu ), i.e., individual groups of integrals (rs tu). For example, each individual centre pair (RS), or centre quadruple (RSTU) in the second case, might define such a group (the symbol R denotes the centre related to the PAO function index r). In the following we employ the notation [rs] for fitting domains, which are specific for individual groups of densities ρ rs, ρ rs = P Ξ P (r). (13) P [rs] The fact that the fitting functions Ξ P for ρ rs are restricted to [rs] is underlined in subsequent formulae by replacing the auxiliary function index P by P [rs]. For fitting domains, which are specific for individual groups of integrals, [rs] would have to be replaced by [rstu]. Using the notation [rs] for the fitting domains the linear equation system (8) takes the form (P [rs] Q [rs] ) Q = (P [rs] rs), [rs] Q [rs] or (P [rs] ρ rs ) = (P [rs] ρ rs ), (14) implying that the single large set of linear equations is replaced by many, small ones. The number of groups and the size of the fitting domains are naturally O(N ) and O(1), respectively, the computational cost for this step is thus O(N ). O(N ) scaling is also obtained for the assembly step even though Dunlap s robust formula (rs tu) = P [rs] P [rs] P [rs] (P [rs] ρ tu ) + (ρ rs ρ tu ) P [rs] (P [rs] ρ tu ) (15) no longer simplifies to the V approximation of Vahtras due to eq. (14), except for the case of identical fitting domains for ρ rs and ρ tu. This is naturally fulfilled for fitting domains which are specific for individual groups of integrals ([rstu] instead of [rs]). A specification of fitting domains based on centre quadruples (RSTU) thus appears to be attractive and actually has been tried in the present work: these centre quadruple specific fitting domains [rstu] = [RSTU] include all fitting functions on the centres RST and U. The number of groups, i.e., of different domains [RSTU] then corresponds to length of the sparse centre quadruples list and thus is O(N ). However, it turned out that the resulting fitting domains according to such a specification are too small, leading to dramatically wrong correlation energies in the 1st iteration and subsequent divergence of the CC calculation. On the one hand the fitting domains have to be larger for a proper fit of the densities, and on the other hand it is desirable to reduce the number of groups and thus the number of equation systems to solve. We therefore propose a centre pair specification of the fitting domains: [rs] = [RS] includes the fitting functions on the centres of all centre quadruples (RSTU) containing the centre pair (RS). Optionally, further centres can be added to [RS] based on a distance criterion (controlled by the parameter R fit ) with respect to any centre already included in [RS] due to the merged centre quadruples. The number of different fitting domains corresponds only to the number of relevant centre pairs in this case, and obviously is also O(N ). The fitting domains for ρ rs and for ρ tu may now be different, implying that eq. (11) no longer is robust. Combining eqs. (14) and (15) yields (rs tu) = (rs R [rs] )(1/J [rs] ) RP (P [rs] tu) + (rs Q [tu] )(1/J [tu] ) QR (R [tu] tu) P [rs] R [rs] R [tu] Q [tu] (rs R [rs] )(1/J [rs] ) RP (P [rs] Q [tu] )(1/J [tu] ) QS (S [tu] tu) (16) P [rs] R [rs] S [tu] Q [tu] as the general robust expression for the integrals (rs tu). In the course of this work we also implemented the sim-
6 6 pler symmetric formula [ (rs tu) = 1 (rs R [rs] )(1/J [rs] ) RP (P [rs] tu) 2 P [rs] R [rs] + (rs Q [tu] )(1/J [tu] ) QR (R [tu] tu) (17), R [tu] Q [tu] which is computationally more efficient but formally nonrobust. Nevertheless, eq. (17) turned out to be very useful, as will be demonstrated in section III. Examining eqs. (16) or (17) it is immediately evident that the PAOs as well as the fitting functions are all spatially close, leading to the desired O(N ) scaling behaviour in the computational cost for the assembly step. The number of 3-index Coulomb integrals (rs Q [tu] ) occurring in eqs. (16) and (17) also is O(N ), a sufficient distribution is specified by introducing the united centre pair (UCP) fitting domains [RS] U. These are defined as the union of all fitting domains [TU] of right centre pairs (TU), which form a relevant centre quadruple (RSTU) with the left centre pair (RS). The 3-index integral set (rs Q [RS] U ) carries all the (rs Q [tu] ) that are required in the assembly step according to eqs. (16) or (17). From the UCP fitting domains [RS] U the united centre (UC) fitting domains [R] U are derived, defined as the union of all [RS] U with common centre R. UCP and UC fitting domains are instrumental to achieve O(N ) scaling also in the 2-index integral transformation (12). Moreover, the UCP domains are employed to pre-compute and store the intermediate quantities Xrs Q = (rs R [rs] )(1/J [rs] ) RP (P [rs] Q) P [rs] R [rs] = P [rs] P [rs] (P [rs] Q), with Q [RS] U, (18) survived the pre-screening. Permutational symmetry between µ and ν is not exploited. A block of half transformed integrals (P rν) can then be kept in memory (all relevant PAOs φ r ), with the second transformation step carried out immediately afterwards. Permutational symmetry in the PAO indices r and s is exploited at the level of centre pairs (RS) and built into the predetermined sparse centre pair list {RS} [13]. Individual centre blocks of fully transformed integrals (P rs) are written to disk. Once all integrals (P rs) are computed the sequence of blocks on the integral file is resorted such that the block index of the fitting functions becomes fastest, and the order of PAO centre pairs (RS) (slow) matches the canonical order of {RS}. This optimal order minimizes non-contiguous read-back from disk during the assembly of the 4-ext integrals according to eqs. (16) or (17). The assembly step is driven by the sparse centre quadruples list {RSTU} and can be carried out either outside the CC iterations, which requires storage of the 4-ext integral set (rs tu), or within the CC iterations on-the-fly during the construction of the external exchange matrices, eq. (1). In order to reduce I/O, subsets of integrals (P rs) and fitting coefficients Q (with r R, s S, P [RS] U, Q [RS]) are cached in memory for as many centre pairs (RS) as possible within the amount of memory that is available (oldest cache entries with respect to the last readout are overwritten first). Since the centre quadruples list {RSTU} is ordered such that spatially close centres are also close in the list, integrals and fitting coefficients of cached centre pairs are likely to be reused in the sequence of the assembly of the (rs tu), by virtue of the sparsity of {RSTU}. The circular caching procedure as described here is remi niscent of the disk caching algorithm used for the triples amplitudes in the local T1 method [14]. which are used to compute the 3rd term of eq. (16) efficiently. The 2-index integral transformation (12) is driven by a loop over centre blocks of fitting functions Ξ P. Lookup tables, generated from the [R] U and [RS] U domains, are used to restrict the PAO centre ranges R and S for a given block of fitting functions in the first and second transformation step, respectively. Additionally, prescreening techniques are used in each step to reduce the PAO centre range further (product pre-screening with integrals (P µν) and (P rν), respectively, and appropriate test densities obtained from the PAO projection matrix). O(N ) scaling in the evaluation of the 3-index integrals (P µν) is achieved by product pre-screening based on the Schwarz estimate of the magnitude of (P µν) (P µν) (P P ) 1 2 (µν µν) 1 2, (19) and by skipping those blocks of fitting functions corresponding to centres that are not included in any of the UC fitting domains [R] U belonging to PAO centres R that III. TEST CALCULATIONS The new modules for the computation of the 4-ext integrals with density fitting were implemented in the context of the O(N ) local CC code [12, 14] of the MOL- PRO [59] program package (development version). The acronyms DF-LCCSD and LDF-LCCSD will be used in the following to distinguish between the non-local DF approach with full fitting basis, and the local DF approach with fitting domains. The latter method is O(N ), as discussed in section II C. In this section we present some test calculations to demonstrate accuracy, as well as scaling behaviour and performance of the DF and LDF approximations in LCCSD. In table I incremental errors in correlation energies are presented for several small test molecules on going from canonical to local CCSD, and by using the DF and LDF approximations for the 4-ext integrals. For all these calculations the cc-pvtz basis set [60] with the corresponding fitting basis optimized by
7 7 TABLE I: Fitting errors of DF-LCCSD (full fitting basis) vs. LCCSD and of LDF-LCCSD (local fitting basis) vs. DF-LCCSD for a number of test molecules (all in µhartree). Total correlation energies (in hartree) for canonical and local CCSD and the deviation of the latter from the former, i.e., LCCSD vs CCSD, is also given, for comparison. nc denotes no convergence in the LCCSD iterations. For all calculations the cc-pvtz basis set together with the corresponding 8s6p5d3f1g/4s3p2d1f fitting basis optimized for DF-MP2 by Weigend et al. [61] was used. E (CCSD) E (LCCSD) a (LCCSD) b (DF LCCSD) c (LDF LCCSD,robust) d (LDF LCCSD,symm) molecule e R fit = 0 R fit = 3 R fit = 0 R fit = 3 isobutene butane pentane hexatriene glycine nc benzenethiol nc alanine oxalic acid nc benzoquinone nc maleic acid nc a Deviation in E corr due to local approximation: E (LCCSD) E (CCSD) b Fitting error: E (DF LCCSD) E (LCCSD) c Error due to local fit domains: E (LDF LCCSD,robust) E (DF LCCSD). The robust formula (16) is used. d Error due to local fit domains: E (LDF LCCSD,symm) E (DF LCCSD). The symmetric formula (17) is used. e All geometries were optimized at the B3LYP/cc-pVTZ(d/p) level. Weigend et al. [61] for DF-MP2 has been used. The LMOs were obtained with Pipek-Mezey orbital localization [41]. The PAO domains were determined by using the Boughton-Pulay procedure [43] with the completeness criterion set to a threshold of 0.985, which is suitable for triple zeta basis sets. It is evident from Table I, that the fitting error inflicted on the LCCSD correlation energy due to the DF approximation is also systematic and considerably smaller than the deviation between canonical and local CCSD. It amounts to some hundred µhartree for the molecules in Table I, and a substantial decrease in this error can be expected if a new fitting basis, optimized for the 4-ext integrals, is used instead of the Weigend DF-MP2 basis. The presently used fitting basis by Weigend et al. is tailored for the fitting of orbital densities involving an occupied and a virtual orbital (as they occur in the 2-ext exchange integrals of MP2), but not for densities involving two virtual orbitals (PAOs). For a fit of the same quality as in DF-MP2 the fitting basis may have to include additional functions of higher angular momentum. The development of new fitting basis sets, adapted for such densities will be the subject of future work and has to await the implementation of DF-3-ext integrals in LCCSD. Table I also shows the incremental error caused by the use of local fitting domains, i.e., the additional fitting error of LDF-LCCSD relative to DF-LCCSD. Calculations using the robust (16) and symmetric (17) formulae with R fit = 0 and R fit = 3 bohr, respectively, are reported. Evidently, this error is again much smaller than the fitting error of DF-LCCSD itself (by one to two orders of magnitude). Furthermore, the formally non-robust symmetric formula (17) yields results virtually identical to those obtained with the robust equation (16). There are even several cases for R fit = 0, where convergence problems were encountered with the robust formula, whereas with the symmetric formula smooth convergence was observed throughout. Possibly, the explicit calculation of the third term in eq. (16), containing fitted densities for both electrons of the Coulomb integral, requires a larger local fitting basis. The origin of the unexpectedly good behaviour of the symmetric formula lies in the specification of the fitting domains [RS], which include the fitting functions on the centres of all centre quadruples (RSTU) containing the centre pair (RS) and therefore introduces some symmetry between the left fitting domain [RS] and the right fitting domain [TU], even though they are not strictly identical. (Recall also, that the densities ρ rs and ρ tu are spatially close in all relevant integrals (rs tu)). As a consequence, the left and right fitting domains [RS] and [TU] are very similar, rendering eq. (17) nearly robust. Eq. (11), on the other hand, does not work, the CC diverges for all the molecules in Table I. Since the left and right fitting domains are not exactly identical the permutational symmetry in the fitted integrals (rs tu) is lost in eq. (11), while it is still conserved in eq. (17), as it is, of course, in eq. (16). The computational savings due to the use of the DF approximation for the 4-ext integrals are substantial even for the small molecules in Table I: for maleic acid the calculation of the (rs tu) integrals in LCCSD takes 2.4 CPU hours on 1.33 GHz Athlon PC, while the calculation
8 8 INDI CALI [GLY] 3 FIG. 1: Molecular geometries of Indinavir (INDI), the warhead of calicheamicin γ I 1 (CALI), and (Gly) 3. of the (rs tu) integrals in DF-LCCSD takes only 5 CPU minutes on the same machine. Of course, computational performance and scaling behaviour of DF-LCCSD, and in particular of LDF-LCCSD, has to be demonstrated for larger molecular systems. Calculations on extended systems are also required to verify the applicability of local fitting domains and of the symmetric formula in LDF-LCCSD. For that purpose we performed DF-LCCSD and LDF-LCCSD calculations (robust and symmetric, with different settings of R fit ) on a set of extended test molecules (cf. Fig. 1), which have been used already in previous work [9, 12 14]: (i) the unavoidable system of the poly-glycine peptide chains (Gly) n HO[C(O)CH 2 NH] n H (n = ), which are highly suitable to demonstrate the scaling behaviour, (ii) the Indinavir (INDI) molecule, a protease inhibitor used in the therapy of HIV infection, and (iii) the warhead of the calicheamicin γ I 1 anti-cancer drug in its pre-trigger state (CALI) [62]. The geometries of all these molecules are identical to those in Ref. [13]. Most of these test calculations were carried out by using the correlation consistent cc-pvdz basis set of Dunning [63] in conjunction with the corresponding fitting basis optimized by Weigend et al. for DF-MP2 [61]. Further calculations involving the cc-pvtz(f/p) basis (no d-functions on H) and the related fitting basis (again optimized for DF-MP2 [61]) were performed for the CALI molecule. For the construction of the PAO domains a value of 0.98 for the Boughton-Pulay completeness criterion was employed. In Table II we report the correlation energies of LCCSD and DF-LCCSD, and the fitting errors of DF- LCCSD and LDF-LCCSD, respectively (in analogy to Table I above) for these molecules. Evidently, the additional error of LDF-LCCSD relative to DF-LCCSD inflicted by the use of local fitting domains, also for these extended molecular systems, is much smaller than the fitting error of DF-LCCSD itself (by one to two orders of magnitude). Furthermore, the extremely close agreement between the symmetric (17) and the robust (16) LDF-LCCSD correlation energies also holds for fitting domains that comprise only a small fraction of the total number of fitting functions, as is the case for these extended molecules. Table III compiles the average sizes of the centre pair fitting domains [RS], the united centre pair fitting do-
9 9 TABLE II: Fitting errors of DF-LCCSD (full fitting basis) vs. LCCSD and of LDF-LCCSD (local fitting basis) vs. DF-LCCSD for a number of extended molecular systems (all in µhartree). Total correlation energies (in hartree) for LCCSD and DF-LCCSD are also given. For all calculations except CALI/VTZ the cc-pvdz basis set together with the corresponding 7s5p4d2f/3s2p1d fitting basis optimized for DF-MP2 by Weigend et al. [61] was used. In contrast to Ref. [13] the multipole approximation for distant pair integrals in the LMP2 calculation was not invoked. E (LCCSD) E (DF LCCSD) a (DF LCCSD) b (LDF LCCSD,robust) c (LDF LCCSD,symm) molecule R fit = 0 R fit = 3 R fit = 0 R fit = 3 (Gly) nc (Gly) (Gly) (Gly) (Gly) (Gly) (Gly) (Gly) INDI CALI nc CALI/VTZ d a Fitting error: E (DF LCCSD) E (LCCSD) b Error due to local fit domains: E (LDF LCCSD,robust) E (DF LCCSD). The robust formula (16) is used. c Error due to local fit domains: E (LDF LCCSD,symm) E (DF LCCSD). The symmetric formula (17) is used. d AO basis set: cc-pvtz(f/p), fitting basis: 8s6p5d3f1g/4s3p2d1f, optimized for DF-MP2 [61]. TABLE III: Number of AO basis and fitting functions, compared to the average size of the different fitting domains for a series of test molecules. For all calculations except CALI/VTZ the cc-pvdz basis set together with the corresponding 7s5p4d2f/3s2p1d fitting basis optimized for DF-MP2 by Weigend et al. [61] was used. molecule a n AO b n FIT n([rs]) c n([rs] U ) d n([r] U ) e R fit = 0 R fit = 3 R fit = 0 R fit = 3 R fit = 0 R fit = 3 (Gly) (Gly) (Gly) (Gly) (Gly) (Gly) (Gly) (Gly) INDI CALI CALI/VTZ f a Number of AO basis functions. b Number of fitting basis functions. c Average size of center pair fitting domains [RS]. d Average size of united center pair fitting domains [RS] U. e Average size of united center fitting domains [R] U. f AO basis set: cc-pvtz(f/p), fitting basis: 8s6p5d3f1g/4s3p2d1f, optimized for DF-MP2 [61]. mains [RS] U, and the united centre fitting domains [R] U, respectively, for these test molecules. [RS] determines directly the computational cost of the solve and the assembly steps, whereas [RS] U and [R] U affect the cost of the 2-index transformation (12). The average size of these fitting domains ([RS] in particular) is clearly much smaller than the total number of fitting functions for all extended molecules and saturates beyond a certain size for the (Gly) n series (which of course is a prerequisite for the achievement of O(N ) scaling in LDF-LCCSD). In Table IV the CPU-cost for the calculation of the 4-ext integrals (rs tu) and (rs tu) in LCCSD and DF- LCCSD/LDF-LCCSD, respectively, is analyzed for the INDI and CALI molecule. Individual timings for (µν σλ)
10 10 TABLE IV: Analysis of CPU-times for INDI and CALI. LCCSD DF-LCCSD LDF-LCCSD,robust LDF-LCCSD,symm R fit = 0 R fit = 3 R fit = 0 R fit = 3 INDI, cc-pvdz a AO integrals c Transformation d Solve Assembly Total 4-ext e CALI, cc-pvdz a AO integrals c Transformation d Solve Assembly Total 4-ext e CALI, cc-pvtz(f/p) b AO integrals c Transformation d Solve Assembly Total 4-ext e a CPU time in seconds on an Athlon/1.2 GHz, compiler: INTEL F90, version 7.0 for LINUX b CPU time in seconds on a HP IA64/900 MHz, compiler: HP F90, version under HPUX c evaluation of integrals (µν σλ), or (P µν) d 4-index transformation (µν σλ) (rs tu), or 2-index transformation (P µν) (P rs) e overall time for generating 4-ext integrals (rs tu), or (rs tu) and (P µν) integral evaluation, 4- and 3-index transformation, and the cost for the solve and assembly steps are compared between LCCSD, DF-LCCSD and LDF- LCCSD. Substantial savings are achieved by virtue of density fitting. For the INDI case the overall CPU-time decreases by a factor of 14 between LCCSD and DF- LCCSD. A further factor of 3 is gained on going to LDF- LCCSD,symm with R fit = 0, which then is about 42 times faster than LCCSD. For comparison, in O(N ) DF- LMP2 [39] a factor of 9 is gained in the calculation of the exchange integrals relative to O(N ) LMP2 [9], for the same molecular system. For the CALI/VDZ molecule DF-LCCSD saves a factor of 28 relative to LCCSD. LDF- LCCSD,symm with R fit = 0, on the other hand, is only slightly faster than DF-LCCSD (611 compared to 747 seconds), and LDF-LCCSD with R fit = 3 bohr is even slower than the DF-LCCSD. The CALI molecule, due to the numerous conjugated double- and triple bonds, is a relatively unfavourable case for local correlation methods. The related LMOs extend over several centres, which also manifests in the length of the centre quadruples list {RSTU}, being only 34 times shorter than in the non-local case, whereas a factor of 344 is saved for the INDI molecule [13]. As a consequence, the fitting domains for CALI are all rather large, and in particular the [RS] U and [R] U domains exhibit only a small reduction in the number of fitting functions relative to the whole fitting basis (cf. Table III). Apparently the CALI molecule is a case where the crossover in CPU cost between DF- LCCSD and LDF-LCCSD is not yet reached. The situation changes somewhat if the bigger cc-pvtz(f/p) basis is used instead. Here, CALI/VTZ LDF-LCCSD,symm with R fit = 0 is about 40% faster than DF-LCCSD. Relative to the exact calculation of the 4-ext integrals in LCCSD LDF-LCCSD,symm with R fit = 0 wins by factors of 90 and 34 for the cc-pvtz(f/p) and cc-pvdz basis sets, respectively. Evidently, the savings due to the use of density fitting become larger for bigger basis sets. The O(N ) scaling behaviour of LDF-LCCSD is demonstrated for the poly-glycine peptide series in Figures 2 and 3. In Fig. 2 the timings of individual steps of the (rs tu) calculation are plotted against the chain length for DF-LCCSD (upper panel) and LDF-LCCSD,symm,R fit = 0 (lower panel), respectively. Without fitting domains, Integral evaluation, transformation, and the assembly step all exhibit O(N 2 ), the solve step even O(N 3 ) scaling, as anticipated from the discussion above. For the case of LDF-LCCSD, on the other hand, O(N ) scaling is observed in all computational steps early on. For the (Gly) 16 chain the (rs tu) calculation took 824 seconds on an 1.2 GHz Athlon, which is more than 5 times faster than without fitting
11 11 CPU-time / s CPU-time / s a) DF-LCCSD AO integrals Transformation Solve Assembly Total 4-ext b) LDF-LCCSD n CPU-time / s CPU-time / s a) DF-LCCSD,robust,R =3 AO integrals Transformation Solve Assembly Total 4-ext b) DF-LCCSD,symm,R = n fit fit FIG. 2: CPU times (in seconds, on an Athlon/1.2 GHz, compiler: INTEL F90, version 7.0 for LINUX) spent for the individual steps of the (rs tu) calculation as a function of the chain length n of the poly-glycine peptides (Gly) n, n = The cc-pvdz basis set together with the corresponding 7s5p4d2f/3s2p1d fitting basis optimized for DF-MP2 by Weigend et al. [61] was used. Upper panel (a): DF-LCCSD (full fitting basis) Lower panel (b): LDF-LCCSD,symm,R fit = 0, plotted to same scale as (a) FIG. 3: CPU times (in seconds, on an Athlon/1.2 GHz, compiler: INTEL F90, version 7.0 for LINUX) spent for the individual steps of the (rs tu) calculation as a function of the chain length n of the poly-glycine peptides (Gly) n, n = The cc-pvdz basis set together with the corresponding 7s5p4d2f/3s2p1d fitting basis optimized for DF- MP2 by Weigend et al. [61] was used. Upper panel (a): LDF-LCCSD,robust,R fit = 3 (using eq. (16) in assembly step) Lower panel (b): LDF-LCCSD,symm,R fit = 3 (using eq. (17) in assembly step) domains, and more than 16 times faster than the exact calculation of the (rs tu) integrals in LCCSD, i.e., without density fitting. Fig. 3 shows analogous plots of LDF-LCCSD,robust,R fit = 3 (upper panel) and LDF- LCCSD,symm,R fit = 3 (lower panel), respectively. With the parameter R fit now set to 3 bohr, which extends the fitting domains substantially (cf. Table III), O(N ) scaling still is clearly recognizable for all computational steps. The sole difference in the timings between LDF- LCCSD,robust and LDF-LCCSD,symm manifests in the more expensive assembly step of the former (by about a factor of 1.7 in these calculations) due to the use of eq. (16) instead of (17). The increase in the total time spent for the calculation of the (rs tu) integrals however is rather small. Fig. 4 finally illustrates the progress that has been made in the present work: O(N ) scaling for the exact computation of the 4-ext integrals was demonstrated in Ref. [13]. The plain use of the DFapproximation for these integrals yields significant com- putational savings, yet O(N ) scaling is lost and replaced by O(N 3 ) scaling (although with a small pre-factor). Finally, introducing the concept of locality also for the fitting functions restores O(N ) scaling again at a very small price with respect to accuracy. IV. CONCLUSIONS The calculation of the exact 4-ext integrals (rs tu), despite being an O(N ) step, constitutes (particularly for larger basis sets) the major bottleneck in our local CCSD method [13]. In the present work, density fitting has been applied for the approximate calculation of these integrals, which is faster by an order of magnitude or more compared to the O(N ) 4-index integral transformation in LCCSD. The additional fitting error in the correlation energy due to the use of approximate 4-ext integrals (rs tu) has been studied for a number of test molecules. It turns
12 12 CPU /s Calculation of 4-ext integrals (rs tu) LCCSD DF-LCCSD LDF-LCCSD n FIG. 4: Overall CPU times (in seconds, on an Athlon/1.2 GHz, compiler: INTEL F90, version 7.0 for LINUX) as a function of the chain length n of the poly-glycine peptides (Gly) n, n = , spent for the calculation of the exact 4-ext integrals (rs tu), and for the approximate 4-ext integrals (rs tu) via DF-LCCSD (full fitting basis) and LDF- LCCSD,symm,R fit = 0, respectively. The cc-pvdz basis set together with the corresponding 7s5p4d2f/3s2p1d fitting basis optimized for DF-MP2 by Weigend et al. [61] was used. out to be systematic and substantially smaller than the error due to the local ansatz. The fitting errors reported here for these DF-LCCSD calculations are slightly larger than those reported in a recent paper by Werner et al. for DF-LMP2 [39], which is due to the use of non-optimal fitting basis sets: the calculations reported here all employed fitting basis sets that are optimal for DF-MP2, i.e., optimized for the fitting of 2-ext, rather than 4-ext integrals. First tentative calculations with some test fitting basis sets already indicate, that a similar accuracy as in DF-LMP2 can be obtained in DF-LCCSD by using tailored fitting basis sets. In order to achieve O(N ) scaling in the context of the DF approximation locality must be exploited for the fitting functions, i.e. the range of fitting functions used to fit a specific local density ρ rs has to be confined to the spatial vicinity of ρ rs. A specification of such fitting domains for individual pairs of centres (RS) turned out to be adequate. Such a domain [RS] then carries all fitting functions on the centres RST and U of all relevant centre quadruples (RSTU) containing the centre pair (RS). Since the number of relevant centre pairs is O(N ), while the size of the fitting domains is O(1), the computational cost of e.g. the inversion of the Coulomb metric reduces from O(N 3 ) to O(N ). In fact, O(N ) scaling for all computational steps of the calculation of the (rs tu) integrals (including evaluation and transformation of the 3-index Coulomb integrals) was achieved within this Local Density Fitting LCCSD (LDF-LCCSD) approach. The use of centre pair specific fitting domains brings an additional difficulty though: since the fitting domains for the left density ρ rs (1) and the right density ρ tu (2) of the integral (rs tu) may now be different, Dunlap s robust expression for the approximate integral (rs tu) no longer simplifies to the usual simple form known as the V approximation. Therefore, in order to compute the (rs tu) to second order with respect to the fitting error in the densities, the full Dunlap formula must be implemented. In practice, however, it turns out, that a simpler symmetric formula, even though formally nonrobust, yields results that are virtually identical to those obtained with the formally robust expression. The reason for the exceptionally good behaviour of the symmetric formula must be related to the specification of the fitting domains. By construction, the domains for the left and right densities must be quite similar, thus rendering the symmetric formula almost robust. The additional fitting error of LDF-LCCSD relative to DF-LCCSD due to the use of local fitting domains is rather small. It is at least an order of magnitude smaller than the original fitting error of DF-LCCSD for all test cases considered here. For extended molecular systems the O(N ) LDF-LCCSD method, being faster than DF- LCCSD by about a factor of 3 4, appears to be method of choice. It is interesting to note that in contrast to the DF- LMP2 case the DF approximation for the 4-ext integrals does not lead to a reduction of the computational complexity with respect to the number of basis functions per centre N atom. The assembly step exhibits the same O(Natom) 5 scaling as the 4-index integral transformation in LCCSD. On the other hand, the assembly step can be performed with high efficiency. The cc-pvdz and cc-pvtz(f/p) calculations on the CALI molecule as reported in the previous section clearly demonstrate, that the overall savings of the DF approximation increase with the basis set: Relative to the exact calculation of the 4- ext integrals in LCCSD speedups by factors of 34 and 90 were observed, respectively, for these two basis sets. In practice, it turns out that for the 4-ext integrals in DF- LCCSD the computational savings due to density fitting are even higher than for the exchange integrals in DF- LMP2. When considering, for the case of the CALI molecule with the cc-pvtz(f/p) basis as an example, the overall CPU times of the whole coupled-cluster calculation (including the construction of the remaining integral sets, as well as the CC iterations and the LMP2 calculation), the use of the DF-approximation for the 4-ext integrals alone (as presented in this paper) lowers the CPU cost by more than a factor of two. Provided that the calculation of the G(E) term (cf. Refs. [12, 13]) is avoided in the CC iterations the construction of the 2-ext and 3-ext integral distributions now is responsible for 90 % of the overall CPU cost. The aim of the next implementation step therefore is to replace the related integral transformations and to compute these integrals also via density fitting, finally making it possible to efficiently employ bigger AO basis sets in local coupled cluster calculations.
On the selection of domains and orbital pairs in local correlation treatments
 On the selection of domains and orbital pairs in local correlation treatments Hans-Joachim Werner and Klaus Pflüger Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569
On the selection of domains and orbital pairs in local correlation treatments Hans-Joachim Werner and Klaus Pflüger Institut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569
Density-Fitting Approximations to the Electron Repulsion Integrals
 Density-Fitting Approximations to the Electron Repulsion Integrals C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Created on 19 July 2010 Here we will follow some
Density-Fitting Approximations to the Electron Repulsion Integrals C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Created on 19 July 2010 Here we will follow some
Methods for Treating Electron Correlation CHEM 430
 Methods for Treating Electron Correlation CHEM 430 Electron Correlation Energy in the Hartree-Fock approximation, each electron sees the average density of all of the other electrons two electrons cannot
Methods for Treating Electron Correlation CHEM 430 Electron Correlation Energy in the Hartree-Fock approximation, each electron sees the average density of all of the other electrons two electrons cannot
The orbital-specific-virtual local coupled cluster singles and doubles method
 The orbital-specific-virtual local coupled cluster singles and doubles method Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, Martin Schütz, and Hans-Joachim Werner Citation: J. Chem. Phys. 136, 144105
The orbital-specific-virtual local coupled cluster singles and doubles method Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, Martin Schütz, and Hans-Joachim Werner Citation: J. Chem. Phys. 136, 144105
The orbital-specific virtual local triples correction: OSV-L(T)
 The orbital-specific virtual local triples correction: OSV-L(T) Martin Schütz, Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, and Hans-Joachim Werner Citation: The Journal of Chemical Physics 138,
The orbital-specific virtual local triples correction: OSV-L(T) Martin Schütz, Jun Yang, Garnet Kin-Lic Chan, Frederick R. Manby, and Hans-Joachim Werner Citation: The Journal of Chemical Physics 138,
Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems
 John von Neumann Institute for Computing Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems Christof Hättig published in Computational Nanoscience: Do It Yourself, J. Grotendorst, S.
John von Neumann Institute for Computing Beyond Hartree-Fock: MP2 and Coupled-Cluster Methods for Large Systems Christof Hättig published in Computational Nanoscience: Do It Yourself, J. Grotendorst, S.
High Accuracy Local Correlation Methods: Computer Aided Implementation
 High Accuracy Local Correlation Methods: Computer Aided Implementation Marcel Nooijen Alexander Auer Princeton University University of Waterloo USA Canada So Hirata, PNNL Supported by: NSF ITR (Information
High Accuracy Local Correlation Methods: Computer Aided Implementation Marcel Nooijen Alexander Auer Princeton University University of Waterloo USA Canada So Hirata, PNNL Supported by: NSF ITR (Information
Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism
 Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism Stéphanie Essafi,*,1 Simone Tomasi, 2 Varinder K. Aggarwal, 1 Jeremy Harvey*,1 1 School of Chemistry,
Homologation of Boronic Esters with Organolithium Compounds: A Computational Assessment of Mechanism Stéphanie Essafi,*,1 Simone Tomasi, 2 Varinder K. Aggarwal, 1 Jeremy Harvey*,1 1 School of Chemistry,
Fast and accurate Coulomb calculation with Gaussian functions
 Fast and accurate Coulomb calculation with Gaussian functions László Füsti-Molnár and Jing Kong Q-CHEM Inc., Pittsburgh, Pennysylvania 15213 THE JOURNAL OF CHEMICAL PHYSICS 122, 074108 2005 Received 8
Fast and accurate Coulomb calculation with Gaussian functions László Füsti-Molnár and Jing Kong Q-CHEM Inc., Pittsburgh, Pennysylvania 15213 THE JOURNAL OF CHEMICAL PHYSICS 122, 074108 2005 Received 8
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
OVERVIEW OF QUANTUM CHEMISTRY METHODS
 OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
OVERVIEW OF QUANTUM CHEMISTRY METHODS Outline I Generalities Correlation, basis sets Spin II Wavefunction methods Hartree-Fock Configuration interaction Coupled cluster Perturbative methods III Density
Introduction to Computational Chemistry
 Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
Introduction to Computational Chemistry Vesa Hänninen Laboratory of Physical Chemistry Chemicum 4th floor vesa.hanninen@helsinki.fi September 10, 2013 Lecture 3. Electron correlation methods September
4 Post-Hartree Fock Methods: MPn and Configuration Interaction
 4 Post-Hartree Fock Methods: MPn and Configuration Interaction In the limit of a complete basis, the Hartree-Fock (HF) energy in the complete basis set limit (ECBS HF ) yields an upper boundary to the
4 Post-Hartree Fock Methods: MPn and Configuration Interaction In the limit of a complete basis, the Hartree-Fock (HF) energy in the complete basis set limit (ECBS HF ) yields an upper boundary to the
Local Approaches to the Simulation of Electron Correlation in complex systems
 Local Approaches to the Simulation of Electron Correlation in complex systems Martin Schütz Institut für Physikalische und Theoretische Chemie, Universität Regensburg Universitätsstraße 31, D-93040 Regensburg
Local Approaches to the Simulation of Electron Correlation in complex systems Martin Schütz Institut für Physikalische und Theoretische Chemie, Universität Regensburg Universitätsstraße 31, D-93040 Regensburg
We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include,
 1 Common Notation We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include, µ, ν, λ, σ: Primary basis functions, i.e., atomic orbitals (AOs) {φ µ ( r)}. The size
1 Common Notation We will use C1 symmetry and assume an RHF reference throughout. Index classes to be used include, µ, ν, λ, σ: Primary basis functions, i.e., atomic orbitals (AOs) {φ µ ( r)}. The size
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Jack Simons, Henry Eyring Scientist and Professor Chemistry Department University of Utah
 1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
1. Born-Oppenheimer approx.- energy surfaces 2. Mean-field (Hartree-Fock) theory- orbitals 3. Pros and cons of HF- RHF, UHF 4. Beyond HF- why? 5. First, one usually does HF-how? 6. Basis sets and notations
Section 3 Electronic Configurations, Term Symbols, and States
 Section 3 Electronic Configurations, Term Symbols, and States Introductory Remarks- The Orbital, Configuration, and State Pictures of Electronic Structure One of the goals of quantum chemistry is to allow
Section 3 Electronic Configurations, Term Symbols, and States Introductory Remarks- The Orbital, Configuration, and State Pictures of Electronic Structure One of the goals of quantum chemistry is to allow
Towards gas-phase accuracy for condensed phase problems
 Towards gas-phase accuracy for condensed phase problems Fred Manby Centre for Computational Chemistry, School of Chemistry University of Bristol STC 2006: Quantum Chemistry Methods and Applications Erkner,
Towards gas-phase accuracy for condensed phase problems Fred Manby Centre for Computational Chemistry, School of Chemistry University of Bristol STC 2006: Quantum Chemistry Methods and Applications Erkner,
Fully Automated Implementation of the Incremental Scheme: Application. to CCSD Energies for Hydrocarbons and Transition Metal Compounds
 Fully Automated Implementation of the Incremental Scheme: Application to CCSD Energies for Hydrocarbons and Transition Metal Compounds Joachim Friedrich 1, Michael Hanrath 1, Michael Dolg 1 1 Institute
Fully Automated Implementation of the Incremental Scheme: Application to CCSD Energies for Hydrocarbons and Transition Metal Compounds Joachim Friedrich 1, Michael Hanrath 1, Michael Dolg 1 1 Institute
Electron Correlation
 Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Local correlation in the virtual space in multireference singles and doubles configuration interaction
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 18 8 MAY 2003 ARTICLES Local correlation in the virtual space in multireference singles and doubles configuration interaction Derek Walter, Arun Venkatnathan,
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 18 8 MAY 2003 ARTICLES Local correlation in the virtual space in multireference singles and doubles configuration interaction Derek Walter, Arun Venkatnathan,
Lecture 5: More about one- Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory.
 Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Lecture 5: More about one- determinant wave functions Final words about the Hartree-Fock theory. First step above it by the Møller-Plesset perturbation theory. Items from Lecture 4 Could the Koopmans theorem
Computational Chemistry I
 Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Exercise 1: Structure and dipole moment of a small molecule
 Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Introduction to computational chemistry Exercise 1: Structure and dipole moment of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the dipole moment of a small
Chemistry 334 Part 2: Computational Quantum Chemistry
 Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Density Functional Theory. Martin Lüders Daresbury Laboratory
 Density Functional Theory Martin Lüders Daresbury Laboratory Ab initio Calculations Hamiltonian: (without external fields, non-relativistic) impossible to solve exactly!! Electrons Nuclei Electron-Nuclei
Density Functional Theory Martin Lüders Daresbury Laboratory Ab initio Calculations Hamiltonian: (without external fields, non-relativistic) impossible to solve exactly!! Electrons Nuclei Electron-Nuclei
Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule. Vesa Hänninen
 Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the electronic energy
Introduction to computational chemistry Exercise I: Structure and electronic energy of a small molecule Vesa Hänninen 1 Introduction In this exercise the equilibrium structure and the electronic energy
( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r)
 Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
Born Oppenheimer Approximation: Ĥ el ( R)Ψ el ( r;r) = E el ( R)Ψ el ( r;r) For a molecule with N electrons and M nuclei: Ĥ el What is E el (R)? s* potential surface Reaction Barrier Unstable intermediate
Performance of Hartree-Fock and Correlated Methods
 Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Chemistry 460 Fall 2017 Dr. Jean M. Standard December 4, 2017 Performance of Hartree-Fock and Correlated Methods Hartree-Fock Methods Hartree-Fock methods generally yield optimized geomtries and molecular
Lecture 4: methods and terminology, part II
 So theory guys have got it made in rooms free of pollution. Instead of problems with the reflux, they have only solutions... In other words, experimentalists will likely die of cancer From working hard,
So theory guys have got it made in rooms free of pollution. Instead of problems with the reflux, they have only solutions... In other words, experimentalists will likely die of cancer From working hard,
Building a wavefunction within the Complete-Active. Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy
 Building a wavefunction within the Complete-Active Active-Space Coupled-Cluster Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy Dmitry I. Lyakh (Karazin Kharkiv
Building a wavefunction within the Complete-Active Active-Space Coupled-Cluster Cluster with Singles and Doubles formalism: straightforward description of quasidegeneracy Dmitry I. Lyakh (Karazin Kharkiv
NWChem: Coupled Cluster Method (Tensor Contraction Engine)
 NWChem: Coupled Cluster Method (ensor Contraction Engine) What we want to solve H Ψ = E Ψ Many Particle Systems Molecular/Atomic Physics, Quantum Chemistry (electronic Schrödinger equations) Solid State
NWChem: Coupled Cluster Method (ensor Contraction Engine) What we want to solve H Ψ = E Ψ Many Particle Systems Molecular/Atomic Physics, Quantum Chemistry (electronic Schrödinger equations) Solid State
Basis sets for electron correlation
 Basis sets for electron correlation Trygve Helgaker Centre for Theoretical and Computational Chemistry Department of Chemistry, University of Oslo, Norway The 12th Sostrup Summer School Quantum Chemistry
Basis sets for electron correlation Trygve Helgaker Centre for Theoretical and Computational Chemistry Department of Chemistry, University of Oslo, Norway The 12th Sostrup Summer School Quantum Chemistry
Ab initio treatment of electron correlations in polymers: Lithium hydride
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 10 8 MARCH 2000 Ab initio treatment of electron correlations in polymers: Lithium hydride chain and beryllium hydride polymer Ayjamal Abdurahman a) Max-Planck-Institut
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 10 8 MARCH 2000 Ab initio treatment of electron correlations in polymers: Lithium hydride chain and beryllium hydride polymer Ayjamal Abdurahman a) Max-Planck-Institut
NWChem: Coupled Cluster Method (Tensor Contraction Engine)
 NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
NWChem: Coupled Cluster Method (Tensor Contraction Engine) Why CC is important?! Correlation effects are important!! CC is size-extensive theory: can be used to describe dissociation processes.! Higher-order
Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner
 JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 21 1 DECEMBER 2001 Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner Helena Larsen,
JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 21 1 DECEMBER 2001 Direct optimization of the atomic-orbital density matrix using the conjugate-gradient method with a multilevel preconditioner Helena Larsen,
Lec20 Fri 3mar17
 564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
Electron Correlation Methods
 Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
Electron Correlation Methods HF method: electron-electron interaction is replaced by an average interaction E HF c = E 0 E HF E 0 exact ground state energy E HF HF energy for a given basis set HF E c
QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory
 Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Electronic structure theory: Fundamentals to frontiers. 1. Hartree-Fock theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley National
Predictive Computing for Solids and Liquids
 Predictive Computing for Solids and Liquids So Hirata Department of Chemistry May 214 Blue Waters Symposium 1 Schrödinger equation for a water molecule 1-particle, 3-dimensional partial differential equation
Predictive Computing for Solids and Liquids So Hirata Department of Chemistry May 214 Blue Waters Symposium 1 Schrödinger equation for a water molecule 1-particle, 3-dimensional partial differential equation
Density functional calculation of nuclear magnetic resonance chemical shifts
 Density functional calculation of nuclear magnetic resonance chemical shifts Christoph van Wüllen Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, D-44780 Bochum, Germany Received 22 August
Density functional calculation of nuclear magnetic resonance chemical shifts Christoph van Wüllen Lehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, D-44780 Bochum, Germany Received 22 August
Fragmentation methods
 Fragmentation methods Scaling of QM Methods HF, DFT scale as N 4 MP2 scales as N 5 CC methods scale as N 7 What if we could freeze the value of N regardless of the size of the system? Then each method
Fragmentation methods Scaling of QM Methods HF, DFT scale as N 4 MP2 scales as N 5 CC methods scale as N 7 What if we could freeze the value of N regardless of the size of the system? Then each method
Coupled Cluster Theory and Tensor Decomposition Techniques
 Coupled Cluster Theory and Tensor Decomposition Techniques Alexander A. Auer Atomistic Modelling Group Interface Chemistry and Surface Engineering Hierarchy of quantum chemical methods Analysis of orbitals
Coupled Cluster Theory and Tensor Decomposition Techniques Alexander A. Auer Atomistic Modelling Group Interface Chemistry and Surface Engineering Hierarchy of quantum chemical methods Analysis of orbitals
Session 1. Introduction to Computational Chemistry. Computational (chemistry education) and/or (Computational chemistry) education
 Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Electronic energy optimisation in ONETEP
 Electronic energy optimisation in ONETEP Chris-Kriton Skylaris cks@soton.ac.uk 1 Outline 1. Kohn-Sham calculations Direct energy minimisation versus density mixing 2. ONETEP scheme: optimise both the density
Electronic energy optimisation in ONETEP Chris-Kriton Skylaris cks@soton.ac.uk 1 Outline 1. Kohn-Sham calculations Direct energy minimisation versus density mixing 2. ONETEP scheme: optimise both the density
MP2[V] A Simple Approximation to Second-Order Møller Plesset Perturbation Theory
![MP2[V] A Simple Approximation to Second-Order Møller Plesset Perturbation Theory MP2[V] A Simple Approximation to Second-Order Møller Plesset Perturbation Theory](/thumbs/77/75214269.jpg) pubs.acs.org/jctc MP2[V] A Simple Approximation to Second-Order Møller Plesset Perturbation Theory Jia Deng, Andrew T. B. Gilbert, and Peter M. W. Gill* Research School of Chemistry, The Australian National
pubs.acs.org/jctc MP2[V] A Simple Approximation to Second-Order Møller Plesset Perturbation Theory Jia Deng, Andrew T. B. Gilbert, and Peter M. W. Gill* Research School of Chemistry, The Australian National
High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules
 High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules Markus Schneider Fritz Haber Institute of the MPS, Berlin, Germany École Polytechnique Fédérale de Lausanne, Switzerland دانشگاه
High-level Quantum Chemistry Methods and Benchmark Datasets for Molecules Markus Schneider Fritz Haber Institute of the MPS, Berlin, Germany École Polytechnique Fédérale de Lausanne, Switzerland دانشگاه
Highly accurate quantum-chemical calculations
 1 Highly accurate quantum-chemical calculations T. Helgaker Centre for Theoretical and Computational Chemistry, Department of Chemistry, University of Oslo, Norway A. C. Hennum and T. Ruden, University
1 Highly accurate quantum-chemical calculations T. Helgaker Centre for Theoretical and Computational Chemistry, Department of Chemistry, University of Oslo, Norway A. C. Hennum and T. Ruden, University
Using BLIS for tensor computations in Q-Chem
 Using BLIS for tensor computations in Q-Chem Evgeny Epifanovsky Q-Chem BLIS Retreat, September 19 20, 2016 Q-Chem is an integrated software suite for modeling the properties of molecular systems from first
Using BLIS for tensor computations in Q-Chem Evgeny Epifanovsky Q-Chem BLIS Retreat, September 19 20, 2016 Q-Chem is an integrated software suite for modeling the properties of molecular systems from first
This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry.
 1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Chapter 3. The (L)APW+lo Method. 3.1 Choosing A Basis Set
 Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
Handbook of Computational Quantum Chemistry. DAVID B. COOK The Department of Chemistry, University of Sheffield
 Handbook of Computational Quantum Chemistry DAVID B. COOK The Department of Chemistry, University of Sheffield Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1998 CONTENTS 1 Mechanics and molecules 1 1.1
Handbook of Computational Quantum Chemistry DAVID B. COOK The Department of Chemistry, University of Sheffield Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1998 CONTENTS 1 Mechanics and molecules 1 1.1
Wave function methods for the electronic Schrödinger equation
 Wave function methods for the electronic Schrödinger equation Zürich 2008 DFG Reseach Center Matheon: Mathematics in Key Technologies A7: Numerical Discretization Methods in Quantum Chemistry DFG Priority
Wave function methods for the electronic Schrödinger equation Zürich 2008 DFG Reseach Center Matheon: Mathematics in Key Technologies A7: Numerical Discretization Methods in Quantum Chemistry DFG Priority
Ab initio calculations for potential energy surfaces. D. Talbi GRAAL- Montpellier
 Ab initio calculations for potential energy surfaces D. Talbi GRAAL- Montpellier A theoretical study of a reaction is a two step process I-Electronic calculations : techniques of quantum chemistry potential
Ab initio calculations for potential energy surfaces D. Talbi GRAAL- Montpellier A theoretical study of a reaction is a two step process I-Electronic calculations : techniques of quantum chemistry potential
C:\Users\Leonardo\Desktop\README_ELMO_NOTES.txt Montag, 7. Juli :48
 *********** * Input * *********** Besides the normal GAMESS-UK input directives, to perform an ELMO calculation one needs to specify the elmo keyword and to provide additional instructions that are contained
*********** * Input * *********** Besides the normal GAMESS-UK input directives, to perform an ELMO calculation one needs to specify the elmo keyword and to provide additional instructions that are contained
Tutorial I: IQ MOL and Basic DFT and MP2 Calculations 1 / 30
 Tutorial I: IQ MOL and Basic DFT and MP2 Calculations Q-Chem User Workshop, Denver March 21, 2015 1 / 30 2 / 30 Introduction to IQMOL DFT and MP2 Calculations 3 / 30 IQMOL and Q-CHEM IQMOL is an open-source
Tutorial I: IQ MOL and Basic DFT and MP2 Calculations Q-Chem User Workshop, Denver March 21, 2015 1 / 30 2 / 30 Introduction to IQMOL DFT and MP2 Calculations 3 / 30 IQMOL and Q-CHEM IQMOL is an open-source
1 Rayleigh-Schrödinger Perturbation Theory
 1 Rayleigh-Schrödinger Perturbation Theory All perturbative techniques depend upon a few simple assumptions. The first of these is that we have a mathematical expression for a physical quantity for which
1 Rayleigh-Schrödinger Perturbation Theory All perturbative techniques depend upon a few simple assumptions. The first of these is that we have a mathematical expression for a physical quantity for which
Post Hartree-Fock: MP2 and RPA in CP2K
 Post Hartree-Fock: MP2 and RPA in CP2K A tutorial Jan Wilhelm jan.wilhelm@chem.uzh.ch 4 September 2015 Further reading MP2 and RPA by Mauro Del Ben, Jürg Hutter, Joost VandeVondele Del Ben, M; Hutter,
Post Hartree-Fock: MP2 and RPA in CP2K A tutorial Jan Wilhelm jan.wilhelm@chem.uzh.ch 4 September 2015 Further reading MP2 and RPA by Mauro Del Ben, Jürg Hutter, Joost VandeVondele Del Ben, M; Hutter,
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 28, December 08, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Chemistry 4560/5560 Molecular Modeling Fall 2014
 Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Divergence in Møller Plesset theory: A simple explanation based on a two-state model
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 22 8 JUNE 2000 Divergence in Møller Plesset theory: A simple explanation based on a two-state model Jeppe Olsen and Poul Jørgensen a) Department of Chemistry,
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 22 8 JUNE 2000 Divergence in Møller Plesset theory: A simple explanation based on a two-state model Jeppe Olsen and Poul Jørgensen a) Department of Chemistry,
6. Iterative Methods for Linear Systems. The stepwise approach to the solution...
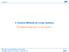 6 Iterative Methods for Linear Systems The stepwise approach to the solution Miriam Mehl: 6 Iterative Methods for Linear Systems The stepwise approach to the solution, January 18, 2013 1 61 Large Sparse
6 Iterative Methods for Linear Systems The stepwise approach to the solution Miriam Mehl: 6 Iterative Methods for Linear Systems The stepwise approach to the solution, January 18, 2013 1 61 Large Sparse
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride. Dimer. Philip Straughn
 Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
Multi-reference Density Functional Theory. COLUMBUS Workshop Argonne National Laboratory 15 August 2005
 Multi-reference Density Functional Theory COLUMBUS Workshop Argonne National Laboratory 15 August 2005 Capt Eric V. Beck Air Force Institute of Technology Department of Engineering Physics 2950 Hobson
Multi-reference Density Functional Theory COLUMBUS Workshop Argonne National Laboratory 15 August 2005 Capt Eric V. Beck Air Force Institute of Technology Department of Engineering Physics 2950 Hobson
Beyond the Hartree-Fock Approximation: Configuration Interaction
 Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
TDDFT in Chemistry and Biochemistry III
 TDDFT in Chemistry and Biochemistry III Dmitrij Rappoport Department of Chemistry and Chemical Biology Harvard University TDDFT Winter School Benasque, January 2010 Dmitrij Rappoport (Harvard U.) TDDFT
TDDFT in Chemistry and Biochemistry III Dmitrij Rappoport Department of Chemistry and Chemical Biology Harvard University TDDFT Winter School Benasque, January 2010 Dmitrij Rappoport (Harvard U.) TDDFT
Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little
 Vinay Ramasesh Getting Involved with Research Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little experience with actual scientific research. As a freshman, I d
Vinay Ramasesh Getting Involved with Research Before I entered the Texas Academy of Mathematics and Science as a junior, I had very little experience with actual scientific research. As a freshman, I d
THE CRYSCOR PROJECT : STATUS AND PROSPECTS
 THE CRYSCOR PROJECT : STATUS AND PROSPECTS ( # ) Cesare PISANI, Massimo BUSSO, Gabriella CAPECCHI, Silvia CASASSA, Roberto DOVESI, Lorenzo MASCHIO (*) Claudio ZICOVICH-WILSON ( ) Vic R. SAUNDERS ( & )
THE CRYSCOR PROJECT : STATUS AND PROSPECTS ( # ) Cesare PISANI, Massimo BUSSO, Gabriella CAPECCHI, Silvia CASASSA, Roberto DOVESI, Lorenzo MASCHIO (*) Claudio ZICOVICH-WILSON ( ) Vic R. SAUNDERS ( & )
Other methods to consider electron correlation: Coupled-Cluster and Perturbation Theory
 Other methods to consider electron correlation: Coupled-Cluster and Perturbation Theory Péter G. Szalay Eötvös Loránd University Institute of Chemistry H-1518 Budapest, P.O.Box 32, Hungary szalay@chem.elte.hu
Other methods to consider electron correlation: Coupled-Cluster and Perturbation Theory Péter G. Szalay Eötvös Loránd University Institute of Chemistry H-1518 Budapest, P.O.Box 32, Hungary szalay@chem.elte.hu
Chemistry 433 Computational Chemistry Fall Semester 2002 Dr. Rainer Glaser
 Chemistry 433 Computational Chemistry Fall Semester 2002 Dr. Rainer Glaser Second 1-Hour Examination Electron Correlation Methods Wednesday, November 6, 2002, 11:00-11:50 Name: Answer Key Question 1. Electron
Chemistry 433 Computational Chemistry Fall Semester 2002 Dr. Rainer Glaser Second 1-Hour Examination Electron Correlation Methods Wednesday, November 6, 2002, 11:00-11:50 Name: Answer Key Question 1. Electron
Calculations of band structures
 Chemistry and Physics at Albany Planning for the Future Calculations of band structures using wave-function based correlation methods Elke Pahl Centre of Theoretical Chemistry and Physics Institute of
Chemistry and Physics at Albany Planning for the Future Calculations of band structures using wave-function based correlation methods Elke Pahl Centre of Theoretical Chemistry and Physics Institute of
Same idea for polyatomics, keep track of identical atom e.g. NH 3 consider only valence electrons F(2s,2p) H(1s)
 XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
Electric properties of molecules
 Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Electric properties of molecules For a molecule in a uniform electric fielde the Hamiltonian has the form: Ĥ(E) = Ĥ + E ˆµ x where we assume that the field is directed along the x axis and ˆµ x is the
Introduction to Computational Quantum Chemistry: Theory
 Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
QUANTUM CHEMISTRY FOR TRANSITION METALS
 QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
QUANTUM CHEMISTRY FOR TRANSITION METALS Outline I Introduction II Correlation Static correlation effects MC methods DFT III Relativity Generalities From 4 to 1 components Effective core potential Outline
with a proper choice of the potential U(r). Clearly, we should include the potential of the ions, U ion (r):.
 The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
Consequently, the exact eigenfunctions of the Hamiltonian are also eigenfunctions of the two spin operators
 VI. SPIN-ADAPTED CONFIGURATIONS A. Preliminary Considerations We have described the spin of a single electron by the two spin functions α(ω) α and β(ω) β. In this Sect. we will discuss spin in more detail
VI. SPIN-ADAPTED CONFIGURATIONS A. Preliminary Considerations We have described the spin of a single electron by the two spin functions α(ω) α and β(ω) β. In this Sect. we will discuss spin in more detail
DFT calculations of NMR indirect spin spin coupling constants
 DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
Q-Chem 4.0: Expanding the Frontiers. Jing Kong Q-Chem Inc. Pittsburgh, PA
 Q-Chem 4.0: Expanding the Frontiers Jing Kong Q-Chem Inc. Pittsburgh, PA Q-Chem: Profile Q-Chem is a high performance quantum chemistry program; Contributed by best quantum chemists from 40 universities
Q-Chem 4.0: Expanding the Frontiers Jing Kong Q-Chem Inc. Pittsburgh, PA Q-Chem: Profile Q-Chem is a high performance quantum chemistry program; Contributed by best quantum chemists from 40 universities
Coupled-Cluster Perturbative Triples for Bond Breaking
 Coupled-Cluster Perturbative Triples for Bond Breaking Andrew G. Taube and Rodney J. Bartlett Quantum Theory Project University of Florida INT CC Meeting Seattle July 8, 2008 Why does chemistry need triples?
Coupled-Cluster Perturbative Triples for Bond Breaking Andrew G. Taube and Rodney J. Bartlett Quantum Theory Project University of Florida INT CC Meeting Seattle July 8, 2008 Why does chemistry need triples?
AN INTRODUCTION TO QUANTUM CHEMISTRY. Mark S. Gordon Iowa State University
 AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
Tensor network simulations of strongly correlated quantum systems
 CENTRE FOR QUANTUM TECHNOLOGIES NATIONAL UNIVERSITY OF SINGAPORE AND CLARENDON LABORATORY UNIVERSITY OF OXFORD Tensor network simulations of strongly correlated quantum systems Stephen Clark LXXT[[[GSQPEFS\EGYOEGXMZMXMIWUYERXYQGSYVWI
CENTRE FOR QUANTUM TECHNOLOGIES NATIONAL UNIVERSITY OF SINGAPORE AND CLARENDON LABORATORY UNIVERSITY OF OXFORD Tensor network simulations of strongly correlated quantum systems Stephen Clark LXXT[[[GSQPEFS\EGYOEGXMZMXMIWUYERXYQGSYVWI
This is called a singlet or spin singlet, because the so called multiplicity, or number of possible orientations of the total spin, which is
 9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
9. Open shell systems The derivation of Hartree-Fock equations (Chapter 7) was done for a special case of a closed shell systems. Closed shell means that each MO is occupied by two electrons with the opposite
T. Helgaker, Department of Chemistry, University of Oslo, Norway. T. Ruden, University of Oslo, Norway. W. Klopper, University of Karlsruhe, Germany
 1 The a priori calculation of molecular properties to chemical accuarcy T. Helgaker, Department of Chemistry, University of Oslo, Norway T. Ruden, University of Oslo, Norway W. Klopper, University of Karlsruhe,
1 The a priori calculation of molecular properties to chemical accuarcy T. Helgaker, Department of Chemistry, University of Oslo, Norway T. Ruden, University of Oslo, Norway W. Klopper, University of Karlsruhe,
CP2K: Selected Developments
 CP2K: Selected Developments Jürg Hutter Department of Chemistry University of Zurich Outline Introduction History and Performance Current and Future Developments Post-Hartree-Fock Methods GW Methods RPA
CP2K: Selected Developments Jürg Hutter Department of Chemistry University of Zurich Outline Introduction History and Performance Current and Future Developments Post-Hartree-Fock Methods GW Methods RPA
Dmitry I. Lyakh * National Center for Computational Sciences, Oak Ridge National Laboratory, Oak Ridge TN 37831
 1 Efficient Electronic Structure Theory via Hierarchical Scale-Adaptive Coupled-Cluster Formalism: I. Theory and Computational Complexity Analysis Dmitry I. Lyakh * National Center for Computational Sciences,
1 Efficient Electronic Structure Theory via Hierarchical Scale-Adaptive Coupled-Cluster Formalism: I. Theory and Computational Complexity Analysis Dmitry I. Lyakh * National Center for Computational Sciences,
Incomplete Cholesky preconditioners that exploit the low-rank property
 anapov@ulb.ac.be ; http://homepages.ulb.ac.be/ anapov/ 1 / 35 Incomplete Cholesky preconditioners that exploit the low-rank property (theory and practice) Artem Napov Service de Métrologie Nucléaire, Université
anapov@ulb.ac.be ; http://homepages.ulb.ac.be/ anapov/ 1 / 35 Incomplete Cholesky preconditioners that exploit the low-rank property (theory and practice) Artem Napov Service de Métrologie Nucléaire, Université
Introduction to Hartree-Fock Molecular Orbital Theory
 Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Origins of Mathematical Modeling in Chemistry Plato (ca. 428-347
Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Origins of Mathematical Modeling in Chemistry Plato (ca. 428-347
Computational Chemistry. An Introduction to Molecular Dynamic Simulations
 Computational Chemistry An Introduction to Molecular Dynamic Simulations Computational chemistry simulates chemical structures and reactions numerically, based in full or in part on the fundamental laws
Computational Chemistry An Introduction to Molecular Dynamic Simulations Computational chemistry simulates chemical structures and reactions numerically, based in full or in part on the fundamental laws
Introduction to multiconfigurational quantum chemistry. Emmanuel Fromager
 Institut de Chimie, Strasbourg, France Page 1 Emmanuel Fromager Institut de Chimie de Strasbourg - Laboratoire de Chimie Quantique - Université de Strasbourg /CNRS M2 lecture, Strasbourg, France. Notations
Institut de Chimie, Strasbourg, France Page 1 Emmanuel Fromager Institut de Chimie de Strasbourg - Laboratoire de Chimie Quantique - Université de Strasbourg /CNRS M2 lecture, Strasbourg, France. Notations
Instructor background for the discussion points of Section 2
 Supplementary Information for: Orbitals Some fiction and some facts Jochen Autschbach Department of Chemistry State University of New York at Buffalo Buffalo, NY 14260 3000, USA Instructor background for
Supplementary Information for: Orbitals Some fiction and some facts Jochen Autschbach Department of Chemistry State University of New York at Buffalo Buffalo, NY 14260 3000, USA Instructor background for
Towards a force field based on density fitting
 THE JOURNAL OF CHEMICAL PHYSICS 124, 104101 2006 Towards a force field based on density fitting Jean-Philip Piquemal a and G. Andrés Cisneros Laboratory of Structural Biology, National Institute of Environmental
THE JOURNAL OF CHEMICAL PHYSICS 124, 104101 2006 Towards a force field based on density fitting Jean-Philip Piquemal a and G. Andrés Cisneros Laboratory of Structural Biology, National Institute of Environmental
CP2K: the gaussian plane wave (GPW) method
 CP2K: the gaussian plane wave (GPW) method Basis sets and Kohn-Sham energy calculation R. Vuilleumier Département de chimie Ecole normale supérieure Paris Tutorial CPMD-CP2K CPMD and CP2K CPMD CP2K http://www.cpmd.org
CP2K: the gaussian plane wave (GPW) method Basis sets and Kohn-Sham energy calculation R. Vuilleumier Département de chimie Ecole normale supérieure Paris Tutorial CPMD-CP2K CPMD and CP2K CPMD CP2K http://www.cpmd.org
MODERN ELECTRONIC STRUCTURE THEORY: Electron Correlation
 5.61 Physical Chemistry Lecture #30 1 MODERN ELECTRONIC STRUCTURE THEORY: Electron Correlation In the previous lecture, we covered all the ingredients necessary to choose a good atomic orbital basis set.
5.61 Physical Chemistry Lecture #30 1 MODERN ELECTRONIC STRUCTURE THEORY: Electron Correlation In the previous lecture, we covered all the ingredients necessary to choose a good atomic orbital basis set.
Oslo node. Highly accurate calculations benchmarking and extrapolations
 Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Physics 202 Laboratory 5. Linear Algebra 1. Laboratory 5. Physics 202 Laboratory
 Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Practical Advice for Quantum Chemistry Computations. C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
 Practical Advice for Quantum Chemistry Computations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Choice of Basis Set STO-3G is too small 6-31G* or 6-31G** 6 probably
Practical Advice for Quantum Chemistry Computations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Choice of Basis Set STO-3G is too small 6-31G* or 6-31G** 6 probably
