Practice Test-3 Solution Physics
|
|
|
- Lorin Barker
- 5 years ago
- Views:
Transcription
1 NEET (ractice aper- ) Sluti. (a) has diesi T, hast ad diesi less.. (d) s a t a t t a t a t t----- () a t a t ractice Test- Sluti hysics t Subtractig = t a t t eliiatig t a a t. d. (b) a d. a () d p x y si gt ta x cs d d x si gt 4. d ta cs. (a) F f f F = g g i =. +. = N. kx 6. (d) w kx k as k k s re wrk is de i secd sprig. 7. (c) a k rt c k r rt k r t = krt wer = F d = a =. = k r t. 8. (a) e eu [cefficiet f restituti] u ' e gh g g Ttal distace = h + e h e h... e h H B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age 4 h e e....e e = h h e e d 9. (c) F 9 x 9 x d 9 x d 4 ( 9 x ) x 9x c, biusly kietic eergy is axiu fr x =. whe 9 x Thus, K.E ax J. (c) g T a T g a g a a a a c =.a g. (b) The bdy will e dw s fricti will be i upward directi. g si g cs a g si f a fr Rllig is withut slippig r.f r f a = r r I r gsi gsi f f f = g/ 6
2 . (d) = N f rtati =. (b) As there is exteral trque ttal agular etu ad ttal eergy is csered. 4. (b) V G G r R V r R R r. stress stress Y (c) Y = strai Y 6. a (d) ta g p p a g h gh a h h d is crrect pti as water will put se pressure at right ed als. d aerage teperature ,,, = thus teperature differece will be i reerse rder thus tie f clig rder is T T T 7. (b) 8. (b) steepess fr cure is less tha. Thus fr l is less tha. Thus pti (b) is crrect. / 9. (d) p = cstat T T. / k k. (b) T rs rs T T T p, keepig lue r cstat. f. (d) Ttal K.E. = RT f f RT RT [ f is degree f freed f xyge ad f is f arg] RT 4 RT = RT NEET (ractice aper- ) Sluti. (a) x = A si wt Whe x = A si t t = T 6 6 Whe x = A Sit = T T T. (c) KA f = a (fr Q) f = a (fr ) KA - f = f KA f = dy 4. (b) particle elcity = / y f x f Wae elcity = f y y f 4f s. (a) as,s t t S after secd bth pulses will cacel their aplitude ad de will be fred. Thus eergy will be kietic ly. 6. (a) E 8x r, E 8 At pit î 7. (b) q = q q t/rc e t / RC e t / RC I e RC R t lg I = lg R RC As graph is startig fr sae pit R is cstat. Sice graph is re bed thus slpe alue decreased thus C icreased. 8. (b) As electric field will exert frce i directi acrss plates, thus etu r elcity alg the plate will be sae thrughut ti f the particle. Thus, cs cs cs cs 9. (c) A B B C q q q q 4 r 4 r 4 r 4r (Assuig surfaces are etallic) r r r r r r r r as rr r r t t B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age
3 . (c) Applig cditi f balaced wheat ste bridge R R R R 4 R R4 R R. (c) Fr steady curret A = cstat A A s. Q p p. (a) Fr Galaeter I, R G R. R 8. R. (a) Due t agetic field trque will be applied bth cils i sae plae. 4. (d) usig Bit - Saart law B = B B [directi it plae] I r. (a) LI Q BA L I I = ML T A 6. (a) iitially iductr will bece shrt circuit. R S, R i,i, i R R R R R i i i 7. (a) u R 4/ 7. 6c / 4/ / u R S iage is i first ediu. 8. (c) Cditi f ttal iteral refracti ccurs at secd refracti. S fr first refracti si4 ( ) si r si( 9 r) ().sir cs r ta r = si r = fr (). / NEET (ractice aper- ) Sluti 9. (a) asr., r, r r r 4 4. (c) s ea life = t = T t N = N e = N N e. 7N e N N S percetage decay = N N N e N = 6% 4. (c) hc i e hc e hc e A B AB 4. (c) AND gate 4. (c) E Eh 44. (a) Light rays ges away after refracti thrugh air bubble iside water s it behaes as ccae lese. 4. (c) Aplitude will be haled I A K.A I I. 4 4 B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age
4 Cheistry 46. Higher perirty grup attached ppsite t duble bds. S bth are E. S pti (d) is crrect 47. Let suppse that chage i teperature is T supplyig 8 J f heat V RT U CVT Wrk de S, = Chage i iteral eergy S crrect aswer is (b). R CV C V R frdiatic NEET (ractice aper- ) Sluti gas 48. Na Ether S pti (d) is crrect. 49. ph lg[h ] S pti (b) is crrect.. All f the will shw geetrical iseris S crrect pti is (d). Rate = k [X] [C] X = k eq [A] [B] S, Rate = k [A] [B] [C]. Reactiity Steric hidrace S crrect pti is (a) [ N] [H ] [NH]. t t t S pti (b) is crrect 4. CN first attack at carb at f carbyl grup, s it is a uclephilic additi reacti S crrect pti is (b). Fe e Fe; E. 6 lt Fe e Fe;E.44 V.. (i) (ii) Fe e Fe ; E?..(iii) (i) (ii) = (iii) S, G G G E E E.6.44 E.88.8 =.77 V S pti (d) is crrect E 6. I Le pair f Nitrge are delcalized i etire rig ad fr a aratic cpud. S it will be least basic because datig electrs (le pair) rig will lse its araticity. S crrect pti is (d) OMgBr Ether CHCHO 7. C6H CH Br Mg C6H CH MgBr C6H CH C CH S pti (b) is crrect MgBr(OH) C 6H CH C CH B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age 4 OH H O
5 NEET (ractice aper- ) Sluti 8. (i) I CH CH C H is re stable tha CH CH CH C H because f hypercjugati. (ii) I CH NH C H ad C H OH structure f this is re stable, CH NH C H (iii) CH O C H is re stable because f resace. B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age is re stable because resatig (i) CH C H CH7 is re stable because f hypercjugatig structures. S crrect pti is (b) O % H SO 4 dilnaoh 9. CH CH HC C H CH C H CH CHO OH HgSO 4 C (Hydrati) (Aldl Re acti) S pti (d) is crrect 6. O zlysis f bezee O O H C C H fred S crrect aswer is (a) 6. Opti (a) is crrect 6. Aical AgNO will gie psitie test with terial alkyes. S crrect pti is (d) 6. Opti (b) is crrect. 64. Lidlr s catalyst yields cis-alkee hydrgeati f alkye. S crrect pti is (b) 6. C-rdiati uber is FCC is S (d) is crrect pti. 66. Le pair Le pair repulsi is strger tha le pair bd pair, ad le pair bd pair is strger tha bd pair bd pair S crrect aswer is (b) 67. Opti (b) is crrect 68. I, cetral at ctai le pair ad bd pair S crrect aswer is (a) 69. Mre electregatie cetral at strger will be acid. 7. Opti (b) is crrect 7. Deac prcess CuCl 4HCl O Cl HO Opti (b) is crrect 7. Cbalt hae c-rdiati 6, s [ C(NH) Cl] Cl s ttal uber f i iizati will be. Opti (c) is crrect 7. H E a E f a b = 9 9 Kcal S pti (c) is crrect 74. Cplexes differ i uber f upaired electrs S, pti (c) is crrect 7. Fr lecular rbital thery Opti (b) is crrect Z Eergy f electric species = Opti (b) is crrect because f iert pair effect 78. Vreal Z, s if Z < the V real < V ideal Videal S pti (b) is crrect S crrect pti is (c)
6 79. pti (d) is crrect 8. Relatie lwerig i apur pressure = Mle fracti f slute S, x B 98 8 M B 8 M B 8 S crrect pti is (c) 8. O the basis f stability f carbcati geerated real f Opti (b) is crrect 8. Equal uber cati & ai are issig S it is schttky effect S crrect pti is (b) 8. KOH NEET (ractice aper- ) Sluti CH CH CH(I)CH CH CH CH(OH)CH CH C C CH CH COOH HO Al O Cl [O] 84. S pti (a) is crrect [B] K, S by puttig alue f [C] K K & K [B] = 76.8% [C] =.7% S crrect pti is (d) S pti (c) is crrect CH COOH CHCOONa = = 9.7 S crrect pti is (c) HCl NaCl 87. Will declrize brie water ad als fr -ebered rig cyclic ahydride. S pti (c) is crrect g f CO ctai.77 g f carb.8 g f H O ctai.4 g f hydrge S calculatig epirical frula 89. (a) satisfy ctet rule 9. +M effect f NH icrease reactiity. Ad M effect f NO decreases reactiity tha bezee S pti (a) is crrect B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age 6
7 NEET (ractice aper- ) Sluti Bilgy 9. b 9. c 9. b 94. c 9. a 96. c 97. d 98. d 99. b. d. d. d. c 4. d. c 6. b 7. d 8. b 9. Bus. c. a. c. b 4. c. a 6. d 7. b 8. d 9. b. d. a. a. c 4. d. d 6. d 7. b 8. b 9. d. d. a. b. d 4. d. c 6. b 7. c 8. a 9. c 4. Bus 4. d 4. a 4. a 44. b 4. c 46. b 47. d 48. d 49. d. c. c. c. b 4. c. c 6. d 7. d 8. d 9. d 6. b 6. d 6. c 6. d 64. b 6. c 66. d 67. d 68. a 69. d 7. c 7. b 7. d 7. b 74. d 7. c 76. c 77. a 78. d 79. d 8. b B- 6/4-46 M, Brahaad Cly, Durgakud, Varaasi; age 7
Identical Particles. We would like to move from the quantum theory of hydrogen to that for the rest of the periodic table
 We wuld like t ve fr the quatu thery f hydrge t that fr the rest f the peridic table Oe electr at t ultielectr ats This is cplicated by the iteracti f the electrs with each ther ad by the fact that the
We wuld like t ve fr the quatu thery f hydrge t that fr the rest f the peridic table Oe electr at t ultielectr ats This is cplicated by the iteracti f the electrs with each ther ad by the fact that the
Hº = -690 kj/mol for ionization of n-propylene Hº = -757 kj/mol for ionization of isopropylene
 Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
Prblem 56. (a) (b) re egative º values are a idicati f mre stable secies. The º is mst egative fr the i-ryl ad -butyl is, bth f which ctai a alkyl substituet bded t the iized carb. Thus it aears that catis
Solutions. Definitions pertaining to solutions
 Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
Slutis Defiitis pertaiig t slutis Slute is the substace that is disslved. It is usually preset i the smaller amut. Slvet is the substace that des the disslvig. It is usually preset i the larger amut. Slubility
ALE 26. Equilibria for Cell Reactions. What happens to the cell potential as the reaction proceeds over time?
 Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
Name Chem 163 Secti: Team Number: AL 26. quilibria fr Cell Reactis (Referece: 21.4 Silberberg 5 th editi) What happes t the ptetial as the reacti prceeds ver time? The Mdel: Basis fr the Nerst quati Previusly,
MATH Midterm Examination Victor Matveev October 26, 2016
 MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
MATH 33- Midterm Examiati Victr Matveev Octber 6, 6. (5pts, mi) Suppse f(x) equals si x the iterval < x < (=), ad is a eve peridic extesi f this fucti t the rest f the real lie. Fid the csie series fr
Every gas consists of a large number of small particles called molecules moving with very high velocities in all possible directions.
 Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
Kietic thery f gases ( Kietic thery was develped by Berlli, Jle, Clasis, axwell ad Bltzma etc. ad represets dyamic particle r micrscpic mdel fr differet gases sice it thrws light the behir f the particles
MATHEMATICS 9740/01 Paper 1 14 Sep hours
 Cadidate Name: Class: JC PRELIMINARY EXAM Higher MATHEMATICS 9740/0 Paper 4 Sep 06 3 hurs Additial Materials: Cver page Aswer papers List f Frmulae (MF5) READ THESE INSTRUCTIONS FIRST Write yur full ame
Cadidate Name: Class: JC PRELIMINARY EXAM Higher MATHEMATICS 9740/0 Paper 4 Sep 06 3 hurs Additial Materials: Cver page Aswer papers List f Frmulae (MF5) READ THESE INSTRUCTIONS FIRST Write yur full ame
Unit -2 THEORY OF DILUTE SOLUTIONS
 Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
Uit - THEORY OF DILUTE SOLUTIONS 1) hat is sluti? : It is a hmgeus mixture f tw r mre cmpuds. ) hat is dilute sluti? : It is a sluti i which slute ccetrati is very less. 3) Give a example fr slid- slid
IIT JEE, 2005 (MAINS) SOLUTIONS CHEMISTRY 1
 IIT JEE, 005 (MAINS) SLUTINS EMISTRY 1 Disclaimer: Tis bklet ctais te questis f IIT-JEE 005, Mai Examiati based te memry recall f studets alg wit slutis prvided by te faculty f Brilliat Tutrials. Sice
IIT JEE, 005 (MAINS) SLUTINS EMISTRY 1 Disclaimer: Tis bklet ctais te questis f IIT-JEE 005, Mai Examiati based te memry recall f studets alg wit slutis prvided by te faculty f Brilliat Tutrials. Sice
cannot commute.) this idea, we can claim that the average value of the energy is the sum of such terms over all points in space:
 Che 441 Quatu Cheistry Ntes May, 3 rev VI. Apprxiate Slutis A. Variati Methd ad Huckel Mlecular Orbital (HMO) Calculatis Refereces: Liberles, Ch. 4, Atkis, Ch. 8, Paulig ad Wils Streitweiser, "MO Thery
Che 441 Quatu Cheistry Ntes May, 3 rev VI. Apprxiate Slutis A. Variati Methd ad Huckel Mlecular Orbital (HMO) Calculatis Refereces: Liberles, Ch. 4, Atkis, Ch. 8, Paulig ad Wils Streitweiser, "MO Thery
Chapter 5: Properties of Solutions
 hapter 5: rperties Slutis 1. Wax is a slid ixture hydrcar cpuds csistig lecules with lg chais car ats. Which slvet wuld yu expect t e st capale disslvig wax? N-plar will disslve i plar slvet a. HOH. H
hapter 5: rperties Slutis 1. Wax is a slid ixture hydrcar cpuds csistig lecules with lg chais car ats. Which slvet wuld yu expect t e st capale disslvig wax? N-plar will disslve i plar slvet a. HOH. H
x 2 x 3 x b 0, then a, b, c log x 1 log z log x log y 1 logb log a dy 4. dx As tangent is perpendicular to the x axis, slope
 The agle betwee the tagets draw t the parabla y = frm the pit (-,) 5 9 6 Here give pit lies the directri, hece the agle betwee the tagets frm that pit right agle Ratig :EASY The umber f values f c such
The agle betwee the tagets draw t the parabla y = frm the pit (-,) 5 9 6 Here give pit lies the directri, hece the agle betwee the tagets frm that pit right agle Ratig :EASY The umber f values f c such
Quantum Mechanics for Scientists and Engineers. David Miller
 Quatum Mechaics fr Scietists ad Egieers David Miller Time-depedet perturbati thery Time-depedet perturbati thery Time-depedet perturbati basics Time-depedet perturbati thery Fr time-depedet prblems csider
Quatum Mechaics fr Scietists ad Egieers David Miller Time-depedet perturbati thery Time-depedet perturbati thery Time-depedet perturbati basics Time-depedet perturbati thery Fr time-depedet prblems csider
A Hartree-Fock Calculation of the Water Molecule
 Chemistry 460 Fall 2017 Dr. Jea M. Stadard Nvember 29, 2017 A Hartree-Fck Calculati f the Water Mlecule Itrducti A example Hartree-Fck calculati f the water mlecule will be preseted. I this case, the water
Chemistry 460 Fall 2017 Dr. Jea M. Stadard Nvember 29, 2017 A Hartree-Fck Calculati f the Water Mlecule Itrducti A example Hartree-Fck calculati f the water mlecule will be preseted. I this case, the water
Examination No. 3 - Tuesday, Nov. 15
 NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
NAME (lease rit) SOLUTIONS ECE 35 - DEVICE ELECTRONICS Fall Semester 005 Examiati N 3 - Tuesday, Nv 5 3 4 5 The time fr examiati is hr 5 mi Studets are allwed t use 3 sheets f tes Please shw yur wrk, artial
2015 Regional Physics Exam Solution Set
 05 Reginal hysics Exa Slutin Set. Crrect answer: D Nte: [quantity] dentes: units f quantity WYSE Acadeic Challenge 05 Reginal hysics Exa SOLUTION SET r F r a lengthass length / tie ass length / tie. Crrect
05 Reginal hysics Exa Slutin Set. Crrect answer: D Nte: [quantity] dentes: units f quantity WYSE Acadeic Challenge 05 Reginal hysics Exa SOLUTION SET r F r a lengthass length / tie ass length / tie. Crrect
Physics 321 Solutions for Final Exam
 Page f 8 Physics 3 Slutins fr inal Exa ) A sall blb f clay with ass is drpped fr a height h abve a thin rd f length L and ass M which can pivt frictinlessly abut its center. The initial situatin is shwn
Page f 8 Physics 3 Slutins fr inal Exa ) A sall blb f clay with ass is drpped fr a height h abve a thin rd f length L and ass M which can pivt frictinlessly abut its center. The initial situatin is shwn
CPT 17. XI-LJ (Date: ) PHYSICS CHEMISTRY MATHEMATICS 1. (B) 31. (A) 61. (A) 2. (B) 32. (B) 62. (C) 3. (D) 33. (D) 63. (B) 4. (B) 34.
 CPT-7 / XI-LJ / NARAYANA I I T A C A D E M Y CPT 7 XI-LJ (Date:.0.7) CODE XI-LJ PHYSICS CHEMISTRY MATHEMATICS. (B). (A) 6. (A). (B). (B) 6. (C). (D). (D) 6. (B). (B). (C) 6. (C) 5. (D) 5. (C) 65. (A) 6.
CPT-7 / XI-LJ / NARAYANA I I T A C A D E M Y CPT 7 XI-LJ (Date:.0.7) CODE XI-LJ PHYSICS CHEMISTRY MATHEMATICS. (B). (A) 6. (A). (B). (B) 6. (C). (D). (D) 6. (B). (B). (C) 6. (C) 5. (D) 5. (C) 65. (A) 6.
BIT Chapters = =
 BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
BIT Chapters 17-0 1. K w = [H + ][OH ] = 9.5 10 14 [H + ] = [OH ] =.1 10 7 ph = 6.51 The slutin is neither acidic nr basic because the cncentratin f the hydrnium in equals the cncentratin f the hydride
Narayana IIT Academy
 -0-8_Sr. IIT_IZ_Ph-I_JEE-ADV_New Model-_P_GTA-9_Key&Sol s Narayaa IIT Academy INDIA Sec: Sr.IIT_IZ GTA-9 Date: -0-8 Time: 0:00 PM to 05:00 PM NEW Model-(P) Ma Marks: 64 KEY & SLUTINS MATHS AB ABC 3 AB
-0-8_Sr. IIT_IZ_Ph-I_JEE-ADV_New Model-_P_GTA-9_Key&Sol s Narayaa IIT Academy INDIA Sec: Sr.IIT_IZ GTA-9 Date: -0-8 Time: 0:00 PM to 05:00 PM NEW Model-(P) Ma Marks: 64 KEY & SLUTINS MATHS AB ABC 3 AB
Axial Temperature Distribution in W-Tailored Optical Fibers
 Axial Temperature Distributi i W-Tailred Optical ibers Mhamed I. Shehata (m.ismail34@yah.cm), Mustafa H. Aly(drmsaly@gmail.cm) OSA Member, ad M. B. Saleh (Basheer@aast.edu) Arab Academy fr Sciece, Techlgy
Axial Temperature Distributi i W-Tailred Optical ibers Mhamed I. Shehata (m.ismail34@yah.cm), Mustafa H. Aly(drmsaly@gmail.cm) OSA Member, ad M. B. Saleh (Basheer@aast.edu) Arab Academy fr Sciece, Techlgy
are specified , are linearly independent Otherwise, they are linearly dependent, and one is expressed by a linear combination of the others
 Chater 3. Higher Order Liear ODEs Kreyszig by YHLee;4; 3-3. Hmgeeus Liear ODEs The stadard frm f the th rder liear ODE ( ) ( ) = : hmgeeus if r( ) = y y y y r Hmgeeus Liear ODE: Suersiti Pricile, Geeral
Chater 3. Higher Order Liear ODEs Kreyszig by YHLee;4; 3-3. Hmgeeus Liear ODEs The stadard frm f the th rder liear ODE ( ) ( ) = : hmgeeus if r( ) = y y y y r Hmgeeus Liear ODE: Suersiti Pricile, Geeral
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
PHYSICS 151 Notes for Online Lecture #23
 PHYSICS 5 Ntes fr Online Lecture #3 Peridicity Peridic eans that sething repeats itself. r exaple, eery twenty-fur hurs, the Earth aes a cplete rtatin. Heartbeats are an exaple f peridic behair. If yu
PHYSICS 5 Ntes fr Online Lecture #3 Peridicity Peridic eans that sething repeats itself. r exaple, eery twenty-fur hurs, the Earth aes a cplete rtatin. Heartbeats are an exaple f peridic behair. If yu
1) When an object is placed at the center of curvature of a concave mirror, the image is.
 ) Whe a bjet is plae at the eter f urature f a ae mirrr, the image is. a) upright a irtual b) ierte a real ) larger a irtual ) Whe a bjet is plae ery far frm the fal pit f a ergig les, the image is. a)
) Whe a bjet is plae at the eter f urature f a ae mirrr, the image is. a) upright a irtual b) ierte a real ) larger a irtual ) Whe a bjet is plae ery far frm the fal pit f a ergig les, the image is. a)
ALL INDIA TEST SERIES
 AIT-FT-V-(Paper-) PM(l)-JEE(Advaced)/5 FIITJEE tudets Frm All Prgrams have agged 3 i Tp, 66 i Tp ad 7 i Tp 5 All Idia Raks. FIITJEE Perfrmace i JEE (Advaced), : 5 FIITJEE tudets frm assrm / Itegrated chl
AIT-FT-V-(Paper-) PM(l)-JEE(Advaced)/5 FIITJEE tudets Frm All Prgrams have agged 3 i Tp, 66 i Tp ad 7 i Tp 5 All Idia Raks. FIITJEE Perfrmace i JEE (Advaced), : 5 FIITJEE tudets frm assrm / Itegrated chl
PHYSICS. Suppose slit width s are equal, so they produces waves of equal intensity say I. Resultant intensity at any point
 PHYSICS. The figure shws fur pairs f plarizig sheets, see face-. Each pair is muted i the path f iitially uplarized light. The plarizig directi f each sheet (idicated by the dashed lie) is refereced t
PHYSICS. The figure shws fur pairs f plarizig sheets, see face-. Each pair is muted i the path f iitially uplarized light. The plarizig directi f each sheet (idicated by the dashed lie) is refereced t
Intermediate Division Solutions
 Itermediate Divisi Slutis 1. Cmpute the largest 4-digit umber f the frm ABBA which is exactly divisible by 7. Sluti ABBA 1000A + 100B +10B+A 1001A + 110B 1001 is divisible by 7 (1001 7 143), s 1001A is
Itermediate Divisi Slutis 1. Cmpute the largest 4-digit umber f the frm ABBA which is exactly divisible by 7. Sluti ABBA 1000A + 100B +10B+A 1001A + 110B 1001 is divisible by 7 (1001 7 143), s 1001A is
Chapter 9 Frequency-Domain Analysis of Dynamic Systems
 ME 43 Systes Dyaics & Ctrl Chapter 9: Frequecy Dai Aalyis f Dyaic Systes Systes Chapter 9 Frequecy-Dai Aalysis f Dyaic Systes 9. INTRODUCTION A. Bazue The ter Frequecy Respse refers t the steady state
ME 43 Systes Dyaics & Ctrl Chapter 9: Frequecy Dai Aalyis f Dyaic Systes Systes Chapter 9 Frequecy-Dai Aalysis f Dyaic Systes 9. INTRODUCTION A. Bazue The ter Frequecy Respse refers t the steady state
Study of Energy Eigenvalues of Three Dimensional. Quantum Wires with Variable Cross Section
 Adv. Studies Ther. Phys. Vl. 3 009. 5 3-0 Study f Eergy Eigevalues f Three Dimesial Quatum Wires with Variale Crss Secti M.. Sltai Erde Msa Departmet f physics Islamic Aad Uiversity Share-ey rach Ira alrevahidi@yah.cm
Adv. Studies Ther. Phys. Vl. 3 009. 5 3-0 Study f Eergy Eigevalues f Three Dimesial Quatum Wires with Variale Crss Secti M.. Sltai Erde Msa Departmet f physics Islamic Aad Uiversity Share-ey rach Ira alrevahidi@yah.cm
M09/4/CHEMI/SPM/ENG/TZ1/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
PART I: MULTIPLE CHOICE (60 POINTS) A m/s B m/s C m/s D m/s E. The car doesn t make it to the top.
 Uit II Test (practice test for fall ) (Ch. 6 8, 0) Name: Physics Notes: the actual test will hae 0 m/c ad - writte problems. Also the test will coer ch. 9 (static equilibrium), which was ot coered o this
Uit II Test (practice test for fall ) (Ch. 6 8, 0) Name: Physics Notes: the actual test will hae 0 m/c ad - writte problems. Also the test will coer ch. 9 (static equilibrium), which was ot coered o this
Solutions to Equilibrium Practice Problems
 Solutios to Equilibrium Practice Problems Chem09 Fial Booklet Problem 1. Solutio: PO 4 10 eq The expressio for K 3 5 P O 4 eq eq PO 4 10 iit 1 M I (a) Q 1 3, the reactio proceeds to the right. 5 5 P O
Solutios to Equilibrium Practice Problems Chem09 Fial Booklet Problem 1. Solutio: PO 4 10 eq The expressio for K 3 5 P O 4 eq eq PO 4 10 iit 1 M I (a) Q 1 3, the reactio proceeds to the right. 5 5 P O
ANSWERS, HINTS & SOLUTIONS PART TEST I PAPER-2 ANSWERS KEY
 AITS-PT-I (Paper-)-PCM-JEE(Advaced)/8 FIITJEE JEE(Advaced)-8 ANSWERS, HINTS & SOLUTIONS PART TEST I PAPER- ANSWERS KEY Q. No. PHYSICS Q. No. CHEMISTRY Q. No. MATHEMATICS ALL INDIA TEST SERIES. D. B 7.
AITS-PT-I (Paper-)-PCM-JEE(Advaced)/8 FIITJEE JEE(Advaced)-8 ANSWERS, HINTS & SOLUTIONS PART TEST I PAPER- ANSWERS KEY Q. No. PHYSICS Q. No. CHEMISTRY Q. No. MATHEMATICS ALL INDIA TEST SERIES. D. B 7.
PROBLEM Copyright McGraw-Hill Education. Permission required for reproduction or display. SOLUTION. v 1 = 4 km/hr = 1.
 PROLEM 13.119 35,000 Mg ocea lier has a iitial velocity of 4 km/h. Neglectig the frictioal resistace of the water, determie the time required to brig the lier to rest by usig a sigle tugboat which exerts
PROLEM 13.119 35,000 Mg ocea lier has a iitial velocity of 4 km/h. Neglectig the frictioal resistace of the water, determie the time required to brig the lier to rest by usig a sigle tugboat which exerts
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpeCurseWare http://cw.mit.edu 5.8 Small-Mlecule Spectrscpy ad Dyamics Fall 8 Fr ifrmati abut citig these materials r ur Terms f Use, visit: http://cw.mit.edu/terms. 5.8 Lecture #33 Fall, 8 Page f
MIT OpeCurseWare http://cw.mit.edu 5.8 Small-Mlecule Spectrscpy ad Dyamics Fall 8 Fr ifrmati abut citig these materials r ur Terms f Use, visit: http://cw.mit.edu/terms. 5.8 Lecture #33 Fall, 8 Page f
Chapter 7 Electrochemistry 7.2 Conductivity and its application
 Chapter 7 Electrocheistry 7.2 Conductivity and its application Out-class extensive reading: Levine: pp. 506-515, 16.5 electric conductivity 16.6 Electrical conductivity of electrolyte solutions Key proble:
Chapter 7 Electrocheistry 7.2 Conductivity and its application Out-class extensive reading: Levine: pp. 506-515, 16.5 electric conductivity 16.6 Electrical conductivity of electrolyte solutions Key proble:
1) When an object is placed outside the center of curvature of a convex mirror, the image is.
 ) Whe a bjet is plae utsie the eter f urature f a ex mirrr, the image is. a) upright a irtual b) ierte a smaller ) larger a real ) Whe a bjet is plae isie the fal pit f a ergig les, the image is. a) upright
) Whe a bjet is plae utsie the eter f urature f a ex mirrr, the image is. a) upright a irtual b) ierte a smaller ) larger a real ) Whe a bjet is plae isie the fal pit f a ergig les, the image is. a) upright
ELEC NCERT. 1. Which cell will measure standard electrode potential of copper electrode? (g,0.1 bar) H + (aq.,1 M) Cu 2+ (aq.
 I. Multiple Choice Questions (Type-I) 1. Which cell will easure standard electrode potential of copper electrode? Pt (s) H 2 (g,0.1 bar) H + (aq.,1 M) Cu 2+ (aq.,1m) Cu Pt(s) H 2 (g, 1 bar) H + (aq.,1
I. Multiple Choice Questions (Type-I) 1. Which cell will easure standard electrode potential of copper electrode? Pt (s) H 2 (g,0.1 bar) H + (aq.,1 M) Cu 2+ (aq.,1m) Cu Pt(s) H 2 (g, 1 bar) H + (aq.,1
Chapter 4. Problem Solutions
 Chapter 4. Prblem Slutis. The great majrity f alpha particles pass thrugh gases ad thi metal fils with deflectis. T what cclusi abut atmic structure des this bservati lead? The fact that mst particles
Chapter 4. Prblem Slutis. The great majrity f alpha particles pass thrugh gases ad thi metal fils with deflectis. T what cclusi abut atmic structure des this bservati lead? The fact that mst particles
Harmonic Motion (HM) Oscillation with Laminar Damping
 Harnic Mtin (HM) Oscillatin with Lainar Daping If yu dn t knw the units f a quantity yu prbably dn t understand its physical significance. Siple HM r r Hke' s Law: F k x definitins: f T / T / Bf x A sin
Harnic Mtin (HM) Oscillatin with Lainar Daping If yu dn t knw the units f a quantity yu prbably dn t understand its physical significance. Siple HM r r Hke' s Law: F k x definitins: f T / T / Bf x A sin
"NEET / AIIMS " SOLUTION (6) Avail Video Lectures of Experienced Faculty.
 07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
07 NEET EXAMINATION SOLUTION (6) Avail Vide Lectures f Exerienced Faculty Page Sl. The lean exressin which satisfies the utut f this lgic gate is C = A., Whichindicates fr AND gate. We can see, utut C
Section 5.2: Calorimetry and Enthalpy Tutorial 1 Practice, page 297
 Sectio 52: Calorietry ad Ethalpy Tutorial 1 Practice, page 297 1 Give: V 60 L ; T iitial 25 C ; T fial 75 C; d H2O(l) H2O(l) 100 g/l Required: theral eergy required, q Aalysis: q cδt Solutio: Step 1: Deterie
Sectio 52: Calorietry ad Ethalpy Tutorial 1 Practice, page 297 1 Give: V 60 L ; T iitial 25 C ; T fial 75 C; d H2O(l) H2O(l) 100 g/l Required: theral eergy required, q Aalysis: q cδt Solutio: Step 1: Deterie
2. Before we answer the question, here are four important terms relating to redox reactions and galvanic cells.
 CHAPTER SEVENTEEN ELECTROCHEMISTRY Fr Review 1. Electrchemistry is the study f the iterchage f chemical ad electrical eergy. A redx (xidati-reducti) reacti is a reacti i which e r mre electrs are trasferred.
CHAPTER SEVENTEEN ELECTROCHEMISTRY Fr Review 1. Electrchemistry is the study f the iterchage f chemical ad electrical eergy. A redx (xidati-reducti) reacti is a reacti i which e r mre electrs are trasferred.
DISTANCE LEARNING PROGRAMME
 DISTANCE EARNING PROGRAMME (Academic Session : - 7) EADER TEST SERIES / JOINT PACKAGE COURSE TARGET : PRE-MEDICA 7 Test Type : A INDIA OPEN TEST (MAJOR) Test Pattern : NEET-UG TEST DATE : - 4-7 ANSWER
DISTANCE EARNING PROGRAMME (Academic Session : - 7) EADER TEST SERIES / JOINT PACKAGE COURSE TARGET : PRE-MEDICA 7 Test Type : A INDIA OPEN TEST (MAJOR) Test Pattern : NEET-UG TEST DATE : - 4-7 ANSWER
CHEM 10123/10125, Exam 2
 CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
Dynamic Response of Second Order Mechanical Systems with Viscous Dissipation forces
 Hadut #a (pp. 1-39) Dyamic Respse f Secd Order Mechaical Systems with Viscus Dissipati frces d X d X + + = ext() t M D K X F dt dt Free Respse t iitial cditis ad F (t) = 0, Uderdamped, Critically Damped
Hadut #a (pp. 1-39) Dyamic Respse f Secd Order Mechaical Systems with Viscus Dissipati frces d X d X + + = ext() t M D K X F dt dt Free Respse t iitial cditis ad F (t) = 0, Uderdamped, Critically Damped
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
ALL INDIA TEST SERIES
 Frm Classrm/Integrated Schl Prgrams 7 in Tp, in Tp, 5 in Tp, 6 in Tp 5 ll India Ranks & Students frm Classrm /Integrated Schl Prgrams & 7 Students frm ll Prgrams have been warded a Rank in JEE (dvanced),
Frm Classrm/Integrated Schl Prgrams 7 in Tp, in Tp, 5 in Tp, 6 in Tp 5 ll India Ranks & Students frm Classrm /Integrated Schl Prgrams & 7 Students frm ll Prgrams have been warded a Rank in JEE (dvanced),
Q1. A string of length L is fixed at both ends. Which one of the following is NOT a possible wavelength for standing waves on this string?
 Term: 111 Thursday, January 05, 2012 Page: 1 Q1. A string f length L is fixed at bth ends. Which ne f the fllwing is NOT a pssible wavelength fr standing waves n this string? Q2. λ n = 2L n = A) 4L B)
Term: 111 Thursday, January 05, 2012 Page: 1 Q1. A string f length L is fixed at bth ends. Which ne f the fllwing is NOT a pssible wavelength fr standing waves n this string? Q2. λ n = 2L n = A) 4L B)
NUPOC STUDY GUIDE ANSWER KEY. Navy Recruiting Command
 NUPOC SUDY GUIDE ANSWER KEY Navy Recruiting Cmmand CHEMISRY. ph represents the cncentratin f H ins in a slutin, [H ]. ph is a lg scale base and equal t lg[h ]. A ph f 7 is a neutral slutin. PH < 7 is acidic
NUPOC SUDY GUIDE ANSWER KEY Navy Recruiting Cmmand CHEMISRY. ph represents the cncentratin f H ins in a slutin, [H ]. ph is a lg scale base and equal t lg[h ]. A ph f 7 is a neutral slutin. PH < 7 is acidic
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1 MEMO. Analytical Chemistry CMY 283
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1. Analytical Chemistry CMY 283. Time: 120 min Marks: 100 Pages: 6
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
SAFE HANDS & IIT-ian's PACE EDT-04 (JEE) Solutions
 ED- (JEE) Slutins Answer : Optin () ass f the remved part will be / I Answer : Optin () r L m (u csθ) (H) Answer : Optin () P 5 rad/s ms - because f translatin ωr ms - because f rtatin Cnsider a thin shell
ED- (JEE) Slutins Answer : Optin () ass f the remved part will be / I Answer : Optin () r L m (u csθ) (H) Answer : Optin () P 5 rad/s ms - because f translatin ωr ms - because f rtatin Cnsider a thin shell
: ) 9) 6 PM, 6 PM
 Physics 101 Sectio 3 Mar. 1 st : Ch. 7-9 review Ch. 10 Aoucemets: Test# (Ch. 7-9) will be at 6 PM, March 3 (6) Lockett) Study sessio Moday eveig at 6:00PM at Nicholso 130 Class Website: http://www.phys.lsu.edu/classes/sprig010/phys101-3/
Physics 101 Sectio 3 Mar. 1 st : Ch. 7-9 review Ch. 10 Aoucemets: Test# (Ch. 7-9) will be at 6 PM, March 3 (6) Lockett) Study sessio Moday eveig at 6:00PM at Nicholso 130 Class Website: http://www.phys.lsu.edu/classes/sprig010/phys101-3/
CHEM 108 (Spring-2008) Exam. 3 (105 pts)
 CHEM 08 (Spring-008) Exam. (05 pts) Name: --------------------------------------------------------------------------, CLID # -------------------------------- LAST NAME, First (Circle the alphabet segment
CHEM 08 (Spring-008) Exam. (05 pts) Name: --------------------------------------------------------------------------, CLID # -------------------------------- LAST NAME, First (Circle the alphabet segment
Concentration 0. 5 M solutions 1. 0 M solutions. Rates Fast Slow. Which factor would account for the faster reaction rate in Experiment 1?
 72. Consider the following experimental results: Experiment 1 Experiment 2 2+ - - 4 2 2 4 aq Reactants Fe ( aq) + MnO4 ( aq) MnO ( aq) + H C O ( ) Temperature 20 C 40 C Concentration 0. 5 M solutions 1.
72. Consider the following experimental results: Experiment 1 Experiment 2 2+ - - 4 2 2 4 aq Reactants Fe ( aq) + MnO4 ( aq) MnO ( aq) + H C O ( ) Temperature 20 C 40 C Concentration 0. 5 M solutions 1.
Difference of 2 kj per mole of propane! E = kj
 Ethaly, H Fr rcesses measured uder cstat ressure cditi, the heat the reacti is q. E = q + w = q P ext V he subscrit remids is that the heat measured is uder cstat ressure cditi. hermdyamics Slve r q q
Ethaly, H Fr rcesses measured uder cstat ressure cditi, the heat the reacti is q. E = q + w = q P ext V he subscrit remids is that the heat measured is uder cstat ressure cditi. hermdyamics Slve r q q
Motor Stability. Plateau and Mesa Burning
 Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
Mtr Stability Recall mass cservati fr steady erati ( =cstat) m eit m b b r b s r m icr m eit Is this cditi (it) stable? ly if rmally use.3
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
JEE-Main. Practice Test-3 Solution. Physics
 JEE-Main Practice Test- Sutin Phsics. (d) Differentia area d = rdr b b q = d 0 rdr g 0 e r a a. (d) s per Gauss aw eectric fu f eectric fied is reated with net charge encsed within Gaussian surface. Which
JEE-Main Practice Test- Sutin Phsics. (d) Differentia area d = rdr b b q = d 0 rdr g 0 e r a a. (d) s per Gauss aw eectric fu f eectric fied is reated with net charge encsed within Gaussian surface. Which
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
15.0 g Cr = 21.9 g Cr O g Cr 4 mol Cr mol Cr O
 WYSE Academic Challenge Sectinal Chemistry Exam 2008 SOLUTION SET 1. Crrect answer: B. Use PV = nrt t get: PV = nrt 2. Crrect answer: A. (2.18 atm)(25.0 L) = n(0.08206 L atm/ml K)(23+273) n = 2.24 ml Assume
WYSE Academic Challenge Sectinal Chemistry Exam 2008 SOLUTION SET 1. Crrect answer: B. Use PV = nrt t get: PV = nrt 2. Crrect answer: A. (2.18 atm)(25.0 L) = n(0.08206 L atm/ml K)(23+273) n = 2.24 ml Assume
SAFE HANDS & IIT-ian's PACE EDT-10 (JEE) SOLUTIONS
 . If their mea positios coicide with each other, maimum separatio will be A. Now from phasor diagram, we ca clearly see the phase differece. SAFE HANDS & IIT-ia's PACE ad Aswer : Optio (4) 5. Aswer : Optio
. If their mea positios coicide with each other, maimum separatio will be A. Now from phasor diagram, we ca clearly see the phase differece. SAFE HANDS & IIT-ia's PACE ad Aswer : Optio (4) 5. Aswer : Optio
KISHORE VAIGYANIK PROTSAHAN YOJANA Date : Duration : 3 Hours Max. Marks : 160 STREAM - SB/SX
 KISHORE VAIGYANIK PROTSAHAN YOJANA - 04 Date : 0--04 Duratio : Hours Max. Marks : 60 GENERAL INSTRUCTIONS STREAM - SB/SX The Test Booklet cosists of 0 questios. There are Two parts i the questio paper.
KISHORE VAIGYANIK PROTSAHAN YOJANA - 04 Date : 0--04 Duratio : Hours Max. Marks : 60 GENERAL INSTRUCTIONS STREAM - SB/SX The Test Booklet cosists of 0 questios. There are Two parts i the questio paper.
[1 & α(t & T 1. ' ρ 1
 NAME 89.304 - IGNEOUS & METAMORPHIC PETROLOGY DENSITY & VISCOSITY OF MAGMAS I. Desity The desity (mass/vlume) f a magma is a imprtat parameter which plays a rle i a umber f aspects f magma behavir ad evluti.
NAME 89.304 - IGNEOUS & METAMORPHIC PETROLOGY DENSITY & VISCOSITY OF MAGMAS I. Desity The desity (mass/vlume) f a magma is a imprtat parameter which plays a rle i a umber f aspects f magma behavir ad evluti.
concentrations (molarity) rate constant, (k), depends on size, speed, kind of molecule, temperature, etc.
 #73 Notes Unit 9: Kinetics and Equilibrium Ch. Kinetics and Equilibriums I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants,
#73 Notes Unit 9: Kinetics and Equilibrium Ch. Kinetics and Equilibriums I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants,
Reaction Kinetics Multiple Choice
 Reaction Kinetics Multiple Choice January 1999 1. Consider the reaction: Ca (s) + 2H 2 O (l) Ca(OH) 2 (aq) + H 2 (g) At a certain temperature, 2.50 g Ca reacts completely in 30.0 seconds. The rate of consumption
Reaction Kinetics Multiple Choice January 1999 1. Consider the reaction: Ca (s) + 2H 2 O (l) Ca(OH) 2 (aq) + H 2 (g) At a certain temperature, 2.50 g Ca reacts completely in 30.0 seconds. The rate of consumption
Name ID# Section # CH 1020 EXAM 3 Spring Form A
 Name ID# Sectio # CH 1020 EXAM 3 Sprig 2016 - Form A Fill i your ame, ID#, ad sectio o this test booklet. Fill i ad bubble i your ame, ID# (bubble 0 for C ), ad sectio o the scatro form. For questio #60
Name ID# Sectio # CH 1020 EXAM 3 Sprig 2016 - Form A Fill i your ame, ID#, ad sectio o this test booklet. Fill i ad bubble i your ame, ID# (bubble 0 for C ), ad sectio o the scatro form. For questio #60
Rates and Mechanisms of Chemical Reactions
 Rates ad Mechaisms f Chemical Reactis Why sme rxs prceed very fast ad thers require days, mths r eve years t prduce a detectable amt f prduct? H (g) + F (g) HF (g) (very fast) 3 H (g) + N (g) NH 3 (g)
Rates ad Mechaisms f Chemical Reactis Why sme rxs prceed very fast ad thers require days, mths r eve years t prduce a detectable amt f prduct? H (g) + F (g) HF (g) (very fast) 3 H (g) + N (g) NH 3 (g)
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
+ve 10 N. Note we must be careful about writing If mass is not constant: dt dt dt
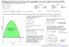 Force /N Moet is defied as the prodct of ass ad elocity. It is therefore a ector qatity. A ore geeral ersio of Newto s Secod Law is that force is the rate of chage of oet. I the absece of ay exteral force,
Force /N Moet is defied as the prodct of ass ad elocity. It is therefore a ector qatity. A ore geeral ersio of Newto s Secod Law is that force is the rate of chage of oet. I the absece of ay exteral force,
LC Oscillations. di Q. Kirchoff s loop rule /27/2018 1
 L Oscillatios Kirchoff s loop rule I di Q VL V L dt ++++ - - - - L 3/27/28 , r Q.. 2 4 6 x 6.28 I. f( x) f( x).. r.. 2 4 6 x 6.28 di dt f( x) Q Q cos( t) I Q si( t) di dt Q cos( t) 2 o x, r.. V. x f( )
L Oscillatios Kirchoff s loop rule I di Q VL V L dt ++++ - - - - L 3/27/28 , r Q.. 2 4 6 x 6.28 I. f( x) f( x).. r.. 2 4 6 x 6.28 di dt f( x) Q Q cos( t) I Q si( t) di dt Q cos( t) 2 o x, r.. V. x f( )
NURTURE COURSE TARGET : JEE (MAIN) Test Type : ALL INDIA OPEN TEST TEST DATE : ANSWER KEY HINT SHEET. 1. Ans.
 Test Type : LL INDI OPEN TEST Paper Code : 0000CT005 00 CLSSROOM CONTCT PROGRMME (cadeic Sessio : 05-06) NURTURE COURSE TRGET : JEE (MIN) 07 TEST DTE : - 0-06 NSWER KEY HINT SHEET Corporate Office : CREER
Test Type : LL INDI OPEN TEST Paper Code : 0000CT005 00 CLSSROOM CONTCT PROGRMME (cadeic Sessio : 05-06) NURTURE COURSE TRGET : JEE (MIN) 07 TEST DTE : - 0-06 NSWER KEY HINT SHEET Corporate Office : CREER
Advanced Placement. Chemistry. Integrated Rates
 Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Mathematics Extension 2
 009 HIGHER SCHOOL CERTIFICATE EXAMINATION Mathematics Etesio Geeral Istructios Readig time 5 miutes Workig time hours Write usig black or blue pe Board-approved calculators may be used A table of stadard
009 HIGHER SCHOOL CERTIFICATE EXAMINATION Mathematics Etesio Geeral Istructios Readig time 5 miutes Workig time hours Write usig black or blue pe Board-approved calculators may be used A table of stadard
ACTIVE FILTERS EXPERIMENT 2 (EXPERIMENTAL)
 EXPERIMENT ATIVE FILTERS (EXPERIMENTAL) OBJETIVE T desig secd-rder lw pass ilters usig the Salle & Key (iite psitive- gai) ad iiite-gai apliier dels. Oe circuit will exhibit a Butterwrth respse ad the
EXPERIMENT ATIVE FILTERS (EXPERIMENTAL) OBJETIVE T desig secd-rder lw pass ilters usig the Salle & Key (iite psitive- gai) ad iiite-gai apliier dels. Oe circuit will exhibit a Butterwrth respse ad the
20 Faraday s Law and Maxwell s Extension to Ampere s Law
 Chapter 20 Faraday s Law and Maxwell s Extensin t Ampere s Law 20 Faraday s Law and Maxwell s Extensin t Ampere s Law Cnsider the case f a charged particle that is ming in the icinity f a ming bar magnet
Chapter 20 Faraday s Law and Maxwell s Extensin t Ampere s Law 20 Faraday s Law and Maxwell s Extensin t Ampere s Law Cnsider the case f a charged particle that is ming in the icinity f a ming bar magnet
BC Calculus Review Sheet. converges. Use the integral: L 1
 BC Clculus Review Sheet Whe yu see the wrds.. Fid the re f the uuded regi represeted y the itegrl (smetimes f ( ) clled hriztl imprper itegrl).. Fid the re f differet uuded regi uder f() frm (,], where
BC Clculus Review Sheet Whe yu see the wrds.. Fid the re f the uuded regi represeted y the itegrl (smetimes f ( ) clled hriztl imprper itegrl).. Fid the re f differet uuded regi uder f() frm (,], where
Fall 2011 CHEM Test 4, Form A
 Fall 2011 CHEM 1110.40413 Test 4, Form A Part I. Multiple Choice: Clearly circle the best answer. (60 pts) Name: 1. The common constituent in all acid solutions is A) H 2 SO 4 B) H 2 C) H + D) OH 2. Which
Fall 2011 CHEM 1110.40413 Test 4, Form A Part I. Multiple Choice: Clearly circle the best answer. (60 pts) Name: 1. The common constituent in all acid solutions is A) H 2 SO 4 B) H 2 C) H + D) OH 2. Which
Sound Absorption Characteristics of Membrane- Based Sound Absorbers
 Purdue e-pubs Publicatis f the Ray W. Schl f Mechaical Egieerig 8-28-2003 Sud Absrpti Characteristics f Membrae- Based Sud Absrbers J Stuart Blt, blt@purdue.edu Jih Sg Fllw this ad additial wrks at: http://dcs.lib.purdue.edu/herrick
Purdue e-pubs Publicatis f the Ray W. Schl f Mechaical Egieerig 8-28-2003 Sud Absrpti Characteristics f Membrae- Based Sud Absrbers J Stuart Blt, blt@purdue.edu Jih Sg Fllw this ad additial wrks at: http://dcs.lib.purdue.edu/herrick
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Chemistry 12. Resource Exam B. Exam Booklet
 Chemistry 12 Resource Exam B Exam Booklet Contents: 21 pages Examination: 2 hours 50 multiple-choice questions in the Exam Booklet Additional Time Permitted: 60 minutes Province of British Columbia PART
Chemistry 12 Resource Exam B Exam Booklet Contents: 21 pages Examination: 2 hours 50 multiple-choice questions in the Exam Booklet Additional Time Permitted: 60 minutes Province of British Columbia PART
D.S.G. POLLOCK: TOPICS IN TIME-SERIES ANALYSIS STATISTICAL FOURIER ANALYSIS
 STATISTICAL FOURIER ANALYSIS The Furier Represetati f a Sequece Accrdig t the basic result f Furier aalysis, it is always pssible t apprximate a arbitrary aalytic fucti defied ver a fiite iterval f the
STATISTICAL FOURIER ANALYSIS The Furier Represetati f a Sequece Accrdig t the basic result f Furier aalysis, it is always pssible t apprximate a arbitrary aalytic fucti defied ver a fiite iterval f the
Phys101 Final Code: 1 Term: 132 Wednesday, May 21, 2014 Page: 1
 Phys101 Final Cde: 1 Term: 1 Wednesday, May 1, 014 Page: 1 Q1. A car accelerates at.0 m/s alng a straight rad. It passes tw marks that are 0 m apart at times t = 4.0 s and t = 5.0 s. Find the car s velcity
Phys101 Final Cde: 1 Term: 1 Wednesday, May 1, 014 Page: 1 Q1. A car accelerates at.0 m/s alng a straight rad. It passes tw marks that are 0 m apart at times t = 4.0 s and t = 5.0 s. Find the car s velcity
] after equilibrium has been established?
![] after equilibrium has been established? ] after equilibrium has been established?](/thumbs/74/71209406.jpg) Chemistry 1 Solubility Equilibrium onster Review 1. A saturated solution forms when a 0. 10 mol of salt is added to 10. L of water. The salt is A. Li S B. CuBr C. Zn( OH) ( ) D. NH CO 4. Consider the following
Chemistry 1 Solubility Equilibrium onster Review 1. A saturated solution forms when a 0. 10 mol of salt is added to 10. L of water. The salt is A. Li S B. CuBr C. Zn( OH) ( ) D. NH CO 4. Consider the following
[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.
![[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34. [ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.](/thumbs/74/69911105.jpg) .. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
.. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
M11/4/CHEMI/SPM/ENG/TZ2/XX CHEMISTRY STANDARD LEVEL PAPER 1. Monday 9 May 2011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Engineering Mechanics Dynamics & Vibrations. Engineering Mechanics Dynamics & Vibrations Plane Motion of a Rigid Body: Equations of Motion
 1/5/013 Egieerig Mechaics Dyaics ad Vibratios Egieerig Mechaics Dyaics & Vibratios Egieerig Mechaics Dyaics & Vibratios Plae Motio of a Rigid Body: Equatios of Motio Motio of a rigid body i plae otio is
1/5/013 Egieerig Mechaics Dyaics ad Vibratios Egieerig Mechaics Dyaics & Vibratios Egieerig Mechaics Dyaics & Vibratios Plae Motio of a Rigid Body: Equatios of Motio Motio of a rigid body i plae otio is
dm dt = 1 V The number of moles in any volume is M = CV, where C = concentration in M/L V = liters. dcv v
 Mg: Pcess Aalyss: Reac ae s defed as whee eac ae elcy lue M les ( ccea) e. dm he ube f les ay lue s M, whee ccea M/L les. he he eac ae beces f a hgeeus eac, ( ) d Usually s csa aqueus eeal pcesses eac,
Mg: Pcess Aalyss: Reac ae s defed as whee eac ae elcy lue M les ( ccea) e. dm he ube f les ay lue s M, whee ccea M/L les. he he eac ae beces f a hgeeus eac, ( ) d Usually s csa aqueus eeal pcesses eac,
Calorimetry, Heat and ΔH Problems
 Calorimetry, Heat and ΔH Problems 1. Calculate the quantity of heat involved when a 70.0g sample of calcium is heated from 22.98 C to 86.72 C. c Ca= 0.653 J/g C q = 2.91 kj 2. Determine the temperature
Calorimetry, Heat and ΔH Problems 1. Calculate the quantity of heat involved when a 70.0g sample of calcium is heated from 22.98 C to 86.72 C. c Ca= 0.653 J/g C q = 2.91 kj 2. Determine the temperature
Solutions to Midterm II. of the following equation consistent with the boundary condition stated u. y u x y
 Sltis t Midterm II Prblem : (pts) Fid the mst geeral slti ( f the fllwig eqati csistet with the bdary cditi stated y 3 y the lie y () Slti : Sice the system () is liear the slti is give as a sperpsiti
Sltis t Midterm II Prblem : (pts) Fid the mst geeral slti ( f the fllwig eqati csistet with the bdary cditi stated y 3 y the lie y () Slti : Sice the system () is liear the slti is give as a sperpsiti
Electrochemistry Redox Half-Reactions
 Electrchemistry Electrchemistry deals with the relatiship betwee chemical chage ad electricity Electrchemical s (tw types) Galvaic s use a sptaeus ( G < 0) reacti t prduce electricity (batteries) Electrlytic
Electrchemistry Electrchemistry deals with the relatiship betwee chemical chage ad electricity Electrchemical s (tw types) Galvaic s use a sptaeus ( G < 0) reacti t prduce electricity (batteries) Electrlytic
Electrochemistry. Reduction: the gaining of electrons. Reducing agent (reductant): species that donates electrons to reduce another reagent.
 Electrchemistry Review: Reductin: the gaining f electrns Oxidatin: the lss f electrns Reducing agent (reductant): species that dnates electrns t reduce anther reagent. Oxidizing agent (xidant): species
Electrchemistry Review: Reductin: the gaining f electrns Oxidatin: the lss f electrns Reducing agent (reductant): species that dnates electrns t reduce anther reagent. Oxidizing agent (xidant): species
Electrostatics. . where,.(1.1) Maxwell Eqn. Total Charge. Two point charges r 12 distance apart in space
 Maxwell Eq. E ρ Electrstatics e. where,.(.) first term is the permittivity i vacuum 8.854x0 C /Nm secd term is electrical field stregth, frce/charge, v/m r N/C third term is the charge desity, C/m 3 E
Maxwell Eq. E ρ Electrstatics e. where,.(.) first term is the permittivity i vacuum 8.854x0 C /Nm secd term is electrical field stregth, frce/charge, v/m r N/C third term is the charge desity, C/m 3 E
Narayana IIT Academy
 Narayaa IIT Academy INDIA XII_IC_SPARK JEE-MAIN Date: 3-7-7 Time : 3 Hours CPT -5 Ma Marks : 36 KEY SHEET PHYSICS ) B ) C 3) A 4) C 5) B 6) A 7) A 8) C 9) D ) A ) B ) C 3) D 4) B 5) A 6) A 7) C 8) C 9)
Narayaa IIT Academy INDIA XII_IC_SPARK JEE-MAIN Date: 3-7-7 Time : 3 Hours CPT -5 Ma Marks : 36 KEY SHEET PHYSICS ) B ) C 3) A 4) C 5) B 6) A 7) A 8) C 9) D ) A ) B ) C 3) D 4) B 5) A 6) A 7) C 8) C 9)
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
Find this material useful? Yu can help ur team t keep this site up and bring yu even mre cntent cnsider dnating via the link n ur site. Still having truble understanding the material? Check ut ur Tutring
L. A. P. T. Quick Look at Slow and Fast Light
 Quick Lk at Slw ad Fast Light Ceter fr Phtic Cmmuicati ad Cmputig Labratry fr Atmic ad Phtic Techlgy Ccept f Phase Velcity f a Mchrmatic Wave Mchrmatic plae wave z z 1 (c/) t 1 z Phase frt E ( ) i( k z
Quick Lk at Slw ad Fast Light Ceter fr Phtic Cmmuicati ad Cmputig Labratry fr Atmic ad Phtic Techlgy Ccept f Phase Velcity f a Mchrmatic Wave Mchrmatic plae wave z z 1 (c/) t 1 z Phase frt E ( ) i( k z
