Dielectric properties of liquid ethanol. A computer simulation study
|
|
|
- Sabrina Lambert
- 5 years ago
- Views:
Transcription
1 Dielectric properties of liquid ethanol. A computer simulation study Leonor Saiz, Elvira Guàrdia, and Joan-Àngel Padró Citation: The Journal of Chemical Physics 113, 2814 (2000); doi: / View online: View Table of Contents: Published by the AIP Publishing Articles you may be interested in Liquid 1-propanol studied by neutron scattering, near-infrared, and dielectric spectroscopy J. Chem. Phys. 140, (2014); / Are dipolar liquids ferroelectric? Simulation studies J. Chem. Phys. 126, (2007); / Free energy of liquid water from a computer simulation via cell theory J. Chem. Phys. 126, (2007); / Dielectric relaxation of supercritical water: Computer simulations J. Chem. Phys. 113, 3499 (2000); / Spectroscopic and dielectric properties of liquid water: A molecular dynamics simulation study J. Chem. Phys. 109, 4911 (1998); /
2 JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 7 15 AUGUST 2000 Dielectric properties of liquid ethanol. A computer simulation study Leonor Saiz Departament de Física Fonamental, Universitat de Barcelona, Diagonal 647, E Barcelona, Spain Elvira Guàrdia Departament de Física i Enginyeria Nuclear, Universitat Politècnica de Catalunya, Sor Eulàlia D Anzizu, Campus Nord, Mòdul B4-B5, E Barcelona, Spain Joan-Àngel Padró Departament de Física Fonamental, Universitat de Barcelona, Diagonal 647, E Barcelona, Spain Received 17 April 2000; accepted 18 May 2000 Static and dynamic dielectric properties of liquid ethanol have been studied as a function of the wave-vector number by computer simulation. Molecular dynamics simulations at room temperature have been performed using the optimized potentials for liquid simulations OPLS potential model proposed by Jorgensen J. Phys. Chem. 90, The time dependent correlation functions of the longitudinal and transverse components of the dipole density as well as the individual and total dipole moment autocorrelation functions have been calculated. The infrared spectra and the dielectric relaxation of the liquid have been also analyzed. Results have been compared with the available experimental data. Special attention has been dedicated to investigate the molecular origin of the different analyzed properties American Institute of Physics. S I. INTRODUCTION The study of dielectric properties is fundamental for a deep understanding of polar liquids. Computer simulations provide a suitable tool to analyze these properties at molecular level, providing a direct route from microscopic details to macroscopic properties of experimental interest. The molecular dynamics MD simulation method is appropriate for this purpose since it enables one to obtain both static and dynamic properties. However, difficulties arise due to the slow relaxation of the time correlation functions of interest and to the sensitivity to the long-range intermolecular interactions of the quantities involved. To overcome the first difficulty, extremely long simulations should be performed whereas fairly large systems are required to overcome the latter. Moreover, statistically accurate calculations of these collective properties will also require very long simulations since only one value of a given collective quantity is obtained at each time step. In the case of polar protic systems, the study of dielectric properties is especially relevant due to the important role played by these fluids as solvents in chemistry for a review on computer simulation of hydrogen bonded liquids, see Ref. 1. Among these liquids, water has focused the interest of most of the computer simulation studies. In the case of alcohols, only the dielectric properties of liquid methanol 2 4 and methanol water mixtures 5 have been analyzed by computer simulation. Many experimental studies of both static and dynamic dielectric properties of hydrogen bonded liquids have been carried out, but in the case of ethanol, the latter are restricted to the microwave region of the spectra 6 and to the infrared region 7 up to about 34 cm 1. To achieve a better understanding of the dielectric properties of monohydric alcohols, we show in this article the results obtained for liquid ethanol by means of MD simulations. We have computed the static dielectric constant and the transverse and longitudinal components of the dielectric permittivity at different wave-vector numbers paying special attention to the low wave-vector limit, which provides an alternative route to calculate the dielectric permittivity. We have also determined the time correlation functions of the longitudinal and transverse components of the dipole density as a function of the wave-vector number, as well as the total dipole moment autocorrelation function and its self- and distinct components. In addition, the dielectric relaxation and the far infrared spectra have been investigated. It is worth emphasizing that previous computer simulations of liquid ethanol were restricted to the computation of individual properties at different thermodynamic states The article is organized as follows. In Sec. II, a theoretical background is provided for the necessary definitions. Details of the simulations are given in Sec. III. Static dielectric properties are reported in Sec. IV. Section V is devoted to the results concerning dynamical dielectric properties, including time correlation functions and frequency spectra. The dynamics underlying the principal dielectric band in the microwave frequency region is analyzed. The infrared spectra is investigated in Sec. VI. Comparison between experimental and simulation data is presented throughout the article. The main results and conclusions are summarized in Sec. VII. II. THEORETICAL FRAMEWORK For an infinite system with cubic or spherical symmetry, the dielectric permittivity and susceptibility tensors are related through /2000/113(7)/2814/9/$ American Institute of Physics
3 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Dielectric properties of liquid ethanol 2815 L k, 0 L k, L k, 1 and T k, T 0 k, for the longitudinal and transverse components, respectively. is the dielectric constant at optical frequencies. For a model system with nonpolarizable molecules, 1. Linear response theory relates the susceptibility tensor 0 (k,) to correlation functions of the Fourier components of the dipole density of the system M (k,t). The two components of M (k,t), M k,tm L k,tm T k,t, can be expressed as follows: N M L k,t kˆ kˆ j t exp ikr CM j t j T k y M T k,0 2 2N 2, 11 where y 2, 12 k B T 0 with the molecular number density and the molecular dipole moment. Another quantity of interest, 16 R (k), results by dividing both sides of Eqs. 10 and 11 R k M T k,0 2, 13 2M L k,0 2 or equivalently R k x T k x L k, 14 where and N M T k,t 1kˆ kˆ j t exp ikr CM j t, 5 j1 where j (t) is the dipole moment of the jth molecule and r CM j (t) the position of its center-of-mass. The components of the susceptibility tensor are given by 14,15 0 A k, M A k,0 2 1i A Vk B T A k,, 6 0 where AL or T with L 1 and T 2, V is the volume of the sample, 0 is the vacuum permittivity, and A (k,) is the Fourier transform, A k, 0 dt A k,t exp it, of the normalized dipole density correlation function A (k,t) defined by A k,t M A k,t M A k,0. 8 M A k,0 2 Because of the short-ranged nature of the correlations which determine the dielectric permittivity, (k,) must become independent of the wave-vector number when k is sufficiently small, that is lim k 0 T k, lim L k,. k 0 The expressions for the components of the static wave-vector dependent dielectric constant can be deduced from Eqs. 1 and 2. For the static case, i.e., 0, they reduce to 7 9 x T k M T k,0 2 2N 2 and 15 x L k M L k,0 2 N In the low k limit the following condition must be fulfilled: lim k 0 Alternatively, L k lim T k. k 0 17 lim R k. 18 k 0 Equations 9 and 17 provide a route to determine and, respectively. However, due to the periodic boundary conditions used during the computer simulations, the k vectors which may be studied are restricted to k 2 L l,m,n, 19 where l, m, n are integers and L is the length of the cubic box. Thus, the smallest wave-vector which may be studied is k min 2/L. Whether the low k limit is reached can be tested through the consistency of the calculated L (k min,) and T (k min,). Another approach for the determination of is based on the calculation of the total dipole moment of the primitive cube of the simulation M (t) N j1 j (t). If the Ewald summation technique is used to handle with the longrange interactions and conducting walls boundary conditions are assumed, the relation between and the total dipole moment correlation is given by 17,18 L k y M L k,0 2 M 0 2 1i, 20 3Vk L k N 2 10 B T 0 where is the Fourier transform of the M autocorrelation and function
4 2816 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Saiz, Guàdia, and Padró t M t M M 0 2 Finally, the static dielectric constant is given by y 3 G k, where G k is the finite system Kirkwood correlation factor 22 G k M 0 2 N G k accounts for the correlations of the total dipole moment. It can be expressed as G k M 0 2 N 2 1 N1 2 i j, 24 where the averages of the product i j are performed for different molecules (i j). G k reflects the degree of correlation between the orientation of neighboring molecules. So, G k 1 if there is no correlation, G k 1 when molecular dipole moments tend to orient themselves parallel to each other, and G k 1 when molecular dipole moments tend to orient themselves in opposite directions. Once has been obtained from Eq. 22 the Kirkwood factor g k which describes orientational correlations in an infinite sample may be obtained by means of the relationship g k 2 G 3 k. 25 III. COMPUTER SIMULATION DETAILS Molecular dynamics simulations of liquid ethanol were performed at room temperature (T298 K) and the experimental density at normal pressure (d g/cm 3 ). The number of molecules in the central box was N125, which corresponds to a cubic box of side length L22.99 Å. Periodic boundary conditions were used. We adopted the optimized potentials for liquid simulations OPLS potential and the molecular model proposed by Jorgensen in Ref. 8. Each ethanol molecule consists of four interaction sites corresponding to the two methyl groups and the oxygen and the hydrogen atoms of the hydroxyl group. Hydrogen atoms of the methyl groups are not explicitly considered. Bond lengths and bond angles are kept constant, and the only intramolecular motions considered in the simulations are the torsions around the central C O bond. Interactions between sites are described by Lennard-Jones and Coulomb terms. The partial charges and molecular geometry yield a molecular dipole moment 2.22 D (1 D ccm) that is significantly larger than the experimental gas-phase value 19 gas 1.69 D. In recent work, 11,13 it has been shown that the OPLS potential reproduces fairly well the thermodynamic properties, the structure, and single particle dynamics of liquid ethanol at different thermodynamic states. The equations of motion were integrated using the algorithm proposed by Berendsen and co-workers in Ref. 20, which consists of a leap-frog Verlet algorithm with a weak TABLE I. Static dielectric constant and Kirkwood g factor. Method G k g k This work a Expt. b Expt. c a Statistal errors have been computed following the blocking method of Ref. 23. b Reference 6. c Reference 7. coupling to a thermal bath. We used a time step of 2.5 fs. Constraints were handled by means of the SHAKE method. 21 We performed two MD runs consisting of an initial equilibration period of about 100 ps and an equilibrium period of 1300 ps during which the properties were calculated. The results reported in the next sections are the average of those obtained from these two independent runs. The short-range forces were truncated at half the box length and the Ewald summation with conducting boundary conditions 22 was used for Coulomb interactions. IV. STATIC DIELECTRIC PROPERTIES Let us first examine the static properties obtained from the MD simulations following the scheme described previously. To this purpose we consider the more relevant quantities, namely, the Kirkwood correlation factor, the static dielectric constant, and the longitudinal and transverse wavevector dependent static dielectric constants. A. kä0 properties As discussed in Sec. II, one of the approaches to the computation of the static dielectric constant by MD simulations is from Eqs. 22, 23, and 25 in our case y The values of G k,, and g k found in this way are given in Table I and they are compared with the available experimental data. The Kirkwood g factor G k obtained from MD was greater than 1, as expected since there is a parallel alignment of the dipole moments of near-neighboring molecules. 11 The convergence of the cumulative average of the static dielectric constant as a function of the simulation FIG. 1. Convergence of the static dielectric constant as a function of the simulation length for each run. The dashed line corresponds to the final value of obtained by averaging the two runs.
5 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Dielectric properties of liquid ethanol 2817 TABLE II. Longitudinal and transverse components of the wave-vector dependent static dielectric constant. (k/k min ) 2 k/å 1 x L (k) x T (k) L (k) T (k) R (k) length is plotted in Fig. 1. This picture shows that the MD simulations are long enough to reach a plateau value for and that this plateau value is quite sensitive to the initial conditions. The value obtained by averaging the two runs, i.e., 162, is lower than the experimental data, but the agreement is similar to that achieved for liquid methanol. 2 4 Since part of the discrepancies are due to the value of the high-frequency dielectric constant, we find it more significant to compare the difference ( ). In this case, the agreement between experimental and MD simulation results is better. It should be noticed that the experimental value of corresponds to a polarizable system i.e., the real fluid, whereas the simulations have been carried out with a system of nonpolarizable molecules for which 1. B. Wave-vector dependent dielectric constant V. DYNAMIC DIELECTRIC PROPERTIES In this section we are concerned with the calculation of the dipole density time correlation functions and their power spectra. Special attention has been dedicated to the investigation of the origin of the different contributions to the total dipole moment autocorrelation function. A thorough comparison with experimental dielectric relaxation data, where collective motions are of special interest, has been also performed. A. Dipole density time correlation functions The normalized time correlation functions of the longitudinal L (k,t) and transverse T (k,t) components of the dipole density defined by Eq. 8 have been computed during the MD simulations for the five k values listed above. In Figs. 2 and 3, the L (k,t) and T (k,t) functions are plotted and a detail of the short-time regime is provided in the inset. The (t) function resulting from the MD simulations, as well as its different contributions are represented in Fig. 4. The relaxation of the longitudinal dipole density plays a central role in solvation dynamics. 28 As can be seen from Fig. 2, the main feature of L (k,t) is a fast initial decay with a strongly oscillatory character. The same behavior was previously observed for methanol 4 and water. 29 The initial decay of L (k,t) has been attributed to inertial and librational dynamics. 30 It is worthy to note that other polar nonhydrogen bonded liquids show a fast decay of L (k,t) to zero and then overdamped oscillations. 16 The other approach to the computation of the static dielectric constant is from the low k limit of the longitudinal and transverse components of the permittivity tensor. In this study, k min Å 1. We have calculated the wave-vector dependent quantities for the three smallest k values, namely, kk min, &k min, and )k min, as well as for k6k min and 3k min. The values of x L and x T found from the MD simulations by using Eqs. 15 and 16, the resulting L (k) and T (k) components of the permittivity tensor computed from Eqs. 10 and 11 and the R (k) values computed from Eq. 14 are collected in Table II. The statistical errors have been evaluated by using the blocking method as described in Ref. 23. The general trends found in the k dependence of the T (k) and L (k) functions agree with previous theoretical results for model polar liquids In the low k limit, we can see that the relation T (k min ) R (k min ) is satisfied within the statistical errors. The good agreement between the results obtained following the two approaches to the calculation of is an indication that the size of the simulated system is sufficiently large to be considered as representing a real isotropic fluid. As was already pointed out by Fonseca and Ladanyi, 2 T (k) provides a route much better than L (k) to determine the dielectric permittivity. While T (k) is a slowly varying function of k near k0, the transition L (k 0) occurs very sharply. Furthermore, L (k) has a singularity at x L (k)1/y and it is negative for yx L (k)1 see Eq. 10. As we can see in Table II, we have been able to obtain L (k) for values of k lower and higher than that corresponding to this singularity. To the best of our knowledge the only FIG. 2. Normalized time correlation function of the longitudinal component of the dipole moment density for (k/k previous computer simulation results showing the divergence min ) 2 1 full line, (k/k min ) 2 2 dotted line, (k/k min ) 2 3 dashed line, (k/k min ) 2 6 long dashed line, and of L (k) at small k are those reported for liquid water in a recent work of Bopp et al. 27 (k/k min ) 2 9 dotted dashed line. The inset shows the short-time behavior of L (k,t).
6 2818 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Saiz, Guàdia, and Padró 2 t j1 A j exp t/ j, t0.5 ps. 26 FIG. 3. Same as Fig. 2 for the transverse component of the dipole moment density T (k,t). Comparison of Figs. 2 and 3 reveals important differences in the relaxation of the transverse and longitudinal components of the dipole density. The decay of the transverse component is slower than that of its longitudinal counterpart. On the other hand, the behavior of the T (k,t) functions is similar to that of the total dipole moment autocorrelation function (t) see Figs. 3 and 4. For the transverse and total functions displayed in Figs. 3 and 4, it is possible to distinguish three separate regimes. The long-time regime which takes place for t1.5 ps can be modeled as an exponential function and describes the relaxation of the principal dielectric band of T (k,t) and (t). This regime takes place when inertial and librational dynamics do not contribute appreciately to the collective reorientation. The short-time regime, i.e., for t0.5 ps, which is shown in detail in the inserts of Figs. 3 and 4, has a fast initial inertial decay and then an oscillatory behavior which is related to the librational dynamics of the molecule. The oscillatory behavior is characteristic of hydrogen bonded liquids and it is absent in polar liquids without hydrogen bonding interactions such as acetonitrile. 16 The fast initial decay and the oscillatory behavior are both mainly due to the individual particle reorientation. This aspect will be discussed in more detail in Sec. V B. The intermediate regime can also be modeled as an exponential function but with a characteristic time smaller than the one corresponding to the long-time regime. The large and intermediate regimes for the different functions, where is any of the (t) or T (k,t) correlation functions, have been fitted to a function of the type For the whole time interval considered, these functions can be expressed by 2 t j1 A j exp t/ j 2 1 j1 A j librational-inertial t. 27 The fitted parameters are summarized in Table III. We observe that the amplitude A 1 of the main relaxation process decreases with increasing k, while that of the second process A 2 increases. Both relaxation times decrease as k increases, but the dependence on k is much more important in the case of 1 than for 2. On the other hand, the relaxation times corresponding to T (k min,t) are smaller than those of (t). The difference is specially significant in the case of 1. The so-called Debye relaxation time D is also reported in Table III. It was obtained from the following expression: 31 D lim 0 i. 28 For a Debye dielectric 32 it should be D 1. According to the results given in Table III, liquid ethanol does not behave like a Debye fluid since D is significantly lower than 1. B. Self- and distinct contributions to the total dipole moment autocorrelation function Decomposing M (t) M (0) into its components arising from the correlations of the dipole moment of a molecule and the correlations between distinct different molecular dipole moments, one obtains the following expression for (t) see Eq. 21 N2 t s t M 0 2 NN12 i 0 u j 0 d t, 29 M 0 2 where s (t) is the reorientational autocorrelation function of the individual dipoles and can be expressed by s t it i 0 i 0 2 u i t u i 0, 30 TABLE III. Two-exponential fit parameters for the dipole density time correlation functions resulting from MD simulations components: self, total, and transverse T, Debye relaxation time, and single-particle correlation time. Component (k/k min ) 2 A 1 1 /ps A 2 2 /ps D /ps s /ps Self Total T T T T T
7 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Dielectric properties of liquid ethanol 2819 TABLE IV. Dielectric relaxation parameters obtained from fitting three superimposed Debye equations to the experimental () and () data of liquid ethanol at room temperature. g 1 g 2 g 3 1 /ps 2 /ps 3 /ps Ref Ref FIG. 4. Normalized total dipole moment autocorrelation function (t) solid line, self-contribution G k 1 s (t) dotted dashed line, and distinct contribution (1G k 1 ) d (t) long dashed line. The inset shows the shorttime behavior of (t) full line and s (t) dotted dashed line. with u i (t) a unit vector along the molecular dipole moment, i (t)u i (t). The d (t) function is given by d t u it u j 0, u i 0 u j 0 i j, 31 and, using Eq. 23, u i 0 u j 0 G k1, i j. 32 N1 Thus, Eq. 29 can be written as t 1 G s k t 1 1 G k d t. 33 ing to our findings, liquid ethanol approximately obeys the Kivelson Madden relationship 14 D / s G k. C. Dielectric relaxation Dielectric relaxation of hydrogen bonded liquids has been extensively studied by experimental techniques. Data for liquid ethanol at room temperature are available in the microwave zone up to 3 cm 1 of the spectra 6 and up to frequencies of THz 34 cm 1 reaching the far infrared region. 7 The experimental dielectric relaxation provides information on the real and imaginary parts of the frequency dependent permittivity ()()i(). In the case of monohydric alcohols, experimental data are usually analyzed by considering an empirical model which consists of three Debye-type processes. 33 With this assumption, can be separated into the real and imaginary parts and j1 j1 n n g j 1 j 2 g j j 1 j 2, In our system, the prefactors of the self and distinct contributions are (1/G k ) and (11/G k )0.65 respectively. In Eqs. 35 and 36, 0.02, respectively. Thus, the main contribution to (t) j are the relaxation times and g corresponds to the correlations among different molecular j ( j j )/( ), j j1, are the weights of processes j contributing to the total dispersion, dipoles. beginning at the static permittivity Figure 4 shows the two contributions to (t). In the 1 and ending at the extrapolated high frequency permittivity inset of the same figure we show the short-time behavior of lim the total and self-autocorrelation functions. The selfcomponent for short times consists of a fast initial inertial (). The two available sets of experimental dielectric relaxation parameters for liquid ethanol at room temperature are decay with oscillations due to librational dynamics. For listed in Table IV. It is worthy to note that Kindt and longer times, s (t) shows a nearly exponential decay. The Schmuttenmaer 7 obtained significantly faster relaxation long-time decay of s (t) is faster than that of the total dipole moment autocorrelation function (t). The distinct times for the second and third Debye processes than those reported by Barthel and co-workers. 6 The component for short times does not present a fast inertial j relaxation times are comparable to the values reported in Table III for the decaying behavior. Yet, d (t) decays more slowly than total dipole moment autocorrelation function. In general, the s (t). Therefore, the behavior of (t) at long times will be relaxation times resulting from MD are smaller than the experimental estimations. The disagreement is especially sig- dominated by d (t). Thus, the long-time behavior of (t) should be associated with collective motions. nificant in the case of the main dispersion As expected, the relaxation times 1 and 2 found for 1 value. A similar discrepancy was observed in the case of methanol. 3,4 On the s (t) according to Eq. 26 are smaller than those corresponding to (t) see Table III. Also given in Table III is 2 value resulting from (t) is quite close to other hand, the the second relaxation time given in Ref. 7. According to our the single-particle correlation time s defined as findings, the relaxation time corresponding to the third Debye process cannot be determined from the decay of (t). s s t dt. 34 This is probably due to the very different order-of-magnitude 0 of 3 and 1. It should thus be determined from the decay of The relation between the total and single-particle correlation a function directly related to the physical origen of the corresponding relaxation process see, for instance, Ref. 34. times is interesting from a theoretical point of view. Accord
8 2820 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Saiz, Guàdia, and Padró FIG. 5. Real () and imaginary () parts of the frequency dependent permittivity. Molecular dynamics results solid line, experimental data from Ref. 6 dotted line and from Ref. 7 dashed line. Experimental lines are calculated from the parameters of Table IV. The arrows indicate the vertical scale corresponding to each curve. The MD results for (), () and the corresponding Cole Cole plot are compared with the experimental data in Figs. 5 and 6, respectively. The deviation of the Cole Cole plot from a semicircle confirms that liquid ethanol is not a Debye fluid. In the high frequency region of the Cole Cole plot, MD results exhibit the typical loops characteristic of fast librational motions. The dependence of the permittivity with frequency is qualitatively reproduced by the MD results, but we can observe some quantitative disagreements between the simulation results and experimental data. These disagreements are partly due to the discrepancies in the values of the static permittivity and the permittivity at optical frequencies. In Figs. 7 and 8, this is overcome by plotting the quantities (() )/( ) and ()/( ). The discrepancies are now effectively reduced. The disagreements in the decay of the (() )/( ) function as well as in the position of the peak of ()/( ) are directly related to the small value of the first relaxation time 1 resulting from MD. The discrepancies in the value of the second relaxation time 2 originate the differences observed in the intermediate frequency region of the Cole Cole plot. VI. INFRARED ABSORPTION The experimental infrared absorption spectrum of a system is given in terms of the infrared absorption coefficient FIG. 7. Same as Fig. 5 with the real and imaginary parts of scaled as (() )/( ) and ()/( ), respectively. and the imaginary part () of the frequency dependent dielectric constant. These quantities are related to the absorption line shape, I(), by exp I, 3Vcn 37 cn 42 1exp I, 3V 38 where (k B T) 1, h/2, h is the Planck constant, c is the speed of light in vacuum, n() is the refractive index of the medium, and I() is the Fourier transform of the unnormalized total dipole moment autocorrelation function I 1 dtm t M 0 exp it For classical systems, M (t) M (0) is real and symmetric, and I() can be written as I 1 0 dtm t M 0 cos t. 40 Equations 37, 38, and 40 can be used to relate infrared data with MD results. In the classical limit ( 0), Eq. 38 has the following form: cn dt t cos t, 0 41 FIG. 6. Cole Cole plot of the frequency dependent permittivity. Molecular dynamics results solid line, experimental data from Ref. 6 dotted line and from Ref. 7 dashed line. Experimental lines are calculated from the parameters of Table IV. FIG. 8. Same as Fig. 6 with the real and imaginary parts of scaled as (() )/( ) and ()/( ), respectively.
9 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Dielectric properties of liquid ethanol 2821 FIG times the real part of the transverse solid line and longitudinal dotted line components of the dipole moment densitity spectrum for k k min. Notice that the curve corresponding to L (k min,) has been multiplied by 0.1. and 750 cm 1 with a peak at approximately 640 cm 1. Similar characteristics are shown by the 2 Re ( L (k min,)) function in the same region. However, the maximum is located at slightly higher frequencies 690 cm 1. On the other hand, the very good agreement between 2 Re ( T (k min,)) and 2 Re (()) corroborates that the k 0 limit has effectively been reached in our simulations. To the best of our knowledge, there are not experimental infrared data to compare with our results. Experimental data corresponding to liquid methanol display a maximum at similar frequencies around 570 cm 1 according to Ref. 37 and around 700 cm 1 according to Ref. 38. To gain insight into the dynamic origin of the spectral features, we have analyzed the self- and distinct contributions to. By using Eq. 33, we obtain the following relation where (t) is defined by Eq. 21 and we have used the definition of given in Eq. 22. Since we are concerned with frequencies higher than that of the principal dispersion band, we neglect the frequency dependence of the refractive index 36 to write 2 Re. 42 An alternative procedure to calculate is through the k 0 limits of L (k,) and T (k,). As in the 0 case see Sec. IV B, the most straightforward route to () is provided by the transversal component. In the k k min limit, Eqs. 2 and 6 lead to Im T k min, Re T k min,. 43 The absorption coefficient is thus proportional to 2 Re ( T (k min,)). Due to the 2 factor, emphasizes the high frequency part of the spectra. Resonant motions lead to maxima in the absorption coefficient whereas exponential relaxations result in slowly increasing functions that reach a constant value for frequencies larger than the inverse of the characteristic time. The MD results for 2 Re ( T (k min,)) and 2 Re (()) are depicted in Figs. 9 and 10, respectively. In Fig. 9, the 2 Re ( L (k min,)) function is also plotted for the sake of completeness. The 2 Re ( T (k min,)) spectrum exhibits a broad maximum at frequencies located between 500 FIG times the real part of the power spectrum of the total dipole moment autocorrelation function solid line, self-contribution G k 1 2 s () dotted dashed line, and distinct contribution (1 G k 1 ) 2 d () dashed line. 2 1 G k 2 s 1 1 G k 2 d. 44 As can be seen from Fig. 10, the self-contribution G 1 k 2 Re ( s ()) has a broad band in the infrared region located between 400 and 800 cm 1, with a maximum at 600 cm 1. This frequency corresponds to an oscillation with a characteristic time of 0.05 ps. This high frequency maximum can be assigned to librational motions of the hydroxyl H atoms around the central methyl oxygen bond and, more specifically, to hydrogen bonded molecules. This assumption is supported by a previous analysis of the spectral densities in the liquid. 11 This analysis shows that the power spectrum of the velocity autocorrelation function of H-bonded hydrogen atoms has a band whose maximum is around 630 cm 1, while the H atoms that do not participate in a hydrogen bond give a band whose maximum is around 370 cm 1. Because of the very low percentage of nonbonded molecules, 11 this band at intermediate frequencies can not be appreciated in Fig. 10. The G 1 k 2 Re ( s ()) function also presents a maximum at low frequencies in the far infrared zone of the spectra. This peak is located at about 70 cm 1. It corresponds to an oscillation with a characteristic time ten times greater than the previous one 0.5 ps. In the case of water, the peak found in this frequency range has been ascribed to different physical origins, namely, hindered motions of the molecules in the cage formed by its neighbors, 39 induced dipole contributions, 40 and bending vibrations of the hydrogen bonds O O O units. 41 In our ethanol simulations, dipole induced contributions have not been taken into account. On the other hand, a maximum at about 70 cm 1 also appears in the power spectra of the velocity autocorrelation functions of the oxygen atoms of liquid ethanol, and this maximum is independent of the hydrogen bonding state of the molecule. 11 We may then conclude that the low frequency peak shown by G 1 k 2 Re ( s ()) can be ascribed to hindered motions of the molecules in the cage formed by its neighbors. The distinct component (1G 1 k ) 2 Re d () see Fig. 10 presents no spectral features until frequencies between 600 and 800 cm 1 with a maximum at 670 cm 1. Thus, the contribution of the distinct component produces a shift to higher frequencies of the librational band of the absorption
10 2822 J. Chem. Phys., Vol. 113, No. 7, 15 August 2000 Saiz, Guàdia, and Padró spectrum. A similar behavior of the distinct component was found in liquid water and it was ascribed to an optical-like mode VII. SUMMARY The static dielectric constant, the dielectric relaxation, as well as the infrared spectra of liquid ethanol at room conditions have been investigated from MD simulations. Two alternative ways to determine the dielectric properties have been considered. One of them consists in computing the total dipole moment fluctuations and time correlation functions. The other consists in obtaining the low k limit of the wavevector dependent dielectric permittivity. The good agreement between the results obtained with the two approaches is an indication that our system can be considered as representing a real isotropic liquid. The behavior as a function of the wave-vector number of the transverse and longitudinal components of the static permittivity is in accordance with theoretical predictions. In particular, we have been able to find the singularity of L (k) at small k. A different behavior for the time correlation functions of the longitudinal and transverse components of the dipole density has been observed. The L (k,t) functions decay faster than their transverse counterparts. For all the k values achieved in the simulations, both functions L (k,t) and T (k,t) show, at short times, an oscillatory behavior which is attributed to the librational dynamics of molecules. Computation of the self- and distinct contributions to the total dipole moment autocorrelation function have allowed us a better understanding of the physical origin of the different motions involved in the relaxation of the total dipole moment. The short-time behavior of (t) is mainly due to single particle reorientations. On the contrary, the long-time decay of (t) should be associated with collective motions. The dielectric relaxation bands computed from the simulations show a qualitative agreement with the experimental data, but the static dielectric constant and the dielectric relaxation times obtained from MD are smaller than the experimental ones. However, our results are in similar accordance with experiments reported for other hydrogen bonding liquids such as water and methanol. The infrared spectra shows a librational band with a peak at approximately 640 cm 1. It is interesting to note that a nonnegligible contribution of the distinct component has been detected for frequencies between 600 and 800 cm 1. In the far infrared region of the spectra, the self-component displays a maximum at frequencies 70 cm 1 which may be ascribed to the hindered motion of molecules in the cage of its neighbors. ACKNOWLEDGMENTS We thank Richard Buchner for helpful discussions. L. S. gratefully acknowledges an FPI fellowship from Ministerio de Educación y Cultura. This work has been supported by DGES through Grant No. PB CO3 and by CIRIT through Grant No. 1999SGR B. M. Ladanyi and M. S. Skaf, Annu. Rev. Phys. Chem. 44, T. Fonseca and B. M. Ladanyi, J. Chem. Phys. 93, J. Casulleras and E. Guàrdia, Mol. Simul. 8, M. S. Skaf, T. Fonseca, and B. M. Ladanyi, J. Chem. Phys. 98, M. S. Skaf and B. M. Ladanyi, J. Chem. Phys. 102, J. Barthel, K. Bachhuber, R. Buchner, and H. Hetzenauer, Chem. Phys. Lett. 165, J. T. Kindt and C. A. Schmuttenmaer, J. Phys. Chem. 100, W. L. Jorgensen, J. Phys. Chem. 90, M. E. van Leeuwen, Mol. Phys. 87, J. Gao, D. Habibollazadeh, and L. Shao, J. Phys. Chem. 99, L. Saiz, J. A. Padró, and E. Guàrdia, J. Phys. Chem. B 101, J. A. Padró, L. Saiz, and E. Guàrdia, J. Mol. Struct. 416, M. A. González, E. Enciso, F. J. Bermejo, and M. Bée, J. Chem. Phys. 110, P. Madden and D. Kivelson, Adv. Chem. Phys. 56, G. Stell, G. N. Patey, and J. S. Høye, Adv. Chem. Phys. 48, D. M. F. Edwards, P. A. Madden, and I. R. McDonald, Mol. Phys. 51, M. Neumann, O. Steinhauser, and G. S. Pawley, Mol. Phys. 52, S. W. de Leeuw, J. W. Perram, and E. R. Smith, Annu. Rev. Phys. Chem. 37, CRC Handbook of Chemistry and Physics, 74thed.CRC, New York, H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, A. DiNola, and J. R. Haak, J. Chem. Phys. 81, J. P. Ryckaert, Mol. Phys. 55, M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids Oxford University Press, Oxford, 1987, Chap H. Flyvbjerg and H. G. Petersen, J. Chem. Phys. 91, See also, D. Frenkel and B. Smit, Understanding Molecular Simulation. From Algorithms to Applications Academic, New York, 1996, Appendix D. 24 A. Chandra and B. Bagchi, J. Chem. Phys. 90, ; 91, P. Attard, D. Wei, and G. N. Patey, Chem. Phys. Lett. 172, F. O. Raineri, H. Resat, and H. L. Friedman, J. Chem. Phys. 96, P. A. Bopp, A. A. Kornyshev, and G. Sutmann, Phys. Rev. Lett. 76, S. J. Rosenthal, R. Jiménez, G. R. Fleming, P. V. Kumer, and M. Maroncelli, J. Mol. Liq. 60, , and references therein. 29 D. Bertolini and A. Tani, Mol. Phys. 75, D. Kivelson and H. L. Friedman, J. Phys. Chem. 93, M. Neumann, J. Chem. Phys. 82, The Debye limit corresponds to high friction and vanishing inertia. 33 R. Buchner and J. Barthel, Annu. Rep. Prog. Chem. Sect. C: Phys. Chem. 91, L. Saiz, J. A. Padró, and E. Guàrdia, Mol. Phys. 97, D. A. McQuarrie, Statistical Mechanics Harper & Row, New York, 1976, Chap C. F. L. Böttcher and P. Bordewijk, Theory of Dielectric Polarization Elsevier, Amsterdam, 1978, Vol B. Guillot, P. Marteau, and J. Obriot, J. Chem. Phys. 93, M. Falk and E. Whalley, J. Chem. Phys. 34, J. Martí, J. A. Padró, and E. Guàrdia, J. Chem. Phys. 105, P. A. Madden and R. W. Impey, Chem. Phys. Lett. 123, G. E. Walrafen, in Water, A Comprehensive Treatise, edited by F. Franks Plenum, New York, 1972, Chap. 5, Vol M. A. Ricci, D. Rocca, G. Ruocco, and R. Vallauri, Phys. Rev. A 40, H. Resat, F. O. Raineri, and H. L. Friedman, J. Chem. Phys. 97, D. Bertolini, A. Tani, and R. Vallauri, Mol. Phys. 73,
Dielectric properties of liquid ethanol. A computer simulation study
 JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 7 15 AUGUST 2 Dielectric properties of liquid ethanol. A computer simulation study Leonor Saiz Departament de Física Fonamental, Universitat de Barcelona,
JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 7 15 AUGUST 2 Dielectric properties of liquid ethanol. A computer simulation study Leonor Saiz Departament de Física Fonamental, Universitat de Barcelona,
Structure and Dynamics of Liquid Ethanol
 78 J. Phys. Chem. B 1997, 101, 78-86 Structure and Dynamics of Liquid Ethanol L. Saiz and J. A. Padró* Departament de Física Fonamental, UniVersitat de Barcelona, Diagonal, 647, E-08028 Barcelona, Spain
78 J. Phys. Chem. B 1997, 101, 78-86 Structure and Dynamics of Liquid Ethanol L. Saiz and J. A. Padró* Departament de Física Fonamental, UniVersitat de Barcelona, Diagonal, 647, E-08028 Barcelona, Spain
Velocity cross-correlations and atomic momentum transfer in simple liquids with different potential cores
 PHYSICAL REVIEW E VOLUME 62, NUMBER 1 JULY 2000 Velocity cross-correlations and atomic momentum transfer in simple liquids with different potential cores A. Verdaguer and J. A. Padró Departament de Física
PHYSICAL REVIEW E VOLUME 62, NUMBER 1 JULY 2000 Velocity cross-correlations and atomic momentum transfer in simple liquids with different potential cores A. Verdaguer and J. A. Padró Departament de Física
Dynamic properties of Lennard-Jones fluids and liquid metals
 PHYSICAL REVIEW E VOLUME 60, NUMBER 1 JULY 1999 Dynamic properties of Lennard-Jones fluids and liquid metals M. Canales Departament de Física i Enginyeria Nuclear, Universitat Politècnica de Catalunya,
PHYSICAL REVIEW E VOLUME 60, NUMBER 1 JULY 1999 Dynamic properties of Lennard-Jones fluids and liquid metals M. Canales Departament de Física i Enginyeria Nuclear, Universitat Politècnica de Catalunya,
Structure of liquid ethylene glycol: A molecular dynamics simulation study with different force fields
 JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 7 15 FEBRUARY 2001 Structure of liquid ethylene glycol: A molecular dynamics simulation study with different force fields L. Saiz a) Center for Molecular
JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 7 15 FEBRUARY 2001 Structure of liquid ethylene glycol: A molecular dynamics simulation study with different force fields L. Saiz a) Center for Molecular
Relaxation dominated by inertia: Solvation dynamics of a small ion in a dipolar solvent
 Proc. Indian Acad. Sci. (Chem. Sci3. Vol. 103, No. I, January 1991. pp. 77-82 ~ Printed in India. Relaxation dominated by inertia: Solvation dynamics of a small ion in a dipolar solvent A CHANDRA and B
Proc. Indian Acad. Sci. (Chem. Sci3. Vol. 103, No. I, January 1991. pp. 77-82 ~ Printed in India. Relaxation dominated by inertia: Solvation dynamics of a small ion in a dipolar solvent A CHANDRA and B
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
I. Background and Fundamentals. III.Other Simple Environments IV. Complex Environments, Biological and Otherwise
 Solvation Dynamics: Fundamentals and A Survey of Results in Simple and Complex Environments I. Background and Fundamentals II. Polar Solvation Dynamics III.ther Simple Environments IV. Complex Environments,
Solvation Dynamics: Fundamentals and A Survey of Results in Simple and Complex Environments I. Background and Fundamentals II. Polar Solvation Dynamics III.ther Simple Environments IV. Complex Environments,
Spectroscopy and dynamics of mixtures of water with acetone, acetonitrile, and methanol
 JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 24 22 DECEMBER 2000 Spectroscopy and dynamics of mixtures of water with acetone, acetonitrile, and methanol Dean S. Venables and Charles A. Schmuttenmaer
JOURNAL OF CHEMICAL PHYSICS VOLUME 113, NUMBER 24 22 DECEMBER 2000 Spectroscopy and dynamics of mixtures of water with acetone, acetonitrile, and methanol Dean S. Venables and Charles A. Schmuttenmaer
Ewald summation technique for interaction site models of polar fluids
 arxiv:physics/9901031v1 [physics.comp-ph] 19 Jan 1999 Ewald summation technique for interaction site models of polar fluids Igor P. Omelyan Institute for Condensed Matter Physics, National Ukrainian Academy
arxiv:physics/9901031v1 [physics.comp-ph] 19 Jan 1999 Ewald summation technique for interaction site models of polar fluids Igor P. Omelyan Institute for Condensed Matter Physics, National Ukrainian Academy
arxiv:cond-mat/ v1 [cond-mat.stat-mech] 8 Oct 1996
![arxiv:cond-mat/ v1 [cond-mat.stat-mech] 8 Oct 1996 arxiv:cond-mat/ v1 [cond-mat.stat-mech] 8 Oct 1996](/thumbs/72/66812395.jpg) December 21, 2013 arxiv:cond-mat/9610066v1 [cond-mat.stat-mech] 8 Oct 1996 Some Finite Size Effects in Simulations of Glass Dynamics Jürgen Horbach, Walter Kob, Kurt Binder Institut für Physik, Johannes
December 21, 2013 arxiv:cond-mat/9610066v1 [cond-mat.stat-mech] 8 Oct 1996 Some Finite Size Effects in Simulations of Glass Dynamics Jürgen Horbach, Walter Kob, Kurt Binder Institut für Physik, Johannes
Roto-translational motion in liquid water and its structural implication
 Volume 215. number 6 CHEMICAL PHYSICS LETTERS 17 December 1993 Roto-translational motion in liquid water and its structural implication I.M. Svishchev and P.G. Kusalik Department of Chemistry, Dalhousie
Volume 215. number 6 CHEMICAL PHYSICS LETTERS 17 December 1993 Roto-translational motion in liquid water and its structural implication I.M. Svishchev and P.G. Kusalik Department of Chemistry, Dalhousie
Using Molecular Dynamics to Compute Properties CHEM 430
 Using Molecular Dynamics to Compute Properties CHEM 43 Heat Capacity and Energy Fluctuations Running an MD Simulation Equilibration Phase Before data-collection and results can be analyzed the system
Using Molecular Dynamics to Compute Properties CHEM 43 Heat Capacity and Energy Fluctuations Running an MD Simulation Equilibration Phase Before data-collection and results can be analyzed the system
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Phase transitions of quadrupolar fluids
 Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Hands-on : Model Potential Molecular Dynamics
 Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Translational and rotational dynamics in supercritical methanol from molecular dynamics simulation*
 Pure Appl. Chem., Vol. 76, No. 1, pp. 203 213, 2004. 2004 IUPAC Translational and rotational dynamics in supercritical methanol from molecular dynamics simulation* Michalis Chalaris and Jannis Samios Physical
Pure Appl. Chem., Vol. 76, No. 1, pp. 203 213, 2004. 2004 IUPAC Translational and rotational dynamics in supercritical methanol from molecular dynamics simulation* Michalis Chalaris and Jannis Samios Physical
Analysis of the simulation
 Analysis of the simulation Marcus Elstner and Tomáš Kubař January 7, 2014 Thermodynamic properties time averages of thermodynamic quantites correspond to ensemble averages (ergodic theorem) some quantities
Analysis of the simulation Marcus Elstner and Tomáš Kubař January 7, 2014 Thermodynamic properties time averages of thermodynamic quantites correspond to ensemble averages (ergodic theorem) some quantities
Structural dynamics of hydrogen bonded methanol oligomers: Vibrational transient hole burning studies of spectral diffusion
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 1 1 JULY 2003 Structural dynamics of hydrogen bonded methanol oligomers: Vibrational transient hole burning studies of spectral diffusion I. R. Piletic, K.
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 1 1 JULY 2003 Structural dynamics of hydrogen bonded methanol oligomers: Vibrational transient hole burning studies of spectral diffusion I. R. Piletic, K.
Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases
 MD Simulation of Diatomic Gases Bull. Korean Chem. Soc. 14, Vol. 35, No. 1 357 http://dx.doi.org/1.51/bkcs.14.35.1.357 Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases Song
MD Simulation of Diatomic Gases Bull. Korean Chem. Soc. 14, Vol. 35, No. 1 357 http://dx.doi.org/1.51/bkcs.14.35.1.357 Molecular Dynamics Simulation Study of Transport Properties of Diatomic Gases Song
Dielectric response of a polar fluid trapped in a spherical nanocavity
 THE JOURNAL OF CHEMICAL PHYSICS 124, 144714 2006 Dielectric response of a polar fluid trapped in a spherical nanocavity Ronald Blaak a and Jean-Pierre Hansen b Department of Chemistry, University of Cambridge,
THE JOURNAL OF CHEMICAL PHYSICS 124, 144714 2006 Dielectric response of a polar fluid trapped in a spherical nanocavity Ronald Blaak a and Jean-Pierre Hansen b Department of Chemistry, University of Cambridge,
Supercritical Water Confined In Graphene Nanochannels
 Superritial Water Confined In Graphene Nanohannels J. Sala*, E. Guàrdia, and J. Martí Departament de Físia i Enginyeria Nulear, Universitat Politènia de Catalunya, B4-B5 Campus Nord, 08034 Barelona, Catalonia,
Superritial Water Confined In Graphene Nanohannels J. Sala*, E. Guàrdia, and J. Martí Departament de Físia i Enginyeria Nulear, Universitat Politènia de Catalunya, B4-B5 Campus Nord, 08034 Barelona, Catalonia,
 Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Multiple time step Monte Carlo
 JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 18 8 NOVEMBER 2002 Multiple time step Monte Carlo Balázs Hetényi a) Department of Chemistry, Princeton University, Princeton, NJ 08544 and Department of Chemistry
JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 18 8 NOVEMBER 2002 Multiple time step Monte Carlo Balázs Hetényi a) Department of Chemistry, Princeton University, Princeton, NJ 08544 and Department of Chemistry
Orientational relaxation and vibrational excitation transfer in methanol carbon tetrachloride solutions
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 5 1 FEBRUARY 2003 Orientational relaxation and vibrational excitation transfer in methanol carbon tetrachloride solutions K. J. Gaffney, I. R. Piletic, and
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 5 1 FEBRUARY 2003 Orientational relaxation and vibrational excitation transfer in methanol carbon tetrachloride solutions K. J. Gaffney, I. R. Piletic, and
MOLECULAR SPECTROSCOPY
 MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models
 JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
Brownian Motion and Langevin Equations
 1 Brownian Motion and Langevin Equations 1.1 Langevin Equation and the Fluctuation- Dissipation Theorem The theory of Brownian motion is perhaps the simplest approximate way to treat the dynamics of nonequilibrium
1 Brownian Motion and Langevin Equations 1.1 Langevin Equation and the Fluctuation- Dissipation Theorem The theory of Brownian motion is perhaps the simplest approximate way to treat the dynamics of nonequilibrium
The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity
 Draft: September 29, 1999 The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity N. G. Fuller 1 and R. L. Rowley 1,2 Abstract The influence of model flexibility upon simulated viscosity
Draft: September 29, 1999 The Effect of Model Internal Flexibility Upon NEMD Simulations of Viscosity N. G. Fuller 1 and R. L. Rowley 1,2 Abstract The influence of model flexibility upon simulated viscosity
Solvation Dynamics in Aqueous Reverse Micelles: A Computer Simulation Study
 Solvation Dynamics in Aqueous Reverse Micelles: A Computer Simulation Study James Faeder and Branka M. Ladanyi* Department of Chemistry, Colorado State UniVersity, Fort Collins, Colorado 80523 ReceiVed:
Solvation Dynamics in Aqueous Reverse Micelles: A Computer Simulation Study James Faeder and Branka M. Ladanyi* Department of Chemistry, Colorado State UniVersity, Fort Collins, Colorado 80523 ReceiVed:
Structure and Dynamics : An Atomic View of Materials
 Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
On the calculation of solvation free energy from Kirkwood- Buff integrals: A large scale molecular dynamics study
 On the calculation of solvation free energy from Kirkwood- Buff integrals: A large scale molecular dynamics study Wynand Dednam and André E. Botha Department of Physics, University of South Africa, P.O.
On the calculation of solvation free energy from Kirkwood- Buff integrals: A large scale molecular dynamics study Wynand Dednam and André E. Botha Department of Physics, University of South Africa, P.O.
Structural study of aqueous solutions of tetrahydrofuran and acetone mixtures using dielectric relaxation technique
 PRAMANA Printed in India Vol. 46, No. 2, journal of February 1996 physics pp. 91 98 Structural study of aqueous solutions of tetrahydrofuran and acetone mixtures using dielectric relaxation technique A
PRAMANA Printed in India Vol. 46, No. 2, journal of February 1996 physics pp. 91 98 Structural study of aqueous solutions of tetrahydrofuran and acetone mixtures using dielectric relaxation technique A
Michael W. Mahoney Department of Physics, Yale University, New Haven, Connecticut 06520
 JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 23 15 DECEMBER 2001 Quantum, intramolecular flexibility, and polarizability effects on the reproduction of the density anomaly of liquid water by simple potential
JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 23 15 DECEMBER 2001 Quantum, intramolecular flexibility, and polarizability effects on the reproduction of the density anomaly of liquid water by simple potential
An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation
 J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
Investigations of Freezing Pure Water
 Investigations of Freezing Pure Water David Meldgin Constanze Kalcher May 2, 2013 Abstract We use a the molecular simulation package LAAMPS to simulate the freezing of water. We analyze the SPC and TIP3P
Investigations of Freezing Pure Water David Meldgin Constanze Kalcher May 2, 2013 Abstract We use a the molecular simulation package LAAMPS to simulate the freezing of water. We analyze the SPC and TIP3P
Kirkwood Buff derived force field for mixtures of acetone and water
 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 23 15 JUNE 2003 Kirkwood Buff derived force field for mixtures of acetone and water Samantha Weerasinghe a) and Paul E. Smith b) Department of Biochemistry,
JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 23 15 JUNE 2003 Kirkwood Buff derived force field for mixtures of acetone and water Samantha Weerasinghe a) and Paul E. Smith b) Department of Biochemistry,
Dielectric Constant and Structure of Liquid 18-Crown-6 Calculated from Molecular Dynamics Simulations
 1024 J. Phys. Chem. B 1997, 101, 1024-1034 Dielectric Constant and Structure of Liquid 18-Crown-6 Calculated from Molecular Dynamics Simulations F. T. H. Leuwerink* and W. J. Briels Chemical Physics Laboratory,
1024 J. Phys. Chem. B 1997, 101, 1024-1034 Dielectric Constant and Structure of Liquid 18-Crown-6 Calculated from Molecular Dynamics Simulations F. T. H. Leuwerink* and W. J. Briels Chemical Physics Laboratory,
Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA)
 Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA) http://dare.uva.nl/document/351205 File ID 351205 Filename 5: Vibrational dynamics of the bending mode of water
Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA) http://dare.uva.nl/document/351205 File ID 351205 Filename 5: Vibrational dynamics of the bending mode of water
Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia
 University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
Monte Carlo simulation of confined water
 Monte Carlo simulation of confined water Author: Guillermo Cámbara Ruiz Advisor: Giancarlo Franzese Facultat de Física, Universitat de Barcelona, Diagonal 645, 08028 Barcelona, Spain. Abstract: In living
Monte Carlo simulation of confined water Author: Guillermo Cámbara Ruiz Advisor: Giancarlo Franzese Facultat de Física, Universitat de Barcelona, Diagonal 645, 08028 Barcelona, Spain. Abstract: In living
Hyeyoung Shin a, Tod A. Pascal ab, William A. Goddard III abc*, and Hyungjun Kim a* Korea
 The Scaled Effective Solvent Method for Predicting the Equilibrium Ensemble of Structures with Analysis of Thermodynamic Properties of Amorphous Polyethylene Glycol-Water Mixtures Hyeyoung Shin a, Tod
The Scaled Effective Solvent Method for Predicting the Equilibrium Ensemble of Structures with Analysis of Thermodynamic Properties of Amorphous Polyethylene Glycol-Water Mixtures Hyeyoung Shin a, Tod
Supplementary Figures
 Supplementary Figures iso ( =2900 cm -1 ) 1.0 0.8 0.6 0.4 0.2 0.0-0.2-0.4 pump cm -1 3450 cm -1 cm -1 cm -1-0.5 0.0 0.5 1.0 1.5 2.0 2.5 delay [ps] Supplementary Figure 1: Raw infrared pump-probe traces.
Supplementary Figures iso ( =2900 cm -1 ) 1.0 0.8 0.6 0.4 0.2 0.0-0.2-0.4 pump cm -1 3450 cm -1 cm -1 cm -1-0.5 0.0 0.5 1.0 1.5 2.0 2.5 delay [ps] Supplementary Figure 1: Raw infrared pump-probe traces.
Development of a simple, self-consistent polarizable model for liquid water
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2003 Development of a simple, self-consistent polarizable model for liquid water Haibo
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2003 Development of a simple, self-consistent polarizable model for liquid water Haibo
New Six-site Acetonitrile Model for Simulations of Liquid Acetonitrile and its Aqueous Mixtures
 New Six-site Acetonitrile Model for Simulations of Liquid Acetonitrile and its Aqueous Mixtures ALEXEI M. NIKITIN, 1,2 ALEXANDER P. LYUBARTSEV 2 1 Engelhardt Institute of Molecular Biology Russian Academy
New Six-site Acetonitrile Model for Simulations of Liquid Acetonitrile and its Aqueous Mixtures ALEXEI M. NIKITIN, 1,2 ALEXANDER P. LYUBARTSEV 2 1 Engelhardt Institute of Molecular Biology Russian Academy
5.74 Introductory Quantum Mechanics II
 MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
Supporting Information for: Physics Behind the Water Transport through. Nanoporous Graphene and Boron Nitride
 Supporting Information for: Physics Behind the Water Transport through Nanoporous Graphene and Boron Nitride Ludovic Garnier, Anthony Szymczyk, Patrice Malfreyt, and Aziz Ghoufi, Institut de Physique de
Supporting Information for: Physics Behind the Water Transport through Nanoporous Graphene and Boron Nitride Ludovic Garnier, Anthony Szymczyk, Patrice Malfreyt, and Aziz Ghoufi, Institut de Physique de
Hidden Breakdown of Linear Response: Projections of Molecular Motions in Nonequilibrium Simulations of Solvation Dynamics
 Copyright 2003 by the American Chemical Society VOLUME 107, NUMBER 24, JUNE 19, 2003 LETTERS Hidden Breakdown of Linear Response: Projections of Molecular Motions in Nonequilibrium Simulations of Solvation
Copyright 2003 by the American Chemical Society VOLUME 107, NUMBER 24, JUNE 19, 2003 LETTERS Hidden Breakdown of Linear Response: Projections of Molecular Motions in Nonequilibrium Simulations of Solvation
Dielectric and thermodynamic response of a generalized reaction field model for liquid state simulations
 Dielectric and thermodynamic response of a generalized reaction field model for liquid state simulations Howard Alper and Ronald M. Levy Department of Chemistry, Rutgers University, Piscataway, New Jersey
Dielectric and thermodynamic response of a generalized reaction field model for liquid state simulations Howard Alper and Ronald M. Levy Department of Chemistry, Rutgers University, Piscataway, New Jersey
Spin Relaxation and NOEs BCMB/CHEM 8190
 Spin Relaxation and NOEs BCMB/CHEM 8190 T 1, T 2 (reminder), NOE T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations
Spin Relaxation and NOEs BCMB/CHEM 8190 T 1, T 2 (reminder), NOE T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations
Discontinuous molecular dynamics for rigid bodies: Applications
 THE JOURNAL OF CHEMICAL PHYSICS 126, 074106 2007 Discontinuous molecular dynamics for rigid bodies: Applications Lisandro Hernández de la Peña, Ramses van Zon, and Jeremy Schofield Chemical Physics Theory
THE JOURNAL OF CHEMICAL PHYSICS 126, 074106 2007 Discontinuous molecular dynamics for rigid bodies: Applications Lisandro Hernández de la Peña, Ramses van Zon, and Jeremy Schofield Chemical Physics Theory
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
 Bull. Mater. Sci., Vol. 31, No. 3, June 2008, pp. 525 532. Indian Academy of Sciences. Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
Bull. Mater. Sci., Vol. 31, No. 3, June 2008, pp. 525 532. Indian Academy of Sciences. Water structure near single and multi-layer nanoscopic hydrophobic plates of varying separation and interaction potentials
FEMTOSECOND MID-INFRARED SPECTROSCOPY OF HYDROGEN-BONDED LIQUIDS
 Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
Phase Transitions in Relaxor Ferroelectrics
 Phase Transitions in Relaxor Ferroelectrics Matthew Delgado December 13, 2005 Abstract This paper covers the properties of relaxor ferroelectrics and considers the transition from the paraelectric state
Phase Transitions in Relaxor Ferroelectrics Matthew Delgado December 13, 2005 Abstract This paper covers the properties of relaxor ferroelectrics and considers the transition from the paraelectric state
Fast and slow dynamics of hydrogen bonds in liquid water. Abstract
 Fast and slow dynamics of hydrogen bonds in liquid water Francis W. Starr 1, Johannes K. Nielsen 1,2 & H. Eugene Stanley 1 1 Center for Polymer Studies, Center for Computational Science, and Department
Fast and slow dynamics of hydrogen bonds in liquid water Francis W. Starr 1, Johannes K. Nielsen 1,2 & H. Eugene Stanley 1 1 Center for Polymer Studies, Center for Computational Science, and Department
SUPPLEMENTARY INFORMATION An Empirical IR Frequency Map for Ester C=O Stretching Vibrations
 SUPPLEMENTARY INFORMATION An Empirical IR Frequency Map for Ester C=O Stretching Vibrations Sean C. Edington, Jennifer C. Flanagan, Carlos R. Baiz* Department of Chemistry, University of Texas at Austin
SUPPLEMENTARY INFORMATION An Empirical IR Frequency Map for Ester C=O Stretching Vibrations Sean C. Edington, Jennifer C. Flanagan, Carlos R. Baiz* Department of Chemistry, University of Texas at Austin
Molecular Dynamics Simulations. Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia
 Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics
 for resubmission Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics Aram Davtyan, 1 Gregory A. Voth, 1 2, a) and Hans
for resubmission Dynamic force matching: Construction of dynamic coarse-grained models with realistic short time dynamics and accurate long time dynamics Aram Davtyan, 1 Gregory A. Voth, 1 2, a) and Hans
On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water
 On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water Orsolya Gereben and László Pusztai Research Institute for
On the accurate calculation of the dielectric constant and the diffusion coefficient from molecular dynamics simulations: the case of SPC/E water Orsolya Gereben and László Pusztai Research Institute for
Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception
 Journal of Physics: Conference Series PAPER OPEN ACCESS Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception To cite this article: D I
Journal of Physics: Conference Series PAPER OPEN ACCESS Density dependence of dielectric permittivity of water and estimation of the electric field for the breakdown inception To cite this article: D I
CHAPTER 2 SOLVENT-SOLUTE INTERACTIONS
 CHAPTER 2 SOLVENT-SOLUTE INTERACTIONS 2.1 INTRODUCTION The photophysical and photochemical properties of a dye molecule are determined by the nature and the energy of its electronically excited states.
CHAPTER 2 SOLVENT-SOLUTE INTERACTIONS 2.1 INTRODUCTION The photophysical and photochemical properties of a dye molecule are determined by the nature and the energy of its electronically excited states.
Edinburgh Research Explorer
 Edinburgh Research Explorer Classical molecular dynamics simulation of microwave heating of liquids: the case of water Citation for published version: Afify, N & Sweatman, M 2018, 'Classical molecular
Edinburgh Research Explorer Classical molecular dynamics simulation of microwave heating of liquids: the case of water Citation for published version: Afify, N & Sweatman, M 2018, 'Classical molecular
Chapter 5: Molecular Scale Models for Macroscopic Dynamic Response. Fluctuation-Dissipation Theorem:
 G. R. Strobl, Chapter 6 "The Physics of Polymers, 2'nd Ed." Springer, NY, (1997). R. B. Bird, R. C. Armstrong, O. Hassager, "Dynamics of Polymeric Liquids", Vol. 2, John Wiley and Sons (1977). M. Doi,
G. R. Strobl, Chapter 6 "The Physics of Polymers, 2'nd Ed." Springer, NY, (1997). R. B. Bird, R. C. Armstrong, O. Hassager, "Dynamics of Polymeric Liquids", Vol. 2, John Wiley and Sons (1977). M. Doi,
Dynamics of Polar Solvation (in Dipolar & Ionic Solvents)
 Dynamics of Polar Solvation (in Dipolar & Ionic Solvents) P L1 M4 Φ=2 KDP S L2 G2 S S 1 t Spectral Response S (t) 1..8.6.4.2. N dipolar CF 3 ionic M3 wg M2 M1 1-13 1-12 1-11 1-1 1-9 1-8 Time / s Sp15 ACS
Dynamics of Polar Solvation (in Dipolar & Ionic Solvents) P L1 M4 Φ=2 KDP S L2 G2 S S 1 t Spectral Response S (t) 1..8.6.4.2. N dipolar CF 3 ionic M3 wg M2 M1 1-13 1-12 1-11 1-1 1-9 1-8 Time / s Sp15 ACS
Water-methanol separation with carbon nanotubes and electric fields
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 215 Supplementary Information: Water-methanol separation with carbon nanotubes and electric fields
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 215 Supplementary Information: Water-methanol separation with carbon nanotubes and electric fields
Structure of water adsorbed on a single graphene sheet
 PHYSICAL REVIEW B 78, 75 8 Structure of water adsorbed on a single graphene sheet M. C. Gordillo* Departamento de Sistemas Físicos, Químicos y Naturales, Facultad de Ciencias Experimentales, Universidad
PHYSICAL REVIEW B 78, 75 8 Structure of water adsorbed on a single graphene sheet M. C. Gordillo* Departamento de Sistemas Físicos, Químicos y Naturales, Facultad de Ciencias Experimentales, Universidad
CHEM Atomic and Molecular Spectroscopy
 CHEM 21112 Atomic and Molecular Spectroscopy References: 1. Fundamentals of Molecular Spectroscopy by C.N. Banwell 2. Physical Chemistry by P.W. Atkins Dr. Sujeewa De Silva Sub topics Light and matter
CHEM 21112 Atomic and Molecular Spectroscopy References: 1. Fundamentals of Molecular Spectroscopy by C.N. Banwell 2. Physical Chemistry by P.W. Atkins Dr. Sujeewa De Silva Sub topics Light and matter
Two-Dimensional Spin-Polarized Hydrogen at Zero
 J Low Temp Phys (2013) 171:685 692 DOI 10.1007/s10909-012-0756-7 Two-Dimensional Spin-Polarized Hydrogen at Zero Temperature L. Vranješ Markić J. Boronat Received: 13 July 2012 / Accepted: 16 September
J Low Temp Phys (2013) 171:685 692 DOI 10.1007/s10909-012-0756-7 Two-Dimensional Spin-Polarized Hydrogen at Zero Temperature L. Vranješ Markić J. Boronat Received: 13 July 2012 / Accepted: 16 September
NMR, the vector model and the relaxation
 NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
Symmetry of the Dielectric Tensor
 Symmetry of the Dielectric Tensor Curtis R. Menyuk June 11, 2010 In this note, I derive the symmetry of the dielectric tensor in two ways. The derivations are taken from Landau and Lifshitz s Statistical
Symmetry of the Dielectric Tensor Curtis R. Menyuk June 11, 2010 In this note, I derive the symmetry of the dielectric tensor in two ways. The derivations are taken from Landau and Lifshitz s Statistical
Kinetic Pathways of Ion Pair Dissociation in Water
 3706 J. Phys. Chem. B 1999, 103, 3706-3710 Kinetic Pathways of Ion Pair Dissociation in Water Phillip L. Geissler, Christoph Dellago, and David Chandler* Department of Chemistry, UniVersity of California,
3706 J. Phys. Chem. B 1999, 103, 3706-3710 Kinetic Pathways of Ion Pair Dissociation in Water Phillip L. Geissler, Christoph Dellago, and David Chandler* Department of Chemistry, UniVersity of California,
Structure of supercooled and glassy water under pressure
 Wesleyan University WesScholar Division III Faculty Publications Natural Sciences and Mathematics 1999 Structure of supercooled and glassy water under pressure F. W. Starr Wesleyan University M. C. Bellissent-Funel
Wesleyan University WesScholar Division III Faculty Publications Natural Sciences and Mathematics 1999 Structure of supercooled and glassy water under pressure F. W. Starr Wesleyan University M. C. Bellissent-Funel
T 1, T 2, NOE (reminder)
 T 1, T 2, NOE (reminder) T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations of the system following perturbation
T 1, T 2, NOE (reminder) T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations of the system following perturbation
Polarization. D =e i E=k i e o E =e o E+ P
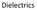 Dielectrics Polarization a perfect insulator or a dielectric is the material with no free charge carriers. if an electric field, E, is applied to the insulator no charge flow. However in the presence of
Dielectrics Polarization a perfect insulator or a dielectric is the material with no free charge carriers. if an electric field, E, is applied to the insulator no charge flow. However in the presence of
Electromagnetic fields and waves
 Electromagnetic fields and waves Maxwell s rainbow Outline Maxwell s equations Plane waves Pulses and group velocity Polarization of light Transmission and reflection at an interface Macroscopic Maxwell
Electromagnetic fields and waves Maxwell s rainbow Outline Maxwell s equations Plane waves Pulses and group velocity Polarization of light Transmission and reflection at an interface Macroscopic Maxwell
Water models in classical simulations
 Water models in classical simulations Maria Fyta Institut für Computerphysik, Universität Stuttgart Stuttgart, Germany Water transparent, odorless, tasteless and ubiquitous really simple: two H atoms attached
Water models in classical simulations Maria Fyta Institut für Computerphysik, Universität Stuttgart Stuttgart, Germany Water transparent, odorless, tasteless and ubiquitous really simple: two H atoms attached
A Comprehensive Liquid Simulation Study of Neat Formic Acid
 J. Phys. Chem. B 2000, 104, 8287-8294 8287 A Comprehensive Liquid Simulation Study of Neat Formic Acid Péter Mináry, Pál Jedlovszky,, Mihaly Mezei, and László Turi*, Department of Physical Chemistry, EötVös
J. Phys. Chem. B 2000, 104, 8287-8294 8287 A Comprehensive Liquid Simulation Study of Neat Formic Acid Péter Mináry, Pál Jedlovszky,, Mihaly Mezei, and László Turi*, Department of Physical Chemistry, EötVös
P. W. Atkins and R. S. Friedman. Molecular Quantum Mechanics THIRD EDITION
 P. W. Atkins and R. S. Friedman Molecular Quantum Mechanics THIRD EDITION Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1997 Introduction and orientation 1 Black-body radiation 1 Heat capacities 2 The
P. W. Atkins and R. S. Friedman Molecular Quantum Mechanics THIRD EDITION Oxford New York Tokyo OXFORD UNIVERSITY PRESS 1997 Introduction and orientation 1 Black-body radiation 1 Heat capacities 2 The
Matthias Lütgens, Frank Friedriszik, and Stefan Lochbrunner* 1 Concentration dependent CARS and Raman spectra of acetic acid in carbon tetrachloride
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 SUPPORTING INFORMATION Direct observation of the cyclic dimer in liquid acetic
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 SUPPORTING INFORMATION Direct observation of the cyclic dimer in liquid acetic
Molecular Dynamics Simulation of Methanol-Water Mixture
 Molecular Dynamics Simulation of Methanol-Water Mixture Palazzo Mancini, Mara Cantoni University of Urbino Carlo Bo Abstract In this study some properties of the methanol-water mixture such as diffusivity,
Molecular Dynamics Simulation of Methanol-Water Mixture Palazzo Mancini, Mara Cantoni University of Urbino Carlo Bo Abstract In this study some properties of the methanol-water mixture such as diffusivity,
Solvation and reorganization energies in polarizable molecular and continuum solvents
 Solvation and reorganization energies in polarizable molecular and continuum solvents Joel S. Bader CuraGen Corporation, 322 East Main Street, Branford, Connecticut 06405 Christian M. Cortis Department
Solvation and reorganization energies in polarizable molecular and continuum solvents Joel S. Bader CuraGen Corporation, 322 East Main Street, Branford, Connecticut 06405 Christian M. Cortis Department
Part 2: Molecular Dynamics. Literature History Practical Issues
 Part 2: Molecular Dynamics Literature History Practical Issues 1 Literature A. K. Hartmann Big Practical Guide to Computer Simulations (World Scientific, 2015) M. P. Allen and D.J. Tildesley Computer Simulations
Part 2: Molecular Dynamics Literature History Practical Issues 1 Literature A. K. Hartmann Big Practical Guide to Computer Simulations (World Scientific, 2015) M. P. Allen and D.J. Tildesley Computer Simulations
Multiple time step Monte Carlo simulations: Application to charged systems with Ewald summation
 JOURNAL OF CHEMICAL PHYSICS VOLUME 11, NUMBER 1 1 JULY 004 Multiple time step Monte Carlo simulations: Application to charged systems with Ewald summation Katarzyna Bernacki a) Department of Chemistry
JOURNAL OF CHEMICAL PHYSICS VOLUME 11, NUMBER 1 1 JULY 004 Multiple time step Monte Carlo simulations: Application to charged systems with Ewald summation Katarzyna Bernacki a) Department of Chemistry
Lecture 12. Electron Transport in Molecular Wires Possible Mechanisms
 Lecture 12. Electron Transport in Molecular Wires Possible Mechanisms In Lecture 11, we have discussed energy diagrams of one-dimensional molecular wires. Here we will focus on electron transport mechanisms
Lecture 12. Electron Transport in Molecular Wires Possible Mechanisms In Lecture 11, we have discussed energy diagrams of one-dimensional molecular wires. Here we will focus on electron transport mechanisms
Molecular dynamics simulation of limiting conductances for LiCl, NaBr, and CsBr in supercritical water
 JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 2 8 JANUARY 2000 Molecular dynamics simulation of limiting conductances for LiCl, NaBr, and CsBr in supercritical water S. H. Lee Department of Chemistry,
JOURNAL OF CHEMICAL PHYSICS VOLUME 112, NUMBER 2 8 JANUARY 2000 Molecular dynamics simulation of limiting conductances for LiCl, NaBr, and CsBr in supercritical water S. H. Lee Department of Chemistry,
Supporting information for the manuscript. Excited state structural evolution during charge-transfer reactions in Betaine-30
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting information for the manuscript Excited state structural evolution during
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting information for the manuscript Excited state structural evolution during
What happens when light falls on a material? Transmission Reflection Absorption Luminescence. Elastic Scattering Inelastic Scattering
 Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
Raman Spectroscopy What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman, Fluorescence and IR Scattering Absorption
Theory of selective excitation in stimulated Raman scattering
 Theory of selective excitation in stimulated Raman scattering S. A. Malinovskaya, P. H. Bucksbaum, and P. R. Berman Michigan Center for Theoretical Physics, FOCUS Center, and Department of Physics, University
Theory of selective excitation in stimulated Raman scattering S. A. Malinovskaya, P. H. Bucksbaum, and P. R. Berman Michigan Center for Theoretical Physics, FOCUS Center, and Department of Physics, University
Lecture 11: Potential Energy Functions
 Lecture 11: Potential Energy Functions Dr. Ronald M. Levy ronlevy@temple.edu Originally contributed by Lauren Wickstrom (2011) Microscopic/Macroscopic Connection The connection between microscopic interactions
Lecture 11: Potential Energy Functions Dr. Ronald M. Levy ronlevy@temple.edu Originally contributed by Lauren Wickstrom (2011) Microscopic/Macroscopic Connection The connection between microscopic interactions
Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation
 Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation Cecilie Ro nne and Lars Thrane a) Department of Chemistry,
Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation Cecilie Ro nne and Lars Thrane a) Department of Chemistry,
Supplementary information for. plasmonic nanorods interacting with J-aggregates.
 Supplementary information for Approaching the strong coupling limit in single plasmonic nanorods interacting with J-aggregates. by Gülis Zengin, Göran Johansson, Peter Johansson, Tomasz J. Antosiewicz,
Supplementary information for Approaching the strong coupling limit in single plasmonic nanorods interacting with J-aggregates. by Gülis Zengin, Göran Johansson, Peter Johansson, Tomasz J. Antosiewicz,
Dipole Solvation: Nonlinear Effects, Density Reorganization, and the Breakdown of the Onsager Saturation Limit
 Dipole Solvation: Nonlinear Effects, Density Reorganization, and the Breakdown of the Onsager Saturation Limit Anatoli Milischuk and Dmitry V. Matyushov* Department of Chemistry and Biochemistry, Arizona
Dipole Solvation: Nonlinear Effects, Density Reorganization, and the Breakdown of the Onsager Saturation Limit Anatoli Milischuk and Dmitry V. Matyushov* Department of Chemistry and Biochemistry, Arizona
Supplementary Materials
 Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Supplementary Figure 1. Spin-spin relaxation curves for three La 1.8-x Eu 0.2 Sr x CuO 4 samples.
 Supplementary Figure 1. Spin-spin relaxation curves for three La 1.8-x Eu 0.2 Sr x CuO 4 samples. The data here are raw nuclear quadrupole resonance (NQR) data multiplied by temperature to compensate for
Supplementary Figure 1. Spin-spin relaxation curves for three La 1.8-x Eu 0.2 Sr x CuO 4 samples. The data here are raw nuclear quadrupole resonance (NQR) data multiplied by temperature to compensate for
Heat capacity of water: a signature of nuclear quantum effects. Abstract
 Heat capacity of water: a signature of nuclear quantum effects C. Vega a, M. M. Conde a, C. McBride a, J. L. F. Abascal a, E. G. Noya b, R. Ramirez c and L. M. Sesé d a Departamento de Química Física,
Heat capacity of water: a signature of nuclear quantum effects C. Vega a, M. M. Conde a, C. McBride a, J. L. F. Abascal a, E. G. Noya b, R. Ramirez c and L. M. Sesé d a Departamento de Química Física,
Rearrangement dynamics of the hydrogen-bonded network of clathrate hydrates encaging polar guest
 Rearrangement dynamics of the hydrogen-bonded network of clathrate hydrates encaging polar guest Kenichiro Koga and Hideki Tanaka Department of Polymer Chemistry, Kyoto University, Sakyo ku, Kyoto 606
Rearrangement dynamics of the hydrogen-bonded network of clathrate hydrates encaging polar guest Kenichiro Koga and Hideki Tanaka Department of Polymer Chemistry, Kyoto University, Sakyo ku, Kyoto 606
Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model
 1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b)
 Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b) and Ward H. Thompson 1) Department of Chemistry, University
Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b) and Ward H. Thompson 1) Department of Chemistry, University
