Ca Calcium. Introduction
|
|
|
- Rosemary Rose
- 6 years ago
- Views:
Transcription
1 Ca Calcium Introduction Calcium belongs to group 2 of the periodic table, along with Be, Mg, Sr and Ba. It has an atomic number of 20, an atomic mass of 40, one oxidation state (+2) and six naturally occurring isotopes ( 40 Ca, 42 Ca, 43 Ca, 44 Ca, 46 Ca and 48 Ca), with 40 Ca accounting for 96.9% and 44 Ca for 2.1% of the total mass (Mittlefehldt 1999a). Calcium is most similar chemically to the heavier alkali earth elements Sr and Ba. Calcium is the fifth most abundant of all the elements, constituting approximately 3% by weight (equivalent to 4.2% CaO) of the upper continental crust and 5.29% (7.41% CaO) for the bulk continental crust (McLennan and Taylor 1999). It is a widespread refractory lithophile element forming several common minerals including calcite CaCO 3, gypsum CaSO 4.2H 2 O, dolomite CaMg(CO 3 ) 2, anhydrite CaSO 4 and fluorite CaF 2. Calcium is also widely distributed in other minerals such as feldspar, amphibole and pyroxene, and is often associated with clay minerals such as illite, chlorite and Ca montmorillonite (Zupancic and Pirc 1999). In igneous rocks, calcium is enriched in anorthosite, which is composed predominantly of calcium-rich plagioclase feldspar, and in mafic rocks. However, rocks such as granite and sandstone, and their metamorphic equivalents, have relatively low total CaO contents. Limestone and dolomite are carbonate sediments that are an important sink for Ca in its geochemical cycle. Levels of Ca in most sedimentary rocks reflect the abundance of carbonate minerals (calcite and dolomite), though sulphate minerals, such as gypsum and anhydrite can also be important, particularly in sandstone and evaporites. Plagioclase is the principal host for Ca in some detrital sediments. Calcium levels in pelite vary widely, some Lower Palaeozoic deep-water shale having less than 0.05% CaO, while some types of calcareous mudstone may have several per cent CaO and grade into impure limestone. Where Ca carbonates or sulphates are important constituents of sedimentary rocks, they supply most of the Ca in stream water; levels are lower in areas where Ca is derived from the slower weathering of minerals, such as plagioclase feldspar. McLennan and Murray (1999) quote average values for river particulates and loess as 2.2 and 0.79% Ca respectively. Estimates for the Ca contents of igneous rocks are very variable, but Mielke (1979) quotes: ultramafic 25,000 mg kg -1 ; basaltic 76,000 mg kg -1 ; high-ca granite 25,300 mg kg -1, low-ca granite 5100 mg kg -1, and syenite 18,000 mg kg -1. Calcium is indicative of calcareous rocks, especially in association with Sr, Mg and Ba. Where Ca and other elements indicate calcareous rocks, and other elements (e.g., REEs) indicate felsic intrusives, bedrock geology may be favourable for skarn type mineralisation. Calcium generally has a high mobility and, except under strongly alkaline conditions, occurs in solution as dissociated Ca ions. Concentrations generally increase with stream order as a result of increasing contact time between water and soil or rock. The most common Ca-bearing minerals in sedimentary rocks, calcite and dolomite, weather on contact with acid solutions, typically carbonic acid (H 2 CO 3) derived from the dissolution of atmospheric CO 2 in rain, releasing Ca or Ca and Mg, respectively. Soil air has higher CO 2 concentrations than the atmosphere, due to the respiration of soil organisms and the decay of organic matter. Soil solutions thus contain higher levels of H2CO 3 than rain, and have a greater capacity to dissolve carbonate minerals. Soluble major ions, such as Ca, influence the quantities of soluble trace elements. Solutions of most soil types contain an excess of Ca, which constitutes more than 90% of the total cation concentration; Ca, is, therefore, the most important cation in governing the solubility of trace elements in soil (Kabata-Pendias 2001). Divalent calcium (Ca ) ions are held more strongly than monovalent ions on the negatively-charged surfaces of clay minerals and organic matter. As soil acidity 3+ increases, Al replaces Ca as the dominant 3+ exchangeable cation, so high Al concentrations in stream water tend to correlate with low Ca levels. TCalcium concentrations in surface water are highly variable; in a study of United Kingdom rivers, Neal and Robson (2000) reported Ca -1 concentrations in the range 10 to 150 mg l. The main anthropogenic activity that would lead to an increase of Ca in the drainage system is 97
2 the long-established agricultural practice of liming land to correct for soil acidity. Other anthropogenic sources of calcium include cement factories, fertilisers and dust, although geogenic sources are much more important than anthropogenic ones in the environment (Reimann and de Caritat 1998). Calcium is an essential nutrient element for plants and animals for the development of bone, nervous system and cells. It is practically nontoxic. TIn upland areas with base-poor rocks and low stream water ph, added Ca has been shown to reduce the toxic effects of high concentrations of Al on aquatic biota such as brown trout (Brown 1983). Table 16 compares the median concentrations of Ca in the FOREGS samples and in some reference datasets. Table 16. Median concentrations of Ca in the FOREGS samples and in some reference data sets. Calsium Origin Source Number of Size fraction Extraction Median (CaO) samples mm % Crust 1) Upper continental n.a. n.a. Total 3.59 Subsoil FOREGS 788 <2.0 Total (XRF) 1.13 Topsoil FOREGS 845 <2.0 Total (XRF) 0.92 Soil 2) World n.a. n.a Total 1.96 Water (Ca) FOREGS 808 Filtered < (mg l -1 ) µm Water (Ca) 3) World n.a. n.a. 12 (mg l -1 ) Stream sediment FOREGS 852 <0.15 Total (XRF) 2.33 Floodplain sediment FOREGS 747 <2.0 Total (XRF) ) Rudnick & Gao 2004, 2) Koljonen 1992, 3) Ivanov CaO in soil The median CaO content in subsoil is 1.13% and in topsoil 0.92%, with a range varying from 0.02 to 51.6% in subsoils and 0.03 to 47.7% in topsoils. The histograms show multimodal populations, with the highest frequency between 0.6 and 1% CaO (low-ca samples). For subsoil a second low-cao mode is present at about 2% CaO. High CaO modes occur at around 15% and 30% in subsoil, and near to 9% and 30% in topsoil. The average ratio topsoil/subsoil is Low CaO values in subsoil (<0.38%) occur in the eastern Iberian peninsula, the Armorican Massif and the Central Massif in France (all Variscan areas), over most of England, Wales and Ireland, and in the glacial drift area of Denmark, northern Germany and Poland. The topsoil pattern is very similar. On the subsoil CaO distribution map, the following calcareous regions show high CaO values (>2.97%): eastern Spain (Triassic limestone in the Baetic Cordillera in southern Spain, Jurassic and Cretaceous limestone in the Cordillera Ibérica in eastern Spain, Cretaceous and Palaeogene limestone and marl in the Pyrenees, Carboniferous and Cretaceous limestone in Cantabria in northern Spain); southern Italy, Greece, the Dalmatian coast, southern France and the western Alps, the eastern part of the Paris Basin and the Charente, western Ireland, and the Baltic states (Estonia, Latvia, Lithuania). In addition, high CaO occurs in western Slovakia (calcareous loess), parts of the Norwegian Caledonides, northern Fennoscandia, and Albania, where greenstone belts and other mafic and ultramafic crystalline rocks with Carich plagioclase account for the higher values. The topsoil CaO distribution map generally shows the same pattern as the subsoil map, with only minor differences. In Latvia and Lithuania, the topsoils are depleted in Ca, reflecting natural leaching in the loose freely drained calcareous soil in a cold climate with abundant precipitation; soils are made up from a mixture of Quaternary (Weichsel) glacial till with Devonian dolomite, Jurassic limestone, Cretaceous chalk, etc. Similar leaching can be observed in Poland, but Polish 98
3 soil types are developed mainly on non-calcareous older deposits of the penultimate glaciation, which were deeply leached after the glaciation. Similar depletion also occurs in the arable soil of northern Germany, but Ca is added there by liming, a practice stopped in the Baltic states after 1990 (Reimann et al. 2003). Some Ca depletion also occurs in topsoil in Slovakia, and Ca enrichment of topsoil occurs in Austria. Topsoil in calcareous areas contains on average less CaO than subsoil: the ratio of topsoil/subsoil is 0.70 for the whole dataset (Map 3). This is mostly due to leaching of carbonates from topsoil by acid rain, with simultaneous enrichment of the clay fraction. From map 3 it can be deduced that the intensity of Ca leaching is independent of the Ca content of the soil, and occurs both in calcareous and non-calcareous areas. This relative uniformity is mathematically expressed in the correlation of between topsoils and subsoils (table 4)" CaO in soil has a strong negative correlation with SiO 2 (-0.78 in subsoil and in topsoil). Because Si and Ca are two major elements in a closed number system, this is an autocorrelation (see introduction). CaO also has a weak negative correlation with Zr, Hf, Al, K, Ba. CaO in soil shows a good positive correlation with ph, and a weak one with Sr. In topsoil, Ca also has a weak positive correlation with Mg. The positive correlation of ph with CaO (0.52 in subsoil and 0.49 in topsoil) does not correspond to a gradual increase in both values, but to a sharp boundary at around ph 6.6 between acidic low-ca soil and neutral to slightly alkaline Ca-bearing soil, as is seen in the scattergram (Figure 9). Figure 9. Scattergram of CaO and ph values in subsoils. Ca in stream water Calcium values in stream water range over three orders of magnitude, from 0.02 to 592 mg l -1, with a median value of 40 mg l -1. Calcium data tend to correlate with ph, conductivity and bicarbonate. The Ca distribution pattern is closely associated with the Major ions high mineralization waters, while it is negatively correlated with the REEs water patterns. Patterns in stream water Ca data are generally markedly different from distributions in the solid sample media and strongly related to ph, with lowest concentrations in the most acid environments throughout most of southern and central Fennoscandia, and highest concentrations in some areas adjacent to the Mediterranean, in calcareous environments of northern France and south-east England, and in Germany, Poland and the Baltic states, possibly associated with intensive agricultural activity. Lowest Ca in stream water (<14 mg l -1 ) are found throughout Fennoscandia, northern Britain and Wales on Precambrian Shield, Laurentian and Caledonian terrains, in north-west Iberian Peninsula, Brittany, Massif Central and Corsica on Variscan and in eastern Switzerland in the Alpidic Orogen. The low Ca values are predominantly associated with acid igneous and metamorphic (schist) rocks. Enhanced Ca concentrations in stream water (>90 mg l -1 ) are found in Baltic countries on Palaeozoic limestone, Poland and parts of north Germany on Precambrian Shield derived glacial drift, in central and south-east England on Mesozoic carbonate rocks, western Ireland on Carboniferous limestone, northern and southwestern France, western Germany, southern and eastern Iberian Peninsula and Sardinia on Variscan with Mesozoic cover rocks, in the Pannonian basin of Hungary and parts of Italy with Sicily on Alpine Orogen. These high Ca values represent water generally derived from calcareous rocks (mainly calcitic clastics, mudstone and chalk). In Spain, high Ca values are associated with Triassic limestone in the Baetic Cordillera, Jurassic and Cretaceous limestone in the Cordillera Ibérica, Cretaceous and Palaeogene limestone and marl in the 99
4 Frequency, % Cumulative frequency, % Calcium Ratio Topsoil / Subsoil U CaO Kilometers CaO (Ratio Topsoil / Subsoil) Number of samples 779 Median CaO % (Topsoil) Geochemical Baseline Mapping Canary Islands Map 3. Ratio of CaO in topsoil vs subsoil. CaO
5 Pyrenees, Carboniferous and Cretaceous limestone in Cantabria; Cambrian limestone in Extremadura and in Galicia-Asturias. In Poland, in addition to the occurrence of carbonate rocks (e.g., Silesia-Krakow and Lublin Uplands), the origin of higher concentrations of Ca (and Sr and Mg) is attributed to Ca-Mg fertilisers used to lime soils. A similar explanation is valid for highly anomalous values in Germany, with Jurassic limestone, loess and fertilisers. A more detailed description on the chemistry of Ca in stream water is given in Annex 1 of this volume by Ander et al. (2006), where the thematic interpretation of stream water chemistry is discussed (see sections on cation predominance, calcite solubility, gypsum solubility and fluorite solubility). CaO in stream sediment The median CaO content in stream sediment is 2.44%, with a range varying from 0.08 to 55.7%. The western Iberian Peninsula stream sediment is low in CaO (<1.18%), as is the French Massif Central, Brittany, the Garonne area, most of Germany, Denmark, central Poland, western and northern Britain, central Sweden and adjacent Norway. High CaO contents in stream sediment (>5.91%) appear over Mesozoic and Caenozoic calcareous rocks in an area extending from southern France and the western Alps through the upper Rhône valley, to the Paris Basin. High values also characterise eastern Spain over Mesozoic limestones, and southern Spain over Palaeozoic calcareous parts of the Baetic Cordillera. The high CaO values over central Ireland reflect the underlying Carboniferous limestone. Mesozoic and Tertiary limestone gives rise to high values in parts of south-western France, most of Italy including Sicily, Greece, Albania, the Dalmatian coast of Croatia, Slovenia, and the eastern Alps. High CaO values are also present over the Italian alkaline volcanic province. Areas above the median value for Ca in stream sediment also occur over Mesozoic limestone in north-eastern Germany, over glacial drift in Lithuania, western Latvia and western Estonia (over calcareous Devonian clay, dolomite and limestone), in western Norway (Proterozoic amphibolite and anorthosite) and northern Norway (greenstone belts with Ca-rich plagioclase). In the eastern part of France, and in the Rhône-Provence Tertiary graben, anthropogenic contribution of Ca by salt brining factories is suspected (CaCl 2 release); this salt possibly has a measurable impact when used as a de-icing medium for road maintenance in winter. CaO in stream sediment has a strong negative correlation with SiO 2 (-0.78), a good negative correlation with Al 2 O 3, Ga, Nb, Ti, K 2 O and Ba, and a weak negative correlation with Na 2 O, Rb, Ta, Tm, Yb, Lu, Hf and Zr. As all these elements are related to normal silicate substrates, the contrast with Ca-bearing limestone is apparent. Calcium has a good positive correlation with Sr (substitution in carbonates and feldspars), and a weak positive correlation with Mg (e.g., dolomite). CaO in floodplain sediment Total CaO values in floodplain sediment vary from <0.05 to 54.4%, with a median of 2.07%. Low CaO values in floodplain sediment (<0.83%) occur in the predominantly granitic areas of central Sweden, a few areas within the glacial drift plains in Germany and Poland; the Bohemian Massif (Czech-German border), southern Scotland (gneiss and sandstone parts), western part of England and Wales (mainly shale, igneous and metamorphic rocks), north-eastern Ireland (granite), Brittany (granite, gneiss), northern Massif Central (granite), and most of the western Iberian Peninsula (Variscan sediments and crystalline rocks). High total CaO values in floodplain sediment (>6.83%) occur over calcareous rocks in Ireland, the Paris and the coastal Charente basins, the French Mediterranean coastal region, the Rhône- Saône River basin draining the limestone of the Jura Mountains and the calcareous rocks of the Western Alps; the mainly calcareous rocks of the eastern half of Spain from the Pyrenees to the 101
6 Baetic Cordillera; the Alpine calcareous rocks covering Switzerland, south Germany and Austria; the calcareous rich sediments of the Po river basin and the Apennine carbonate rocks of Italy, including Sicily; the Dalmatian coast of Croatia with limestone; and the calcareous and ophiolitic rocks of Albania and Greece. Calcium in floodplain sediment shows a good positive correlation with Sr (0.55), a weak correlation with MgO (0.32); a very strong negative correlation with SiO2 (-0.81), and a good negative correlation with Zr (-0.49) and K (-0.41). In conclusion, most of the CaO patterns in floodplain sediment are due to the calcareous lithology, and there are no discernible influences from anthropogenic activities concerned with addition of phosphate fertilisers or liming of noncalcareous agricultural soil in humid climates. However, in the eastern part of France, and in the Rhône-Provence Tertiary graben, an anthropogenic contribution has been reported that may have influenced the natural patterns, i.e., introduction of Ca by salt brining factories is suspected (CaCl 2 release); this salt possibly has a measurable impact when used as a de-icing medium for road maintenance in winter. Ca comparison between sample media Patterns in Ca distribution between all solid sample media are broadly similar, although higher Ca is present in stream sediment throughout the coastal region of Croatia (presence of limestone particles) - this is the opposite to trends observed in Al, Fe and related elements. Floodplain and topsoil distributions in the Baltic states are similar, with less Ca than observed in subsoil and stream sediments (agricultural soils used for soil sampling). In Ireland, Ca is higher in stream and floodplain sediments compared to soil. A boxplot comparing CaO variation in subsoil, topsoil, stream sediment and floodplain sediment is presented in Figure 10. Patterns in stream water Ca data are, in the main, markedly different from distributions in the solid sample media and strongly related to ph, with lowest concentrations in the most acid environments throughout most of southern and central Fennoscandia, and highest concentrations in some areas adjacent to the Mediterranean, in calcareous environments of northern France and south-east England, and in Germany, Poland and the Baltic states, possibly associated with intensive agricultural activity. Figure 10. Boxplot comparison of CaO variation in subsoil, topsoil, stream sediment and floodplain sediment. 102
Introduction. Table 27. Median concentrations of Eu in the FOREGS samples and in some reference data sets.
 Eu Europium Introduction See section on Rare Earth Elements (REEs). Table 27 compares the median concentrations of Eu in the FOREGS samples and in some reference datasets. Table 27. Median concentrations
Eu Europium Introduction See section on Rare Earth Elements (REEs). Table 27 compares the median concentrations of Eu in the FOREGS samples and in some reference datasets. Table 27. Median concentrations
Hf Hafnium. Introduction
 Hf Hafnium Introduction Hafnium is a member of group 4 of the periodic table, along with Ti and Zr. It has an atomic number of 72, an atomic mass of 178, one oxidation state (+4) and five naturally occurring
Hf Hafnium Introduction Hafnium is a member of group 4 of the periodic table, along with Ti and Zr. It has an atomic number of 72, an atomic mass of 178, one oxidation state (+4) and five naturally occurring
Ga Gallium. Introduction
 Ga Gallium Introduction Gallium is a member of group 13 of the periodic table, which also includes B, Al, In and Tl. The element has an atomic number of 31, an atomic mass of 70, one main oxidation state
Ga Gallium Introduction Gallium is a member of group 13 of the periodic table, which also includes B, Al, In and Tl. The element has an atomic number of 31, an atomic mass of 70, one main oxidation state
Sr Strontium. Introduction
 Sr Strontium Introduction Strontium belongs to the group 2 elements of the periodic table, along with Be, Mg, Ca and Ba. It has an atomic number of 38, an atomic mass of 88, one oxidation state (+2) and
Sr Strontium Introduction Strontium belongs to the group 2 elements of the periodic table, along with Be, Mg, Ca and Ba. It has an atomic number of 38, an atomic mass of 88, one oxidation state (+2) and
La Lanthanum. Introduction
 La Lanthanum Introduction Lanthanum is one of the rare earth elements (REEs), which is a collective term for the elements from lanthanum to lutetium, atomic numbers 57 71, in the periodic table. The element
La Lanthanum Introduction Lanthanum is one of the rare earth elements (REEs), which is a collective term for the elements from lanthanum to lutetium, atomic numbers 57 71, in the periodic table. The element
Be Beryllium. Introduction
 Be Beryllium Introduction Beryllium is the lightest of the group 2 elements that include Mg, Ca, Sr and Ba, although its chemistry has little in common with these more typical alkaline earth metals. The
Be Beryllium Introduction Beryllium is the lightest of the group 2 elements that include Mg, Ca, Sr and Ba, although its chemistry has little in common with these more typical alkaline earth metals. The
U Uranium. Introduction
 U Uranium Introduction Uranium is a member of the actinide series of elements, along with Th and human-made elements such as Pu. The element has an atomic number of 92, an atomic mass of 238, five main
U Uranium Introduction Uranium is a member of the actinide series of elements, along with Th and human-made elements such as Pu. The element has an atomic number of 92, an atomic mass of 238, five main
Ta Tantalum. Introduction
 Ta Tantalum Introduction Tantalum belongs to group 5 of the periodic table, along with V and Nb. The element has an atomic number of 73, an atomic mass of 181, one oxidation state (+5), and two stable
Ta Tantalum Introduction Tantalum belongs to group 5 of the periodic table, along with V and Nb. The element has an atomic number of 73, an atomic mass of 181, one oxidation state (+5), and two stable
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
DISTRIBUTION OF ELEMENTS IN SUBSOIL AND TOPSOIL
 DISTRIBUTION OF ELEMENTS IN SUBSOIL AND TOPSOIL W. De Vos 1, V. Gregorauskiene 2, K. Marsina 3, R. Salminen 4, I. Salpeteur 5, T. Tarvainen 4, P.J. O Connor 6, A. Demetriades 7, S. Pirc 8, M. J. Batista
DISTRIBUTION OF ELEMENTS IN SUBSOIL AND TOPSOIL W. De Vos 1, V. Gregorauskiene 2, K. Marsina 3, R. Salminen 4, I. Salpeteur 5, T. Tarvainen 4, P.J. O Connor 6, A. Demetriades 7, S. Pirc 8, M. J. Batista
PHYSICAL FEATURES OF EUROPE. Europe Unit
 PHYSICAL FEATURES OF EUROPE Europe Unit PENINSULA OF PENINSULAS Europe is a large peninsula that consists of many smaller peninsulas Most places in Europe are no more than 300 miles from an ocean or sea
PHYSICAL FEATURES OF EUROPE Europe Unit PENINSULA OF PENINSULAS Europe is a large peninsula that consists of many smaller peninsulas Most places in Europe are no more than 300 miles from an ocean or sea
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
The Baltic Soil Survey
 The Baltic Soil Survey C. Reimann, U. Siewers, T. Tarvainen, L. Bityukova, J. Erikson, A. Gilucis, V. Gregorauskiene, V. Lukashev, N. Matinian & A. Pasieczna PROJECT AIM: create a comparable database
The Baltic Soil Survey C. Reimann, U. Siewers, T. Tarvainen, L. Bityukova, J. Erikson, A. Gilucis, V. Gregorauskiene, V. Lukashev, N. Matinian & A. Pasieczna PROJECT AIM: create a comparable database
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
GEMAS: A European scale geochemical atlas for environmental management and mineral exploration
 GEMAS PROJECT GEMAS: A European scale geochemical atlas for environmental management and mineral exploration Clemens Reimann 40 Years Listening to the Beat of the Earth 2007: Eurometaux contacts EuroGeoSurveys:
GEMAS PROJECT GEMAS: A European scale geochemical atlas for environmental management and mineral exploration Clemens Reimann 40 Years Listening to the Beat of the Earth 2007: Eurometaux contacts EuroGeoSurveys:
Quiz 1. 3) Which of the following planetary bodies has the least number of impact craters on its surface? A) Mercury B) Mars C) the Moon D) Earth
 Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
Module 9 Sedimentary Rocks
 Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Practice Test Rocks and Minerals. Name. Page 1
 Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
As compaction and cementation of these sediments eventually occur, which area will become siltstone? A) A B) B C) C D) D
 1. A student obtains a cup of quartz sand from a beach. A saltwater solution is poured into the sand and allowed to evaporate. The mineral residue from the saltwater solution cements the sand grains together,
1. A student obtains a cup of quartz sand from a beach. A saltwater solution is poured into the sand and allowed to evaporate. The mineral residue from the saltwater solution cements the sand grains together,
CEE 437 Lecture 10 Rock Classification. Thomas Doe
 CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS
 LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Practice Questions for Lecture 5 Geology 1200
 Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur
 Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur Lecture - 01 Rock Cycle Good morning. I welcome you to this
Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur Lecture - 01 Rock Cycle Good morning. I welcome you to this
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK
 REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK Paul A. Morris 1 1 Geological Survey of Western Australia, 100 Plain Street, East Perth 6004, Western Australia;
REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK Paul A. Morris 1 1 Geological Survey of Western Australia, 100 Plain Street, East Perth 6004, Western Australia;
On the Major and Trace Elements Distribution in Two Different Egyptian Geologic Units: Nile Valley and Siwa Oasis
 On the Major and Trace Elements Distribution in Two Different Egyptian Geologic Units: Nile Valley and Siwa Oasis O.G. Duliu 1,2, Marina V. Frontasyeva 2, E. Steinnes 3, W.M. Badawy 4 1 University of Bucharest,
On the Major and Trace Elements Distribution in Two Different Egyptian Geologic Units: Nile Valley and Siwa Oasis O.G. Duliu 1,2, Marina V. Frontasyeva 2, E. Steinnes 3, W.M. Badawy 4 1 University of Bucharest,
Hg Mercury. Introduction
 Hg Mercury Introduction Mercury is a heavy metal from group 12 of the periodic table, along with Zn and Cd. Gold and Tl are its period neighbours. The element has an atomic number of 80, an atomic mass
Hg Mercury Introduction Mercury is a heavy metal from group 12 of the periodic table, along with Zn and Cd. Gold and Tl are its period neighbours. The element has an atomic number of 80, an atomic mass
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING
 LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
Minerals and Rocks. Environmental Learning Community CORC 1332 Sept 21, 2010
 Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
The Composition of the Continental Crust
 The Composition of the Continental Crust Roberta L. Rudnick Geochemistry Laboratory Department of Geology University of Maryland Apollo 17 view of Earth Rationale: Why is studying crust composition important?
The Composition of the Continental Crust Roberta L. Rudnick Geochemistry Laboratory Department of Geology University of Maryland Apollo 17 view of Earth Rationale: Why is studying crust composition important?
GEOL Introductory Geology: Exploring Planet Earth Fall 2010 Test #2 October 18, 2010
 GEOL 101 - Introductory Geology: Exploring Planet Earth Fall 2010 Test #2 October 18, 2010 Name KEY ID# KEY Multiple choice questions (2 points each). 1. What type of metamorphic rock is formed over large
GEOL 101 - Introductory Geology: Exploring Planet Earth Fall 2010 Test #2 October 18, 2010 Name KEY ID# KEY Multiple choice questions (2 points each). 1. What type of metamorphic rock is formed over large
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Rocks. 3.1 The Rock Cycle. 3.1 The Rock Cycle. 3.1 The Rock Cycle. The Rock Cycle. I. Rocks
 Rocks Tarbuck Lutgens 3.1 The Rock Cycle 3.1 The Rock Cycle I. Rocks Rocks are any solid mass of mineral or mineral-like matter occurring naturally as part of our planet. Types of Rocks 1. Igneous rock
Rocks Tarbuck Lutgens 3.1 The Rock Cycle 3.1 The Rock Cycle I. Rocks Rocks are any solid mass of mineral or mineral-like matter occurring naturally as part of our planet. Types of Rocks 1. Igneous rock
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks
 GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
Prentice Hall EARTH SCIENCE
 Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 3 Rocks 3.1 The Rock Cycle Rocks Rocks are any solid mass of mineral or mineral-like matter occurring naturally as part of our planet. Types of Rocks
Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 3 Rocks 3.1 The Rock Cycle Rocks Rocks are any solid mass of mineral or mineral-like matter occurring naturally as part of our planet. Types of Rocks
Lab: Metamorphism: minerals, rocks and plate tectonics!
 Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Chapter 10. Chapter Rocks and the Rock Cycle. Rocks. Section 1 Rocks and the Rock Cycle
 Chapter 10 Rocks 1 Chapter 10 Section 1 Rocks and the Rock Cycle 2 10.1 Rocks and the Rock Cycle Magma is the parent material for all rocks. Once the magma cools and hardens, many changes can occur. Geology:
Chapter 10 Rocks 1 Chapter 10 Section 1 Rocks and the Rock Cycle 2 10.1 Rocks and the Rock Cycle Magma is the parent material for all rocks. Once the magma cools and hardens, many changes can occur. Geology:
Answers. Rocks. Year 8 Science Chapter 8
 Answers Rocks Year 8 Science Chapter 8 p171 1 Rocks are made up of minerals such as quartz, feldspars, micas, and calcite. Different rocks are made up of different combinations of minerals. 2 Igneous,
Answers Rocks Year 8 Science Chapter 8 p171 1 Rocks are made up of minerals such as quartz, feldspars, micas, and calcite. Different rocks are made up of different combinations of minerals. 2 Igneous,
Chapter 5: Weathering and Soils. Fig. 5.14
 Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
Chapter 5: Weathering and Soils Fig. 5.14 OBJECTIVES Recognize that weathering breaks down minerals and rocks and occurs as a result of both mechanical and chemical processes. Explain the processes that
Rocks and the Rock Cycle notes from the textbook, integrated with original contributions
 Rocks and the Rock Cycle notes from the textbook, integrated with original contributions Alessandro Grippo, Ph.D. Gneiss (a metamorphic rock) from Catalina Island, California Alessandro Grippo review Rocks
Rocks and the Rock Cycle notes from the textbook, integrated with original contributions Alessandro Grippo, Ph.D. Gneiss (a metamorphic rock) from Catalina Island, California Alessandro Grippo review Rocks
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
THE ROCK CYCLE & ROCKS. Subtitle
 THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
Rock Cycle and Rock Types Homework
 Rock Cycle and Rock Types Homework Completion Complete each statement. 1. A(n) is a solid mass of mineral or mineral-like matter that occurs naturally. 2. Rocks are generally classified as igneous,, or
Rock Cycle and Rock Types Homework Completion Complete each statement. 1. A(n) is a solid mass of mineral or mineral-like matter that occurs naturally. 2. Rocks are generally classified as igneous,, or
Weathering Cycle Teacher Notes
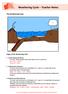 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
WEATHERING. Weathering breakdown of rock materials Erosion transport of broken-down materials
 WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Surface Processes on the Earth. Rocks, Weathering, Erosion and Soil
 Surface Processes on the Earth Rocks, Weathering, Erosion and Soil ROCKS AND ROCK CYCLE Rock types Three main types of rock Igneous Metamorphic Sedimentary Igneous Form when magma or lava cools and hardens
Surface Processes on the Earth Rocks, Weathering, Erosion and Soil ROCKS AND ROCK CYCLE Rock types Three main types of rock Igneous Metamorphic Sedimentary Igneous Form when magma or lava cools and hardens
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite.
 1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
Page 1. Name:
 Name: 1) What is the approximate density of a mineral with a mass of 262.2 grams that displaces 46 cubic centimeters of water? A) 6.1 g/cm 3 C) 1.8 g/cm 3 B) 5.7 g/cm 3 D) 12.2 g/cm 3 2) In which two Earth
Name: 1) What is the approximate density of a mineral with a mass of 262.2 grams that displaces 46 cubic centimeters of water? A) 6.1 g/cm 3 C) 1.8 g/cm 3 B) 5.7 g/cm 3 D) 12.2 g/cm 3 2) In which two Earth
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Sedimentary Environments Chapter 8
 Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
CEE 437 Lecture 11 Rock Classification. Thomas Doe
 CEE 437 Lecture 11 Rock Classification Thomas Doe Translation of Mineral Properties to Rock Properties Comparison of mineral properties to rock properties Rocks have lower strength, especially tensile
CEE 437 Lecture 11 Rock Classification Thomas Doe Translation of Mineral Properties to Rock Properties Comparison of mineral properties to rock properties Rocks have lower strength, especially tensile
Evolution of the Earth
 Evolution of the Earth http://static.newworldencyclopedia.org/f/fe/geologic_clock.jpg Evolution of the Earth Solar system, 4.6 byr Collapse of a nebula Star forms as gravity concentrates material at center
Evolution of the Earth http://static.newworldencyclopedia.org/f/fe/geologic_clock.jpg Evolution of the Earth Solar system, 4.6 byr Collapse of a nebula Star forms as gravity concentrates material at center
Page 1. Name: 1) Which diagram best shows the grain size of some common sedimentary rocks?
 Name: 1) Which diagram best shows the grain size of some common sedimentary rocks? 1663-1 - Page 1 5) The flowchart below illustrates the change from melted rock to basalt. 2) Which processes most likely
Name: 1) Which diagram best shows the grain size of some common sedimentary rocks? 1663-1 - Page 1 5) The flowchart below illustrates the change from melted rock to basalt. 2) Which processes most likely
Rocks. Rocks are composed of 1 or more minerals. Rocks are classified based on how they formed (origin). 3 classes of rocks:
 ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
1. What is the most important agent of chemical weathering on Earth? a. oxygen b. salt c. carbon dioxide d. carbonic acid e. water
 Geology 1-2nd Exam Spring 2013 Prof. Phil Stoffer 1. What is the most important agent of chemical weathering on Earth? a. oxygen b. salt c. carbon dioxide d. carbonic acid e. water 2. Igneous rocks are
Geology 1-2nd Exam Spring 2013 Prof. Phil Stoffer 1. What is the most important agent of chemical weathering on Earth? a. oxygen b. salt c. carbon dioxide d. carbonic acid e. water 2. Igneous rocks are
Rocks Rock- A group of minerals, glass, mineroid bound together in some way.
 Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Lecture series outline
 Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Water Quality 2 Lecture series outline Lecture 1a Water uses, water quality guidelines; physical, chemical and biological parameters Lecture 1b The origins of the constituents of water quality through
Name. 4. The diagram below shows a soil profile formed in an area of granite bedrock. Four different soil horizons, A, B, C, and D, are shown.
 Name 1. In the cross section of the hill shown below, which rock units are probably most resistant to weathering? 4. The diagram below shows a soil profile formed in an area of granite bedrock. Four different
Name 1. In the cross section of the hill shown below, which rock units are probably most resistant to weathering? 4. The diagram below shows a soil profile formed in an area of granite bedrock. Four different
PHYSIOGRAPHIC REGIONS OF THE LOWER 48 UNITED STATES
 PHYSIOGRAPHIC REGIONS OF THE LOWER 48 UNITED STATES LAURENTIAN UPLAND 1. Superior Upland ATLANTIC PLAIN 2. Continental Shelf (not on map) 3. Coastal Plain a. Embayed section b. Sea Island section c. Floridian
PHYSIOGRAPHIC REGIONS OF THE LOWER 48 UNITED STATES LAURENTIAN UPLAND 1. Superior Upland ATLANTIC PLAIN 2. Continental Shelf (not on map) 3. Coastal Plain a. Embayed section b. Sea Island section c. Floridian
Chapter 2. Regional Landscapes and the Hydrologic Cycle
 Chapter 2. Regional Landscapes and the Hydrologic Cycle W. Lee Daniels Department of Crop and Soil Environmental Sciences, Virginia Tech Table of Contents Introduction... 23 Soils and landscapes of the
Chapter 2. Regional Landscapes and the Hydrologic Cycle W. Lee Daniels Department of Crop and Soil Environmental Sciences, Virginia Tech Table of Contents Introduction... 23 Soils and landscapes of the
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 GLS100-01 Quiz#7 chapters 5 and 6 Fall 2009 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Clay minerals formed from gabbro or diorite bedrock
GLS100-01 Quiz#7 chapters 5 and 6 Fall 2009 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Clay minerals formed from gabbro or diorite bedrock
Sediment. Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface
 Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
A rock is a naturally occurring solid mixture of one or more minerals, or organic matter
 A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified by how they are formed, their composition, and texture Rocks change over time through the rock
A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified by how they are formed, their composition, and texture Rocks change over time through the rock
Geology 229 Engineering Geology. Lecture 6. Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5)
 Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion
 Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Adsorption of ions Ion exchange CEC& AEC Factors influencing ion exchange- Significance. Adsorption of ions Ion adsorption and subsequent exchange are important processes that take place between soil colloidal
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Earth History Exam. The remains of an early dinosaur could be found at reference point A. A B. B C. C D. D. page 1
 Name: Date: 1. Base your answer(s) to the following question(s) on the Earth Science Reference Tables and your knowledge of Earth science. The accompanying cross section shows undisturbed sedimentary bedrock.
Name: Date: 1. Base your answer(s) to the following question(s) on the Earth Science Reference Tables and your knowledge of Earth science. The accompanying cross section shows undisturbed sedimentary bedrock.
Ecoregions Glossary. 7.8B: Changes To Texas Land Earth and Space
 Ecoregions Glossary Ecoregions The term ecoregions was developed by combining the terms ecology and region. Ecology is the study of the interrelationship of organisms and their environments. The term,
Ecoregions Glossary Ecoregions The term ecoregions was developed by combining the terms ecology and region. Ecology is the study of the interrelationship of organisms and their environments. The term,
PROSPECTIVE AREAS FOR CRITICAL RAW MATERIALS IN THE EU
 This project has received funding from the European Union s Horizon 2020 research and innovation programme under grant agreement No. 776487 Ref. Ares(2017)5879386-30/11/2017 PROSPECTIVE AREAS FOR CRITICAL
This project has received funding from the European Union s Horizon 2020 research and innovation programme under grant agreement No. 776487 Ref. Ares(2017)5879386-30/11/2017 PROSPECTIVE AREAS FOR CRITICAL
Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p ) Workbook Chapter 4, 5 WEATHERING
 Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p. 95-125) Workbook Chapter 4, 5 THE BIG PICTURE: Weathering, erosion and deposition are processes that cause changes to rock material
Topic 6: Weathering, Erosion and Erosional-Deposition Systems (workbook p. 95-125) Workbook Chapter 4, 5 THE BIG PICTURE: Weathering, erosion and deposition are processes that cause changes to rock material
L.O: HOW GEOLOGISTS SEQUENCE EVENTS IN EARTH'S GEOLOGIC HISTORY IF NOT OVERTURNED, OLDEST ON BOTTOM, YOUNGEST ON TOP
 L.O: HOW GEOLOGISTS SEQUENCE EVENTS IN EARTH'S GEOLOGIC HISTORY IF NOT OVERTURNED, OLDEST ON BOTTOM, YOUNGEST ON TOP 1. Unless a series of sedimentary rock layers has been overturned, the bottom rock layer
L.O: HOW GEOLOGISTS SEQUENCE EVENTS IN EARTH'S GEOLOGIC HISTORY IF NOT OVERTURNED, OLDEST ON BOTTOM, YOUNGEST ON TOP 1. Unless a series of sedimentary rock layers has been overturned, the bottom rock layer
Paleoclimate indicators
 Paleoclimate indicators Rock types as indicators of climate Accumulation of significant thicknesses of limestone and reef-bearing limestone is restricted to ~20º + - equator Gowganda tillite, Ontario
Paleoclimate indicators Rock types as indicators of climate Accumulation of significant thicknesses of limestone and reef-bearing limestone is restricted to ~20º + - equator Gowganda tillite, Ontario
Laboratory 5 Sedimentary and Metamorphic Rocks a.
 Laboratory 5 Sedimentary and Metamorphic Rocks a. LAB 5 provides samples of all three principal groupings of rocks including: 1) Igneous (plutonic and extrusive felsic, intermediate, and mafic varieties)
Laboratory 5 Sedimentary and Metamorphic Rocks a. LAB 5 provides samples of all three principal groupings of rocks including: 1) Igneous (plutonic and extrusive felsic, intermediate, and mafic varieties)
Pacific Northwest Rock Lab, Part II. Igneous Rocks. Name Per.
 Name Per. Pacific Northwest Rock Lab, Part II After you ve classified all your rocks, place their numbers next to the names and read the information about the rock. Check the classifications here (igneous,
Name Per. Pacific Northwest Rock Lab, Part II After you ve classified all your rocks, place their numbers next to the names and read the information about the rock. Check the classifications here (igneous,
Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE Instructions: Read each question carefully before selecting the BEST answer Provide specific and detailed
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE Instructions: Read each question carefully before selecting the BEST answer Provide specific and detailed
Mammoth Cave National Park, Kentucky
 Mammoth Cave National Park, Kentucky Objectives of Today s Lecture Refresher on Sedimentary Depositional Systems and Rock Classifications Transgressive and Regressive Marine Environments Carbonate Depositional
Mammoth Cave National Park, Kentucky Objectives of Today s Lecture Refresher on Sedimentary Depositional Systems and Rock Classifications Transgressive and Regressive Marine Environments Carbonate Depositional
6.3 Geography. The student uses geographic tools to answer geographic questions. The student is expected to:
 Europe as a Region 1 6.3 Geography. The student uses geographic tools to answer geographic questions. The student is expected to: (A) pose and answer geographic questions, including: Where is it located?
Europe as a Region 1 6.3 Geography. The student uses geographic tools to answer geographic questions. The student is expected to: (A) pose and answer geographic questions, including: Where is it located?
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
9/4/2015. Feldspars White, pink, variable Clays White perfect Quartz Colourless, white, red, None
 ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
A. IGNEOUS Rocks formed by cooling and hardening of hot molten rock called magma (within crust or at its surface).
 EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type
 Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre
Discrimination between Archean A-type granitoids and sanukitoid suites using tectonic setting, geochemistry, and fertility type ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre
Sedimentary Rocks. Weathering. Mechanical & Chemical Weathering. Sediments. Lithification. Deposition. Transport. Erosion.
 Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
23/9/2013 ENGINEERING GEOLOGY. Chapter 2: Rock classification:
 ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
Liz LaRosa Images from Geology.com unless otherwise noted
 Liz LaRosa http://www.middleschoolscience.com 2010 Images from Geology.com unless otherwise noted A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified
Liz LaRosa http://www.middleschoolscience.com 2010 Images from Geology.com unless otherwise noted A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified
GEOLOGY OF THAILAND (METAMORPHIC ROCKS)
 GEOLOGY OF THAILAND (METAMORPHIC ROCKS) High-Grade Metamorphic Rocks (Precambrian?) Low-Grade Metamorphic Rocks (Lower Paleozoic) 1 THAILAND EXPLANATION Lower Paleozoic Rocks (Low Grade) Precambrian (?)
GEOLOGY OF THAILAND (METAMORPHIC ROCKS) High-Grade Metamorphic Rocks (Precambrian?) Low-Grade Metamorphic Rocks (Lower Paleozoic) 1 THAILAND EXPLANATION Lower Paleozoic Rocks (Low Grade) Precambrian (?)
DOMINANT SEDIMENTS TYPE IN ROCK Loose fragments of rocks or minerals broken off of bedrock Mineral crystals that precipitate directly out of water
 LAST NAME (ALL IN CAPS): FIRST NAME: 7. SEDIMENTARY PROCESSES, ROCKS, AND ENVIRONMENTS Instructions: Refer to Laboratory 6 in your Lab Book on pages 153-186 to answer the questions in this work sheet.
LAST NAME (ALL IN CAPS): FIRST NAME: 7. SEDIMENTARY PROCESSES, ROCKS, AND ENVIRONMENTS Instructions: Refer to Laboratory 6 in your Lab Book on pages 153-186 to answer the questions in this work sheet.
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
From Atoms to Minerals to Rocks: The building blocks of the Earth
 From Atoms to Minerals to Rocks: The building blocks of the Earth 1 Questions your students might ask? What are rocks made of? What are minerals? What are minerals? What is the difference between Fool
From Atoms to Minerals to Rocks: The building blocks of the Earth 1 Questions your students might ask? What are rocks made of? What are minerals? What are minerals? What is the difference between Fool
