PUBLICATIONS. Journal of Geophysical Research: Atmospheres
|
|
|
- Moris Stewart
- 5 years ago
- Views:
Transcription
1 PUBLICATIONS Journal of Geophysical Research: Atmospheres RESEARCH ARTICLE 1.12/213JD2461 Key Points: Frost flower δ 34 S values measured for the first time Apportionment of frost flower, sea salt and NSS sulfate in Arctic aerosols Frost flower quantification for size segregated aerosols at Alert Supporting Information: Readme Supplemental data Table S1 Table S2 Table S3 Table S4 Correspondence to: A.-L. Norman, alnorman@ucalgary.ca Citation: Seguin, A. M., A.-L. Norman, and L. Barrie (214), Evidence of sea ice source in aerosol sulfate loading and size distribution in the Canadian High Arctic from isotopic analysis, J. Geophys. Res. Atmos., 119, , doi:1.12/ 213JD2461. Received 11 JUL 213 Accepted 25 DEC 213 Accepted article online 4 JAN 214 Published online 31 JAN 214 Evidence of sea ice source in aerosol sulfate loading and size distribution in the Canadian High Arctic from isotopic analysis Alison Michelle Seguin 1,2,3, Ann-Lise Norman 2, and Leonard Barrie 4 1 Department of Chemistry, University of Calgary, Calgary, Alberta, Canada, 2 Department of Physics and Astronomy, University of Calgary, Calgary, Alberta, Canada, 3 RWDI AIR Inc., Calgary, Alberta, Canada, 4 Bolin Centre for Climate Research, Department of Geological Sciences, Stockholm University, Stockholm, Sweden Abstract The influence of frost flowers and seawater brine on ion chemistry in snow, snowpack, ice cores, and aerosols is detected when a lower sulfate to sodium ratio than in seawater is present in polar regions. This evidence can be masked when large amounts of non-sea-salt sulfate are present from other sources such as biogenic and anthropogenic sulfate. Frost flower δ 34 S values were measured for the first time in frost flower sulfates and did not differ significantly from the sea salt δ 34 Svaluesof+21. A method using stable isotopes is introduced to determine the limit of contributions from sea salt and sea ice sources (including frost flowers and brine) on sulfate concentrations in aerosol samples from Alert, Canada. Knowledge of the range of values of δ 34 S nss and the SO 4 /Na ratio found in sea ice sources (i.e., frost flowers) is used to quantitatively constrain the contributions from frost flowers and sea salt in the Arctic aerosol mass during the onset of winter in 27 and 28, allowing for quantification of non-sea-salt sulfate amounts during times when frost flowers are present. Frost flower and/or brine influence was found predominantly in the coarse-mode aerosols (>.95 μm). This method to determine the contributions from sea salt and sea ice sources can be carried over to future studies with snow and ice cores. 1. Introduction Sea ice sources including frost flowers and brine have been proposed to be significant sources of sodium in aerosols and snow during the Antarctic winter [Wagenbach et al., 1998; Rankin et al., 22] with as much as 6% of the yearly sodium in aerosols at coastal sites originating from frost flowers or brine [Rankin and Wolff, 23]. An estimate of 2.7% of the Antarctic sea surface at any one time is covered in frost flowers during the winter months [Rankin et al., 22]. Evidence of frost flowers contributing to ion loading in ice cores, precipitation, and aerosols has been observed in polar regions [i.e., Minikin et al., 1998; Rankin et al., 22; Rankin and Wolff, 23]. The role of snow on sea ice has also been studied as another possible source of elevated concentrations of sodium in the snowpack. Snow lying on sea ice can be influenced by frost flowers, migration of brine within the snow, and sea salt aerosols from the surrounding open leads [Dominé et al., 24; Wasiuta et al., 26; Yang et al., 28]. All of these influences could cause the ion chemistry of aerosol to deviate from typical SO 4 /Na sea salt ratios. [i.e., Alvarez- Aviles et al., 28; Kunasek et al., 21] Recent studies of frost flowers and snow found on sea ice have focused on their role in atmospheric reactions such as mercury depletion events [Douglas et al., 25; Sherman et al., 212] and their possible impact on halogen chemistry in the atmosphere [e.g., Morin et al., 28; Piot and von Glasow, 28; Yang et al., 28; Douglas et al., 212; Prattetal., 213]. Frost flowers are found on rapidly freezing open seawater. Small nodules are formed on new sea ice only a few centimetres thick [Perovich and Richter-Menge, 1994]. A large temperature gradient above the seawater surface leads to water vapor saturation which can result in water vapor condensing onto the nodules. On time scales of a few hours, sea ice and frost flowers form and brine on the sea ice surface can be drawn or wicked up onto the frost flowers. Concentrations of major ions are elevated immediately following formation because of the brine [e.g., Douglas et al., 212]. As the brine cools and looses water to the atmosphere by evaporation, salts remain on the frost flower or on the surface brine and precipitate out. At 8 C, sodium and sulfate precipitate out as mirabilite (Na 2 SO 4 1H 2 O) [Alvarez-Aviles et al., 28]. This can lead to a low sulfate/sodium ratio in sea ice sources (frost flowers and brine) relative to sea salt and can lead to an overestimate of sea salt sulfate (SO 4ss ) in aerosols, SEGUIN ET AL American Geophysical Union. All Rights Reserved. 187
2 1.12/213JD2461 snow, snowpack or glacial firn, and ice that are influenced by sea ice sources when using a sea salt correction that assumes no fractionation such as SO 4ss ¼ Na SS ratio (1) where Na is the total weight of sodium determined in the sample and SS ratio is the ratio of sulfate and sodium in seawater (.25) [Maidment, 1993]. The presence of frost flowers and/or brine is indicated when calculated non-sea-salt sulfate (SO 4nss ), the difference between the measured sulfate and the calculated sea salt sulfate, is found to be negative [Rankin and Wolff, 23]. The presence of other types of non-sea-salt sulfate (such as biogenic and anthropogenic) masks the negative SO 4nss signature. Anthropogenic sulfate has a much larger contribution to the Arctic atmosphere relative to the Antarctic. Frost flower sea salt depleted in sulfate has been observed in Arctic aerosols [Norman et al., 1999], but frost flower/brine signature in aerosols has been observed much more often in the Antarctic where vast refrozen seas form and the influence of anthropogenic sulfate has been minimal leading to larger calculated negative SO 4nss concentrations [e.g., Wagenbach et al., 1998; Rankin et al., 23; Hara et al., 24]. Besides ion concentration, the analysis of sulfur isotopes can give additional information. Sulfur isotopes have been used to determine the presence of frost flowers in aerosols and ice cores in previous studies. Norman et al. [1999] found a linear relationship between the fraction of calculated SO 4nss and calculated δ 34 S nss. They determined that a trend toward lighter calculated δ 34 S nss with an increasing fraction of sea salt was an indication that frost flowers, a source with a depleted SO 4 /Na ratio relative to the sea salt ratio, were present in some of the samples. Jonsell et al. [25] and Kunasek et al. [21] looked at different values for k (SO 4 /Na ratios expected from a combination of frost flowers and sea salt). These previous isotope studies have taken one of two approaches: either the frost flower and sea salt sulfate were treated as a single source and extreme values of k were used to calculate a range of δ 34 S nss values [Jonsell et al., 25; Kunasek et al., 21], or samples with possible high frost flower content were removed from the sample set when further analysis was conducted [Norman et al., 1999]. Direct measurements of the δ 34 S value of frost flower sulfate have not previously been conducted. Kunasek et al. [21] assumed no fractionation when determining background δ 34 S values, but noted that if frost flower growth fractionated sulfur isotopes, non-sea-salt sulfate (such as from volcanic and stratospheric sources) would be underestimated. The work presented here is the first to measure δ 34 S in sulfate from frost flowers. The study then quantifies a sea ice source (frost flower and/or brine) relative to sea salt contribution to aerosol sulfate based on the measured δ 34 S values. This assumes no isotopic fractionation during mirabilite formation. This allows for the apportionment of sea salt, frost flower, and NSS SO 4. It has been noted that snow influence by brine is an equally possible source of deviation from the SO 4 /Na ratio of sea salt [Dominé et al., 24; Alvarez-Aviles et al., 28; Yang et al., 28]. Elevated salinity in snowpack and aerosols have been attributed to blowing snow [Dominé et al., 24; Wasiuta et al., 26], and it is possible that snow on the sea surface would carry a depletion of the SO 4 /Na ratio relative to sea salt if it is heavily influenced by frost flowers and/or brine migration. The term frost flowers throughout this paper will be used to encompass both the frost flower and brine components of the sea ice source. The term sea salt source refers to both direct sources from seawater but may also include sea salt that is present in blowing snow that retains the SO 4 /Na ratio of sea salt. It is possible to calculate the maximum and minimum contribution of sulfate from frost flowers versus sea salt. The total amount of sodium (Na T ) in the sample is expected to come from either sea salt (Na ss ) or a sea ice source (frost flowers or brine) (Na ff ) and not from other sources (e.g., lithospheric [Sirois and Barrie, 1999]) and therefore, Na T ¼ Na ff þ Na ss (2) whereas sulfate (SO 4T ) comes from multiple sources. SO 4T ¼ SO 4ff þ SO 4ss þ SO 4nss (3) where SO 4nss is the NSS and nonfrost flower contribution of sulfate. This originates from biogenic and anthropogenic sources with the possibility of minor or episodic contributions from lithospheric sources [Norman et al., 1999]. The mass ratio of SO 4 /Naforseasalt(SS ratio ) is well known to be.25 [Maidment, 1993]. The mass SO 4 /Na ratio for frost flowers (FF ratio ) is more variable and has a ratio between.5 and.1 [Rankin et al., 22]. Although some studies have determined a larger FF ratio range [Alvarez-Aviles et al., 28], the values within the range stated by Rankin et al. [22] has been found by numerous SEGUIN ET AL American Geophysical Union. All Rights Reserved. 188
3 1.12/213JD2461 others [e.g., Wagenbach et al., 1998; Jonsell et al., 25; Beaudon and Moore, 29]. This ratio may also be associated with snow on the sea ice surface [Dominé et al., 24; Yang et al., 28]. A value of.7 that is closer to the lower end of the estimated range was used in the study by Kunasek et al. [21] and will be used throughout this work. Since the frost flower ratio can vary, sensitivity tests using.5 and.1 will be also considered. Maximum and minimum contributions of frost flower to atmospheric sulfate can be determined as follows: The maximum sulfate contribution from frost flowers or brine will be the minimum of either the total amount of sulfate present in the sample or the total amount of sodium in the sample multiplied by the frost flower ratio (i.e., either all sodium or all sulfate is from frost flowers). The minimum contribution of frost flower sulfate is zero unless the SO 4 /Na ratio is less than that of sea salt. In the later case, SO 4nss is zero (see Supporting Information), and the frost flower contribution is obtained as follows: SO 4ff min ¼ SO 4T SS ratio Na T 1 SSratio FF ratio : (4) 2. Isotope Constraints on Frost Flowers We know from isotope values that δ 34 S T SO 4T ¼ X δ 34 S i SO 4i (5) where subscripts indicate total (T), and i represents the different individual sources of sulfate. In polar regions, this translates to δ 34 S T SO 4T ¼ δ 34 S ff SO 4ff þ δ 34 S ss SO 4ss þ δ 34 S nss SO 4nss (6) where frost flower (ff), sea salt (ss) and non-sea-salt (nss) are the sources of sulfate in these regions. The isotope value for sea salt sulfate is well known +21 ±.1.[Rees et al., 1978]. The non-sea-salt component could range from being dominantly biogenic (+18 ± 1.5 ), [Patris et al., 2; Sanusi et al., 26] influenced to being completely anthropogenic (anthropogenic sources determined at Alert: +4.4 ±.4, Norman et al., Aerosol Sulfate from DMS in the Arctic: Evidence for a Biotic Feedback to Warming?, manuscript in preparation, 214). The amount of sulfate from the sea ice source (SO 4ff )canbecalculated from equations 2, 3, and 6 along with the sea salt and frost flower ratios (see Supporting Information) to give the following equation SO 4ff ¼ N at SS Ratio δ 34 S nss δ 34 S ss þ SO4T δ 34 S T δ 34 S nss δ 34 S ff δ 34 S nss þ SSratio FF ratio δ 34 S nss δ 34 : (7) S ss 3. Methods Five frost flower samples were collected for ion concentrations and δ 34 S values during the Surface Heat Budget of the Arctic Ocean (SHEBA) program. The details of the SHEBA program have been described by others [i.e., Perovich et al., 1999; Pinto et al., 23]. The samples were collected between 7 March and 29 April 1998 upwind from the ship. Frost flowers were placed in storage bottles with a polycarbonate scoop and PVC rod (tamper). All frost flower samples were taken within 5 m of the ship and are taken upwind of all prevailing storms. Samples were frozen until ion concentrations (January 2) and sulfur isotope values (26) were determined in the laboratory. Two high-volume aerosol samplers were deployed at Alert, Nunavut in the fall of 27 (12 October 1 December 1) and 28 (19 September 18 October). One high-volume sampler was used to collect size-segregated samples (aerodynamic diameter <.49 μm, μm, μm, μm, μm, and >7.2 μm) located at N, W (elevation:191 m). The impact of blowing snow was mitigated by a specially designed housing to exclude snow as much as possible around the high-volume sampler. The second high-volume sampler located approximately 2 m away ( N, W; elevation:18 m) was used to collect total aerosol sulfate. Total aerosol samples from this high-volume sampler may include blowing snow since this sampler was not contained within the protective housing. The total aerosol high-volume sampler was not running between 18 October and 2 November 27 due to SEGUIN ET AL American Geophysical Union. All Rights Reserved. 189
4 1.12/213JD2461 Table 1. Sodium and Sulfate Ions, Ratios, and δ 34 S Values of Frost Flowers Collected During the SHEBA Mission [Na + ] 2 [SO 4 ] Mass Ratio Date Collected (ppm) (ppm) ([SO 2 4 ]/[Na + ]) δ 34 S( ) Description 27/3/ New frost flowers and slush 24/4/ Old frost flower layer 24/4/ Old frost flower layer against ice 29/4/ Frost flowers 29/4/ Frost flowers mechanical failure. Apart from this period, the high-volume samplers were run continuously with samples collected two or three times each week. Filters from the high-volume sampling program were processed by dissolving anion and cation species in distilled deionized water [Seguin et al., 211]. Ion concentrations were determined from a portion of the sample either by a Dionex 1 or Dionex ICS-3 liquid ion chromatograph. Sulfur isotope analysis was carried out by the addition of BaCl 2 and HCl to the remaining sample solution to precipitate barium sulfate. Sulfur isotopes were determined by a VG Prism II continuous flow isotope mass spectrometer [Giesemann et al., 1994]. Sulfur isotope measurements are reported as δ 34 S values in parts per thousand ( ) with respect to Vienna Cañon Diablo Triolite. Instrumental uncertainty was determined from replicate laboratory standards to be ±.4. Field blanks were taken into consideration for correction of δ 34 Svaluesandionamountsonthe high-volume filter [Seguin et al., 211]. 4. δ 34 S Values of Frost Flowers Ion concentrations and isotope values are displayed in Table 1 for the five frost flower samples. The first sample, collected on 27 March 1998 was taken as frost flowers were forming. Both the ion ratio and the δ 34 S value were representatives of sea salt, and it is believed that the slush at the base of the frost flower may have been included in the sample. The next two frost flower samples were collected on 24 April 1998 and were representatives of older frost flowers. These were considered older since frost flowers had been partially buried by drifting snow. The sulfate/sodium ratio for these two samples was similar to sea salt, and δ 34 S values were similar to seawater (Table 1). Blowing snow and/or sea salt may have contaminated these older samples. The remaining two samples collected on 29 April 1998 had lower ratios of sulfate/sodium (average.13, Table 1) compared to sea salt (.25). This indicates that some mirabilite precipitated from the frost flower at the time of sampling and if significant isotopic fractionation occurred in this process, it should be observed in the δ 34 S values measured in these two samples. The δ 34 S values of these two samples averaged +2.8 (individual measurements, 2.4 and 21.2, see Table 1). Therefore, little to no sulfur isotope fractionation occurred during the precipitation of mirabilite in frost flowers and the average δ 34 S value of the measured frost flowers is within error (.4 )ofthe seasaltvalue.thisisthefirst time δ 34 S values of frost flowers have been directly measured. 5. Evidence of Frost Flower Contribution From δ 34 S Values If frost flowers or brine do not significantly contribute to aerosol samples, the δ 34 S value will approach the δ 34 S value of sea salt as SO 4 /Na ratio approaches that of sea salt (i.e.,.25). The mixing between sea salt and a non-sea-salt component leads to a linear relationship with a y-intercept of δ 34 S nss when plotting the inverse of this ratio (i.e., Na/SO 4 ) versus δ 34 S. The δ 34 S values for aerosol samples collected at Alert are compared to the Na/SO 4 in the sample in Figure 1. The limits of the mixing triangle (solid border) in Figure 1 represent the three-point mixing of aerosol sulfate with source points: δ 34 S values of +18 ± 1.5 for biogenic [Patris et al., 2; Sanusi et al., 26], +4.4 ±.4 for anthropogenic (Norman et al., manuscript in preparation, 214), and +21 ±.1 [Rees et al., 1978] for sea salt sulfate. If no frost flowers are present, values should fall within the solid triangular region. However, the observations for aerosol SO 4 in total and size-fractionated aerosol show that a large number of points lie outside the mixing triangle, indicative of another source of sulfate in Alert aerosols. Although other sources of sulfate have been found in the Arctic [Rempillo et al., 211], no significant sources, besides the three mentioned above and the frost flowers/brine, are expected at Alert [Norman et al., 1999]. A second dashed triangle in Figure 1 is added to indicate the mixing of anthropogenic, biogenic, and frost flowers. Frost flower δ 34 S values are not significantly different from sea salt (+21 ). With the addition of the frost flower source, all samples fit within error of the second triangle (Figure 1) SEGUIN ET AL American Geophysical Union. All Rights Reserved. 19
5 1.12/213JD m and >7.2 m m and m m <.49 m Total Aerosol Na/SO 4 mass ratio Figure 1. The δ 34 S of the sample versus Na/SO 4 mass ratio of Alert aerosols. If no sea ice influence is present, samples would lie within the mixing triangle with a solid border where the vertices correspond to biogenic (, 18 ), anthropogenic (, 4.4 ), and sea salt (.25 1,21 ) sulfate. This is not the case at Alert. With the addition of sea ice sulfate (frost flowers/brine) (.7 1,21 ), a second mixing triangle (dashed boarder) can be overlaid. All samples (within error) during this study can now be accounted for. indicating that frost flowers can be a significant source of sodium and sulfate at Alert and that the assumption of FF ratio is approximately.7 (.7 1 = 14.29) is reasonable. Higher FF ratio (SO 4 /Na ratios in frost flowers) can be ruled out for a number of samples in this data set since this would lead to the samples falling out of the frost flower mixing triangle (dashed triangle, Figure 1). 6. Frost Flower and Brine Contribution to Aerosol Loading at Alert Sulfate, sodium, and δ 34 S T values were measured at Alert and were used to determine the contribution from frost flowers and/or brine. A time series of these three measurements are displayed in Figure 2. Because the aerosol NSS SO 4 is a mixture of anthropogenic and biogenic sulfate at Alert, the δ 34 S nss value is not known exactly. Instead, the δ 34 S nss values are constrained to fall between +4.4 and +18. due to the endpoints of Total Aerosol SO 4 and Na concentrations (ng/m 3 ) Sep-19 Sep-29 Oct-9 Oct-19 Sulfate Sodium 34 S of sample Oct-14 Oct-21 Oct-28 Nov-4 Nov-11 Nov-18 Nov-25 Dec-2 Dec-9 Date (27) Figure 2. Time series of total aerosol sulfate and sodium concentrations along with the measured δ 34 S T value (secondary axis) of the same for 27 and 28 (insert). SEGUIN ET AL American Geophysical Union. All Rights Reserved. 191
6 1.12/213JD2461 Total Aerosol SO 4 frost flower concentration (ng/m 3 ) Sep-21 Oct-1 Oct-11 Oct-14 Oct-21 Oct-28 Nov-4 Nov-11 Date (27) Nov-18 Nov-25 Dec-2 Dec-9 Figure 3. Limits of the total aerosol sulfate concentration from frost flowers at Alert calculated using equation 7 and δ 34 S nss restraints of +18 and +4.4 along with nonisotopic constraints (i.e., equation 4 and total sulfate and sodium measured). Dark gray represents the limits of concentrations when frost flower SO 4 /Na mass ratio is constrained to.7. A sensitivity test of frost flower ratios between.5 and.1 is illustrated with the light gray. anthropogenic (+4.4 ) and biogenic (+18. ) sulfate. Sulfate associated with lithospheric sources at Alert are expected to be minimal [Norman et al., 1999]. The contributions from frost flowers at Alert for the total sulfate aerosol burden during the fall of 27 and 28 are shown in Figure 3. This takes into consideration both the range of possible δ 34 S nss values and the use of equation 7 along the constraints when considering minimum (equation 4) and maximum (either all sodium or all sulfate is from sea ice sources) contributions from sea ice sources. See Supporting Information for an example of these considerations. A value of.7 for the FF ratio isusedinthebaselinescenario(whereonlyδ 34 SO 4nss values are varied). A sensitivity test of the FF ratio between.5 and.1 is also demonstrated. In 28, the detectable influence from sea ice sources is not significantly different than zero (Figure 3), which can be explained by the warmer temperatures observed at Alert at this time. At 8 C, sodium and sulfate precipitate out as mirabilite (Na 2 SO 4 1H 2 O) [Alvarez-Aviles et al., 28] and atmospheric temperatures just above the surface of the water where frost flowers form can be warmer by as much as 6ºC relative to ambient air temperature [Martin et al., 1996]. Hourly temperatures at Alert recorded by Environment Canada (www. climate.weatheroffice.gc.ca) were averaged for sampling periods in 28 and ranged between 8 and 19 C with maximum temperatures for sampling periods ranging between 2 and 12 C. Therefore, during the majority of sampling periods in 28, temperatures were warmer than the conditions at which frost flowers are expected to form. 7. Source Apportionment We now can determine the contribution of aerosol sulfate from sea salt as follows: SO 4ss ¼ SS Ratio Na T SO 4ff : (8) FF ratio Non-sea-salt (and nonfrost flower) sulfate can then be calculated by equation 3. The source apportionment at Alert for total aerosol sulfate is presented in Figure 4 for 27, assuming a constant frost flower ratio of.7. At Alert, since δ 34 S nss can range between +4.4 (anthropogenic) and +18 (biogenic), the calculations are carried out twice using each end point. This will lead to a minimum (Figure 4a) and maximum (Figure 4b) frost flower contribution for source apportionment. As expected, NSS SO 4 is the largest contribution to the sulfate loading at Alert. The presence of frost flower signature in aerosols is dependent on wind speed, the origin of the air mass, and the presence of frost flowers along the back trajectory of the air mass with frost SEGUIN ET AL American Geophysical Union. All Rights Reserved. 192
7 1.12/213JD2461 Total Aerosol SO 4 (ng/m 3 ) Sep-21 Oct-1 Oct-11 non sea salt sea salt frost flower a Oct-14 Oct-21 Oct-28 Nov-4 Nov-11 Date (27) Nov-18 Nov-25 Dec-2 Dec-9 Total Aerosol SO 4 (ng/m 3 ) Sep-21 Oct-1 Oct-11 b Oct-14 Oct-21 Oct-28 Nov-4 Nov-11 Nov-18 Nov-25 Dec-2 Dec-9 Date (27) Figure 4. Sulfate source apportionment of total aerosol sulfate at Alert. (a) Minimum and (b) maximum frost flower contribution scenarios are displayed (FF ratio =.7). Minimum and maximum frost flower contribution is due to the range of possible δ 34 S nss values (+4.4 to +18. ) and other physical properties (see Supporting Information) as illustrated in Figure 3. Total Aerosol SO 4 frost flower concentration (ng/m 3 ) Oct-14 Oct-21 Oct-28 Nov-4 Nov-11 Date (27) Nov-18 Nov-25 Dec-2 Dec-9 Figure 5. Sum of the minimum frost flower and/or brine contribution to aerosol sulfate concentrations for the different size-segregated samples. Total aerosol (<1 μm in diameter) frost flower and brine contributions are plotted as points for comparison. Blowing snow may be present in total aerosol filters November and 3 5 December.Maximum frost flower and/or brine contribution is displayed in the insert for comparison. The minimum and maximum frost flower contribution is due to the range of possible δ 34 S nss values (+4.4 to +18. ). SEGUIN ET AL American Geophysical Union. All Rights Reserved. 193
8 1.12/213JD2461 flower formation itself dependent on temperature and wind conditions [Alvarez-Aviles et al., 28; Piot and von Glasow, 28; Obbard et al., 29; Roscoe et al., 211]. Therefore, the frost flower influence in aerosols is expected to occur episodically and not be present during all sampling periods. Contributions from sea ice sources are expected to be zero for the majority of the sampling time, and the lower limit (Figure 4a) can usually be expected when estimating frost flower contributions to the sulfate burden. This may lead to an underestimation of sea ice sources, an overestimation of sea salt sources, and an underestimation of biogenic sources during times of significant frost flower events, but would be more accurate during the remainder of the sampling period when frost flower contributions are minimal. Assuming the lower limit would also indicate that δ 34 S nss values lay closer to that of the anthropogenic end member (of +4.4 ) relative to that of a biogenic source (+18 ). As illustrated in Figure 1, this is a valid assumption for the majority of the samples. Frost flower contributions to sulfate aerosols in different size ranges are displayed in Figure 5, along with the total aerosol sulfate concentration. Total aerosol sulfate concentration significantly exceeded the sum of the sulfate concentrations in the size-segregated samples between November and 3 5 December 27. This can be attributed to the fact that the high-volume samplers were not colocated; samples were collected over different sampling periods and sulfate from blowing snow may have been included in the total aerosol samples. Frost flowers and sea salt were found predominantly in the coarser aerosols (>.95 μm): an observation shared by others [Rankin and Wolff, 23]. The highest possible contribution of sulfate from frost flowers in the smallest size range (<.49 μm) is 1% or 19 ng/m 3. This is using the maximum frost flower contribution (i.e., FF ratio =.1) on the day when other size fractions show large amounts of frost flower contribution (see insert of Figure 5). Similarly, the contribution of sea salt sulfate in the smallest size range is at most 48 ng/m 3.Frost flower contributions are minimal in most cases in the <.49 μm size range (minimum frost flower contribution is %). This finding indicates that frost flower contributions inland would be minimal relative to coastal areas since coarse aerosols tend to have a much shorter lifetime than fine aerosols. Concentrations of sodium (maximum 19 ng/m 3 ) in the smallest size range (<.49 μm) found at Alert are comparable to concentrations found in previous studies at sites located away from the coast [Jourdain et al., 28] and is consistent with studies that have found frost flower signatures in aerosols close to the coast but not inland [Hara et al., 24]. 8. Conclusions The sulfur isotopic composition of frost flower sulfate was measured directly for the first time from samples taken near the SHEBA ship. The value of +2.8 was within error (±.4 ) of sea salt (+21 ). This leads to the conclusion that sulfate isotopes in frost flowers do not significantly differ from sea salt δ 34 S values and that fractionation of sulfur isotopes in frost flowers is not significant. A novel way to distinguish between frost flower/brine and sea salt contributions in aerosols is introduced. Frost flower and/or brine influence in aerosols less than.49 μm in diameter was found to be negligible during the study period with most frost flower and brine contribution being found in aerosols larger than.95 μm in diameter. In some cases, sea ice sources contribute greater than 5% of the sulfate in these course-mode aerosols. This suggests local sources of frost flowers or blowing snow influenced by brine in the area. This method can be used in other applications such as precipitation and ice core analysis if the δ 34 S value is measured. This method, combining isotope analysis and a constraint on the sulfate to sodium ratio, determines the extent of the frost flower and brine impact on the total aerosol sulfate loading in the atmosphere and allows for amounts of non-sea-salt (and nonfrost flower) sulfate to be determined even during times when there is influence from frost flowers. Although the δ 34 S values can be used as constraints for some of the contributions from frost flowers, they do have limitations. The possible range of values in both δ 34 S nss and the ratio of FF ratio used leads to uncertainty in the calculation of frost flower sulfate concentrations. Both anthropogenic and biogenic sulfate end points must be considered when looking at frost flower contributions at Alert. It is more likely as water freezes with the onset of winter, that the anthropogenic end point becomes increasingly significant as the biogenic sulfur declines. Although δ 34 S nss values have been constrained to lie between +4.4 and +18 in this study, there would be less error introduced in calculation of the frost flower aerosol concentration if a consistent background sulfur isotope signature was present. Calculations could commence on not only the SEGUIN ET AL American Geophysical Union. All Rights Reserved. 194
9 1.12/213JD2461 concentrations from each source if the δ 34 S nss was known as a single value, but could also be used to study the sea ice source SO 4 /Na ratio. Frost flower ratios are dependent on atmospheric conditions at the time of formation [Perovich and Richter-Menge, 1994; Martin et al., 1996; Rankin et al., 22]. The method presented here may lead to further understanding of temporal variations of sea ice sources, including frost flowers and brine found in aerosols, ice cores, or snowpacks. This could be especially significant in areas where δ 34 S nss values are expected to be relatively consistent over time such as the Antarctic or in preindustrial ice cores where biogenic sulfate is likely the largest source of non-sea-salt sulfate, and anthropogenic sulfate is expected to be minimal [Rankin and Wolff, 23; Patris et al., 22]. Acknowledgments This project was funded through the Arctic-SOLAS program through IPY and NSERC. The authors would like to thank Environment Canada and the Science and Technology Branch Climate Research for their assistance with sample collection. References Alvarez-Aviles, L., W. R. Simpson, T. A. Douglas, M. Sturm, D. Perovich, and F. Dominé (28), Frost flower chemical composition during growth and its implications for aerosol production and bromine activation, J. Geophys. Res., 113, D2134, doi:1.129/28jd1277. Beaudon, E., and J. Moore (29), Frost flower chemical signature in winter snow on Vestfonna ice cap, Nordaustlandet, Svalbard, The Cryosphere, 3, Dominé, F., R. Sparapani, A. Ianniello, and H. J. Beine (24), The origin of sea salt in snow on Arctic sea ice and in coastal regions, Atmos. Chem. Phys., 4, Douglas, T., M. Sturm, W. Simpson, S. Brooks, S. Lindberg, and D. Perovich (25), Elevated mercury measured in snow and frost flowers near Arctic sea ice leads, Geophys. Res. Lett., 32, L452, doi:1.129/24gl Douglas, T. A., et al. (212), Frost flowers growing in the Arctic ocean-atmosphere sea ice snow interface: 1. Chemical composition, J. Geophys. Res., 117, DR9, doi:1.129/211jd1646. Giesemann, A., H.-J. Jäger, A. L. Norman, H. R. Krouse, and W. A. Brand (1994), Online sulfur-isotope determination using an elemental analyzer coupled to a mass spectrometer, Anal. Chem., 66(18), Hara, K., K. Osada, M. Kido, M. Hayashi, K. Matsunaga, Y. Iwasaka, T. Yamanouchi, G. Hashida, and T. Fukatsu (24), Chemistry of sea-salt particles and inorganic halogen species in Antarctic regions: Compositional differences between coastal and inland stations, J. Geophys. Res., 19, D228, doi:1.129/24jd4713. Jonsell, U., M. E. Hansson, C.-M. Morth, and P. Torssander (25), Sulfur isotopic signals in two shallow ice cores from Dronning Maud Land, Antarctica, Tellus, 57B, Jourdain, B., S. Preunkert, O. Cerri, H. Casterbrunet, R. Udisti, and M. Legrand (28), Year-round record of size-segregated aerosol composition in central Antarctica (Concordia station): Implications for the degree of fractionation of sea salt particles, J. Geophys. Res., 113, D1438, doi:1.129/27jd9584. Kunasek, S. A., B. Alexander, E. J. Steig, E. D. Sofen, T. L. Jackson, M. H. Thiemens, J. R. McConnell, D. J. Gleason, and H. M. Amos (21), Sulfate sources and oxidation chemistry over the past 23 years from sulfur and oxygen isotopes of sulfate in a West Antarctic ice core, J. Geophys. Res., 115, D18313, doi:1.129/21jd Maidment, D. R. (Ed) (1993), Handbook of Hydrology, McGraw-Hill, New York. Martin, S., Y. Yu, and R. Drucker (1996), The temperature dependence of frost flower growth on laboratory sea ice and the effect of the flowers on infrared observations of the surface, J. Geophys. Res., 11(C5), 12,111 12,125, doi:1.129/96jc28. Minikin, A., M. Legrand, J. Hall, D. Wagenbach, C. Kleefeld, E. Wolff, E. C. Pasteur, and F. Ducroz (1998), Sulfur-containing species (sulfate and methanesulfonate) in coastal Antarctic aerosol and precipitation, J. Geophys. Res., 13(D9), 1,975 1,99, doi:1.129/98jd249. Morin, S., G. M. Marion, R. von Glasow, D. Voisin, J. Bouchez, and J. Savarino (28), Precipitation of salts in freezing seawater and ozone depletion events: a status report, Atmos. Chem. Phys., 8, , doi:1.5194/acp Norman, A. L., L. A. Barrie, D. Toom-Sauntry, A. Sirois, H. R. Krouse, S. M. Li, and S. Sharma (1999), Sources of aerosol sulfate at Alert: Apportionment using stable isotopes, J. Geophys. Res., 14, 11,619 11,631, doi:1.129/1999jd978. Obbard, R. W., H. K. Roscoe, E. W. Wolff, and H. M. Atkinson (29), Frost flower surface area and chemistry as a function of salinity and temperature, J. Geophys. Res., 114, D235, doi:1.129/29jd Patris, N., R. J. Delmas, and J. Jouzel (2), Isotopic signatures of sulfur in shallow Antarctic ice cores, J. Geophys. Res., 15, , doi:1.129/1999jd9974. Patris, N., R. J. Delmas, M. Legrand, M. De Angelis, F. A. Ferron, M. Stièvenard, and J. Jouzel (22), First sulfur isotope measurements in central Greenland ice cores along the preindustrial periods, J. Geophys. Res., 17, D672, doi:1.129/21jd672. Perovich, D. K., and J. A. Richter-Menge (1994), Surface characteristics of lead ice, J. Geophys. Res., 99(C8), 16,341 16,35, doi:1.129/ 94JC1194. Perovich, D. K., et al. (1999), Year on ice gives climate insights, Eos Trans. AGU, 8(481), , doi:1.129/eo8i41p Pinto, J. O., A. Alam, J. A. Maslanik, J. A. Curry, and R. S. Stone (23), Surface characteristics and atmospheric footprint of springtime Arctic leads at SHEBA, J. Geophys. Res., 18(C4), 851, doi:1.129/2jc473. Piot, M., and R. von Glasow (28), The potential importance of frost flowers, recycling on snow, and open leads for ozone depletion events, Atmos. Chem. Phys., 8, , doi:1.5194/acp Pratt, K. A., et al. (213), Photochemical production of molecular bromine in Arctic surface snowpacks, Nat. Geosci., 6, 351, doi:1.138/ NGE1779. Rankin, A. M., and E. W. Wolff (23), A year-long record of size-segregated aerosol composition at Halley, Antarctica, J. Geophys. Res., 18(D24), 4775, doi:1.129/23jd3993. Rankin, A. M., E. W. Wolff, and S. Martin (22), Frost flowers: Implications for tropospheric chemistry and ice core interpretation, J. Geophys. Res., 17(D23), 4683, doi:1.129/22jd2492. Rees, C. E., W. J. Jenkins, and J. Monster (1978), Sulphur isotopic composition of ocean water sulfate, Geochim. Cosmochim. Ac., 42, Rempillo, O. T., et al. (211), DMS air-sea fluxes and biogenic sulfur as a source of new aerosols in the Arctic fall, J. Geophys. Res., 116, DS4, doi:1.129/211jd Roscoe, H. K., B. Brooks, A. V. Jackson, M. H. Smith, S. J. Walker, R. W. Obbard, and E. W. Wolff (211), Frost flowers in the laboratory: Growth, characteristics, aerosol, and the underlying sea ice, J. Geophys. Res., 116, D1231, doi:1.129/21jd Sanusi, A. A., A. L. Norman, C. Burridge, M. Wadleigh, and W. W. Tang (26), Determination of the S isotope composition of methanesulfonic acid, Anal. Chem., 78, , doi:1.121/ac648. SEGUIN ET AL American Geophysical Union. All Rights Reserved. 195
10 1.12/213JD2461 Seguin, A. M., A. L. Norman, S. Eaton, and M. Wadleigh (211), Seasonality in size segregated biogenic, anthropogenic and sea salt sulfate aerosols over the North Atlantic, Atmos. Environ., 45, , doi:1.116/j.atmosenv Sherman, L. S., J. D. Blum, T. A. Douglas, and A. Steffen (212), Frost flowers growing in the Arctic ocean atmosphere sea ice snow interface: 2. Mercury exchange between the atmosphere, snow, and frost flowers, J. Geophys. Res., 117, DR1, doi:1.129/211jd Sirois, A., and L. A. Barrie (1999), Arctic lower tropospheric aerosol trends and composition at Alert, Canada: , J. Geophys. Res., 14(D9), 11,599 11,618, doi:1.129/1999jd977. Wagenbach, D., F. Ducroz, R. Mulvaney, L. Keck, A. Minikinm, M. Legrand, J. S. Hall, and E. M. Wolff (1998), Sea-salt aerosol in coastal Antarctic regions, J. Geophys. Res., 13(D9), 1,961 1,974, doi:1.129/97jd184. Wasiuta, V., A. L. Norman, and S. Marshall (26), Spatial patterns and seasonal variation of snowpack sulfate isotopes of the Prince of Wales Icefield, Ellesmere Island, Canada, Ann. Glaciol., 43, , doi:1.3189/ Yang, X., J. A. Pyle, and R. A. Cox (28), Sea salt aerosol production and bromine release: Role of snow on sea ice, J. Geophys. Res. Let, 35, L16815, doi:1.129/28gl SEGUIN ET AL American Geophysical Union. All Rights Reserved. 196
Arctic Halogen Chemistry Part II
 Arctic Halogen Chemistry Part II Kerri A. Pratt Department of Chemistry Dept. of Earth & Environmental Sciences University of Michigan 2017 Connaught Summer Institute on Arctic Science Ozone Reminders
Arctic Halogen Chemistry Part II Kerri A. Pratt Department of Chemistry Dept. of Earth & Environmental Sciences University of Michigan 2017 Connaught Summer Institute on Arctic Science Ozone Reminders
Arctic Oxidation Chemistry
 19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
SOURCES AND TRANSPORT PATHWAYS OF MARINE AEROSOL SPECIES INTO WEST ANTARCTICA. S.B. Sneed 1, M.J. Handley 1. Orono, ME USA
 SOURCES AND TRANSPORT PATHWAYS OF MARINE AEROSOL SPECIES INTO WEST ANTARCTICA S. Kaspari 1, P.A. Mayewski 1, D.A. Dixon 1, S.B. Sneed 1, M.J. Handley 1 1 Climate Change Institute, Department of Earth Sciences,
SOURCES AND TRANSPORT PATHWAYS OF MARINE AEROSOL SPECIES INTO WEST ANTARCTICA S. Kaspari 1, P.A. Mayewski 1, D.A. Dixon 1, S.B. Sneed 1, M.J. Handley 1 1 Climate Change Institute, Department of Earth Sciences,
Supplement of Nitrate deposition and preservation in the snowpack along a traverse from coast to the ice sheet summit (Dome A) in East Antarctica
 Supplement of The Cryosphere, 12, 1177 1194, 2018 https://doi.org/10.5194/tc-12-1177-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
Supplement of The Cryosphere, 12, 1177 1194, 2018 https://doi.org/10.5194/tc-12-1177-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
Frost flower chemical composition during growth and its implications for aerosol production and bromine activation
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010277, 2008 Frost flower chemical composition during growth and its implications for aerosol production and bromine
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010277, 2008 Frost flower chemical composition during growth and its implications for aerosol production and bromine
Sea Salt Aerosol from Blowing Snow above Sea Ice - Production Mechanisms and Parametrisations
 Sea Salt Aerosol from Blowing Snow above Sea Ice - Production Mechanisms and Parametrisations Xin Yang British Antarctic Survey, Natural Environment Research Council, UK BAS-INI, 18 September, 2017 1:
Sea Salt Aerosol from Blowing Snow above Sea Ice - Production Mechanisms and Parametrisations Xin Yang British Antarctic Survey, Natural Environment Research Council, UK BAS-INI, 18 September, 2017 1:
Frost flowers: Implications for tropospheric chemistry and ice core interpretation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D23, 4683, doi:10.1029/2002jd002492, 2002 Frost flowers: Implications for tropospheric chemistry and ice core interpretation Andrew M. Rankin and Eric W.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D23, 4683, doi:10.1029/2002jd002492, 2002 Frost flowers: Implications for tropospheric chemistry and ice core interpretation Andrew M. Rankin and Eric W.
East Antarctic ice core sulfur isotope measurements over a complete glacial-interglacial cycle
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D24, 4786, doi:10.1029/2003jd003513, 2003 East Antarctic ice core sulfur isotope measurements over a complete glacial-interglacial cycle B. Alexander 1 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D24, 4786, doi:10.1029/2003jd003513, 2003 East Antarctic ice core sulfur isotope measurements over a complete glacial-interglacial cycle B. Alexander 1 and
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1153966/dc1 Supporting Online Material for The Sensitivity of Polar Ozone Depletion to Proposed Geoengineering Schemes Simone Tilmes,* Rolf Müller, Ross Salawitch *To
www.sciencemag.org/cgi/content/full/1153966/dc1 Supporting Online Material for The Sensitivity of Polar Ozone Depletion to Proposed Geoengineering Schemes Simone Tilmes,* Rolf Müller, Ross Salawitch *To
A study of the sea-salt chemistry using size-segregated aerosol measurements at coastal Antarctic station Neumayer
 A study of the sea-salt chemistry using size-segregated aerosol measurements at coastal Antarctic station Neumayer K. Teinilä a,, A. Frey a, R. Hillamo a, H. C. Tülp b,c, R. Weller b a Finnish Meteorological
A study of the sea-salt chemistry using size-segregated aerosol measurements at coastal Antarctic station Neumayer K. Teinilä a,, A. Frey a, R. Hillamo a, H. C. Tülp b,c, R. Weller b a Finnish Meteorological
Arctic Climate Change. Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013
 Arctic Climate Change Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013 When was this published? Observational Evidence for Arctic
Arctic Climate Change Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013 When was this published? Observational Evidence for Arctic
Sources and distribution of sea salt aerosol from the Tropics to the Poles
 Sources and distribution of sea salt aerosol from the Tropics to the Poles Lyatt Jaeglé Department of Atmospheric Sciences University of Washington, Seattle contributions from present and past graduate
Sources and distribution of sea salt aerosol from the Tropics to the Poles Lyatt Jaeglé Department of Atmospheric Sciences University of Washington, Seattle contributions from present and past graduate
A reinterpretation of sea-salt records in Greenland and Antarctic ice cores?
 276 Annals of Glaciology 39 2004 A reinterpretation of sea-salt records in Greenland and Antarctic ice cores? Andrew M. RANKIN, Eric W. WOLFF, Robert MULVANEY British Antarctic Survey, Natural Environment
276 Annals of Glaciology 39 2004 A reinterpretation of sea-salt records in Greenland and Antarctic ice cores? Andrew M. RANKIN, Eric W. WOLFF, Robert MULVANEY British Antarctic Survey, Natural Environment
Brita Horlings
 Knut Christianson Brita Horlings brita2@uw.edu https://courses.washington.edu/ess431/ Natural Occurrences of Ice: Distribution and environmental factors of seasonal snow, sea ice, glaciers and permafrost
Knut Christianson Brita Horlings brita2@uw.edu https://courses.washington.edu/ess431/ Natural Occurrences of Ice: Distribution and environmental factors of seasonal snow, sea ice, glaciers and permafrost
Volcanoes and climate change
 Volcanoes and climate change Volcanic fallout reveals secrets of past eruptions IMPORTANT INFORMATION about a past volcanic eruption's impact on climate is provided by determining the height of the eruption.
Volcanoes and climate change Volcanic fallout reveals secrets of past eruptions IMPORTANT INFORMATION about a past volcanic eruption's impact on climate is provided by determining the height of the eruption.
SUPPLEMENTARY INFORMATION
 Supplementary discussion S1. Factors determining Δ O in atmospheric nitrate Atmospheric nitrate is the oxidation product of NO x (NO x NO + NO 2 ). NO x is emitted from natural sources including biomass
Supplementary discussion S1. Factors determining Δ O in atmospheric nitrate Atmospheric nitrate is the oxidation product of NO x (NO x NO + NO 2 ). NO x is emitted from natural sources including biomass
Sea salt aerosol response to climate change: last glacial maximum, pre-industrial,
 Sea salt aerosol response to climate change: last glacial maximum, pre-industrial, and doubled-carbon dioxide climates To be submitted to GRL, Natalie M. Mahowald 1, Jean-François Lamarque 1, Xue Xi Tie
Sea salt aerosol response to climate change: last glacial maximum, pre-industrial, and doubled-carbon dioxide climates To be submitted to GRL, Natalie M. Mahowald 1, Jean-François Lamarque 1, Xue Xi Tie
Pacific Decadal Oscillation ( PDO ):
 Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2015-2016. El Nino / La Nina: When looking
Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2015-2016. El Nino / La Nina: When looking
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
First-year sea-ice contact predicts bromine monoxide (BrO) levels at Barrow, Alaska better than potential frost flower contact
 Atmos. Chem. Phys., 7, 1 7, 7 www.atmos-chem-phys.net/7/1/7/ Author(s) 7. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics First-year sea-ice contact predicts bromine
Atmos. Chem. Phys., 7, 1 7, 7 www.atmos-chem-phys.net/7/1/7/ Author(s) 7. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics First-year sea-ice contact predicts bromine
Introduction to Climate Change
 Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
The Arctic Energy Budget
 The Arctic Energy Budget The global heat engine [courtesy Kevin Trenberth, NCAR]. Differential solar heating between low and high latitudes gives rise to a circulation of the atmosphere and ocean that
The Arctic Energy Budget The global heat engine [courtesy Kevin Trenberth, NCAR]. Differential solar heating between low and high latitudes gives rise to a circulation of the atmosphere and ocean that
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
From Isotopes to Temperature: Using Ice Core Data!
 From Isotopes to Temperature: Using Ice Core Data! Spruce W. Schoenemann schoes@uw.edu UWHS Atmospheric Sciences 211 May 2013 Dept. of Earth and Space Sciences University of Washington Seattle http://www.uwpcc.washington.edu
From Isotopes to Temperature: Using Ice Core Data! Spruce W. Schoenemann schoes@uw.edu UWHS Atmospheric Sciences 211 May 2013 Dept. of Earth and Space Sciences University of Washington Seattle http://www.uwpcc.washington.edu
Climate Roles of Land Surface
 Lecture 5: Land Surface and Cryosphere (Outline) Climate Roles Surface Energy Balance Surface Water Balance Sea Ice Land Ice (from Our Changing Planet) Surface Albedo Climate Roles of Land Surface greenhouse
Lecture 5: Land Surface and Cryosphere (Outline) Climate Roles Surface Energy Balance Surface Water Balance Sea Ice Land Ice (from Our Changing Planet) Surface Albedo Climate Roles of Land Surface greenhouse
The origin of sea salt in snow on Arctic sea ice and in coastal regions
 The origin of sea salt in snow on Arctic sea ice and in coastal regions F. Domine, R. Sparapani, A. Ianniello, H. J. Beine To cite this version: F. Domine, R. Sparapani, A. Ianniello, H. J. Beine. The
The origin of sea salt in snow on Arctic sea ice and in coastal regions F. Domine, R. Sparapani, A. Ianniello, H. J. Beine To cite this version: F. Domine, R. Sparapani, A. Ianniello, H. J. Beine. The
A Changing Climate: Past, Present and Future. What is Climate?
 A Changg Climate: Past, Present and Future AT 351 Lab 14 April 30, 2008 What is Climate? The slowly varyg aspects of the atmosphere hydrosphere land surface system Climate is often considered to be an
A Changg Climate: Past, Present and Future AT 351 Lab 14 April 30, 2008 What is Climate? The slowly varyg aspects of the atmosphere hydrosphere land surface system Climate is often considered to be an
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Winter. Here s what a weak La Nina usually brings to the nation with tempseraures:
 2017-2018 Winter Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2017-2018. El Nino /
2017-2018 Winter Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2017-2018. El Nino /
Observing Arctic Sea Ice Change. Christian Haas
 Observing Arctic Sea Ice Change Christian Haas Decreasing Arctic sea ice extent in September Ice extent is decreasing, but regional patterns are very different every year The Cryosphere Today, http://arctic.atmos.uiuc.edu;
Observing Arctic Sea Ice Change Christian Haas Decreasing Arctic sea ice extent in September Ice extent is decreasing, but regional patterns are very different every year The Cryosphere Today, http://arctic.atmos.uiuc.edu;
IMPACTS OF A WARMING ARCTIC
 The Earth s Greenhouse Effect Most of the heat energy emitted from the surface is absorbed by greenhouse gases which radiate heat back down to warm the lower atmosphere and the surface. Increasing the
The Earth s Greenhouse Effect Most of the heat energy emitted from the surface is absorbed by greenhouse gases which radiate heat back down to warm the lower atmosphere and the surface. Increasing the
Exemplar for Internal Achievement Standard. Mathematics and Statistics Level 3
 Exemplar for internal assessment resource Mathematics and Statistics for Achievement Standard 91580 Exemplar for Internal Achievement Standard Mathematics and Statistics Level 3 This exemplar supports
Exemplar for internal assessment resource Mathematics and Statistics for Achievement Standard 91580 Exemplar for Internal Achievement Standard Mathematics and Statistics Level 3 This exemplar supports
The Dynamic Earth Section 3. Chapter 3 The Dynamic Earth Section 3: The Hydrosphere and Biosphere DAY 1
 Chapter 3 The Dynamic Earth Section 3: The Hydrosphere and Biosphere DAY 1 The Hydrosphere The hydrosphere includes all of the water on or near the Earth s surface. This includes water in the oceans, lakes,
Chapter 3 The Dynamic Earth Section 3: The Hydrosphere and Biosphere DAY 1 The Hydrosphere The hydrosphere includes all of the water on or near the Earth s surface. This includes water in the oceans, lakes,
Autonomous "OBuoy" observations of the Arctic atmosphere
 Autonomous "OBuoy" observations of the Arctic atmosphere William Simpson, University of Alaska Fairbanks, wrsimpson@alaska.edu Paty Matrai, Bigelow Laboratory for Ocean Sciences Francisco Chavez, Monterey
Autonomous "OBuoy" observations of the Arctic atmosphere William Simpson, University of Alaska Fairbanks, wrsimpson@alaska.edu Paty Matrai, Bigelow Laboratory for Ocean Sciences Francisco Chavez, Monterey
The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany
 The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany History of Neumayer In March 1981, the Georg von Neumayer Station (70 37 S, 8 22
The Meteorological Observatory from Neumayer Gert König-Langlo, Bernd Loose Alfred-Wegener-Institut, Bremerhaven, Germany History of Neumayer In March 1981, the Georg von Neumayer Station (70 37 S, 8 22
CHAPTER IV THE RELATIONSHIP BETWEEN OCEANOGRAPHY AND METEOROLOGY
 CHAPTER IV THE RELATIONSHIP BETWEEN OCEANOGRAPHY AND METEOROLOGY THE relationship between oceanography and meteorology is of an order different from that between it and geology or biology, because meteorologic
CHAPTER IV THE RELATIONSHIP BETWEEN OCEANOGRAPHY AND METEOROLOGY THE relationship between oceanography and meteorology is of an order different from that between it and geology or biology, because meteorologic
Land Surface Sea Ice Land Ice. (from Our Changing Planet)
 Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice (from Our Changing Planet) Earth s s Climate System Solar forcing Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry
Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice (from Our Changing Planet) Earth s s Climate System Solar forcing Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry
Earth s Climate System. Surface Albedo. Climate Roles of Land Surface. Lecture 5: Land Surface and Cryosphere (Outline) Land Surface Sea Ice Land Ice
 Lecture 5: Land Surface and Cryosphere (Outline) Earth s Climate System Solar forcing Land Surface Sea Ice Land Ice Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry Cycles (from Our Changing
Lecture 5: Land Surface and Cryosphere (Outline) Earth s Climate System Solar forcing Land Surface Sea Ice Land Ice Atmosphere Ocean Land Solid Earth Energy, Water, and Biochemistry Cycles (from Our Changing
Year-round chemical aerosol records in continental Antarctica obtained by automatic samplings
 YEAR-ROUND CHEMICAL AEROSOL RECORDS IN CONTINENTAL ANTARCTICA 1 Year-round chemical aerosol records in continental Antarctica obtained by automatic samplings ROLF WELLER* 1 and DIETMAR WAGENBACH 2, 1 Alfred
YEAR-ROUND CHEMICAL AEROSOL RECORDS IN CONTINENTAL ANTARCTICA 1 Year-round chemical aerosol records in continental Antarctica obtained by automatic samplings ROLF WELLER* 1 and DIETMAR WAGENBACH 2, 1 Alfred
January 25, Summary
 January 25, 2013 Summary Precipitation since the December 17, 2012, Drought Update has been slightly below average in parts of central and northern Illinois and above average in southern Illinois. Soil
January 25, 2013 Summary Precipitation since the December 17, 2012, Drought Update has been slightly below average in parts of central and northern Illinois and above average in southern Illinois. Soil
THE EARTH S CLIMATE SYSTEM
 THE EARTH S CLIMATE SYSTEM Earth s Climate System is driven by interactions between the parts of our biosphere So.what is the Biosphere? a relatively thin layer of Earth that has conditions suitable for
THE EARTH S CLIMATE SYSTEM Earth s Climate System is driven by interactions between the parts of our biosphere So.what is the Biosphere? a relatively thin layer of Earth that has conditions suitable for
Winter sea-ice melt in the Canada Basin, Arctic Ocean
 GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2011gl050219, 2012 Winter sea-ice melt in the Canada Basin, Arctic Ocean Jennifer M. Jackson, 1,2 William J. Williams, 3 and Eddy C. Carmack 3 Received
GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2011gl050219, 2012 Winter sea-ice melt in the Canada Basin, Arctic Ocean Jennifer M. Jackson, 1,2 William J. Williams, 3 and Eddy C. Carmack 3 Received
Will a warmer world change Queensland s rainfall?
 Will a warmer world change Queensland s rainfall? Nicholas P. Klingaman National Centre for Atmospheric Science-Climate Walker Institute for Climate System Research University of Reading The Walker-QCCCE
Will a warmer world change Queensland s rainfall? Nicholas P. Klingaman National Centre for Atmospheric Science-Climate Walker Institute for Climate System Research University of Reading The Walker-QCCCE
Arctic Chemistry And Climate
 21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... )
 Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
M. Mielke et al. C5816
 Atmos. Chem. Phys. Discuss., 14, C5816 C5827, 2014 www.atmos-chem-phys-discuss.net/14/c5816/2014/ Author(s) 2014. This work is distributed under the Creative Commons Attribute 3.0 License. Atmospheric
Atmos. Chem. Phys. Discuss., 14, C5816 C5827, 2014 www.atmos-chem-phys-discuss.net/14/c5816/2014/ Author(s) 2014. This work is distributed under the Creative Commons Attribute 3.0 License. Atmospheric
APPENDIX B PHYSICAL BASELINE STUDY: NORTHEAST BAFFIN BAY 1
 APPENDIX B PHYSICAL BASELINE STUDY: NORTHEAST BAFFIN BAY 1 1 By David B. Fissel, Mar Martínez de Saavedra Álvarez, and Randy C. Kerr, ASL Environmental Sciences Inc. (Feb. 2012) West Greenland Seismic
APPENDIX B PHYSICAL BASELINE STUDY: NORTHEAST BAFFIN BAY 1 1 By David B. Fissel, Mar Martínez de Saavedra Álvarez, and Randy C. Kerr, ASL Environmental Sciences Inc. (Feb. 2012) West Greenland Seismic
Energy and Seasons A B1. 9. Which graph best represents the general relationship between latitude and average surface temperature?
 Energy and Seasons A B1 1. Which type of surface absorbs the greatest amount of electromagnetic energy from the Sun? (1) smooth, shiny, and light colored (2) smooth, shiny, and dark colored (3) rough,
Energy and Seasons A B1 1. Which type of surface absorbs the greatest amount of electromagnetic energy from the Sun? (1) smooth, shiny, and light colored (2) smooth, shiny, and dark colored (3) rough,
Day 1 of Global Warming. Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
The Climate of Kiowa County
 The Climate of Kiowa County Kiowa County is part of the Central Great Plains, encompassing some of the best agricultural land in Oklahoma. Average annual precipitation ranges from about 24 inches in northwestern
The Climate of Kiowa County Kiowa County is part of the Central Great Plains, encompassing some of the best agricultural land in Oklahoma. Average annual precipitation ranges from about 24 inches in northwestern
Sulfur isotopic measurements from a West Antarctic ice core: implications for sulfate source and transport
 Sulfur isotopic measurements from a West Antarctic ice core: implications for sulfate source and transport Lee E. Pruett 1, Karl J. Kreutz 1 *, Moire Wadleigh 2, Paul A. Mayewski 1, Andrei Kurbatov 1 1
Sulfur isotopic measurements from a West Antarctic ice core: implications for sulfate source and transport Lee E. Pruett 1, Karl J. Kreutz 1 *, Moire Wadleigh 2, Paul A. Mayewski 1, Andrei Kurbatov 1 1
Grade 9 Geography Chapter 11 - Climate Connections
 Grade 9 Geography Chapter 11 - Climate Connections 1. Define: Weather. 2. In what way has weather affected your activities in the last two weeks? 3. Define: Climate. 4. Canada s climate is a function of
Grade 9 Geography Chapter 11 - Climate Connections 1. Define: Weather. 2. In what way has weather affected your activities in the last two weeks? 3. Define: Climate. 4. Canada s climate is a function of
University of Bristol - Explore Bristol Research
 Pausata, F. S. R., LeGrande, A. N., & Roberts, W. H. G. (2016). How Will Sea Ice Loss Affect the Greenland Ice Sheet? On the Puzzling Features of Greenland Ice-Core Isotopic Composition; Copenhagen, Denmark,
Pausata, F. S. R., LeGrande, A. N., & Roberts, W. H. G. (2016). How Will Sea Ice Loss Affect the Greenland Ice Sheet? On the Puzzling Features of Greenland Ice-Core Isotopic Composition; Copenhagen, Denmark,
The Climate of Payne County
 The Climate of Payne County Payne County is part of the Central Great Plains in the west, encompassing some of the best agricultural land in Oklahoma. Payne County is also part of the Crosstimbers in the
The Climate of Payne County Payne County is part of the Central Great Plains in the west, encompassing some of the best agricultural land in Oklahoma. Payne County is also part of the Crosstimbers in the
Website Lecture 3 The Physical Environment Part 1
 Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
The Climate of Bryan County
 The Climate of Bryan County Bryan County is part of the Crosstimbers throughout most of the county. The extreme eastern portions of Bryan County are part of the Cypress Swamp and Forest. Average annual
The Climate of Bryan County Bryan County is part of the Crosstimbers throughout most of the county. The extreme eastern portions of Bryan County are part of the Cypress Swamp and Forest. Average annual
Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2009jd012134, 2009 Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland Julia C. Jarvis, 1 Meredith G. Hastings, 2 Eric J. Steig,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2009jd012134, 2009 Isotopic ratios in gas-phase HNO 3 and snow nitrate at Summit, Greenland Julia C. Jarvis, 1 Meredith G. Hastings, 2 Eric J. Steig,
Here s what a weak El Nino usually brings to the nation with temperatures:
 Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2018-2019. El Nino / La Nina: When looking
Time again for my annual Winter Weather Outlook. Here's just a small part of the items I considered this year and how I think they will play out with our winter of 2018-2019. El Nino / La Nina: When looking
The Climate of Texas County
 The Climate of Texas County Texas County is part of the Western High Plains in the north and west and the Southwestern Tablelands in the east. The Western High Plains are characterized by abundant cropland
The Climate of Texas County Texas County is part of the Western High Plains in the north and west and the Southwestern Tablelands in the east. The Western High Plains are characterized by abundant cropland
Supplement of Carbon stocks and fluxes in the high latitudes: using site-level data to evaluate Earth system models
 Supplement of Biogeosciences, 14, 5143 5169, 2017 https://doi.org/10.5194/bg-14-5143-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Supplement of Biogeosciences, 14, 5143 5169, 2017 https://doi.org/10.5194/bg-14-5143-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
World Geography Chapter 3
 World Geography Chapter 3 Section 1 A. Introduction a. Weather b. Climate c. Both weather and climate are influenced by i. direct sunlight. ii. iii. iv. the features of the earth s surface. B. The Greenhouse
World Geography Chapter 3 Section 1 A. Introduction a. Weather b. Climate c. Both weather and climate are influenced by i. direct sunlight. ii. iii. iv. the features of the earth s surface. B. The Greenhouse
Satellites, Weather and Climate Module??: Polar Vortex
 Satellites, Weather and Climate Module??: Polar Vortex SWAC Jan 2014 AKA Circumpolar Vortex Science or Hype? Will there be one this year? Today s objectives Pre and Post exams What is the Polar Vortex
Satellites, Weather and Climate Module??: Polar Vortex SWAC Jan 2014 AKA Circumpolar Vortex Science or Hype? Will there be one this year? Today s objectives Pre and Post exams What is the Polar Vortex
Today. Events. Terrestrial Planet Atmospheres (continued) Homework DUE
 Today Terrestrial Planet Atmospheres (continued) Events Homework DUE Sources of Gas Outgassing from volcanoes 2014 Pearson Education, Inc. Evaporation of surface liquid; sublimation of surface ice (cometary
Today Terrestrial Planet Atmospheres (continued) Events Homework DUE Sources of Gas Outgassing from volcanoes 2014 Pearson Education, Inc. Evaporation of surface liquid; sublimation of surface ice (cometary
Turmoil in the Global Biosphere A View from Space
 BIOTECHNO 2011 - Keynote Venice/Mestre, Italy, May 2011 Turmoil in the Global Biosphere A View from Space S. V. Nghiem 1, P. Clemente-Colón 2, I. G. Rigor 3, D. K. Perovich 4, and G. Neumann 1 1 NASA Jet
BIOTECHNO 2011 - Keynote Venice/Mestre, Italy, May 2011 Turmoil in the Global Biosphere A View from Space S. V. Nghiem 1, P. Clemente-Colón 2, I. G. Rigor 3, D. K. Perovich 4, and G. Neumann 1 1 NASA Jet
LONG-TERM FAST-ICE VARIABILITY OFF DAVIS AND MAWSON STATIONS, ANTARCTICA
 Ice in the Environment: Proceedings of the 16th IAHR International Symposium on Ice Dunedin, New Zealand, 2nd 6th December 2002 International Association of Hydraulic Engineering and Research LONG-TERM
Ice in the Environment: Proceedings of the 16th IAHR International Symposium on Ice Dunedin, New Zealand, 2nd 6th December 2002 International Association of Hydraulic Engineering and Research LONG-TERM
Meltdown Evidence of Climate Change from Polar Science. Eric Wolff
 Meltdown Evidence of Climate Change from Polar Science Eric Wolff (ewwo@bas.ac.uk) Why are the polar regions important for climate? Heat engine Why are the polar regions important for climate? Heat engine
Meltdown Evidence of Climate Change from Polar Science Eric Wolff (ewwo@bas.ac.uk) Why are the polar regions important for climate? Heat engine Why are the polar regions important for climate? Heat engine
Grade 8 Science. Unit 1: Water Systems on Earth Chapter 1
 Grade 8 Science Unit 1: Water Systems on Earth Chapter 1 Effects of Water? Churchill River Large Ocean Wave How do you use water? House Hold Use Personal Use Recreational Activities Water Distribution
Grade 8 Science Unit 1: Water Systems on Earth Chapter 1 Effects of Water? Churchill River Large Ocean Wave How do you use water? House Hold Use Personal Use Recreational Activities Water Distribution
NSIDC Sea Ice Outlook Contribution, 31 May 2012
 Summary NSIDC Sea Ice Outlook Contribution, 31 May 2012 Julienne Stroeve, Walt Meier, Mark Serreze, Ted Scambos, Mark Tschudi NSIDC is using the same approach as the last 2 years: survival of ice of different
Summary NSIDC Sea Ice Outlook Contribution, 31 May 2012 Julienne Stroeve, Walt Meier, Mark Serreze, Ted Scambos, Mark Tschudi NSIDC is using the same approach as the last 2 years: survival of ice of different
The Climate of Grady County
 The Climate of Grady County Grady County is part of the Central Great Plains, encompassing some of the best agricultural land in Oklahoma. Average annual precipitation ranges from about 33 inches in northern
The Climate of Grady County Grady County is part of the Central Great Plains, encompassing some of the best agricultural land in Oklahoma. Average annual precipitation ranges from about 33 inches in northern
University of Bristol - Explore Bristol Research
 Pausata, F., LeGrande, A., & Roberts, W. H. G. (2016). How Will Sea Ice Loss Affect the Greenland Ice Sheet?: On the Puzzling Features of Greenland Ice-Core Isotopic Composition; Copenhagen, Denmark, 26
Pausata, F., LeGrande, A., & Roberts, W. H. G. (2016). How Will Sea Ice Loss Affect the Greenland Ice Sheet?: On the Puzzling Features of Greenland Ice-Core Isotopic Composition; Copenhagen, Denmark, 26
6. What has been the most effective erosive agent in the climate system? a. Water b. Ice c. Wind
 Multiple Choice. 1. Heinrich Events a. Show increased abundance of warm-water species of planktic foraminifera b. Show greater intensity since the last deglaciation c. Show increased accumulation of ice-rafted
Multiple Choice. 1. Heinrich Events a. Show increased abundance of warm-water species of planktic foraminifera b. Show greater intensity since the last deglaciation c. Show increased accumulation of ice-rafted
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/315/5808/84/dc1 Supporting Online Material for Mass-Independent Sulfur Isotopic Compositions in Stratospheric Volcanic Eruptions Mélanie Baroni,* Mark H. Thiemens, Robert
www.sciencemag.org/cgi/content/full/315/5808/84/dc1 Supporting Online Material for Mass-Independent Sulfur Isotopic Compositions in Stratospheric Volcanic Eruptions Mélanie Baroni,* Mark H. Thiemens, Robert
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Physical Oceanography
 Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
Physical Oceanography SECTION 15.1 The Oceans In your textbook, read about modern oceanography. For each item in Column A, write the letter of the matching item in Column B. e b c d a Column A 1. German
CLIMATE READY BOSTON. Climate Projections Consensus ADAPTED FROM THE BOSTON RESEARCH ADVISORY GROUP REPORT MAY 2016
 CLIMATE READY BOSTON Sasaki Steering Committee Meeting, March 28 nd, 2016 Climate Projections Consensus ADAPTED FROM THE BOSTON RESEARCH ADVISORY GROUP REPORT MAY 2016 WHAT S IN STORE FOR BOSTON S CLIMATE?
CLIMATE READY BOSTON Sasaki Steering Committee Meeting, March 28 nd, 2016 Climate Projections Consensus ADAPTED FROM THE BOSTON RESEARCH ADVISORY GROUP REPORT MAY 2016 WHAT S IN STORE FOR BOSTON S CLIMATE?
Coupling Climate to Clouds, Precipitation and Snow
 Coupling Climate to Clouds, Precipitation and Snow Alan K. Betts akbetts@aol.com http://alanbetts.com Co-authors: Ray Desjardins, Devon Worth Agriculture and Agri-Food Canada Shusen Wang and Junhua Li
Coupling Climate to Clouds, Precipitation and Snow Alan K. Betts akbetts@aol.com http://alanbetts.com Co-authors: Ray Desjardins, Devon Worth Agriculture and Agri-Food Canada Shusen Wang and Junhua Li
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
LET NOT THAT ICE MELT IN SVALBARD S. RAJAN, INCOIS NEELU SINGH, NCAOR
 LET NOT THAT ICE MELT IN SVALBARD S. RAJAN, INCOIS NEELU SINGH, NCAOR 1. Arctic (surface air) temperatures are rising twice as fast as the temperatures in the rest of the world (Amplification). The Arctic
LET NOT THAT ICE MELT IN SVALBARD S. RAJAN, INCOIS NEELU SINGH, NCAOR 1. Arctic (surface air) temperatures are rising twice as fast as the temperatures in the rest of the world (Amplification). The Arctic
ATOC OUR CHANGING ENVIRONMENT Class 19 (Chp 6) Objectives of Today s Class: The Cryosphere [1] Components, time scales; [2] Seasonal snow
![ATOC OUR CHANGING ENVIRONMENT Class 19 (Chp 6) Objectives of Today s Class: The Cryosphere [1] Components, time scales; [2] Seasonal snow ATOC OUR CHANGING ENVIRONMENT Class 19 (Chp 6) Objectives of Today s Class: The Cryosphere [1] Components, time scales; [2] Seasonal snow](/thumbs/95/126309683.jpg) ATOC 1060-002 OUR CHANGING ENVIRONMENT Class 19 (Chp 6) Objectives of Today s Class: The Cryosphere [1] Components, time scales; [2] Seasonal snow cover, permafrost, river and lake ice, ; [3]Glaciers and
ATOC 1060-002 OUR CHANGING ENVIRONMENT Class 19 (Chp 6) Objectives of Today s Class: The Cryosphere [1] Components, time scales; [2] Seasonal snow cover, permafrost, river and lake ice, ; [3]Glaciers and
Seasonality of Arctic Black Carbon Processes in the AMAP Multi-Model Ensemble
 Seasonality of Arctic Black Carbon Processes in the AMAP Multi-Model Ensemble R. Mahmood 1,2, K. von Salzen 3, M.G. Flanner 4, M. Sand 5, J. Langner 6, H. Wang 7, and L. Huang 8 1 School of Earth and Ocean
Seasonality of Arctic Black Carbon Processes in the AMAP Multi-Model Ensemble R. Mahmood 1,2, K. von Salzen 3, M.G. Flanner 4, M. Sand 5, J. Langner 6, H. Wang 7, and L. Huang 8 1 School of Earth and Ocean
SC-WACCM! and! Problems with Specifying the Ozone Hole
 SC-WACCM! and! Problems with Specifying the Ozone Hole R. Neely III, K. Smith2, D. Marsh,L. Polvani2 NCAR, 2Columbia Thanks to: Mike Mills, Francis Vitt and Sean Santos Motivation To design a stratosphere-resolving
SC-WACCM! and! Problems with Specifying the Ozone Hole R. Neely III, K. Smith2, D. Marsh,L. Polvani2 NCAR, 2Columbia Thanks to: Mike Mills, Francis Vitt and Sean Santos Motivation To design a stratosphere-resolving
FCAT Review Earths Systems
 FCAT Review Earths Systems PARTS OF EARTHS SYSTEMS The Earth system has 5 main spheres: 1) Atmosphere The layer of gases that forms Earth s outermost layer. It is a mixture of gases- mostly nitrogen and
FCAT Review Earths Systems PARTS OF EARTHS SYSTEMS The Earth system has 5 main spheres: 1) Atmosphere The layer of gases that forms Earth s outermost layer. It is a mixture of gases- mostly nitrogen and
The German Contribution to the WMO GAW Programme:
 WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH The German Contribution to the WMO GAW Programme: Upon the 225 th Anniversary of GAW Hohenpeissenberg Observatory No. 167 Monitoring and Research
WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH The German Contribution to the WMO GAW Programme: Upon the 225 th Anniversary of GAW Hohenpeissenberg Observatory No. 167 Monitoring and Research
The Climate of Marshall County
 The Climate of Marshall County Marshall County is part of the Crosstimbers. This region is a transition region from the Central Great Plains to the more irregular terrain of southeastern Oklahoma. Average
The Climate of Marshall County Marshall County is part of the Crosstimbers. This region is a transition region from the Central Great Plains to the more irregular terrain of southeastern Oklahoma. Average
Norsk-amerikansk forskningsekspedisjon på innlandsisen i Antarktis. Kirsty Langley Norsk Polarinstitutt
 Norsk-amerikansk forskningsekspedisjon på innlandsisen i Antarktis Kirsty Langley Norsk Polarinstitutt The Antarctic ice sheet Area: ca. 14. 10 6 km 2 90 % of Earth s s ice Complete melting: 60 m sea-level
Norsk-amerikansk forskningsekspedisjon på innlandsisen i Antarktis Kirsty Langley Norsk Polarinstitutt The Antarctic ice sheet Area: ca. 14. 10 6 km 2 90 % of Earth s s ice Complete melting: 60 m sea-level
The Distribution of Cold Environments
 The Distribution of Cold Environments Over 25% of the surface of our planet can be said to have a cold environment, but defining what we actually mean by that can be very challenging. This is because cold
The Distribution of Cold Environments Over 25% of the surface of our planet can be said to have a cold environment, but defining what we actually mean by that can be very challenging. This is because cold
Name Per Date Earth Science Climate & Insolation Test
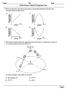 Name Per Date Earth Science Climate & Insolation Test 1) Which graph best represents the general relationship between latitude and average surface temperature? 2) The diagram below shows the apparent path
Name Per Date Earth Science Climate & Insolation Test 1) Which graph best represents the general relationship between latitude and average surface temperature? 2) The diagram below shows the apparent path
Analysis of gross alpha, gross beta activities and beryllium-7 concentrations in surface air: their variation and statistical prediction model
 Iran. J. Radiat. Res., 2006; 4 (3): 155-159 Analysis of gross alpha, gross beta activities and beryllium-7 concentrations in surface air: their variation and statistical prediction model F.Arkian 1*, M.
Iran. J. Radiat. Res., 2006; 4 (3): 155-159 Analysis of gross alpha, gross beta activities and beryllium-7 concentrations in surface air: their variation and statistical prediction model F.Arkian 1*, M.
The spatial distribution and radiative effects of soot in the snow and sea ice during the SHEBA experiment
 The spatial distribution and radiative effects of soot in the snow and sea ice during the SHEBA experiment Thomas C. Grenfell and Bonnie Light Department of Atmospheric Sciences, Box 35164, University
The spatial distribution and radiative effects of soot in the snow and sea ice during the SHEBA experiment Thomas C. Grenfell and Bonnie Light Department of Atmospheric Sciences, Box 35164, University
ATMS 321: Natural Climate Variability Chapter 11
 ATMS 321: Natural Climate Variability Chapter 11 Solar Variability: Total solar irradiance variability is relatively small about a tenth of a percent. Ultraviolet variability is larger, and so could affect
ATMS 321: Natural Climate Variability Chapter 11 Solar Variability: Total solar irradiance variability is relatively small about a tenth of a percent. Ultraviolet variability is larger, and so could affect
Correction to Evaluation of the simulation of the annual cycle of Arctic and Antarctic sea ice coverages by 11 major global climate models
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2006jc003949, 2006 Correction to Evaluation of the simulation of the annual cycle of Arctic and Antarctic sea ice coverages by 11 major global climate
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2006jc003949, 2006 Correction to Evaluation of the simulation of the annual cycle of Arctic and Antarctic sea ice coverages by 11 major global climate
L.O Students will learn about factors that influences the environment
 Name L.O Students will learn about factors that influences the environment Date 1. At the present time, glaciers occur mostly in areas of A) high latitude or high altitude B) low latitude or low altitude
Name L.O Students will learn about factors that influences the environment Date 1. At the present time, glaciers occur mostly in areas of A) high latitude or high altitude B) low latitude or low altitude
The importance of long-term Arctic weather station data for setting the research stage for climate change studies
 The importance of long-term Arctic weather station data for setting the research stage for climate change studies Taneil Uttal NOAA/Earth Systems Research Laboratory Boulder, Colorado Things to get out
The importance of long-term Arctic weather station data for setting the research stage for climate change studies Taneil Uttal NOAA/Earth Systems Research Laboratory Boulder, Colorado Things to get out
Response to Reviewer s comments
 Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Table of Contents. Chapter: Atmosphere. Section 1: Earth's Atmosphere. Section 2: Energy Transfer in the Atmosphere. Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
Single-particle characterization of the Antarctic aerosols collected at King George Island, Chile
 Single-particle characterization of the Antarctic aerosols collected at King George Island, Chile Shila Maskey¹, Hae-Jin Jung¹, BoHwa Kim¹, Hyeok Jung², Kang-Ho Ahn², and Chul-Un Ro¹ ¹Department of Chemistry,
Single-particle characterization of the Antarctic aerosols collected at King George Island, Chile Shila Maskey¹, Hae-Jin Jung¹, BoHwa Kim¹, Hyeok Jung², Kang-Ho Ahn², and Chul-Un Ro¹ ¹Department of Chemistry,
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
1 What Is Climate? TAKE A LOOK 2. Explain Why do areas near the equator tend to have high temperatures?
 CHAPTER 17 1 What Is Climate? SECTION Climate BEFORE YOU READ After you read this section, you should be able to answer these questions: What is climate? What factors affect climate? How do climates differ
CHAPTER 17 1 What Is Climate? SECTION Climate BEFORE YOU READ After you read this section, you should be able to answer these questions: What is climate? What factors affect climate? How do climates differ
Components of the Climate System. Lecture 2: Earth s Climate System. Pop Quiz. Sub-components Global cycles What comes in What goes out
 Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
