REVISION BOOKLET DO NOT WRITE IN THIS BOOKLET!!!!! Year 10 SCIENCE INSTRUCTIONS:
|
|
|
- Melanie McDaniel
- 5 years ago
- Views:
Transcription
1 REVISION BOOKLET DO NOT WRITE IN THIS BOOKLET!!!!! Year 10 SCIENCE INSTRUCTIONS: Aim to spend about minutes on each section. There are 5 sections and you can do them in any order. There is an Information Page with a Periodic Table, some chemical reactions and formula on page 2. SECTIONS: A. PLANTS AND HUMAN BIOLOGY B. SCIENCE IN SPORT C. SCIENCE IN THE SUPERMARKET D. FAMILY TREES E. ELECTRICITY When you have finished go back over all your answers. Make sure you have attempted all questions. Even the Excellence questions have an Achieved level answer There are no marks taken off for wrong answers. Marks are only awarded for correct answers. If you are unsure of an answer then at least have a go.
2 Year 10 Science Information Page MOTION FORMULAE F = m x a Weight = m x g g = 10 N kg -1 Distance change in speed Speed = time acceleration = time E k = ½ mv 2 Work = Force x distance E p = m x g x h PERIODIC TABLE- The first 20 Elements 1 H 1 4 He 2 7 Li 3 9 Be 4 11 B 5 12 C 6 14 N 7 16 O 8 19 F 9 20 Ne Na Mg Al Si P S Cl Ar K Ca An ACID plus a BASE reacts to produce a METAL SALT plus WATER. Acid + Base Salt + Water 2. An ACID plus a METAL reacts to produce a METAL SALT plus HYDROGEN. Acid + Metal Metal Salt + Hydrogen 3. An ACID plus a METAL CARBONATE reacts to produce a METAL SALT plus CARBON DIOXIDE plus WATER. Acid + Metal Carbonate Metal Salt + Carbon Dioxide + Water When they react they produce: Sulfuric Acid produces sulfates. Hydrochloric Acid produces chlorides. Nitric acid produces nitrates. Bases: Sodium hydroxide Potassium hydroxide Calcium hydroxide Magnesium hydroxide Metals: Sodium Potassium Calcium Magnesium Zinc Iron Copper
3 Section A PLANTS AND HUMAN BIOLOGY Objective: To show understanding of the parts of plants and their functions Question 1 Here is a diagram of a typical seed. The plumule is already labelled. 1. (a) Label the parts of the bean seed, using the following labels: Radicle, cotyledon, testa Plumule (shoot) Plumule (shoot) b) What role do the cotyledons play in seed germination? c) List TWO conditions needed for a seed to germinate. d) Is sunlight needed for germination? Explain your answer
4 e) Once it has successfully germinated the plant continues to develop its three main parts, - the leaves, the roots and the stem. Describe the function of the roots and stem Roots Stem e) The leaf is the part of the plant that carries out photosynthesis. Describe in your own words the process of photosynthesis and explain why it is important to the plant. f) Complete the word equation for photosynthesis: Carbon dioxide + Water + g) In the equation above, something should be written over the arrow. State what is missing and explain why this is important for photosynthesis to happen. h) Discuss how the leaves get the carbon dioxide and water that they need to carry out photosynthesis and then what it does with the products of photosynthesis.
5 i) To find out if a leaf has been photosynthesising it can be tested for starch. Name the solution used to test for starch in the leaf and state the colour it should go if starch is present. SOLUTION: COLOUR: j) Explain why testing for starch indicates whether a leaf has been photosynthesising. Objective: To show understanding of the process of plant reproduction Question 2 There are two types of pollination, insect/animal and wind. a) Complete the table below to match each characteristic with the most likely type of pollination. Write either WIND or INSECT. Characteristic Distinctive smell Small, light pollen Large amounts of pollen Colourful flowers Contain nectar Wind or Insect pollinated b) Discuss the advantages and disadvantages, to the plant, of each type of pollination
6 c) Explain what pollination is and describe what is formed as a result. d) Seeds can be dispersed (spread) by exploding as shown below. Describe TWO other ways seeds can be dispersed. Plants can reproduce using either sexual or asexual reproduction. Pollination is involved in the sexual reproduction process. e) Give two methods of how plants can reproduce without sex (asexually). f) Explain one advantage and one disadvantage of asexual reproduction. Advantage Disadvantage
7 Objective: To show understanding of the digestive system Question 3 a) Explain, giving a one or two sentence summary, what the purpose of digestion is. After food leaves the stomach it passes through the intestines. After the small intestine comes the large intestine. c) What is the role of the small intestine and explain why it is important that it is working properly. (i.e. what are the problems you would have if it didn t work properly?) Objective: To show understanding of the circulatory system Question 4 There are 3 types of blood vessels: Arteries, Veins and capillaries. a) Which of these three types takes blood away from the heart? b) When you are measuring your pulse, what type of blood vessel are you pressing your fingers against and what are you actually measuring? c) Discuss why the walls of capillaries are much thinner than the walls of arteries.
8 d) Discuss how the circulatory system (heart, lungs and blood vessels) work together to keep the body alive.
9 Section B SCIENCE IN SPORT Objective: To show an understanding of motion-time graphs and motion calculations Question 1 The Tour de France is a cycle race that is held each year across parts of France. The following were the results for one of the legs: Time (min) Distance (km/h) (a) Use this data to complete the graph below Distance (km) b) Calculate the speed of the rider over the first 60 minutes. c) State when the rider is moving the quickest. Time (mins) Between minutes and minutes.
10 d) Explain how you knew the answer to c) e) Comment on the motion of the rider during his journey. Objective: To show an understanding of forces Question 2 Below is the diagram of the rider from the Tour de France. a) Draw FOUR arrows with all the forces acting on the rider labelled. b) Explain TWO ways cyclists can reduce friction acting on them and their bike. c) What type of energy is produced when friction acts?
11 d) Forces can be divided into two groups, contact and non-contact forces. Place the following into their correct column: Contact forces magnetism, electrostatic, friction, buoyancy, gravity Non-contact forces The All Black s scrum is 904 kilograms. e) Calculate the weight of the scrum. F gravity = m x g g= 10 Nkg -1 f) Describe the effects a force can have on an object. g) Compare the weight and mass of a person on the earth and on the moon. h) Starting from rest, an 80 kg man accelerates uniformly to 12 m s -1 in a time of 2 seconds. Calculate his acceleration. Give the correct unit. acceleration = Unit
12 i) Calculate the force acting on the man while he accelerates. i) Calculate the kinetic energy of the man while he is travelling at 8 m s -1. Give the correct unit for your answer. Kinetic energy = Unit i) While the man is running a headwind comes and up his speed decreases to a constant speed of 8 m s -1. Discuss what this tells us relative size of the forces when the man has reached a steady speed. Objective: To demonstrate an understanding of motion calculations Question 3 Usain Bolt is a Jamaican sprinter who has set records for the men s 100 metres and 200 metre sprints. His time for the 100 m is 9.58 s. a) Calculate his average speed for each distance. (speed = d/t) b) If Usain ran the 400 metres at speed of 9.4 m s -1, calculate if he would be able to beat the world record holder Michael Johnson of USA who has a time of 43.18s for the 400 metres. (t = d/speed)
13 Objective: To demonstrate an understanding of energy. Question 4 You may need to use the formula sheet on page 2 to answer some of these questions A 2 kg medicine ball is rolled down a ramp and reaches a speed of 6 m s -1. a) What type of, and how much, energy does the ball have at the bottom of the ramp? Type of Energy amount of energy = b) The ball travels along the flat until it stops rolling. Explain what has happened to the energy that the ball had at the bottom of the ramp. In all sports you have to do work. (d) Force is measured in Newtons, distance is measured in metres, what are the units of work? (e) A woman pushes a wheelbarrow with a force of 250 N, over a distance of 20 m. Calculate the work done by the woman. Show the formula you used and your working.
14 Section C SCIENCE IN THE SUPERMARKET Objective: To describe the parts of atoms and their relation to ion formation Question 1 a) Write the name or symbols of the following elements. Li Boron Ca Iron K There are 3 particles which make up an atom. b) Name these 3 particles c) The nucleus of an atom contains almost all of the mass of the atom. Which two of the particles listed in b) make up the nucleus? d) Beryllium has an atomic number of 4. Fluorine has an atomic number of 9. Complete the table below using this information. Symbol Beryllium Number of electrons 4 Fluorine Electron arrangement 2,7 Number of electrons lost or gained to form an ion Symbol of the ion formed Be 2+ e) Explain why sodium, which has 11 electrons, forms a positive ion but chlorine, which has 17 electrons, forms a negative ion. F
15 f) Complete the diagram to show how the electrons would be arranged around the nucleus of an argon atom. (Use the periodic table given on page 2) Ar g) What is the electron arrangement of Aluminium? h) What is the electron arrangement of the fluorine ion (F -1 )? g) Complete the following chart about the elements in Zinc chloride - MgCl 2 Element Symbol Element Name Number of atoms of each element in the formula Total number of atoms in the molecule of the compound h) Complete the following chart about Iron hydroxide - Fe(OH) 3 Element Symbol Element Name Number of atoms of each element in the formula Total number of atoms in the molecule of the compound i) What is the name of K 2 CO 3? j) Use the list of ions given in the box to write the formula of the following ionic compounds. Zn 2+ zinc O 2- oxide Na + sodium OH - hydroxide Fe 2+ iron Cl - chloride (i) Copper chloride (ii) Magnesium carbonate Cu 2+ copper NO 3 - Mg 2+ magnesium SO 4 2- Al 3+ aluminium CO 3 2- nitrate sulfate carbonate (iii) Iron nitrate (iv) Aluminium sulfate
16 k) A sodium atom has 11 protons, 12 neutrons and 11 electrons. A fluoride ion has 11 protons, 12 neutrons and 10 electrons. Discuss how and why an atom changes into an ion and how this change leads to an ability to form compounds. In your answer you should talk about: The electron arrangements of the sodium atom and the sodium ion What happens to an atom when it changes into an ion (and why) Why ions form compounds which have no charge
17 Objective: To demonstrate abilities in writing formulae and equations To demonstrate understanding of acids, their properties and reactions Question 2 a) Organise the following substances into acids and bases. Write your answers in the table below. Soap (8.5) Oven cleaner (ph 12) Blood (ph 7.4) Sodium hydroxide Lemonade (ph 4) Shampoo (ph 5.5) ACID BASE Use the table below to answer the questions that follow. Substance tested With red litmus, paper it became: With Universal indicator it went: A BLUE BLUE B RED GREEN C RED YELLOW b) Which substance is an acid? c) Which substance is a base? d) i. Which substance is neither an acid nor a base? ii. What would be the approximate ph of this substance? A substance found in a bathroom cupboard was tested with blue litmus paper. It stayed blue. e) What does this tell you about the substance? Explain your answer.
18 Sarah puts some Iron metal into a test tube and adds sulfuric acid. A chemical reaction occurs at once. Answer these questions about it: f) Write down the formula for sulfuric acid. g) How could Sarah know that a chemical reaction had happened? Use the information given on the second page of this booklet to complete word equations for each of the following reactions by naming the products produced. h) Iron + sulfuric + acid + (i) Magnesium + hydrochloric hydroxide acid + (j) Copper + nitric carbonate acid (k) Write a balanced formula equation for reaction (j) (Use the table of ions given three pages back) Question 3 Protein one of the types of nutrients we get from food. a) Describe how you would test for protein and what you would expect to see if the test was positive Method Result (if positive)
19 Mass of NaOH (g) Question 4 John did an experiment to find out how much sodium hydroxide was needed to neutralise various solutions of sulfuric acid. His results are presented below with a graph Neutralisation of Sulfuric acid using Sodium Hydroxide Concentration of Sulfuric acid (g L -1 ) a) From the graph determine how much sodium hydroxide is needed to neutralise a solution with 140 grams per litre of sulfuric acid. b) By extending the graph determine how much sodium hydroxide is needed to neutralise a solution with a concentration of 200 grams per litre of sulfuric acid. Question 5 Two metals magnesium and aluminium were placed into two separate test tubes containing hydrochloric acid. The magnesium appeared to react quicker as there were more bubbles immediately produced. State two ways that you could speed up the reaction happening between both metals and the acid.
20 Section D FAMILY TREES Objective: To demonstrate an understanding of basic terms and definitions Question 1 a) Complete the following table: Word Definition/ Meaning Cell division for making sex cells Heterozygous Homozygous An alternative form of a gene A gene that will only be expressed if the dominant allele is not present What a feature looks like (physical characteristic) b) Use the following words to complete the paragraph: recessive dominant heterozygous homozygous phenotype Sarah s dad has brown eyes which is a characteristic but her mum does not so her mum must be for that feature. Her dad can be homozygous dominant or. The brown eyes are part of his Objective: To demonstrate an understanding of different types of variation Question 2 Give two examples of continuous variation, two examples of either/or variation and two of noninherited variation. Complete the table. continuous variation either/or variation not inherited at all Objective: To show an understanding of the methods of inheritance and genetics Question 3 A dominant red daschund dog (MM) was bred with a recessive black dog (mm). a) Complete a punnett square to show the puppies colours. b) State the ratio of red puppies to black puppies.
21 c) Use another punnett square to explain what would happen if one of these puppies was then bred (mated) with a black dog. d) Talia s mum can roll her tongue which is dominant but her dad cannot. Talia can roll her tongue too but her sister cannot. Draw a punnett square and explain how this is possible. Use the letters L and l for the punnett square. Punnett square e) A family has three girls. The parents decide to have one more child and hope they will have a boy. Use a punnett square to discuss the probability of them having a boy. Space for a diagram if needed
22 Question 4 Human females have 46 chromosomes in each of their body cells. A housefly has only 24 chromosomes in each of its body cells while a tomato plant has 8 chromosomes in its pollen cells. (a) How many chromosomes are there in a human egg cell? (b) How many chromosomes are there in a fly s egg? (c) How many chromosomes are there in a fertilised fly s egg (a zygote)? (d) How many chromosomes are there in a tomato leaf cell? (b) What is one important difference between a gamete and a zygote? Discuss why this difference is important. Scientists working to improve human nutrition used selective breeding to increase the yield of tomato plants. In selective breeding, breeders only keep plants with the best characteristics for the trait they want. Soon they only have plants with "good" genes. When using selection with self-pollinating plants it takes only a few years to find the best combinations of good genes. If the "good" genes are recessive, they can often be quickly identified. Another group of scientists added a new gene to tomato plants so that it was able to produce a chemical which killed off the insects that damage it. Normal tomato plants cannot produce this chemical. c) Discuss why selective breeding cannot be used to make tomato plants that produce this chemical.
23 Objective: To show understanding of mitosis and meiosis Question 5 Pine trees are very easy to clone. Name the type of cell division involved here. a) Name of cell division: b) Explain the possible advantages of using this method of producing new trees. c) What are some of the disadvantages of this type of reproduction? d) Discuss the differences between the two types of cell division Mitosis and Meiosis. In your answer you should consider: The reason for each type of cell division The effect on chromosome number after the process The number of daughter cells.
24 Section E ELECTRICITY Objective: To describe the nature of static electricity Question 1 1. Kelsey loves to bounce on her trampoline on sunny days. After bouncing for several minutes her hair sticks up on end. a) Name the type of electricity that causes her hair to stick up. b) Discuss why Kelsey s hair begins to stick up. c) When Kelsey s sister also gets onto the trampoline, Kelsey likes to give her a small electric shock by touching her hand. Explain why this happens. d) Kelsey s sister says that it is similar to a bolt of lightning. (i) (ii) On the diagram, draw where a lightning bolt is most likely to occur. Use the diagram to explain what causes lightning.
25 Question 2 a) (i) Describe what is meant by an electrical conductor. ii) Give an example of a substance that can be used as an electrical conductor. b) (i) Describe what is meant by an electrical insulator. ii) Give an example of a substance that can be used as an electrical insulator. Objective: To describe the parts and functions of electrical components Question 3. a) The table below shows symbols for circuit components. Complete the table below by writing the correct name next to each symbol Name The following descriptions are describing various electrical components. b) Give the matching name for each description. Description A device which counts the number of electrons of current flowing past a point per second A device which converts electrical energy into light energy Name A device which stops the circuit operating by breaking the circuit A device which stops the circuit operating by melting when the current is too high A device which resists electric current flowing around a circuit A device which measures the energy supplied or used by a part in the circuit
26 c) In the spaces below draw circuit diagrams, using the correct symbols for: A single cell for the power supply, connected to a resistor and a bulb in series. There is a voltmeter measuring the voltage of the bulb. A 9 volt battery connected to two bulbs. There are two switches, so that each bulb can be turned on or off without affecting the other bulb. There is an ammeter to measure the total current of the circuit.
Coimisiún na Scrúduithe Stáit State Examinations Commission
 Coimisiún na Scrúduithe Stáit State Examinations Commission 2015. S26 JUNIOR CERTIFICATE EXAMINATION, 2015 SCIENCE HIGHER LEVEL THURSDAY, 11 JUNE MORNING, 9.30 to 11.30 INSTRUCTIONS 1. Write your examination
Coimisiún na Scrúduithe Stáit State Examinations Commission 2015. S26 JUNIOR CERTIFICATE EXAMINATION, 2015 SCIENCE HIGHER LEVEL THURSDAY, 11 JUNE MORNING, 9.30 to 11.30 INSTRUCTIONS 1. Write your examination
Science 1.5 AS Demonstrate understanding of aspects of acids and bases WORKBOOK. Working to Excellence
 Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
What is the resultant force acting on the object? BASE jumpers jump from very high buildings and mountains for sport.
 Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
Q1. As the world population increases there is a greater demand for fertilisers.
 Q1. As the world population increases there is a greater demand for fertilisers. (a) Explain what fertilisers are used for............. (b) The amount of nitrogen in a fertiliser is important. How many
Q1. As the world population increases there is a greater demand for fertilisers. (a) Explain what fertilisers are used for............. (b) The amount of nitrogen in a fertiliser is important. How many
2. Name the part of the brain which controls posture and balance of the body?
 . Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
. Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
Final Revision. Complete the following sentences:
 Final Revision Complete the following sentences: 1. Medicine, -------------- and ------------- are examples of the outcomes of some chemical reactions. 2. The gas evolves on thermal decomposition of metal
Final Revision Complete the following sentences: 1. Medicine, -------------- and ------------- are examples of the outcomes of some chemical reactions. 2. The gas evolves on thermal decomposition of metal
...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]
![...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1] ...[1] (ii) Name two elements from group 0....[2] (b)(i) Which box best represents particles from group 0 elements?...[1]......[1]](/thumbs/76/74051985.jpg) High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
High Demand Questions QUESTIONSHEET 1 The boxes represent particles of different gases. One box shows the particles of elements in group 0 (group 8). A B C D What name is given to group 0 (8) elements?
10. How many chromosomes are in human gametes (reproductive cells)? 23
 Name: Key Block: Define the following terms: 1. Dominant Trait-characteristics that are expressed if present in the genotype 2. Recessive Trait-characteristics that are masked by dominant traits unless
Name: Key Block: Define the following terms: 1. Dominant Trait-characteristics that are expressed if present in the genotype 2. Recessive Trait-characteristics that are masked by dominant traits unless
The electrolysis of sodium chloride solution produces useful substances. covalent ionic non-metallic
 1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
SCIENCE Year 10 Examination C 40 marks
 NAME: SCIENCE TEACHER: (circle code) 10C SCIENCE Year 10 Examination 2012 10 C 40 marks Make sure that you have answered all the questions in Paper 10B before you start this paper Time allowed for both
NAME: SCIENCE TEACHER: (circle code) 10C SCIENCE Year 10 Examination 2012 10 C 40 marks Make sure that you have answered all the questions in Paper 10B before you start this paper Time allowed for both
Choose words from the list to complete the sentences below. In an atom, the particles with a negative charge are called...
 Q1 This question is about the structure of atoms (a) Choose words from the list to complete the sentences below electrons ions neutrons protons In an atom, the particles with a negative charge are called
Q1 This question is about the structure of atoms (a) Choose words from the list to complete the sentences below electrons ions neutrons protons In an atom, the particles with a negative charge are called
CBSE QUESTION PAPER CLASS-X SCIENCE
 CBSE QUESTION PAPER CLASS-X SCIENCE SECTION - A Q.1. State the effect of a magnetic field on the path of a moving charged particle. 1 Mark Q.2. Which system facilitates the communication between central
CBSE QUESTION PAPER CLASS-X SCIENCE SECTION - A Q.1. State the effect of a magnetic field on the path of a moving charged particle. 1 Mark Q.2. Which system facilitates the communication between central
Atoms What subatomic particles make up the atom?
 Atoms What subatomic particles make up the atom? What are the masses of the subatomic particles? What do atomic and mass number represent? What does 7 3 Li represent? How are elements arranged in the periodic
Atoms What subatomic particles make up the atom? What are the masses of the subatomic particles? What do atomic and mass number represent? What does 7 3 Li represent? How are elements arranged in the periodic
Name Class Date. KEY CONCEPT Gametes have half the number of chromosomes that body cells have.
 Section 1: Chromosomes and Meiosis KEY CONCEPT Gametes have half the number of chromosomes that body cells have. VOCABULARY somatic cell autosome fertilization gamete sex chromosome diploid homologous
Section 1: Chromosomes and Meiosis KEY CONCEPT Gametes have half the number of chromosomes that body cells have. VOCABULARY somatic cell autosome fertilization gamete sex chromosome diploid homologous
D.C.H.S BIOLOGY DEPARTMENT
 D.C.H.S BIOLOGY DEPARTMENT NAT 5 Homework Booklet Unit 2 Multicellular Organisms 1 HOMEWORK 1- Cells, Tissues and Organs 1. (a) Multicellular organisms are composed of different types of cells which are
D.C.H.S BIOLOGY DEPARTMENT NAT 5 Homework Booklet Unit 2 Multicellular Organisms 1 HOMEWORK 1- Cells, Tissues and Organs 1. (a) Multicellular organisms are composed of different types of cells which are
Chemistry Assessment Unit AS 1
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education 2014 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry ML [AC112] MONDAY
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education 2014 Chemistry Assessment Unit AS 1 assessing Basic Concepts in Physical and Inorganic Chemistry ML [AC112] MONDAY
Relative electrical conductivity (1= low, 10= high) [2] [3]
![Relative electrical conductivity (1= low, 10= high) [2] [3] Relative electrical conductivity (1= low, 10= high) [2] [3]](/thumbs/89/97804380.jpg) 1 Kylie is choosing a metal to make a base for a saucepan. base Look at the information about some metals. Metal Melting point in C Relative electrical conductivity (1= low, 10= high) Relative conductivity
1 Kylie is choosing a metal to make a base for a saucepan. base Look at the information about some metals. Metal Melting point in C Relative electrical conductivity (1= low, 10= high) Relative conductivity
C2.6 Quantitative Chemistry Foundation
 C2.6 Quantitative Chemistry Foundation 1. Relative masses Use the periodic table to find the relative masses of the elements below. (Hint: The top number in each element box) Hydrogen Carbon Nitrogen Oxygen
C2.6 Quantitative Chemistry Foundation 1. Relative masses Use the periodic table to find the relative masses of the elements below. (Hint: The top number in each element box) Hydrogen Carbon Nitrogen Oxygen
C2.6 Quantitative Chemistry Foundation
 C2.6 Quantitative Chemistry Foundation 1. Relative masses Use the periodic table to find the relative masses of the elements below. (Hint: The top number in each element box) Hydrogen Carbon Nitrogen Oxygen
C2.6 Quantitative Chemistry Foundation 1. Relative masses Use the periodic table to find the relative masses of the elements below. (Hint: The top number in each element box) Hydrogen Carbon Nitrogen Oxygen
Additional Science 1 AS1FP. (Jan13AS1FP01) General Certificate of Secondary Education Foundation Tier January Unit 5
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Additional Science 1 Unit 5 Tuesday 22 January 2013 For this paper you must have: a ruler a
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Additional Science 1 Unit 5 Tuesday 22 January 2013 For this paper you must have: a ruler a
Draw one line from each solution to the ph value of the solution. Solution ph value of the solution
 1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
A student investigated the effect of changing ph and temperature on the digestion of lactose in milk
 Q1. Fresh milk is a mixture of compounds including fat, protein and about 5 % lactose sugar. Lactose must be digested by the enzyme lactase, before the products can be absorbed. Lactase can be added to
Q1. Fresh milk is a mixture of compounds including fat, protein and about 5 % lactose sugar. Lactose must be digested by the enzyme lactase, before the products can be absorbed. Lactase can be added to
*Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats.
 Plant Reproduction *Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats. Reproduction In Plants Plant reproduction is the production of new
Plant Reproduction *Modifications in reproduction were key adaptations enabling plants to spread into a variety of terrestrial habitats. Reproduction In Plants Plant reproduction is the production of new
Orchard School. New Document 1 Name: Class: Date: 129 minutes. Time: 126 marks. Marks: Comments: Page 1
 New Document Name: Class: Date: Time: Marks: 29 minutes 26 marks Comments: Page Q. The ph scale is a measure of the acidity or alkalinity of a solution. (a) Solution Draw one line from each solution to
New Document Name: Class: Date: Time: Marks: 29 minutes 26 marks Comments: Page Q. The ph scale is a measure of the acidity or alkalinity of a solution. (a) Solution Draw one line from each solution to
SCIENCE HIGHER LEVEL
 J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
Second Term. Questions. Unit (1)
 (1) Complete the following: Questions Unit (1) 1) Nitrogen pentoxide breaks up into.. and.. gas. 2) At the beginning of the reaction, the concentration of reactants is... 3) The speed of a chemical reaction
(1) Complete the following: Questions Unit (1) 1) Nitrogen pentoxide breaks up into.. and.. gas. 2) At the beginning of the reaction, the concentration of reactants is... 3) The speed of a chemical reaction
THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY AT Myton School
 THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY AT Myton School Introduction Before you start the AS Chemistry course in September you should have completed this new bridging course for Chemists. It has been
THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY AT Myton School Introduction Before you start the AS Chemistry course in September you should have completed this new bridging course for Chemists. It has been
Science Unit Learning Summary
 Learning Summary Inheritance, variation and evolution Content Sexual and asexual reproduction. Meiosis leads to non-identical cells being formed while mitosis leads to identical cells being formed. In
Learning Summary Inheritance, variation and evolution Content Sexual and asexual reproduction. Meiosis leads to non-identical cells being formed while mitosis leads to identical cells being formed. In
Trilogy Quantitative chemistry
 Trilogy Quantitative chemistry Foundation revision questions Name: Class: Date: Time: 6 minutes Marks: 6 marks Comments: Page of 23 (a) Formulae and equations are used to describe chemical reactions. Aluminium
Trilogy Quantitative chemistry Foundation revision questions Name: Class: Date: Time: 6 minutes Marks: 6 marks Comments: Page of 23 (a) Formulae and equations are used to describe chemical reactions. Aluminium
Q1. Methane and oxygen react together to produce carbon dioxide and water.
 Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
3 rd Quarter Study Guide Name
 3 rd Quarter Study Guide Name Life Science - You are responsible to know: 1. Identify various parts of the cell & their functions. 2. Distinguish between Prokaryotic and Eukaryotic cells 3. State differences
3 rd Quarter Study Guide Name Life Science - You are responsible to know: 1. Identify various parts of the cell & their functions. 2. Distinguish between Prokaryotic and Eukaryotic cells 3. State differences
In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium salt to produce potassium
 Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
7.1.2 Cell Functions. 104 minutes. 137 marks. Page 1 of 30
 7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
Indicate the answer choice that best completes the statement or answers the question.
 Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
In the exam you will be asked to tackle questions such as the one below. Mitosis or meiosis?
 Get started AO1 Cell division This unit will help you to recognise when cells divide by mitosis and when they divide by meiosis. t will also help you to understand the importance of cell division in the
Get started AO1 Cell division This unit will help you to recognise when cells divide by mitosis and when they divide by meiosis. t will also help you to understand the importance of cell division in the
Asexual & Plant Reproduction
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ sexual & Plant Reproduction Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ sexual & Plant Reproduction Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
Part 2- Biology Paper 2 Inheritance and Variation Knowledge Questions
 Part 2- Biology Paper 2 Inheritance and Variation Knowledge Questions AQA TRILOGY Biology (8464) from 2016 Topic T4.6 Inheritance, variation and evolution Topic Student Checklist R A G Describe features
Part 2- Biology Paper 2 Inheritance and Variation Knowledge Questions AQA TRILOGY Biology (8464) from 2016 Topic T4.6 Inheritance, variation and evolution Topic Student Checklist R A G Describe features
2. 2 Complete this table of the parts of an atom: Particle Charge Location in atom Proton. Negative
 Q Outcome 1. 1 Give a definition of the term matter 2. 2 Complete this table of the parts of an atom: Particle Charge Location in atom Proton In the nucleus Negative 3. 2 Draw an atom and label all particles.
Q Outcome 1. 1 Give a definition of the term matter 2. 2 Complete this table of the parts of an atom: Particle Charge Location in atom Proton In the nucleus Negative 3. 2 Draw an atom and label all particles.
Name Class Date. Pearson Education, Inc., publishing as Pearson Prentice Hall. 33
 Chapter 11 Introduction to Genetics Chapter Vocabulary Review Matching On the lines provided, write the letter of the definition of each term. 1. genetics a. likelihood that something will happen 2. trait
Chapter 11 Introduction to Genetics Chapter Vocabulary Review Matching On the lines provided, write the letter of the definition of each term. 1. genetics a. likelihood that something will happen 2. trait
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
UNIT 9A Inheritance and Selection
 UNIT 9A Inheritance and Selection Know what inheritance means Know what characteristics are Know that sexual reproduction needs two parents Know that animals and plants can be bred for their characteristics
UNIT 9A Inheritance and Selection Know what inheritance means Know what characteristics are Know that sexual reproduction needs two parents Know that animals and plants can be bred for their characteristics
NATIONAL 5 CHEMISTRY
 Farr High School NATIONAL 5 CHEMISTRY Unit 1 Chemical Changes and Structure Question Booklet 1 Rates of Reaction 1. Explain how the following would affect the rate of the reaction between 1.0 g of magnesium
Farr High School NATIONAL 5 CHEMISTRY Unit 1 Chemical Changes and Structure Question Booklet 1 Rates of Reaction 1. Explain how the following would affect the rate of the reaction between 1.0 g of magnesium
THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY Birchwood High School
 THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY Birchwood High School Mrs Ryan Chemistry Please also access the website below which is a link to a really good PPT that will help to bridge the gap between GCSE
THE BRIDGING COURSE TO SIXTH FORM CHEMISTRY Birchwood High School Mrs Ryan Chemistry Please also access the website below which is a link to a really good PPT that will help to bridge the gap between GCSE
MULTIPLE CHOICE ANSWER SHEET
 STUDENT NAME: MULTIPLE CHOICE ANSWER SHEET Circle the letter indicating the best answer. 1 A B C D 2 A B C D 3 A B C D 4 A B C D 5 A B C D 6 A B C D 7 A B C D 8 A B C D 9 A B C D 10 A B C D 11 A B C D
STUDENT NAME: MULTIPLE CHOICE ANSWER SHEET Circle the letter indicating the best answer. 1 A B C D 2 A B C D 3 A B C D 4 A B C D 5 A B C D 6 A B C D 7 A B C D 8 A B C D 9 A B C D 10 A B C D 11 A B C D
Assessment Schedule 2017 Biology: Demonstrate understanding of biological ideas relating to the life cycle of flowering plants (90928)
 NCEA Level 1 Biology (90928) 2017 page 1 of 5 Assessment Schedule 2017 Biology: Demonstrate understanding of biological ideas relating to the life cycle of flowering plants (90928) Evidence Statement QUESTION
NCEA Level 1 Biology (90928) 2017 page 1 of 5 Assessment Schedule 2017 Biology: Demonstrate understanding of biological ideas relating to the life cycle of flowering plants (90928) Evidence Statement QUESTION
a. capture sunlight and absorb CO 2
 BIO 274-01 Exam 1 Name Matching (10 pts) 1. Match each plant part with its function: root c a. capture sunlight and absorb CO 2 for photosynthesis leaves a b. provides support, conducts water and nutrients
BIO 274-01 Exam 1 Name Matching (10 pts) 1. Match each plant part with its function: root c a. capture sunlight and absorb CO 2 for photosynthesis leaves a b. provides support, conducts water and nutrients
Chapter 5. Heredity. Table of Contents. Section 1 Mendel and His Peas. Section 2 Traits and Inheritance. Section 3 Meiosis
 Heredity Table of Contents Section 1 Mendel and His Peas Section 2 Traits and Inheritance Section 3 Meiosis Section 1 Mendel and His Peas Objectives Explain the relationship between traits and heredity.
Heredity Table of Contents Section 1 Mendel and His Peas Section 2 Traits and Inheritance Section 3 Meiosis Section 1 Mendel and His Peas Objectives Explain the relationship between traits and heredity.
Semester Question and Answer Booklet
 YEAR 10 SCIENCE EXAMINATION Semester 1 2016 Question and Answer Booklet STUDENT NAME: TEACHER: DATE: Time allowed for this paper: 1 hour and 30 minutes Materials required: Pens, pencils, eraser, ruler,
YEAR 10 SCIENCE EXAMINATION Semester 1 2016 Question and Answer Booklet STUDENT NAME: TEACHER: DATE: Time allowed for this paper: 1 hour and 30 minutes Materials required: Pens, pencils, eraser, ruler,
No Brain Too Small. Credits: Four
 No Brain Too Small Level 1 Science 2015 90944 Demonstrate understanding of aspects of acids and bases Credits: Four Achievement Achievement with Merit Achievement with Excellence Demonstrate understanding
No Brain Too Small Level 1 Science 2015 90944 Demonstrate understanding of aspects of acids and bases Credits: Four Achievement Achievement with Merit Achievement with Excellence Demonstrate understanding
SCIENCE (REVISED SYLLABUS) HIGHER LEVEL
 2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
SPECIMEN. Candidate Number
 Advanced Subsidiary GCE CHEMISTRY A F321 QP Unit F321: Atoms, Bonds and Groups Specimen Paper Candidates answer on the question paper. Additional Materials: Data Sheet for Chemistry (Inserted) Scientific
Advanced Subsidiary GCE CHEMISTRY A F321 QP Unit F321: Atoms, Bonds and Groups Specimen Paper Candidates answer on the question paper. Additional Materials: Data Sheet for Chemistry (Inserted) Scientific
NATIONAL 4 CHEMISTRY
 Farr High School NATIONAL 4 CHEMISTRY Unit 1 Chemical Changes and Structure Question Booklet 1 Energy Changes of Chemical Reactions 1. What is meant by: (b) an exothermic reaction an endothermic reaction.
Farr High School NATIONAL 4 CHEMISTRY Unit 1 Chemical Changes and Structure Question Booklet 1 Energy Changes of Chemical Reactions 1. What is meant by: (b) an exothermic reaction an endothermic reaction.
Final Exam Review. 1. Arrange the 7 levels of Linnaean classification from most general (ie: kingdom) to most specific (ie: species)
 SBI 3U1 Final Exam Review Diversity 1. Arrange the 7 levels of Linnaean classification from most general (ie: kingdom) to most specific (ie: species) 2. a) Explain how the structure of prokaryotic cells
SBI 3U1 Final Exam Review Diversity 1. Arrange the 7 levels of Linnaean classification from most general (ie: kingdom) to most specific (ie: species) 2. a) Explain how the structure of prokaryotic cells
16+ ENTRANCE EXAMINATION
 ST EDWARD S OXFORD 16+ ENTRANCE EXAMINATION For entry in September 2015 CHEMISTRY Time: 1 hour Candidates Name: St Edward's School 1 1. Complete the table below. St Edward's School 2 Element calcium Symbol
ST EDWARD S OXFORD 16+ ENTRANCE EXAMINATION For entry in September 2015 CHEMISTRY Time: 1 hour Candidates Name: St Edward's School 1 1. Complete the table below. St Edward's School 2 Element calcium Symbol
Level 789 Pathway: Combined Science Double Award
 Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
The drawing shows a container of a compound called magnesium chloride. How many elements are joined together to form magnesium chloride?
 Bonding part 5 Q1. The drawing shows a container of a compound called magnesium chloride. How many elements are joined together to form magnesium chloride? Magnesium chloride is an ionic compound. What
Bonding part 5 Q1. The drawing shows a container of a compound called magnesium chloride. How many elements are joined together to form magnesium chloride? Magnesium chloride is an ionic compound. What
ANSWERS: Atoms and Ions
 ANSWERS: Atoms and Ions 1) Available in April 2014 2) a) Atom Atomic No Electron arrangement of atom Electron arrangement of ion Ion symbol Ca 20 2,8,8,2 2,8,8 Ca 2+ F 9 2,7 2,8 F Cl 17 2,8,7 2,8,8 Cl
ANSWERS: Atoms and Ions 1) Available in April 2014 2) a) Atom Atomic No Electron arrangement of atom Electron arrangement of ion Ion symbol Ca 20 2,8,8,2 2,8,8 Ca 2+ F 9 2,7 2,8 F Cl 17 2,8,7 2,8,8 Cl
Review for Biochemistry
 Review for Biochemistry A student records the ph values of three samples and is asked to predict the ph of a fourth sample. The student is told that Sample Z is less acidic than Sample X but more acidic
Review for Biochemistry A student records the ph values of three samples and is asked to predict the ph of a fourth sample. The student is told that Sample Z is less acidic than Sample X but more acidic
1 Mendel and His Peas
 CHAPTER 3 1 Mendel and His Peas SECTION Heredity BEFORE YOU READ After you read this section, you should be able to answer these questions: What is heredity? How did Gregor Mendel study heredity? National
CHAPTER 3 1 Mendel and His Peas SECTION Heredity BEFORE YOU READ After you read this section, you should be able to answer these questions: What is heredity? How did Gregor Mendel study heredity? National
Q1. The electronic structure of the atoms of five elements are shown in the figure below.
 Q1. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Q1. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
St Robert of Newminster Catholic School and Sixth Form College
 St Robert of Newminster Catholic School and Sixth Form College Year 12 Pre-Course Tasks: CHEMISTRY Exercise Mark Grade Atomic structure Chemical bonding Chemical equations Maths for chemists Moles Name:
St Robert of Newminster Catholic School and Sixth Form College Year 12 Pre-Course Tasks: CHEMISTRY Exercise Mark Grade Atomic structure Chemical bonding Chemical equations Maths for chemists Moles Name:
Name: Period: Score: Everything About Chemical Formulas
 Name: Period: Score: Everything About Formulas Compounds have unique names that identify them for us when we study chemical properties and changes. Chemists have devised a shorthand way of representing
Name: Period: Score: Everything About Formulas Compounds have unique names that identify them for us when we study chemical properties and changes. Chemists have devised a shorthand way of representing
1. What is a dot diagram? 2. Drawing dot diagrams. Name:
 Name: Skill Sheet 29.2 Dot Diagrams You have learned that atoms are composed of protons, neutrons, electrons. The electrons occupy energy levels that surround the nucleus in the form of an electron cloud.
Name: Skill Sheet 29.2 Dot Diagrams You have learned that atoms are composed of protons, neutrons, electrons. The electrons occupy energy levels that surround the nucleus in the form of an electron cloud.
Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment
 Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment 1. Which of these shows the correct hierarchical sequence? A. organs cells tissues organ systems B. cells tissues organs organ systems
Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment 1. Which of these shows the correct hierarchical sequence? A. organs cells tissues organ systems B. cells tissues organs organ systems
NCEA Level 1 Science (90944) 2017 page 1 of 6. Q Evidence Achievement Achievement with Merit Achievement with Excellence
 Assessment Schedule 2017 NCEA Level 1 Science (90944) 2017 page 1 of 6 Science: Demonstrate understanding of aspects of acids and bases (90944) Evidence Point Q Evidence Achievement Achievement with Merit
Assessment Schedule 2017 NCEA Level 1 Science (90944) 2017 page 1 of 6 Science: Demonstrate understanding of aspects of acids and bases (90944) Evidence Point Q Evidence Achievement Achievement with Merit
SC10F Exam Review. Sample Multiple Choice Questions. I. Reproduction. 1. Mitosis is
 SC10F Exam Review Sample Multiple Choice Questions I. Reproduction 1. Mitosis is ) the production of gametes. B) the division of one cell into two genetically identical cells. C) the production of cells
SC10F Exam Review Sample Multiple Choice Questions I. Reproduction 1. Mitosis is ) the production of gametes. B) the division of one cell into two genetically identical cells. C) the production of cells
1 Mendel and His Peas
 CHAPTER 5 1 Mendel and His Peas SECTION Heredity BEFORE YOU READ After you read this section, you should be able to answer these questions: What is heredity? How did Gregor Mendel study heredity? National
CHAPTER 5 1 Mendel and His Peas SECTION Heredity BEFORE YOU READ After you read this section, you should be able to answer these questions: What is heredity? How did Gregor Mendel study heredity? National
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level. Paper 1 Multiple Choice October/November 2006
 UNIVERSITY OF MRIGE INTERNTIONL EXMINTIONS General ertificate of Education Ordinary Level SIENE (PHYSIS, IOLOGY) 5125/01 Paper 1 Multiple hoice October/November 2006 dditional Materials: Multiple hoice
UNIVERSITY OF MRIGE INTERNTIONL EXMINTIONS General ertificate of Education Ordinary Level SIENE (PHYSIS, IOLOGY) 5125/01 Paper 1 Multiple hoice October/November 2006 dditional Materials: Multiple hoice
Labs 7 and 8: Mitosis, Meiosis, Gametes and Genetics
 Biology 107 General Biology Labs 7 and 8: Mitosis, Meiosis, Gametes and Genetics In Biology 107, our discussion of the cell has focused on the structure and function of subcellular organelles. The next
Biology 107 General Biology Labs 7 and 8: Mitosis, Meiosis, Gametes and Genetics In Biology 107, our discussion of the cell has focused on the structure and function of subcellular organelles. The next
IGCSE Double Award Extended Coordinated Science
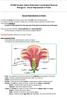 IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
REVIEW OF BASIC CHEMISTRY ANSWER KEY
 REVIEW OF BASIC CHEMISTRY ANSWER KEY 1. Name the following elements. Spelling counts: 2. Write the symbols for the following elements. H hydrogen sodium Na S sulphur phosphorus P Cl chlorine fluorine F
REVIEW OF BASIC CHEMISTRY ANSWER KEY 1. Name the following elements. Spelling counts: 2. Write the symbols for the following elements. H hydrogen sodium Na S sulphur phosphorus P Cl chlorine fluorine F
Quantitative Chemistry. AQA Chemistry topic 3
 Quantitative Chemistry AQA Chemistry topic 3 3.1 Conservation of Mass and Balanced Equations Chemical Reactions A chemical reaction is when atoms are basically rearranged into something different. For
Quantitative Chemistry AQA Chemistry topic 3 3.1 Conservation of Mass and Balanced Equations Chemical Reactions A chemical reaction is when atoms are basically rearranged into something different. For
Reinforcement Unit 3 Resource Book. Meiosis and Mendel KEY CONCEPT Gametes have half the number of chromosomes that body cells have.
 6.1 CHROMOSOMES AND MEIOSIS KEY CONCEPT Gametes have half the number of chromosomes that body cells have. Your body is made of two basic cell types. One basic type are somatic cells, also called body cells,
6.1 CHROMOSOMES AND MEIOSIS KEY CONCEPT Gametes have half the number of chromosomes that body cells have. Your body is made of two basic cell types. One basic type are somatic cells, also called body cells,
Science Class 9 th ATOMS AND MOLECULES. Symbols of Atoms of Different Elements. Atomic Mass. Molecules. Ions. Mole Concept. Finish Line & Beyond
 Science Class 9 th ATOMS AND MOLECULES Symbols of Atoms of Different Elements Atomic Mass Molecules Ions Mole Concept Atom An atom is a particle of matter that uniquely defines a chemical element. An atom
Science Class 9 th ATOMS AND MOLECULES Symbols of Atoms of Different Elements Atomic Mass Molecules Ions Mole Concept Atom An atom is a particle of matter that uniquely defines a chemical element. An atom
THE WORK OF GREGOR MENDEL
 GENETICS NOTES THE WORK OF GREGOR MENDEL Genetics-. - Austrian monk- the father of genetics- carried out his work on. Pea flowers are naturally, which means that sperm cells fertilize the egg cells in
GENETICS NOTES THE WORK OF GREGOR MENDEL Genetics-. - Austrian monk- the father of genetics- carried out his work on. Pea flowers are naturally, which means that sperm cells fertilize the egg cells in
Half Yearly Exam 2015
 GOZO COLLEGE Secondary School KULLEĠĠ TA GĦAWDEX Skola Sekondarja Half Yearly Exam 015 Year 9 Track 3 CHEMISTRY Time: 1½ hours Name: Class: Useful Data: Atomic numbers and relative atomic masses are given
GOZO COLLEGE Secondary School KULLEĠĠ TA GĦAWDEX Skola Sekondarja Half Yearly Exam 015 Year 9 Track 3 CHEMISTRY Time: 1½ hours Name: Class: Useful Data: Atomic numbers and relative atomic masses are given
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS
 CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
London Examinations IGCSE
 Centre No. Candidate No. Paper Reference(s) 4437/5 London Examinations IGCSE Science (Double Award) Chemistry Paper 5 igher Tier Tuesday 6 November 2007 Morning Time: 1 hour 30 minutes Materials required
Centre No. Candidate No. Paper Reference(s) 4437/5 London Examinations IGCSE Science (Double Award) Chemistry Paper 5 igher Tier Tuesday 6 November 2007 Morning Time: 1 hour 30 minutes Materials required
Complete this study guide to receive 5 bonus points on your test. Only study guides that are complete will receive the bonus.
 CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
CHEMISTRY AND PERIODIC TABLE STUDY GUIDE Assigned: Thursday, 09 10 14 Due: Thursday, 09 18 14 Test Day: Friday, 09 19 14 Complete this study guide to receive 5 bonus points on your test. Only study guides
National 4 Unit Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes. Homework
 National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
Coimisiún na Scrúduithe Stáit State Examinations Commission
 2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
ESA Study Guide Year 10 Science
 Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
Then and now Questions from pages 26, 27 of ESA Study Guide Year 10 Science 1. Which early scientist thought atoms would combine to form new substances? 2. Which New Zealand scientist found that most of
ST EDWARD S OXFORD. Lower Sixth Entrance Assessment. November Chemistry. 1 Hour. Candidates name:... St Edward's School 1
 ST EDWARD S OXFORD Lower Sixth Entrance Assessment November 2013 Chemistry 1 Hour Candidates name:... St Edward's School 1 St Edward's School 2 1. Complete the table below. Element calcium Symbol Pb S
ST EDWARD S OXFORD Lower Sixth Entrance Assessment November 2013 Chemistry 1 Hour Candidates name:... St Edward's School 1 St Edward's School 2 1. Complete the table below. Element calcium Symbol Pb S
Biology. Revisiting Booklet. 6. Inheritance, Variation and Evolution. Name:
 Biology 6. Inheritance, Variation and Evolution Revisiting Booklet Name: Reproduction Name the process by which body cells divide:... What kind of cells are produced this way? Name the process by which
Biology 6. Inheritance, Variation and Evolution Revisiting Booklet Name: Reproduction Name the process by which body cells divide:... What kind of cells are produced this way? Name the process by which
Sexual Reproduction and Genetics
 Chapter Test A CHAPTER 10 Sexual Reproduction and Genetics Part A: Multiple Choice In the space at the left, write the letter of the term, number, or phrase that best answers each question. 1. How many
Chapter Test A CHAPTER 10 Sexual Reproduction and Genetics Part A: Multiple Choice In the space at the left, write the letter of the term, number, or phrase that best answers each question. 1. How many
SNC2D SNC2D Exam Review Name:
 CHEMISTRY 1. Element with 9 protons: 2. Element with 7 neutrons: 3. Horizontal row in the periodic table: 4. Vertical column in the periodic table: 5. The name of the elements in group #1: 6. The name
CHEMISTRY 1. Element with 9 protons: 2. Element with 7 neutrons: 3. Horizontal row in the periodic table: 4. Vertical column in the periodic table: 5. The name of the elements in group #1: 6. The name
Biology I Level - 2nd Semester Final Review
 Biology I Level - 2nd Semester Final Review The 2 nd Semester Final encompasses all material that was discussed during second semester. It s important that you review ALL notes and worksheets from the
Biology I Level - 2nd Semester Final Review The 2 nd Semester Final encompasses all material that was discussed during second semester. It s important that you review ALL notes and worksheets from the
Bullers Wood School. Chemistry Department. Transition to A Level Chemistry Workbook. June 2018
 Bullers Wood School Chemistry Department Transition to A Level Chemistry Workbook June 2018 This booklet contains questions for you to work through and answer over the summer to prepare for the A level
Bullers Wood School Chemistry Department Transition to A Level Chemistry Workbook June 2018 This booklet contains questions for you to work through and answer over the summer to prepare for the A level
Atoms and Ions Junior Science
 2018 Version Atoms and Ions Junior Science 1 http://msutoday.msu.edu Introduction Chemistry is the study of matter and energy and the interaction between them. The elements are the building blocks of all
2018 Version Atoms and Ions Junior Science 1 http://msutoday.msu.edu Introduction Chemistry is the study of matter and energy and the interaction between them. The elements are the building blocks of all
UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION TIER SAMPLE ASSESSMENT MATERIALS
 GCSE CHEMISTRY Sample Assessment Materials 71 Surname Other Names Centre Number Candidate Number GCSE CHEMISTRY UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION
GCSE CHEMISTRY Sample Assessment Materials 71 Surname Other Names Centre Number Candidate Number GCSE CHEMISTRY UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION
MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 0620 CHEMISTRY. 0620/21 Paper 2 (Core Theory), maximum raw mark 80
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 0620 CHEMISTRY
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 0620 CHEMISTRY
Mapping our Genes. Gregor Johann Mendel observed that organisms inherit traits through discrete units of inheritance which are now called genes.
 13. C h a p t e r at G l a n c e Heredity is defined as the transmission of traits, physical or mental, from parents to offspring. Gregor Johann Mendel observed that organisms inherit traits through discrete
13. C h a p t e r at G l a n c e Heredity is defined as the transmission of traits, physical or mental, from parents to offspring. Gregor Johann Mendel observed that organisms inherit traits through discrete
RELEASED. Spring 2013 North Carolina Measures of Student Learning: NC s Common Exams. Physical Science
 Released Form Spring 2013 North arolina Measures of Student Learning: N s ommon Exams Physical Science RELESE Public Schools of North arolina State oard of Education epartment of Public Instruction Raleigh,
Released Form Spring 2013 North arolina Measures of Student Learning: N s ommon Exams Physical Science RELESE Public Schools of North arolina State oard of Education epartment of Public Instruction Raleigh,
SARALA BIRLA ACADEMY BANGALORE FINAL EXAMINATIONS Chemistry (One and Half Hours) Grade VII Tuesday, 17/032/2009
 SARALA BIRLA ACADEMY BANGALORE FINAL EXAMINATIONS 2008 2009 Chemistry (One and Half Hours) Grade VII Tuesday, 17/032/2009 Answers to this Paper must be written on this paper itself. You will not be allowed
SARALA BIRLA ACADEMY BANGALORE FINAL EXAMINATIONS 2008 2009 Chemistry (One and Half Hours) Grade VII Tuesday, 17/032/2009 Answers to this Paper must be written on this paper itself. You will not be allowed
Lecture 2: The Chemistry of Life
 Lecture 2: The Chemistry of Life In this lecture: Matter, atoms, and the periodic table Chemical bonding Ionic vs. covalent bonds Hydrogen bonds and Van der Waals forces Polarity Electronegativity What
Lecture 2: The Chemistry of Life In this lecture: Matter, atoms, and the periodic table Chemical bonding Ionic vs. covalent bonds Hydrogen bonds and Van der Waals forces Polarity Electronegativity What
Physics/Additional Science
 Write your name here Surname Other names Centre Number Candidate Number Edexcel GCSE Physics/Additional Science Unit 2: Physics for Your Future Thursday 24 May 2012 Morning Time: 1 hour You must have:
Write your name here Surname Other names Centre Number Candidate Number Edexcel GCSE Physics/Additional Science Unit 2: Physics for Your Future Thursday 24 May 2012 Morning Time: 1 hour You must have:
MV18. Double Award Science: Chemistry. Unit C1 Higher Tier [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING
![MV18. Double Award Science: Chemistry. Unit C1 Higher Tier [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING MV18. Double Award Science: Chemistry. Unit C1 Higher Tier [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING](/thumbs/85/91312857.jpg) General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier MV18 [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING TIME 1 hour, plus your additional time allowance.
General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier MV18 [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING TIME 1 hour, plus your additional time allowance.
Q1. The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence.
 Q1. The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
Q1. The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
Q1. The diagram shows some of the cell divisions that occur during human reproduction.
 Q. The diagram shows some of the cell divisions that occur during human reproduction. (a) (i) Name the type of cell division that produces cell D from cell B. () Which organ in the male body produces cell
Q. The diagram shows some of the cell divisions that occur during human reproduction. (a) (i) Name the type of cell division that produces cell D from cell B. () Which organ in the male body produces cell
