SCIENCE Year 10 Examination C 40 marks
|
|
|
- Lauren Lillian Turner
- 6 years ago
- Views:
Transcription
1 NAME: SCIENCE TEACHER: (circle code) 10C SCIENCE Year 10 Examination C 40 marks Make sure that you have answered all the questions in Paper 10B before you start this paper Time allowed for both examinations: 2 hours Answer all questions in the spaces provided on the paper. You may use a calculator. Show all your working in calculations; marks are awarded for it. Give units for all answers (e.g. kg or m) unless they are already provided. For Teacher Use Question Total Marks gained Marks available ANSWER ALL THE QUESTIONS IN THE SPACES PROVIDED Page 1
2 Question One: READY STEADY GROW [4 marks] A student carried out an experiment to find out what conditions some lettuce seeds needed in order to germinate. The table shows his results. Which conditions did the lettuce seeds need for germination? (b) State one factor that the student should have kept constant in his experiment. The diagram shows two pollen tubes growing from pollen grains on the stigma of an insect-pollinated flower. (c) The pollen grains from which pollen tubes are growing came from the anthers of other flowers on the same plant as this flower. Is this an example of self-pollination or cross-pollination? Explain your answer. Type of pollination: Reason: (d) Two of the pollen grains shown in the diagram have not grown pollen tubes. These pollen grains were blown by the wind onto the stigma of this flower from a different species of plant. State two ways in which the flower from which these pollen grains were blown would differ from the flower shown above Page 2
3 Question Two: GREEN IS GREAT! [6 marks] Chlorophyll is a green pigment found in plants. It absorbs light, which is used in photosynthesis. Where, in a leaf cell, would you expect to find chlorophyll? The graph shows the rate of photosynthesis of a plant when exposed to different colours of light. Different colours of light have different wavelengths. (b) (i) Which light produces the greatest rate of photosynthesis? Colour: Wavelength: nm (ii) What colour(s) of light give a rate of photosynthesis of 50 units? (c) Describe the effect on the rate of photosynthesis you would expect if green light is shone on the leaf instead of blue light. Page 3
4 Students investigating the effect of changing light intensity on the rate of photosynthesis cut discs from a leaf. The discs sank when put in beakers of sodium hydrogen carbonate solution. Bubbles of gas formed on the leaf discs and after a while the discs rose to the surface. The beaker was placed at different distances from the light and the results are shown below. Distance from light (cm) Average time taken for all discs to rise (minutes) (d) Plot the results above on the graph below, and join the points with a suitable freehand line. (e) Using these results, write down the relationship (pattern) between the rate of photosynthesis and the distance that the light source is away from the discs. Page 4
5 Question Three: NUTS [3 marks] The diagram shows the two forces which act on a coconut as it falls from a tall palm tree. There is no wind. On the diagram name the forces acting on the coconut as it falls. Choose from: air resistance buoyancy gravity up thrust weight (b) Explain in terms of the forces involved why the coconut accelerates as it just begins to fall. (c) What happens to size of the upward force as the coconut accelerates? Page 5
6 Question Four: IT S ATOMIC! [2 marks] Every atomic nucleus can be represented like this. Use the letters A, X and Z to complete the table. Letter Atomic number Mass number Chemical symbol Some elements can exist as isotopes. Isotopes are the atoms of the same element, however their mass numbers are different. The diagram represents four atoms W,X, Y, and Z. (b) Which two of the atoms are isotopes of the same element? Give a reason for your answer. Atoms and Reason: Page 6
7 Question Five: WATER, WATER, EVERYWHERE!!! [3 marks] The diagram shows a negatively charged plastic rod held close to a thin stream of water. The water is attracted towards the rod. How could the rod be given a charge? A company that produces bottles of mouthwash found a problem with the automatic filling system. As the bottles go towards the filler, they move around on the conveyer belt and become electrostatically charged. This causes the stream of mouthwash to move sideways, missing the open top of the bottle. (b) The company came up with a solution to the problem. Before the bottles reach the filler, they pass through a stream of ionised air. Ions are charged particles. The ions in the air neutralise the charge on the bottles. Explain why the plastic bottles become charged. (c) Earthing the conveyor belt with a conducting wire (attaching a wire to the rubber conveyer belt) would not have solved this problem. Give a reason why. Page 7
8 Question Six: A LOT OF HOT AIR [3 marks] Diagram 1 shows a hairdryer. Diagram 2 shows how the heaters and fan of the hairdryer are connected to a plug. All the switches are shown in the OFF position. Which switch or switches have to be ON to make: (circle your answer(s)) (i) only the fan work S 1 S 2 S 3 (ii) heater 2 work? S 1 S 2 S 3 (b) The heaters can only be switched on when the fan is also switched on. Explain why, in terms of the circuit diagram. Page 8
9 Question Seven: SMOKING THE COOL KILLER! [5 marks] When tobacco is burned in cigarettes, carbon monoxide is formed. A device called a Smokerlyzer measures the percentage of carbon monoxide in a person s breath. This indicates the percentage of carbon monoxide in the person s blood. Four people tested their breath using a Smokerlyzer. They repeated the test every two hours during one day at work. The results are shown in the table. Name Percentage of carbon monoxide in the blood 9 am 11 am 1 pm 3 pm Richard Alan Jenny Elizabeth Which two people are most likely to have smoked tobacco before 9 am? Red blood cells transport oxygen from the lungs to the muscles. The oxygen is used in respiration to release energy which the muscles use to shorten and lengthen. If the air we breathe in contains carbon monoxide, the red blood cells will take up carbon monoxide instead of oxygen. (b) Use this information to explain why, when they are running, many smokers become out of breath sooner than non-smokers do. (c) Alveoli are structures found in the lungs. Name the gases moving into and out of the alveoli. alveoli Page 9 Gas moving into the alveoli : Gas moving out of the alveoli:
10 Nicotine is a substance found in cigarettes. The diagram shows a nicotine molecule. It contains atoms of three elements. (d) Nicotine is a compound. How does the diagram show this? (e) Use the key to write down the chemical formula of nicotine. Page 10
11 Question Eight: ROCK ON! [7 marks] The picture shows ripple marks on a piece of sedimentary rock. These ripple marks were formed when the sediments were first laid down. Explain how these ripple marks could have been formed. Study the two diagrams below. A (b) Explain how basalt ended up in the different layers in the diagram. (c) What type of rock could be forming at A as a result from basalt forming there? Explain your answer. Type of rock: Reason: The Earth s crust (lithosphere) is broken up into huge plates. The diagram below shows two plate boundaries A and B. (d) Name the process that occurs at plate boundary A. Page 11
12 The two types of plate have different densities as shown in the table. (e) Explain what is occuring at plate boundary B, and why. Type of plate Density (g/cm 3 ) Continental 2.7 Oceanic 3.0 The graph shows how the P, S and Surface waves spread out from the epicentre of an earthquake. A and B are seismic stations. (f) Use the graph to find the time delay between the arrival of the P and S waves at station A. (g) Explain how the graph shows that the speed of the surface waves is constant. Page 12
13 Question Nine: WE ARE WATCHING YOU! [7 marks] During a police investigation, a forensic scientist tests a powder she suspects contains the compound called potassium chloride. The forensic scientist carries out some tests on the powder. She identifies the type of salt by comparing her results to the table below. Procedure Expected observation Type of compound Add hydrochloric acid Carbon dioxide is given off Carbonate Add nitric acid and then silver nitrate solution Thick white precipitate Chloride Add iron(ii)sulfate solution followed by sulfuric acid Brown ring forms Nitrate Add barium chloride solution White precipitate Sulfate State which procedure could be used to show the powder is a chloride. (b) The forensic scientist finds blood in some samples of the powder collected. State two pieces of information that can be obtained from the blood. (c) The SoCO can also carry out chromatography on the blood to see if the suspect has been taking drugs. Describe the process of paper chromatography. You may use a diagram to help answer your question. Page 13
14 DNA fingerprinting can be used to identify people. One example of the use of DNA fingerprinting is to find out which man is the father of a child. The diagram shows the DNA fingerprints of a child, the child s mother and two men who claim to be the child s father. The numbers refer to the bars on the DNA fingerprints. Only half the bars of the child s DNA fingerprint match the mother s DNA fingerprint. (d) Which man, A or B, is more likely to be the father of the child? Use the numbers on the DNA fingerprints to explain your choice. In your answer you should refer to all four people. END OF PAPER C CHECK YOUR ANSWERS Page 14
SCIENCE. Year 10 Examination A 40 marks. Make sure that you have answered all the questions in paper 10B before you start this paper.
 NAME: SCIENCE TEACHER: (circle code) 10A SCIENCE Year 10 Examination 2013 10A 40 marks Make sure that you have answered all the questions in paper 10B before you start this paper. Time allowed for both
NAME: SCIENCE TEACHER: (circle code) 10A SCIENCE Year 10 Examination 2013 10A 40 marks Make sure that you have answered all the questions in paper 10B before you start this paper. Time allowed for both
SCIENCE. Year 10 Examination A 40 marks. Make sure that you have answered all the questions in paper 10B before you start this paper
 NAME: SCIENCE TEACHER: (circle code) 10A SCIENCE Year 10 Examination 2012 10A 40 marks Make sure that you have answered all the questions in paper 10B before you start this paper Time allowed for both
NAME: SCIENCE TEACHER: (circle code) 10A SCIENCE Year 10 Examination 2012 10A 40 marks Make sure that you have answered all the questions in paper 10B before you start this paper Time allowed for both
Paper 1. Science test. First name. Last name. School KEY STAGE TIER
 Sc KEY STAGE 3 Science test Paper 1 TIER 5 7 2003 Please read this page, but do not open the booklet until your teacher tells you to start. Write your name and the name of your school in the spaces below.
Sc KEY STAGE 3 Science test Paper 1 TIER 5 7 2003 Please read this page, but do not open the booklet until your teacher tells you to start. Write your name and the name of your school in the spaces below.
Additional Science Unit Physics P2. Physics Unit Physics P2 PHY2F. (Jan11PHY2F01)
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Additional Science Unit Physics P2 Physics Unit Physics P2 General Certificate of Secondary
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Additional Science Unit Physics P2 Physics Unit Physics P2 General Certificate of Secondary
SCIENCE TEACHER: (circle code) SCIENCE. Year 10 Examination C 40 marks. Time allowed for both examinations: 2 hours
 NAME: SCIENCE TEACHER: (circle code) 10C SCIENCE Year 10 Examination 2011 10 C 40 marks Make sure that you have answered all the questions in Paper 10B before you start this paper Time allowed for both
NAME: SCIENCE TEACHER: (circle code) 10C SCIENCE Year 10 Examination 2011 10 C 40 marks Make sure that you have answered all the questions in Paper 10B before you start this paper Time allowed for both
Q1. The diagram shows the apparatus for an experiment. Hydrated copper sulphate crystals were heated. They became anhydrous copper sulphate.
 Q1. The diagram shows the apparatus for an experiment. Hydrated copper sulphate crystals were heated. They became anhydrous copper sulphate. (a) Name a suitable piece of equipment to heat tube A.... (b)
Q1. The diagram shows the apparatus for an experiment. Hydrated copper sulphate crystals were heated. They became anhydrous copper sulphate. (a) Name a suitable piece of equipment to heat tube A.... (b)
Draw one line from each solution to the ph value of the solution. Solution ph value of the solution
 1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
1 Look at the image on the photo support sheet, the micrograph shows a chloroplast.
 1 Look at the image on the photo support sheet, the micrograph shows a chloroplast. a Give the letter of the site of the light-dependent reactions of photosynthesis. b i ATP and reduced NADP are products
1 Look at the image on the photo support sheet, the micrograph shows a chloroplast. a Give the letter of the site of the light-dependent reactions of photosynthesis. b i ATP and reduced NADP are products
Name: Date: Class: 2. The diagram below shows the Moon revolving around Earth as viewed from space. (6.L.2.1)
 Name: Date: Class: 6 th Grade Science MSL Practice 1. The diagram below shows the rock cycle. (6.E.2.3) Which two processes result in the formation of igneous rocks? A. melting and solidification B. sedimentation
Name: Date: Class: 6 th Grade Science MSL Practice 1. The diagram below shows the rock cycle. (6.E.2.3) Which two processes result in the formation of igneous rocks? A. melting and solidification B. sedimentation
7.1.2 Cell Functions. 104 minutes. 137 marks. Page 1 of 30
 7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
Paper 1. Science test. Remember. First name. Last name. School KEY STAGE 3 TIER 5 7
 Sc KEY STAGE 3 Science test TIER 5 7 Paper 1 First name Last name School 2007 Remember The test is 1 hour long. You will need: pen, pencil, rubber, ruler, protractor and calculator. The test starts with
Sc KEY STAGE 3 Science test TIER 5 7 Paper 1 First name Last name School 2007 Remember The test is 1 hour long. You will need: pen, pencil, rubber, ruler, protractor and calculator. The test starts with
Life Science. Structure of a plant; Plants are living organisms just like. animals and humans. Like all living. things they need key things to live;
 6.6.3 Life Science Structure of a plant; Plants are living organisms just like animals and humans. Like all living things they need key things to live; water, sunlight, oxygen and food. Plants are different
6.6.3 Life Science Structure of a plant; Plants are living organisms just like animals and humans. Like all living things they need key things to live; water, sunlight, oxygen and food. Plants are different
The vertical distance from the top to the bottom of the slide is 2.5 metres
 Q1.The figure below shows a slide in a children s playground. (a) A child of mass 18 kilograms goes down the slide. The vertical distance from the top to the bottom of the slide is 2.5 metres. Calculate
Q1.The figure below shows a slide in a children s playground. (a) A child of mass 18 kilograms goes down the slide. The vertical distance from the top to the bottom of the slide is 2.5 metres. Calculate
(a) Write a balanced chemical symbol equation for photosynthesis. (2)
 1 Photosynthesis is the process by which plants obtain nutrition. (a) Write a balanced chemical symbol equation for photosynthesis. (b) Leaves can be tested for starch to show that photosynthesis has taken
1 Photosynthesis is the process by which plants obtain nutrition. (a) Write a balanced chemical symbol equation for photosynthesis. (b) Leaves can be tested for starch to show that photosynthesis has taken
Cell parts. nucleus cytoplasm cell surface membrane. cell wall vacuole chloroplast
 7Ab/12 Cell parts nucleus cytoplasm cell surface membrane cell wall vacuole chloroplast found in plant cells only found in plant cells only found in plant cells only found in animal and plant cells found
7Ab/12 Cell parts nucleus cytoplasm cell surface membrane cell wall vacuole chloroplast found in plant cells only found in plant cells only found in plant cells only found in animal and plant cells found
What is the resultant force acting on the object? BASE jumpers jump from very high buildings and mountains for sport.
 Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
1. Take a polythene rod (AB), hold it at its centre and rub both ends with a cloth.
 Q1. A pupil did an experiment following the instructions below. 1. Take a polythene rod (AB), hold it at its centre and rub both ends with a cloth. 2. Suspend the rod, without touching the ends, from a
Q1. A pupil did an experiment following the instructions below. 1. Take a polythene rod (AB), hold it at its centre and rub both ends with a cloth. 2. Suspend the rod, without touching the ends, from a
... + water (3)
 1 (a) Complete the equation for photosynthesis.... + water... +... (3) (b) The rate of photosynthesis in a plant depends on several factors in the environment. These factors include light intensity and
1 (a) Complete the equation for photosynthesis.... + water... +... (3) (b) The rate of photosynthesis in a plant depends on several factors in the environment. These factors include light intensity and
SCIENCE (REVISED SYLLABUS) HIGHER LEVEL
 2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
2. Name the part of the brain which controls posture and balance of the body?
 . Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
. Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
Year 10 Chemistry Exam June 2011 Multiple Choice. Section A Mulltiple Choice
 Year 10 Chemistry Exam June 2011 Multiple Choice Section A Mulltiple Choice 1. An aqueous solution is obtained when: a. a substance dissolves in any liquid b. a substance is dissolved in water c. when
Year 10 Chemistry Exam June 2011 Multiple Choice Section A Mulltiple Choice 1. An aqueous solution is obtained when: a. a substance dissolves in any liquid b. a substance is dissolved in water c. when
UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION TIER SAMPLE ASSESSMENT MATERIALS
 GCSE CHEMISTRY Sample Assessment Materials 71 Surname Other Names Centre Number Candidate Number GCSE CHEMISTRY UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION
GCSE CHEMISTRY Sample Assessment Materials 71 Surname Other Names Centre Number Candidate Number GCSE CHEMISTRY UNIT 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS AND ORGANIC CHEMISTRY FOUNDATION
SCIENCE. Year 9 Examination 2011
 NAME: SCIENCE TEACHER: (circle code) 2011 9A 9A SCIENCE Year 9 Examination 2011 9A 40 marks Make sure that you have answered all the questions in paper 9B before you start this paper Time allowed for both
NAME: SCIENCE TEACHER: (circle code) 2011 9A 9A SCIENCE Year 9 Examination 2011 9A 40 marks Make sure that you have answered all the questions in paper 9B before you start this paper Time allowed for both
1 Forming New Substances
 CHAPTER 9 1 Forming New Substances SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a What are exothermic reactions? What are
CHAPTER 9 1 Forming New Substances SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a What are exothermic reactions? What are
Chemistry Unit Test 1 Review
 Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
SCIENCE. Year 10 Examination B 80 marks. Make sure you have answered all the questions in this paper before you start 10A or 10C
 NAME: SCIENCE TEACHER: (circle code) 10B SCIENCE Year 10 Examination 2013 10B 80 marks Make sure you have answered all the questions in this paper before you start 10A or 10C Time allowed for both examinations:
NAME: SCIENCE TEACHER: (circle code) 10B SCIENCE Year 10 Examination 2013 10B 80 marks Make sure you have answered all the questions in this paper before you start 10A or 10C Time allowed for both examinations:
In the exam you will be asked to tackle questions such as the one below.
 Get started AO3 2 Preparing salts This unit will help you to plan, describe and understand an experiment to prepare a salt. In the exam you will be asked to tackle questions such as the one below. Exam-style
Get started AO3 2 Preparing salts This unit will help you to plan, describe and understand an experiment to prepare a salt. In the exam you will be asked to tackle questions such as the one below. Exam-style
Q1. As the world population increases there is a greater demand for fertilisers.
 Q1. As the world population increases there is a greater demand for fertilisers. (a) Explain what fertilisers are used for............. (b) The amount of nitrogen in a fertiliser is important. How many
Q1. As the world population increases there is a greater demand for fertilisers. (a) Explain what fertilisers are used for............. (b) The amount of nitrogen in a fertiliser is important. How many
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are oxygen and...
 Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
glossary 6 of. boxes with and units. question. the syllabus. to see if. nature. Day. Teacher. Pre readings. Topic. Mr Stocker.
 This revision booklet contains questions from the Coordinated examinations from 2007 2011. At the end of each question you will find a code corresponding to the year that the question came from. example
This revision booklet contains questions from the Coordinated examinations from 2007 2011. At the end of each question you will find a code corresponding to the year that the question came from. example
Name: P2 STATIC CHARGE. Class: Practice Questions. Date: 56 minutes. Time: 56 marks. Marks: GCSE PHYSICS ONLY. Comments: Page 1 of 26
 P2 STATIC CHARGE Practice Questions Name: Class: Date: Time: 56 minutes Marks: 56 marks Comments: GCSE PHYSICS ONLY Page of 26 The figure below shows a slide in a children s playground. A child of mass
P2 STATIC CHARGE Practice Questions Name: Class: Date: Time: 56 minutes Marks: 56 marks Comments: GCSE PHYSICS ONLY Page of 26 The figure below shows a slide in a children s playground. A child of mass
Draw a ring around the correct answer to complete each sentence. The energy needed for photosynthesis comes from
 Q1. (a) Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +... (b) Draw a ring around the correct answer to complete each sentence. (i) The energy needed for photosynthesis
Q1. (a) Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +... (b) Draw a ring around the correct answer to complete each sentence. (i) The energy needed for photosynthesis
The Chemistry of Respiration and Photosynthesis
 The Chemistry of Respiration and Photosynthesis Objective- You should be able to write balanced equations for respiration and photosynthesis and explain how the two equations are related. Directions :
The Chemistry of Respiration and Photosynthesis Objective- You should be able to write balanced equations for respiration and photosynthesis and explain how the two equations are related. Directions :
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Answer ALL questions.
 Answer ALL questions. 01. The three states of matter are solid, liquid and gas. The diagram shows how the particles are arranged in each of these states. (a) Use words from the box to show the changes
Answer ALL questions. 01. The three states of matter are solid, liquid and gas. The diagram shows how the particles are arranged in each of these states. (a) Use words from the box to show the changes
Describe in full the colour change at the end-point of this titration. ... (1)
 Q1. (a) A solution of barium hydroxide is often used for the titration of organic acids. A suitable indicator for the titration is thymol blue. Thymol blue is yellow in acid and blue in alkali. In a titration
Q1. (a) A solution of barium hydroxide is often used for the titration of organic acids. A suitable indicator for the titration is thymol blue. Thymol blue is yellow in acid and blue in alkali. In a titration
Orchard School. New Document 1 Name: Class: Date: 129 minutes. Time: 126 marks. Marks: Comments: Page 1
 New Document Name: Class: Date: Time: Marks: 29 minutes 26 marks Comments: Page Q. The ph scale is a measure of the acidity or alkalinity of a solution. (a) Solution Draw one line from each solution to
New Document Name: Class: Date: Time: Marks: 29 minutes 26 marks Comments: Page Q. The ph scale is a measure of the acidity or alkalinity of a solution. (a) Solution Draw one line from each solution to
Paper Reference(s) 5PH2H/01 Edexcel GCSE
 Paper Reference(s) 5PH2H/01 Edexcel GCSE Physics/Additional Science Unit 2: Physics for Your Future Higher Tier Thursday 24 May 2012 Morning Time: 1 hour plus your additional time allowance INSTRUCTIONS
Paper Reference(s) 5PH2H/01 Edexcel GCSE Physics/Additional Science Unit 2: Physics for Your Future Higher Tier Thursday 24 May 2012 Morning Time: 1 hour plus your additional time allowance INSTRUCTIONS
GCSE COMBINED SCIENCE: SYNERGY
 GCSE COMBINED SCIENCE: SYNERGY Higher Tier Paper 4H H Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
GCSE COMBINED SCIENCE: SYNERGY Higher Tier Paper 4H H Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown in the bar chart.
 Q1. (a) The diagram shows the Earth s layered structure. Name parts (i) and (ii). (b) The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown
Q1. (a) The diagram shows the Earth s layered structure. Name parts (i) and (ii). (b) The bar chart shows the composition of a sample of dry air from the Earth s atmosphere. Name the three gases shown
AQA GCSE Physics. 60 minutes. 60 marks. Q1 to Q4 to be worked through with tutor. Q5 to Q8 to be worked through independently.
 AQA GCSE Physics Electricity 4.2.5: Static Electricity & Electric Fields Name: Class: Date: Time: 60 minutes Marks: 60 marks Comments: Q to Q4 to be worked through with tutor. Q5 to Q8 to be worked through
AQA GCSE Physics Electricity 4.2.5: Static Electricity & Electric Fields Name: Class: Date: Time: 60 minutes Marks: 60 marks Comments: Q to Q4 to be worked through with tutor. Q5 to Q8 to be worked through
(a) What name is given to this method? (1) (b) Which piece of apparatus should be used to measure the 25.0cm 3 of KOH?
 1 This apparatus can be used in a method to find the volume of sulfuric acid required to neutralise a solution of potassium hydroxide (KOH). burette containing 0.100mol/dm 3 H 2 SO 4 conical flask 25.0cm
1 This apparatus can be used in a method to find the volume of sulfuric acid required to neutralise a solution of potassium hydroxide (KOH). burette containing 0.100mol/dm 3 H 2 SO 4 conical flask 25.0cm
The table shows the results of some tests carried out on three solutions, A, B and C. Hydrochloric acid is added. Solution
 1 Chemical tests can be used to identify compounds. The table shows the results of some tests carried out on three solutions, A, B and C. Solution Flame Test Hydrochloric acid is added Sodium hydroxide
1 Chemical tests can be used to identify compounds. The table shows the results of some tests carried out on three solutions, A, B and C. Solution Flame Test Hydrochloric acid is added Sodium hydroxide
Additional Science Chemistry
 Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Plants and photosynthesis/plants for food
 Medway LEA Advisory Service Plants and photosynthesis/plants for food 9C & 9D 31 min 33 marks Q1-L4, Q2-L5, Q3-L5, Q4-L6, Q5-L6 1. The drawing shows a plant called Tillandsia. (a) (i) The leaves of this
Medway LEA Advisory Service Plants and photosynthesis/plants for food 9C & 9D 31 min 33 marks Q1-L4, Q2-L5, Q3-L5, Q4-L6, Q5-L6 1. The drawing shows a plant called Tillandsia. (a) (i) The leaves of this
IGCSE Double Award Extended Coordinated Science
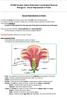 IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
IGCSE Double Award Extended Coordinated Science Biology 8.2 - Sexual Reproduction in Plants Sexual Reproduction in Plants In a plant, the flower is the sexual organ, and it has both male and females parts.
Photosynthesis Revision 1
 Photosynthesis Revision 73 minutes 73 marks Page of 35 Q. This question is about photosynthesis. (a) Plants make glucose during photosynthesis. Some of the glucose is changed into insoluble starch. What
Photosynthesis Revision 73 minutes 73 marks Page of 35 Q. This question is about photosynthesis. (a) Plants make glucose during photosynthesis. Some of the glucose is changed into insoluble starch. What
plant cell tissue nerve cell paramecium
 plant or animal cell? these cells carry what cell? what cell? animal cell red blood oxygen sperm root hair cell (of plant) plant or animal cell? cell, tissue or organ? what cell? paramecium, amoeba or
plant or animal cell? these cells carry what cell? what cell? animal cell red blood oxygen sperm root hair cell (of plant) plant or animal cell? cell, tissue or organ? what cell? paramecium, amoeba or
Page 2. Q1.Green plants can make glucose. Plants need energy to make glucose. How do plants get this energy? (2)
 Q1.Green plants can make glucose. (a) Plants need energy to make glucose. How do plants get this energy? (b) Plants can use the glucose they have made to supply them with energy. Give four other ways in
Q1.Green plants can make glucose. (a) Plants need energy to make glucose. How do plants get this energy? (b) Plants can use the glucose they have made to supply them with energy. Give four other ways in
Choose words from the list to complete the sentences below. electrical heat light kinetic. an endothermic an exothermic a neutralisation a reduction
 Q1. The diagram shows some magnesium ribbon burning. (a) Choose words from the list to complete the sentences below. electrical heat light kinetic an endothermic an exothermic a neutralisation a reduction
Q1. The diagram shows some magnesium ribbon burning. (a) Choose words from the list to complete the sentences below. electrical heat light kinetic an endothermic an exothermic a neutralisation a reduction
ST EDWARD S OXFORD. Lower Sixth Entrance Assessment. November Chemistry. 1 Hour. Candidates name:... St Edward's School 1
 ST EDWARD S OXFORD Lower Sixth Entrance Assessment November 2013 Chemistry 1 Hour Candidates name:... St Edward's School 1 St Edward's School 2 1. Complete the table below. Element calcium Symbol Pb S
ST EDWARD S OXFORD Lower Sixth Entrance Assessment November 2013 Chemistry 1 Hour Candidates name:... St Edward's School 1 St Edward's School 2 1. Complete the table below. Element calcium Symbol Pb S
Which part of an atom is most directly involved in chemical bonding?
 1 Which part of an atom is most directly involved in chemical bonding? nucleus electron proton neutron 1 Which part of an atom is most directly involved in chemical bonding? nucleus electron proton neutron
1 Which part of an atom is most directly involved in chemical bonding? nucleus electron proton neutron 1 Which part of an atom is most directly involved in chemical bonding? nucleus electron proton neutron
Scholarship Examination
 Write your name here Surname Other names Scholarship Examination Subject: Chemistry Time: 45 minutes You must have: Calculator Ruler Total Marks /45 Instructions Use black ink or ball-point pen. Fill in
Write your name here Surname Other names Scholarship Examination Subject: Chemistry Time: 45 minutes You must have: Calculator Ruler Total Marks /45 Instructions Use black ink or ball-point pen. Fill in
YEAR 10 SCIENCE EXAMINATION Semester 2, 2017 QUESTION AND ANSWER BOOKLET
 STUDENT NAME: TEACHER NAME: DATE: YEAR 10 SCIENCE EXAMINATION Semester 2, 2017 QUESTION AND ANSWER BOOKLET TIME ALLOWED FOR THIS PAPER: Reading time before commencing work: Working time for this paper:
STUDENT NAME: TEACHER NAME: DATE: YEAR 10 SCIENCE EXAMINATION Semester 2, 2017 QUESTION AND ANSWER BOOKLET TIME ALLOWED FOR THIS PAPER: Reading time before commencing work: Working time for this paper:
Haytham found a plant that had leaves with some green areas and some white areas. Leaves like this are called variegated leaves.
 Exercise 1.1 Variegated leaves You ll find this exercise easier to do if you have tried Activity 1.1 first, because you need to understand how to test a leaf for starch. You will also need to think about
Exercise 1.1 Variegated leaves You ll find this exercise easier to do if you have tried Activity 1.1 first, because you need to understand how to test a leaf for starch. You will also need to think about
FIFTH GRADE-SCIENCE (SCIENCE5_1)
 Name: Date: FIFTH GRADE-SCIENCE (SCIENCE5_1) 1. Joanne wanted to know how far she could throw a ball. She threw it ten times and measured the distance each time. She always got a different measurement.
Name: Date: FIFTH GRADE-SCIENCE (SCIENCE5_1) 1. Joanne wanted to know how far she could throw a ball. She threw it ten times and measured the distance each time. She always got a different measurement.
Which farm is likely to have been using too much fertiliser on its land? farm C. farm B
 1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
How many protons are there in the nucleus of the atom?... What is the mass number of the atom?... (Total 2 marks)
 Q1. The diagram shows an atom. How many protons are there in the nucleus of the atom?... What is the mass number of the atom?... (Total 2 marks) Page 1 of 53 Q2. The picture shows a man at work in a factory
Q1. The diagram shows an atom. How many protons are there in the nucleus of the atom?... What is the mass number of the atom?... (Total 2 marks) Page 1 of 53 Q2. The picture shows a man at work in a factory
Q1.A student investigated the rate of reaction between sodium thiosulfate solution and dilute hydrochloric acid, as shown in Figure 1.
 Q1.A student investigated the rate of reaction between sodium thiosulfate solution and dilute hydrochloric acid, as shown in Figure 1. The reaction produced a precipitate, which made the mixture turn cloudy.
Q1.A student investigated the rate of reaction between sodium thiosulfate solution and dilute hydrochloric acid, as shown in Figure 1. The reaction produced a precipitate, which made the mixture turn cloudy.
Class X. Exercises solution
 Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
4-4 Bioenergetics Biology
 4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
Elements, compounds, Mixtures
 Elements, compounds, Mixtures Model Answers 1 Level IGCSE(9-1) Subject Chemistry Exam Board Edexcel IGCSE Module Double Award (Paper 1C) Topic Principles of Chemistry Sub-Topic Booklet Elements, Compounds,
Elements, compounds, Mixtures Model Answers 1 Level IGCSE(9-1) Subject Chemistry Exam Board Edexcel IGCSE Module Double Award (Paper 1C) Topic Principles of Chemistry Sub-Topic Booklet Elements, Compounds,
Sri Lankan School Muscat
 Sri Lankan School Muscat Withdrawal Examination 2015/2016 Class : Subject : Paper : Duration: Year 11B/R Physics Name :... 1 2 Hours Q.No. Allocated 1 11 2 12 3 12 4 10 5 06 6 08 7 06 8 12 9 10 10 04 11
Sri Lankan School Muscat Withdrawal Examination 2015/2016 Class : Subject : Paper : Duration: Year 11B/R Physics Name :... 1 2 Hours Q.No. Allocated 1 11 2 12 3 12 4 10 5 06 6 08 7 06 8 12 9 10 10 04 11
Asexual & Plant Reproduction
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ sexual & Plant Reproduction Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ sexual & Plant Reproduction Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
MASTERY PRACTICE BOOK. Learn to apply knowledge and get higher grades.
 1 MASTERY PRACTICE BOOK Learn to apply knowledge and get higher grades. MASTERY PRACTICE BOOK Learn to apply knowledge and get higher grades in science. What will get you really high grades in science
1 MASTERY PRACTICE BOOK Learn to apply knowledge and get higher grades. MASTERY PRACTICE BOOK Learn to apply knowledge and get higher grades in science. What will get you really high grades in science
ST EDWARD S OXFORD. 14+ Entrance Assessment 2014 Science 1 hour. Candidate Name:.
 ST EDWARD S OXFORD 14+ Entrance Assessment 2014 Science 1 hour Candidate Name: PHYSICS 1 Electricity may be produced from a number of different energy resources (a) (i) Complete the table below The first
ST EDWARD S OXFORD 14+ Entrance Assessment 2014 Science 1 hour Candidate Name: PHYSICS 1 Electricity may be produced from a number of different energy resources (a) (i) Complete the table below The first
Chemical Reactions and Equations
 Chemical Reactions and Equations Question 1: Why should a magnesium ribbon be cleaned before burning in air? Magnesium is very reactive metal. When stored it reacts with oxygen to form a layer magnesium
Chemical Reactions and Equations Question 1: Why should a magnesium ribbon be cleaned before burning in air? Magnesium is very reactive metal. When stored it reacts with oxygen to form a layer magnesium
Applications in Forensic Science. T. Trimpe
 Applications in Forensic Science T. Trimpe 2006 http://sciencespot.net/ What is chromatography? From Wikipedia... Chromatography (from Greek word for chromos for colour) is the collective term for a family
Applications in Forensic Science T. Trimpe 2006 http://sciencespot.net/ What is chromatography? From Wikipedia... Chromatography (from Greek word for chromos for colour) is the collective term for a family
Figure 1 shows the charges on the acetate rod and cloth before and after rubbing. Figure 1
 A student rubs an acetate rod with a cloth. Figure shows the charges on the acetate rod and cloth before and after rubbing. Figure (a) Explain how rubbing an acetate rod with a cloth causes the rod and
A student rubs an acetate rod with a cloth. Figure shows the charges on the acetate rod and cloth before and after rubbing. Figure (a) Explain how rubbing an acetate rod with a cloth causes the rod and
The grade 5 English science unit, Plants, meets the academic content standards set in the Korean curriculum, which state students should:
 This unit deals with the structures and functions of plant organs including roots, stems, leaves, and flowers. Students learn that a plant is sustained by the systematic functioning of all its organs.
This unit deals with the structures and functions of plant organs including roots, stems, leaves, and flowers. Students learn that a plant is sustained by the systematic functioning of all its organs.
Physical Sciences: Matter & Energy. What is physical science? A. Physical science is a field of science that studies matter and energy.
 Physical Sciences: Matter & Energy What is physical science? A. Physical science is a field of science that studies matter and energy. B. Physical science has 2 main branches: 1.PHYSICS: the study of how
Physical Sciences: Matter & Energy What is physical science? A. Physical science is a field of science that studies matter and energy. B. Physical science has 2 main branches: 1.PHYSICS: the study of how
Year 10 Chemistry. Practice questions. Topics
 Year 10 Chemistry Practice questions Topics 1 Group 1 2 Group 7 3 Reactivity series 4 Air and Water 5 Rates of reaction 6 Electrolysis 7 Acids, Alkali and Salts Objective: Evaluate group 1 & 7 reactivity
Year 10 Chemistry Practice questions Topics 1 Group 1 2 Group 7 3 Reactivity series 4 Air and Water 5 Rates of reaction 6 Electrolysis 7 Acids, Alkali and Salts Objective: Evaluate group 1 & 7 reactivity
CHAPTER 1: Chemistry, An Introduction
 CHAPTER 1: Chemistry, An Introduction science: the study of nature to explain what one observes 1.4 THE SCIENTIFIC METHOD: How Chemists Think Applying the Scientific Method 1. Make an observation, and
CHAPTER 1: Chemistry, An Introduction science: the study of nature to explain what one observes 1.4 THE SCIENTIFIC METHOD: How Chemists Think Applying the Scientific Method 1. Make an observation, and
Material cycles and energy: photosynthesis
 7 Material cycles and energy: photosynthesis Remember: Plants are living organisms and can carry out all the life processes. Plants must be able to make foods. The foods provide raw materials for growth
7 Material cycles and energy: photosynthesis Remember: Plants are living organisms and can carry out all the life processes. Plants must be able to make foods. The foods provide raw materials for growth
NCEA Level 1 Science (90944) 2017 page 1 of 6. Q Evidence Achievement Achievement with Merit Achievement with Excellence
 Assessment Schedule 2017 NCEA Level 1 Science (90944) 2017 page 1 of 6 Science: Demonstrate understanding of aspects of acids and bases (90944) Evidence Point Q Evidence Achievement Achievement with Merit
Assessment Schedule 2017 NCEA Level 1 Science (90944) 2017 page 1 of 6 Science: Demonstrate understanding of aspects of acids and bases (90944) Evidence Point Q Evidence Achievement Achievement with Merit
The table lists some functions of parts of a plant. Match the part of the plant (A, B, C or D) to its function by writing the letters in the table.
 Low Demand Questions QUESTIONSHEET 1 The diagram shows a flowering plant. A Name the parts labelled A, B, C and D. (c) (d) B C D A... B C... D [4] The table lists some functions of parts of a plant. Match
Low Demand Questions QUESTIONSHEET 1 The diagram shows a flowering plant. A Name the parts labelled A, B, C and D. (c) (d) B C D A... B C... D [4] The table lists some functions of parts of a plant. Match
Website: Page 1. Page 14»Exercise» Page 15» Question 1:
 Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
1. (a) The diagrams below show the arrangement of atoms or molecules in five different substances A, B, C, D and E.
 Level 7 Chemistry Questions 1. (a) The diagrams below show the arrangement of atoms or molecules in five different substances A, B, C, D and E. Each of the circles, and represents an atom of a different
Level 7 Chemistry Questions 1. (a) The diagrams below show the arrangement of atoms or molecules in five different substances A, B, C, D and E. Each of the circles, and represents an atom of a different
The electrolysis of sodium chloride solution produces useful substances. covalent ionic non-metallic
 1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
London Examinations IGCSE
 Centre No. Candidate No. Paper Reference 4 3 3 5 2 H Paper Reference(s) 4335/2H London Examinations IGCSE Chemistry Paper 2H Higher Tier Wednesday 21 May 2008 Afternoon Time: 2 hours Surname Signature
Centre No. Candidate No. Paper Reference 4 3 3 5 2 H Paper Reference(s) 4335/2H London Examinations IGCSE Chemistry Paper 2H Higher Tier Wednesday 21 May 2008 Afternoon Time: 2 hours Surname Signature
5 th Inquiry Review 2010, 2009, 2008, 2007, , 2004, 2003
 1 A student examined a rock sample and described it as having particles of various colors that were 1 millimeter to 12 millimeters in size. The student was making (1) an inference (3) a prediction (2)
1 A student examined a rock sample and described it as having particles of various colors that were 1 millimeter to 12 millimeters in size. The student was making (1) an inference (3) a prediction (2)
Name: Photosynthesis. Class: Date: 76 minutes. Time: 76 marks. Marks: level 1, 2 and 3. Increasing demand. Comments:
 Photosynthesis Name: Class: Date: Time: 76 minutes Marks: 76 marks Comments: level, 2 and 3. Increasing demand Q. Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +
Photosynthesis Name: Class: Date: Time: 76 minutes Marks: 76 marks Comments: level, 2 and 3. Increasing demand Q. Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +
AP Chemistry Unit 2 Test (Chapters 3 and 4)
 AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
Page 2. Q1.A student investigated food dyes using paper chromatography. This is the method used.
 Q1.A student investigated food dyes using paper chromatography. This is the method used. 1. Put a spot of food colouring X on the start line. 2. Put spots of four separate dyes, A, B, C and D, on the start
Q1.A student investigated food dyes using paper chromatography. This is the method used. 1. Put a spot of food colouring X on the start line. 2. Put spots of four separate dyes, A, B, C and D, on the start
Section 3. What Drives the Plates? What Do You See? Think About It. Investigate. Learning Outcomes
 Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
EXAMINATION QUESTIONS (6)
 1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
earth live neutral (ii) What is the colour of the insulation around the wire labelled T? blue brown green and yellow
 Q. (a) The diagram shows the inside of a three-pin plug. What name is given to the wire labelled S? Draw a ring around the correct answer. earth live neutral () What is the colour of the insulation around
Q. (a) The diagram shows the inside of a three-pin plug. What name is given to the wire labelled S? Draw a ring around the correct answer. earth live neutral () What is the colour of the insulation around
GCSE Additional Science
 GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
GCSE Additional Science Module C5 Chemicals of the Natural Environment: What you should know Name: Science Group: Teacher: each of the statements to help focus your revision: R = Red: I don t know this
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 *4495225485* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November
*4495225485* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November
Naming salts. Metal Acid Salt. Sodium hydroxide reacts with Hydrochloric acid to make Sodium chloride
 Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
Naming salts A salt is any compound formed by the neutralisation of an acid by a base. The name of a salt has two parts. The first part comes from the metal, metal oxide or metal carbonate. The second
International Advanced Level Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry
 Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Tuesday 22 May
Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Tuesday 22 May
The characteristic Properties of Acids and
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ The haracteristic Properties of cids and ases Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ The haracteristic Properties of cids and ases Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry
Plants can be either herbaceous or woody.
 Plant Structure Plants can be either herbaceous or woody. Herbaceous plants are plants with growth which dies back to the ground each year, in contrast with woody plants Most herbaceous plants have stems
Plant Structure Plants can be either herbaceous or woody. Herbaceous plants are plants with growth which dies back to the ground each year, in contrast with woody plants Most herbaceous plants have stems
Biology Y9 HY Page 1 of 12
 S E C O N D A R Y S C H O O L - M R I E Ħ E L HALF-YEARLY EXAMINATIONS 2017/2018 YEAR 9 Biology Time: 2 hours Name: Class: Section A: Answer ALL the questions in this section in the space provided. This
S E C O N D A R Y S C H O O L - M R I E Ħ E L HALF-YEARLY EXAMINATIONS 2017/2018 YEAR 9 Biology Time: 2 hours Name: Class: Section A: Answer ALL the questions in this section in the space provided. This
Chemistry CH3FP Unit Chemistry C3 Written Paper Monday 20 May pm to 2.30 pm For this paper you must have: Time allowed Instructions all
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier June 2013 1 2 Chemistry
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier June 2013 1 2 Chemistry
IGCSE (9-1) Edexcel - Chemistry
 IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Chemical Formulae, Equations and Calculations NOTES 1.25: Write word equations and balanced chemical equations (including state symbols): For reactions
IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Chemical Formulae, Equations and Calculations NOTES 1.25: Write word equations and balanced chemical equations (including state symbols): For reactions
