Molecular dynamics modeling of irradiation damage in highly coordinated mineral structures
|
|
|
- Quentin Morgan
- 5 years ago
- Views:
Transcription
1 Molecular dynamics modeling of irradiation damage in highly coordinated mineral structures Grechanovsky A.E., Brik A.B. M.P. Semenenko Institute of Geochemistry, Mineralogy and Ore Formation of NAS of Ukraine, 34 Palladina av., Kyiv, Ukraine Abstract. The radiation stability of zirconolite CaZrTi 2 O 7, pyrochlore Gd 2 Zr 2 O 7 and periclase MgO has been studied by computer simulations methods. Computer simulation of zircon ZrSiO 4 also has been performed for comparison with these structures. These calculations were performed in grid- environment using «GEOPARD» virtual organization. The number of Frenkel pairs after propagation of the primary knock-оn atom of thorium with a kinetic energy of 20 kev (analogue of recoil atom arising due to the alpha decay of actinides) has been characterized by molecular dynamics method. Calculation of the effective charge of oxygen atoms has been performed using density functional theory and B3LYP hybrid functional. It is established that the radiation stability of these minerals depends significantly from two main factors: type of structure and the degree of chemical bonds covalency of the structures (or effective charge of oxygen atoms). The results of computer simulations show that structures with high bond iconicity and high coordination number of cations (periclase, pyrochlore) are characterized by high radiation resistance to amorphization. Keywords Radiation resistance of minerals, semiempirical interatomic potential method, grid-calculations, computer simulation, method of molecular dynamics 1 Introduction Over recent decades, in a number of countries there has been traced a tendency to increase the use of electricity generated by nuclear power plants. In particular, according to the International Atomic Energy Agency (IAEA) data, in 2009 the share of electricity generated by nuclear power plants is 75% in France, 49% in Ukraine, 20% in the United States, and 18% in the Russian Federation [1]. On the other hand, the prospects for the development of nuclear power engineering are associated with the effective management of nuclear waste. The development of nuclear power engineering raises a number of problems relating to the disposal of long-lived radioactive waste and plutonium. One of the main problems in this respect is the choice of radiation resistant matrices, which, in contact with long-lived high-level radioactive waste, for a long time will not change their physical and chemical properties. At present, aluminophosphate or borosilicate glasses have been used as matrices for spent fuel. However, high-level radioactive waste can be stored in these matrices for a time of no longer than years. This is the reason that the search for matrices with efficient performance characteristics has been actively continued. It has been found that crystalline ceramic materials are significantly better suited for the utilization of high-level radioactive waste. To date, a number of ceramic materials have been developed for the disposal of high-level radioactive waste and plutonium. Extensive studies have been performed on materials such as zircon ZrSiO 4, pyrochlores Gd 2 Ti 2 O 7 and Gd 2 Zr 2 O 7, monazites (La,Ce,Nd)PO 4, zirconolite CaZrTi 2 O 7, perovskite CaTiO 3, and other complex oxides, as well as rutile TiO 2 and baddeleyite ZrO 2. Many researchers have considered zircon as a promising matrix for the disposal of nuclear fuel and weapon-grade plutonium [2 5]. However, over geological time, the alpha decay of uranium and thorium atoms leads to the damage of the structure of zircon and its transition from the crystalline state to the X-ray amorphous (metamict) state. Each act of alpha decay results in the formation of an alpha particle and a heavy recoil atom [5]. Alpha particles with an energy of MeV, as was noted in [4], displace approximately 100 atoms in the end of the path with a length of μm, whereas heavy recoil atoms with an energy of kev displace several thousand atoms within an interval of 20 nm. A promising replacement of zircon can be minerals with highly coordinated structure. In contrast to zircon, these compounds are characterized by high radiation stability [6]. In this respect, the purpose of the present work was to -156-
2 investigate the relation between the radiation stability of zirconolite CaZrTi 2 O 7, compounds Gd 2 Zr 2 O 7 with the structure of pyrochlore and periclase MgO and characteristics of its crystalline structures using method of molecular dynamics (MD). MD simulation of zircon structure also has been performed for comparison with these structures. 2 Simulation technique The molecular dynamics (MD) method consists in calculating trajectories of the motion of all atoms involved in a system on the basis of Newton s second law. The initial data are taken as the initial coordinates and velocities of all the atoms and the interatomic interaction potentials. In the majority of such model experiments, the atoms are endowed with some effective charges. The magnitude of these charges depends on the degree of covalency of interatomic bonds and can vary from zero (for covalent compounds) to values of formal charges of ions (for ionic crystals). In addition to the Coulomb interactions of all electrostatic charges between themselves, the interatomic interaction potential takes into account the repulsion of electron shells of the atoms and the dipole dipole interaction between the atoms in terms of the short-range interaction potentials of the following form: a) the Buckingham potential 6 V ( r) = A exp( r / ρ ) С r, (1) where r is the distance between two atoms (Å), A is the pre-exponential factor for the term characterizing the repulsion (ev), ρ is the stiffness parameter (Å), and C is the force parameter of the van der Waals interaction (ev Å 6 ); b) the Morse potential V r) = D [ exp( 2α ( r r )) 2exp( α ( r ))], (2) ( 0 r0 where D is the dissociation energy of the bond between the atoms (ev), α is the softness parameter (Å -1 ), r 0 is the standard bond length between the atoms (Å). Parameters specified in (1) and (2) were taken from works [7-11].Optimization of these structures was made using experimental values of unit-cell parameters, atom coordinates, elastic constants, and thermodynamic properties. In the structure of a mineral, we chose a fragment containing approximately million atoms. One of atoms was replaced by a thorium atom. At the preliminary stage of the simulation, the structural fragment was brought into the thermal equilibrium state for 10 ps at the temperature of modeling T mod (300 K) with the use of an NPT ensemble (here, the number of atoms N in the structural fragment, the pressure P on the walls of the fragment, and the temperature T are constant). At small interatomic distances (less than 1 Å), we used the internuclear repulsion potential ZBL, which was introduced to correctly take into account the strong internuclear repulsion [6]. The simulation time step was 0.5 fs. The main stage of the simulation was performed using the microcanonical ensemble NVE (here, the number of atoms N in the structural fragment, the volume of the structure V, and the energy E are constant). At the beginning of this stage, we specified the direction of motion and the velocity of the thorium atom, which corresponded to a particular kinetic energy. This energy was limited by the number of atoms in the fragment (25 50 atoms per electron-volt, depending on the elastic properties of the mineral). Keeping this in mind, energy of 20 kev has been chosen for the structure fragment consisting of million atoms. Limitations of computation capability prevented us from considering a greater structure fragment. As a result of critical consideration of various programs, we dwelt on the DL_POLY program complex [12], elaborated for simulation of structural fragments of minerals, macromolecules, polymers, and ion systems. This program complex gives an opportunity to of radiation mineralogy (investigation of structures due to alpha decay of actinides), study of processes in minerals, study of forming migrations of point defects in these minerals. For the calculation of the effective charge of oxygen atoms in the minerals we have performed quantum-chemical calculations using density functional theory and the B3LYP hybrid functional. We have used the PC GAMESS code [13] for this purpose. For realization of calculations the web-sites of uagrid.org.ua and grid.inpracom.kiev.ua were used. All calculations were executed in the virtual organization «GEOPARD», organized by Glushkov Institute of Cybernetic of NAS of Ukraine, M.P. Semenenko Institute of Geochemistry, Mineralogy and Ore Formation of NAS of Ukraine and S.I. Subbotin Institute of Geophysics of NAS of Ukraine
3 3 Results and discussion The motion of a primary knock-on atom leads to its collision with other atoms of the system. These atoms are displaced from their equilibrium positions, begin to move, and, in turn, displace other atoms. This stage can be referred to as ballistic. This process results in the creation of an amorphous zone surrounded by relatively undistorted regions (point defects). A substantial fraction of displaced atoms returns to their original positions during a period of several picoseconds. Other atoms form a displacement cascade. Radiation damage, produced by Th recoil with energy of 20 kev in zircon, zirconolite and pyrochlore at the peak of the formation of defect region and after structure recovery of minerals shows in Figure. The results of performed MDand DFT simulations are given in Table. The following quantities are indicated in the table: the mineral and its chemical formula, the effective charge of oxygen atoms Q(O), the number of Frenkel pairs at the end of simulation N FP, and the critical temperature of amorphization T c (if T > T c, then a mineral cannot be amorphized), obtained from the ion-beam irradiation experiments with 800 kev-1.5 MeV Kr + ions [6, 14]. Figure. Radiation damage, produced by 20 kev Th recoil in zircon (a), zirconolite (b) and pyrochlore (c) at the peak of the damage (left column) and after structure relaxation (right column)
4 Table. Results of MD- and DFT simulations of studied minerals Mineral and its chemical formula Q(O), e 0 N FP T c, K Zircon ZrSiO Zirconolite CaZrTi 2 O Pyrochlore Gd 2 Zr 2 O Periclase MgO The results of computer simulations show that periclase MgO and chemical compound Gd 2 Zr 2 O 7 with the structure of pyrochlore are characterized by high bond ionicity (high value of oxygen effective charge) and high radiation stability, obtained from MD simulation and from experimental data. Zirconolite structure CaZrTi 2 O 7 shows the intermediate both bond ionicity and critical temperatures of amorphization T c. Zircon structure ZrSiO 4 is characterized by low bond ionicity (the value of oxygen effective charge is equal to e 0 ) and low radiation stability. In simple terms, the relevance of the type of interatomic forces for resistance to amorphization can be discussed as follows. After the displacement of atoms due to propagating heavy ion, the rearrangement of atoms needed to regain coherence with the crystalline lattice involves significant atomic motion. In a covalent structure, the interactions can be thought of as short-range directional constraints, due to the substantial electronic charge being localized between the neighbouring atoms. Therefore cooperative atomic motion is hooked by the electrons between neighbouring atoms, and requires breaking directional covalent bonds with associated energy cost. On the other hand, highly ionic structure can be viewed as a collection of charged ions. The cooperative rolling of spheres which are only electrostatically charged, does not require additional activation energy, giving damaged ionic structure better chances to re-establish coherence with crystalline lattice. 4 Conclusions The mechanisms of radiation-induced damages in zircon ZrSiO 4, zirconolite CaZrTi 2 O 7, pyrochlore Gd 2 Zr 2 O 7 and periclase MgO structures as a result of the alpha decay due to the recoil of the nucleus have been investigated using the molecular dynamics computer simulation. All calculations were performed in grid-environment using «GEOPARD» virtual organization. The results of researches show that the radiation stability of studied minerals caused by two main factors: type of structure and the degree of chemical bonds covalency of the structures (or effective charge of oxygen atoms). It should be noted that the type of structure and the effective charge of oxygen atoms mainly are interconnected. Highly coordinated structures (periclase, pyrochlore) are characterized by large values of both the effective charge of oxygen atoms and the radiation stability. Structures with a small coordination number of cations (zircon) are characterized by small values of both the effective charge of oxygen atoms and the radiation stability. Results of this study can be used for solving fundamental and practice tasks connected with immobilization and disposal of a high-level waste. In particular, these results can be used for the assessment of radiation stability of matrices, proposed for immobilization of the high-level waste. Our computer simulations permit to analyze and predicted matrices reliability under radiation damage. Using computer simulation methods can save timing and money budgets and promotes to choice of the appropriated matrix. 5 Acknowledgments This research was supported by Government scientific and technical program of introduction and application of gridtechniques in , project 38/13 «Application of grid technology for research of the radiation-enhanced processes, phase transformations and isomorphic substitutions in minerals in connection with the applied problems decision»
5 References [1] Nuclear power reactors in the World. Reference data series No. 2. International atomic energy agency. Vienna, P. 77 [2] Ewing R.C. Zircon: A host phase for the disposal of weapons plutonium // J. Mater. Res Vol. 10. P [3] Shpak A.P., Grechanovsky A.E., Lytovchenko A.S., Legkova G.V., Sayenko S.Yu. Influence of temperature and uranium on the radiation stability of zircon // J. Nucl. Mater V N 1-2. P [4] Grechanovsky A.E. Radiation Stability of Natural and Man-Made Mineral Matrices for Long-Term and Environmentally Safe Disposal of High Level Radioactive Waste [in Russian] Kiev: Logos, p. [5] Robinson M.T. Basic physics of radiation damage production // J. Nucl. Mater Vol. 216, N 1. P [6] Trachenko K., Pruneda J.M., Artacho E., Dove M.T. How the nature of the chemical bond governs resistance to amorphization by radiation damage // Phys. Rev. B V. 71. N 18. P [7] Yu J., Devanathan R., Weber W.J. Molecular dynamics simulation of defect production in collision cascades in zircon // J. Mater. Chem V. 19. Issue 23. P [8] Kramer G.J., Farragher N.P., van Beest B.W.H., van Santen R.A. Interatomic force fields for silicas, aluminophosphates, and zeolites: Derivation based on ab initio calculations // Phys. Rev. B V. 43. N 6. P [9] Qun-bo Fan. Molecular dynamics calculation of thermal expansion coefficient of a series of rare-earth zirconates // J. Comp. Mat. Sci Vol. 46. P [10] Veiller L. Molecular dynamics simulation of the α-recoil nucleus displacement cascade in zirconolite // J. Nucl. Mat Vol. 306, Iss. 1. P [11] Shukla P. Thermal transport properties of MgO and Nd 2 Zr 2 O 7 pyrochlore by molecular dynamics simulation // J. Nucl. Mat Vol P. 1 7 [12] Todorov I.T., Smith W. DL_POLY_3: the CCP5 national UK code for molecular-dynamics simulations // Phil. Trans. Royal Soc. A V P [13] Nemukhin A.V. Molecular modelling by using the PC GAMESS program: From diatomic molecules to enzymes // Moscow Univ. Chem. Bull Vol. 45, N 2. P [14] Meldrum A. A comparison of radiation effects in crystalline ABO 4 -type phosphates and silicates // Miner. Mag Vol. 64, N 2. P
The effect of point defects in zircon
 aterials for nuclear waste immobilization: The effect of point defects in zircon iguel Pruneda Department of Earth Sciences University of Centre for Ceramic Immobilisation Radiation damage process α-decay
aterials for nuclear waste immobilization: The effect of point defects in zircon iguel Pruneda Department of Earth Sciences University of Centre for Ceramic Immobilisation Radiation damage process α-decay
ELECTRONIC STRUCTURE OF Pu 3+ AND Pu 4+ IMPURITY CENTERS IN ZIRCON INTRODUCTION
 Journal of Structural Chemistry. Vol. 51, No. 1, pp. 1-8, 2010 Original Russian Text Copyright 2010 by M. V. Ryzhkov, A. L. Ivanovskii, A. V. Porotnikov, Yu. V. Shchapova, and S. L. Votyakov ELECTRONIC
Journal of Structural Chemistry. Vol. 51, No. 1, pp. 1-8, 2010 Original Russian Text Copyright 2010 by M. V. Ryzhkov, A. L. Ivanovskii, A. V. Porotnikov, Yu. V. Shchapova, and S. L. Votyakov ELECTRONIC
Atomistic Simulation of Nuclear Materials
 BEAR Launch 2013 24 th June 2013 Atomistic Simulation of Nuclear Materials Dr Mark S D Read School of Chemistry Nuclear Education and Research Centre www.chem.bham.ac.uk Birmingham Centre for Nuclear Education
BEAR Launch 2013 24 th June 2013 Atomistic Simulation of Nuclear Materials Dr Mark S D Read School of Chemistry Nuclear Education and Research Centre www.chem.bham.ac.uk Birmingham Centre for Nuclear Education
A COMPARATIVE STUDY OF OXYGEN VACANCY MIGRATION PATHWAYS IN CRYSTALLINE POLYMORPHS OF SILICA
 A COMPARATIVE STUDY OF OXYGEN VACANCY MIGRATION PATHWAYS IN CRYSTALLINE POLYMORPHS OF SILICA L. René Corrales Pacific Northwest National Laboratory Richland, WA 99352 Corresponding author: rene.corrales@pnl.gov
A COMPARATIVE STUDY OF OXYGEN VACANCY MIGRATION PATHWAYS IN CRYSTALLINE POLYMORPHS OF SILICA L. René Corrales Pacific Northwest National Laboratory Richland, WA 99352 Corresponding author: rene.corrales@pnl.gov
Potentials, periodicity
 Potentials, periodicity Lecture 2 1/23/18 1 Survey responses 2 Topic requests DFT (10), Molecular dynamics (7), Monte Carlo (5) Machine Learning (4), High-throughput, Databases (4) NEB, phonons, Non-equilibrium
Potentials, periodicity Lecture 2 1/23/18 1 Survey responses 2 Topic requests DFT (10), Molecular dynamics (7), Monte Carlo (5) Machine Learning (4), High-throughput, Databases (4) NEB, phonons, Non-equilibrium
STUDY ON PHASE RELATION AND SYNTHESIS OF PYROCHLORE IN THE SYSTEM OF Ca-Ce-Zr-O
 STUDY ON PHASE RELATION AND SYNTHESIS OF PYROCHLORE IN THE SYSTEM OF Ca-Ce-Zr-O S. C. Chae, Y. N. Jang, I. K. Bae Korea Institute of Geoscience and Mineral Resources 30 Gajeong-dong, Yuseong-gu, Daejeon,
STUDY ON PHASE RELATION AND SYNTHESIS OF PYROCHLORE IN THE SYSTEM OF Ca-Ce-Zr-O S. C. Chae, Y. N. Jang, I. K. Bae Korea Institute of Geoscience and Mineral Resources 30 Gajeong-dong, Yuseong-gu, Daejeon,
A STUDY OF THERMAL PROPERTIES OF PEROVSKITE CERAMIC MATERIALS VIA MOLECULAR DYNAMICS SIMULATION
 A STUDY OF THERMAL PROPERTIES OF PEROVSKITE CERAMIC MATERIALS VIA MOLECULAR DYNAMICS SIMULATION Wen Fong Goha*, Sohail Aziz Khana and Tiem Leong Yoona School of Physics, Universiti Sains Malaysia, 11800,
A STUDY OF THERMAL PROPERTIES OF PEROVSKITE CERAMIC MATERIALS VIA MOLECULAR DYNAMICS SIMULATION Wen Fong Goha*, Sohail Aziz Khana and Tiem Leong Yoona School of Physics, Universiti Sains Malaysia, 11800,
Interaction of ion beams with matter
 Interaction of ion beams with matter Introduction Nuclear and electronic energy loss Radiation damage process Displacements by nuclear stopping Defects by electronic energy loss Defect-free irradiation
Interaction of ion beams with matter Introduction Nuclear and electronic energy loss Radiation damage process Displacements by nuclear stopping Defects by electronic energy loss Defect-free irradiation
Conduction Modeling in Mixed Alkali Borate Glasses
 International Journal of Pure & Applied Physics ISSN 0973-1776 Vol.1 No.2 (2005), pp. 191-197 Research India Publications http://www.ripub lication.com/ijpap.htm Conduction Modeling in Mixed Alkali Borate
International Journal of Pure & Applied Physics ISSN 0973-1776 Vol.1 No.2 (2005), pp. 191-197 Research India Publications http://www.ripub lication.com/ijpap.htm Conduction Modeling in Mixed Alkali Borate
Chapter V: Interactions of neutrons with matter
 Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Binding Energy and Mass defect
 Binding Energy and Mass defect Particle Relative Electric Charge Relative Mass Mass (kg) Charge (C) (u) Electron -1-1.60 x 10-19 5.485779 x 10-4 9.109390 x 10-31 Proton +1 +1.60 x 10-19 1.007276 1.672623
Binding Energy and Mass defect Particle Relative Electric Charge Relative Mass Mass (kg) Charge (C) (u) Electron -1-1.60 x 10-19 5.485779 x 10-4 9.109390 x 10-31 Proton +1 +1.60 x 10-19 1.007276 1.672623
Jie Lian Department of Nuclear Engineering and Radiological Sciences, University of Michigan, Ann Arbor, Michigan
 JOURNAL OF APPLIED PHYSICS VOLUME 95, NUMBER 11 1 JUNE 2004 APPLIED PHYSICS REVIEWS FOCUSED REVIEW Nuclear waste disposal pyrochlore A 2 B 2 O 7 : Nuclear waste form for the immobilization of plutonium
JOURNAL OF APPLIED PHYSICS VOLUME 95, NUMBER 11 1 JUNE 2004 APPLIED PHYSICS REVIEWS FOCUSED REVIEW Nuclear waste disposal pyrochlore A 2 B 2 O 7 : Nuclear waste form for the immobilization of plutonium
The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals.
 Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
Radiation Damage Modeling of Fused Silica in Fusion Systems
 1 Radiation Damage Modeling of Fused Silica in Fusion Systems F. Mota 1), M.J. Caturla 2), J.M. Perlado 1), A. Ibarra 3), M. León 3), J.Mollá 3) 1) Instituto de Fusion Nuclear (DENIM) / ETSII / Universidad
1 Radiation Damage Modeling of Fused Silica in Fusion Systems F. Mota 1), M.J. Caturla 2), J.M. Perlado 1), A. Ibarra 3), M. León 3), J.Mollá 3) 1) Instituto de Fusion Nuclear (DENIM) / ETSII / Universidad
Defects in TiO 2 Crystals
 , March 13-15, 2013, Hong Kong Defects in TiO 2 Crystals Richard Rivera, Arvids Stashans 1 Abstract-TiO 2 crystals, anatase and rutile, have been studied using Density Functional Theory (DFT) and the Generalized
, March 13-15, 2013, Hong Kong Defects in TiO 2 Crystals Richard Rivera, Arvids Stashans 1 Abstract-TiO 2 crystals, anatase and rutile, have been studied using Density Functional Theory (DFT) and the Generalized
Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions
 CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atoms, Molecules and Solids (selected topics)
 Atoms, Molecules and Solids (selected topics) Part I: Electronic configurations and transitions Transitions between atomic states (Hydrogen atom) Transition probabilities are different depending on the
Atoms, Molecules and Solids (selected topics) Part I: Electronic configurations and transitions Transitions between atomic states (Hydrogen atom) Transition probabilities are different depending on the
Chemical bonding in solids from ab-initio Calculations
 Chemical bonding in solids from ab-initio Calculations 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India & Center for Materials Science and Nanotechnology, University
Chemical bonding in solids from ab-initio Calculations 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India & Center for Materials Science and Nanotechnology, University
Classification of Solids, Fermi Level and Conductivity in Metals Dr. Anurag Srivastava
 Classification of Solids, Fermi Level and Conductivity in Metals Dr. Anurag Srivastava Web address: http://tiiciiitm.com/profanurag Email: profanurag@gmail.com Visit me: Room-110, Block-E, IIITM Campus
Classification of Solids, Fermi Level and Conductivity in Metals Dr. Anurag Srivastava Web address: http://tiiciiitm.com/profanurag Email: profanurag@gmail.com Visit me: Room-110, Block-E, IIITM Campus
ATOMIC BONDING Atomic Bonding
 ATOMIC BONDING Atomic Bonding Primary Bonds Secondary Bonds Ionic Covalent Metallic van der Waals 1. IONIC BONDING q 11 Na & 17 Cl These two ions are attracted to eachother by the electrostatic force developed
ATOMIC BONDING Atomic Bonding Primary Bonds Secondary Bonds Ionic Covalent Metallic van der Waals 1. IONIC BONDING q 11 Na & 17 Cl These two ions are attracted to eachother by the electrostatic force developed
Hands-on : Model Potential Molecular Dynamics
 Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Molecular dynamics modelling of radiation damage in normal, partly inverse and inverse spinels
 Loughborough University Institutional Repository Molecular dynamics modelling of radiation damage in normal, partly inverse and inverse spinels This item was submitted to Loughborough University's Institutional
Loughborough University Institutional Repository Molecular dynamics modelling of radiation damage in normal, partly inverse and inverse spinels This item was submitted to Loughborough University's Institutional
How the nature of the chemical bond governs resistance to amorphization by radiation damage
 How the nature of the chemical bond governs resistance to amorphization by radiation damage Kostya Trachenko, 1 J. M. Pruneda, 1,2 Emilio Artacho, 1 and Martin T. Dove 1 1 Department of Earth Sciences,
How the nature of the chemical bond governs resistance to amorphization by radiation damage Kostya Trachenko, 1 J. M. Pruneda, 1,2 Emilio Artacho, 1 and Martin T. Dove 1 1 Department of Earth Sciences,
Fission Enhanced diffusion of uranium in zirconia
 Fission Enhanced diffusion of uranium in zirconia N. Bérerd, A. Chevarier, N. Moncoffre, Institut de Physique Nucléaire de Lyon, 4, rue Enrico Fermi, 69622 Villeurbanne Cedex, France, Ph. Sainsot, Institut
Fission Enhanced diffusion of uranium in zirconia N. Bérerd, A. Chevarier, N. Moncoffre, Institut de Physique Nucléaire de Lyon, 4, rue Enrico Fermi, 69622 Villeurbanne Cedex, France, Ph. Sainsot, Institut
Control of the fission chain reaction
 Control of the fission chain reaction Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 April 8, 2011 NUCS 342 (Lecture 30) April 8, 2011 1 / 29 Outline 1 Fission chain reaction
Control of the fission chain reaction Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 April 8, 2011 NUCS 342 (Lecture 30) April 8, 2011 1 / 29 Outline 1 Fission chain reaction
Chapter 10: Liquids and Solids
 Chapter 10: Liquids and Solids Chapter 10: Liquids and Solids *Liquids and solids show many similarities and are strikingly different from their gaseous state. 10.1 Intermolecular Forces Intermolecular
Chapter 10: Liquids and Solids Chapter 10: Liquids and Solids *Liquids and solids show many similarities and are strikingly different from their gaseous state. 10.1 Intermolecular Forces Intermolecular
Chapter 3. Crystal Binding
 Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Chapter 3. The structure of crystalline solids 3.1. Crystal structures
 Chapter 3. The structure of crystalline solids 3.1. Crystal structures 3.1.1. Fundamental concepts 3.1.2. Unit cells 3.1.3. Metallic crystal structures 3.1.4. Ceramic crystal structures 3.1.5. Silicate
Chapter 3. The structure of crystalline solids 3.1. Crystal structures 3.1.1. Fundamental concepts 3.1.2. Unit cells 3.1.3. Metallic crystal structures 3.1.4. Ceramic crystal structures 3.1.5. Silicate
Physics Important Terms and their Definitions
 Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
 lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
Chapter 10. Answers to examination-style questions. Answers Marks Examiner s tips. 1 (a) (i) 238. (ii) β particle(s) 1 Electron antineutrinos 1
 (a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
(a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
MockTime.com. Ans: (b) Q6. Curie is a unit of [1989] (a) energy of gamma-rays (b) half-life (c) radioactivity (d) intensity of gamma-rays Ans: (c)
![MockTime.com. Ans: (b) Q6. Curie is a unit of [1989] (a) energy of gamma-rays (b) half-life (c) radioactivity (d) intensity of gamma-rays Ans: (c) MockTime.com. Ans: (b) Q6. Curie is a unit of [1989] (a) energy of gamma-rays (b) half-life (c) radioactivity (d) intensity of gamma-rays Ans: (c)](/thumbs/95/125098222.jpg) Chapter Nuclei Q1. A radioactive sample with a half life of 1 month has the label: Activity = 2 micro curies on 1 8 1991. What would be its activity two months earlier? [1988] 1.0 micro curie 0.5 micro
Chapter Nuclei Q1. A radioactive sample with a half life of 1 month has the label: Activity = 2 micro curies on 1 8 1991. What would be its activity two months earlier? [1988] 1.0 micro curie 0.5 micro
Structure and Dynamics : An Atomic View of Materials
 Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
Emphasis on what happens to emitted particle (if no nuclear reaction and MEDIUM (i.e., atomic effects)
 LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
A new method to acquire nuclear fission data using heavy ion reactions a way to understand the fission phenomenon
 press release date Friday 26 August 15:00 (material distribution) Education, Culture, Sports, Science Press conf., Nuclear Regulatory Agency Press conf., Ibaraki Pref.. Government press conf., Osaka Science
press release date Friday 26 August 15:00 (material distribution) Education, Culture, Sports, Science Press conf., Nuclear Regulatory Agency Press conf., Ibaraki Pref.. Government press conf., Osaka Science
Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science Syllabus and correlation/alignment with Standards
 Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science www.synapse.co.uk/alchemy Syllabus and correlation/alignment with Standards Here you will find 1. a simple syllabus of the course
Principles of Alchemy (Chemistry) by Dr Jamie Love from Merlin Science www.synapse.co.uk/alchemy Syllabus and correlation/alignment with Standards Here you will find 1. a simple syllabus of the course
REVIEW : INTRODUCTION TO THE MOLECULAR ORIGINS OF MECHANICAL PROPERTIES QUANTITATIVE TREATMENT OF INTERATOMIC BONDING : THE LENNARD-JONES POTENTIAL
 LECTURE #19 : 3.11 MECANICS OF MATERIALS F3 INSTRUCTOR : Professor Christine Ortiz OFFICE : 13-422 PONE : 452-384 WWW : http://web.mit.edu/cortiz/www REVIEW : INTRODUCTION TO TE MOLECULAR ORIGINS OF MECANICAL
LECTURE #19 : 3.11 MECANICS OF MATERIALS F3 INSTRUCTOR : Professor Christine Ortiz OFFICE : 13-422 PONE : 452-384 WWW : http://web.mit.edu/cortiz/www REVIEW : INTRODUCTION TO TE MOLECULAR ORIGINS OF MECANICAL
California Science Content Standards Chemistry Grades 9-12
 California Science Content Standards Chemistry Grades 9-12 Standards that all students are expected to achieve in the course of their studies are unmarked. Standards that all students should have the opportunity
California Science Content Standards Chemistry Grades 9-12 Standards that all students are expected to achieve in the course of their studies are unmarked. Standards that all students should have the opportunity
Atomic structure & interatomic bonding. Chapter two
 Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Radioactivity - Radionuclides - Radiation
 Content of the lecture Introduction Particle/ion-atom atom interactions - basic processes on on energy loss - stopping power, range Implementation in in Nucleonica TM TM Examples Origin and use of particles
Content of the lecture Introduction Particle/ion-atom atom interactions - basic processes on on energy loss - stopping power, range Implementation in in Nucleonica TM TM Examples Origin and use of particles
Radioactivity is the spontaneous disintegration of nuclei. The first radioactive. elements discovered were the heavy atoms thorium and uranium.
 Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Write down the nuclear equation that represents the decay of neptunium 239 into plutonium 239.
 Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
Chemistry Unit Overview and Pacing Guide
 Chemistry Unit Overview and Pacing Guide This document provides teachers with an overview of each unit in the Chemistry/Chemistry Honors curriculum. The Curriculum Engine provides additional information
Chemistry Unit Overview and Pacing Guide This document provides teachers with an overview of each unit in the Chemistry/Chemistry Honors curriculum. The Curriculum Engine provides additional information
2 Energy from the Nucleus
 CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
Supplementary Information for. Universal elastic-hardening-driven mechanical instability in α-quartz and quartz. homeotypes under pressure
 Supplementary Information for Universal elastic-hardening-driven mechanical instability in α-quartz and quartz homeotypes under pressure Juncai Dong, Hailiang Zhu, and Dongliang Chen * Beijing Synchrotron
Supplementary Information for Universal elastic-hardening-driven mechanical instability in α-quartz and quartz homeotypes under pressure Juncai Dong, Hailiang Zhu, and Dongliang Chen * Beijing Synchrotron
Swelling Mechanism of Lattice with the Ingrowth of the. Defects in UO 2
 Swelling Mechanism of Lattice with the Ingrowth of the Defects in UO Seçkin D. Günay * Yıldız Technical University, Department of Physics, Faculty of Science, Esenler, 3410, Istanbul, Turkey Swelling of
Swelling Mechanism of Lattice with the Ingrowth of the Defects in UO Seçkin D. Günay * Yıldız Technical University, Department of Physics, Faculty of Science, Esenler, 3410, Istanbul, Turkey Swelling of
Multiscale modelling of D trapping in W
 CMS Multiscale modelling of D trapping in W Kalle Heinola, Tommy Ahlgren and Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki, Finland Contents Background Plasma-wall
CMS Multiscale modelling of D trapping in W Kalle Heinola, Tommy Ahlgren and Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki, Finland Contents Background Plasma-wall
Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2
 Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2 D.G. Lee, G.H. Jeong, W.Z. Oh, K.W. Lee Korea Atomic Energy Research Institute Korea ABSTRACT Irradiated
Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2 D.G. Lee, G.H. Jeong, W.Z. Oh, K.W. Lee Korea Atomic Energy Research Institute Korea ABSTRACT Irradiated
Computer Simulation of Shock Waves in Condensed Matter. Matthew R. Farrow 2 November 2007
 Computer Simulation of Shock Waves in Condensed Matter Matthew R. Farrow 2 November 2007 Outline of talk Shock wave theory Results Conclusion Computer simulation of shock waves Shock Wave Theory Shock
Computer Simulation of Shock Waves in Condensed Matter Matthew R. Farrow 2 November 2007 Outline of talk Shock wave theory Results Conclusion Computer simulation of shock waves Shock Wave Theory Shock
A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane
 A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane Supplementary Information: Simulation Procedure and Physical Property Analysis Simulation Procedure The molecular
A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane Supplementary Information: Simulation Procedure and Physical Property Analysis Simulation Procedure The molecular
RADIATION EFFECTS AND DAMAGE
 RADIATION EFFECTS AND DAMAGE The detrimental consequences of radiation are referred to as radiation damage. To understand the effects of radiation, one must first be familiar with the radiations and their
RADIATION EFFECTS AND DAMAGE The detrimental consequences of radiation are referred to as radiation damage. To understand the effects of radiation, one must first be familiar with the radiations and their
How materials work. Compression Tension Bending Torsion
 Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
Johns Hopkins University What is Engineering? M. Karweit MATERIALS
 Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
6. Computational Design of Energy-related Materials
 6. Computational Design of Energy-related Materials Contents 6.1 Atomistic Simulation Methods for Energy Materials 6.2 ab initio design of photovoltaic materials 6.3 Solid Ion Conductors for Fuel Cells
6. Computational Design of Energy-related Materials Contents 6.1 Atomistic Simulation Methods for Energy Materials 6.2 ab initio design of photovoltaic materials 6.3 Solid Ion Conductors for Fuel Cells
Ionic Bonding - Electrostatic Interactions and Polarization
 Ionic Bonding - Electrostatic Interactions and Polarization Chemistry 754 Solid State Chemistry Dr. Patrick Woodward Lecture #13 Born-Haber Cycle for NaCl It is energetically unfavorable for Na metal and
Ionic Bonding - Electrostatic Interactions and Polarization Chemistry 754 Solid State Chemistry Dr. Patrick Woodward Lecture #13 Born-Haber Cycle for NaCl It is energetically unfavorable for Na metal and
1. Introduction to Clusters
 1. Introduction to Clusters 1.1 The Field of Clusters Atomic clusters are aggregates of atoms containing from few to a few thousand atoms. Due to their small size, the properties of the clusters are, in
1. Introduction to Clusters 1.1 The Field of Clusters Atomic clusters are aggregates of atoms containing from few to a few thousand atoms. Due to their small size, the properties of the clusters are, in
Earth Solid Earth Rocks Minerals Atoms. How to make a mineral from the start of atoms?
 Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
Lattice energy of ionic solids
 1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
Structure-Property Correlation [2] Atomic bonding and material properties
![Structure-Property Correlation [2] Atomic bonding and material properties Structure-Property Correlation [2] Atomic bonding and material properties](/thumbs/84/89142752.jpg) MME 297: Lecture 05 Structure-Property Correlation [2] Atomic bonding and material properties Dr. A. K. M. Bazlur Rashid Professor, Department of MME BUET, Dhaka Topics to discuss today... Review of atomic
MME 297: Lecture 05 Structure-Property Correlation [2] Atomic bonding and material properties Dr. A. K. M. Bazlur Rashid Professor, Department of MME BUET, Dhaka Topics to discuss today... Review of atomic
A COMPUTATIONAL INVESTIGATION OF MIGRATION ENTHALPIES AND ELECTRONIC STRUCTURE IN SrFeO 3-δ
 A COMPUTATIONAL INVESTIGATION OF MIGRATION ENTHALPIES AND ELECTRONIC STRUCTURE IN SrFeO 3-δ A. Predith and G. Ceder Massachusetts Institute of Technology Department of Materials Science and Engineering
A COMPUTATIONAL INVESTIGATION OF MIGRATION ENTHALPIES AND ELECTRONIC STRUCTURE IN SrFeO 3-δ A. Predith and G. Ceder Massachusetts Institute of Technology Department of Materials Science and Engineering
Chemistry-Integrated Year-at-a-Glance ARKANSAS STATE SCIENCE STANDARDS
 Chemistry-Integrated Year-at-a-Glance ARKANSAS STATE SCIENCE STANDARDS FIRST SEMESTER FIRST/SECOND SECOND SEMESTER Unit 1 Motion and Matter Unit 2 Atomic Trends and Behavior Unit 3 Chemical Reactions Unit
Chemistry-Integrated Year-at-a-Glance ARKANSAS STATE SCIENCE STANDARDS FIRST SEMESTER FIRST/SECOND SECOND SEMESTER Unit 1 Motion and Matter Unit 2 Atomic Trends and Behavior Unit 3 Chemical Reactions Unit
Ciclo combustibile, scorie, accelerator driven system
 Ciclo combustibile, scorie, accelerator driven system M. Carta, C. Artioli ENEA Fusione e Fissione Nucleare: stato e prospettive sulle fonti energetiche nucleari per il futuro Layout of the presentation!
Ciclo combustibile, scorie, accelerator driven system M. Carta, C. Artioli ENEA Fusione e Fissione Nucleare: stato e prospettive sulle fonti energetiche nucleari per il futuro Layout of the presentation!
O 3. : Er nanoparticles prospective system for energy convertors
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Interband optical transitions in Gd 2 O 3 : Er nanoparticles prospective system for energy convertors To cite this article: A
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Interband optical transitions in Gd 2 O 3 : Er nanoparticles prospective system for energy convertors To cite this article: A
Lecture 6 - Bonding in Crystals
 Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
SOURCES of RADIOACTIVITY
 Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Atomic and Nuclear Physics. Topic 7.3 Nuclear Reactions
 Atomic and Nuclear Physics Topic 7.3 Nuclear Reactions Nuclear Reactions Rutherford conducted experiments bombarding nitrogen gas with alpha particles from bismuth-214. He discovered that fast-moving particles
Atomic and Nuclear Physics Topic 7.3 Nuclear Reactions Nuclear Reactions Rutherford conducted experiments bombarding nitrogen gas with alpha particles from bismuth-214. He discovered that fast-moving particles
Some nuclei are unstable Become stable by ejecting excess energy and often a particle in the process Types of radiation particle - particle
 Radioactivity George Starkschall, Ph.D. Lecture Objectives Identify methods for making radioactive isotopes Recognize the various types of radioactive decay Interpret an energy level diagram for radioactive
Radioactivity George Starkschall, Ph.D. Lecture Objectives Identify methods for making radioactive isotopes Recognize the various types of radioactive decay Interpret an energy level diagram for radioactive
Structures of Solids. Unit Cells - Not(?) Chapter 4 Ionic and Other Inorganic Solids. CHEM 462 Wednesday, September 22 T.
 Chapter 4 Ionic and Other Inorganic Solids CHEM 462 Wednesday, September 22 T. Hughbanks Structures of Solids Many dense solids are described in terms of packing of atoms or ions. Although these geometric
Chapter 4 Ionic and Other Inorganic Solids CHEM 462 Wednesday, September 22 T. Hughbanks Structures of Solids Many dense solids are described in terms of packing of atoms or ions. Although these geometric
An introduction to Molecular Dynamics. EMBO, June 2016
 An introduction to Molecular Dynamics EMBO, June 2016 What is MD? everything that living things do can be understood in terms of the jiggling and wiggling of atoms. The Feynman Lectures in Physics vol.
An introduction to Molecular Dynamics EMBO, June 2016 What is MD? everything that living things do can be understood in terms of the jiggling and wiggling of atoms. The Feynman Lectures in Physics vol.
MATERIALS. Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle?
 MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
RDCH 702 Lecture 8: Accelerators and Isotope Production
 RDCH 702 Lecture 8: Accelerators and Isotope Production Particle generation Accelerator Direct Voltage Linear Cyclotrons Synchrotrons Photons * XAFS * Photonuclear Heavy Ions Neutrons sources Fission products
RDCH 702 Lecture 8: Accelerators and Isotope Production Particle generation Accelerator Direct Voltage Linear Cyclotrons Synchrotrons Photons * XAFS * Photonuclear Heavy Ions Neutrons sources Fission products
Students are required to bring these definitions HAND written on separate 3 in X 5 in index cards by chapters, the first week of school
 Students are required to bring these definitions HAND written on separate 3 in X 5 in index cards by chapters, the first week of school 2015-2016 Have a Great Summer!!! Ms. Charles LAB SAFETY/Vocabulary
Students are required to bring these definitions HAND written on separate 3 in X 5 in index cards by chapters, the first week of school 2015-2016 Have a Great Summer!!! Ms. Charles LAB SAFETY/Vocabulary
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
Nuclear Chemistry Unit
 Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Isotopes. An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons.
 Nuclear Chemistry Isotopes An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Nuclear Chemistry Isotopes An isotope is an atom of the same element (same number of protons) that varies in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Isotopes. An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons.
 Nuclear Chemistry Isotopes An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Nuclear Chemistry Isotopes An isotope is an atoms of the same element (same number of protons) that vary in the number of neutrons. Most elements have several isotopes Some are unstable and emit radiation
Structure of Cement Phases from ab initio Modeling Crystalline C-S-HC
 Structure of Cement Phases from ab initio Modeling Crystalline C-S-HC Sergey V. Churakov sergey.churakov@psi.ch Paul Scherrer Institute Switzerland Cement Phase Composition C-S-H H Solid Solution Model
Structure of Cement Phases from ab initio Modeling Crystalline C-S-HC Sergey V. Churakov sergey.churakov@psi.ch Paul Scherrer Institute Switzerland Cement Phase Composition C-S-H H Solid Solution Model
Today, I will present the first of two lectures on neutron interactions.
 Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
FACTS WHY? C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei
 C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
Physics 107 Final Exam May 6, Your Name: 1. Questions
 Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
Modern Physics Departmental Exam Last updated November 2013
 Modern Physics Departmental Exam Last updated November 213 87 1. Recently, 2 rubidium atoms ( 37 Rb ), which had been compressed to a density of 113 atoms/cm 3, were observed to undergo a Bose-Einstein
Modern Physics Departmental Exam Last updated November 213 87 1. Recently, 2 rubidium atoms ( 37 Rb ), which had been compressed to a density of 113 atoms/cm 3, were observed to undergo a Bose-Einstein
Chemical bonds. In some minerals, other (less important) bond types include:
 Chemical bonds Chemical bond: force of attraction between two or more atoms/ions Types of bonds in crystals: Ionic bond: electrostatic attraction between two oppositely charged ions. This type of bond
Chemical bonds Chemical bond: force of attraction between two or more atoms/ions Types of bonds in crystals: Ionic bond: electrostatic attraction between two oppositely charged ions. This type of bond
Preview. Subatomic Physics Section 1. Section 1 The Nucleus. Section 2 Nuclear Decay. Section 3 Nuclear Reactions. Section 4 Particle Physics
 Subatomic Physics Section 1 Preview Section 1 The Nucleus Section 2 Nuclear Decay Section 3 Nuclear Reactions Section 4 Particle Physics Subatomic Physics Section 1 TEKS The student is expected to: 5A
Subatomic Physics Section 1 Preview Section 1 The Nucleus Section 2 Nuclear Decay Section 3 Nuclear Reactions Section 4 Particle Physics Subatomic Physics Section 1 TEKS The student is expected to: 5A
CHAPTER 2 INTERATOMIC FORCES. atoms together in a solid?
 CHAPTER 2 INTERATOMIC FORCES What kind of force holds the atoms together in a solid? Interatomic Binding All of the mechanisms which cause bonding between the atoms derive from electrostatic interaction
CHAPTER 2 INTERATOMIC FORCES What kind of force holds the atoms together in a solid? Interatomic Binding All of the mechanisms which cause bonding between the atoms derive from electrostatic interaction
Physics of Materials: Classification of Solids On The basis of Geometry and Bonding (Intermolecular forces)
 Physics of Materials: Classification of Solids On The basis of Geometry and Bonding (Intermolecular forces) Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement,
Physics of Materials: Classification of Solids On The basis of Geometry and Bonding (Intermolecular forces) Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement,
SnO 2 Physical and Chemical Properties due to the Impurity Doping
 , March 13-15, 2013, Hong Kong SnO 2 Physical and Chemical Properties due to the Impurity Doping Richard Rivera, Freddy Marcillo, Washington Chamba, Patricio Puchaicela, Arvids Stashans Abstract First-principles
, March 13-15, 2013, Hong Kong SnO 2 Physical and Chemical Properties due to the Impurity Doping Richard Rivera, Freddy Marcillo, Washington Chamba, Patricio Puchaicela, Arvids Stashans Abstract First-principles
Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model
 1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
The effect of the enhanced. primordial abundance of 7Li. Chapter 2. 8Li (a, n) 11 B reaction rate on
 Chapter 2 The effect of the enhanced 8Li (a, n) 11 B reaction rate on primordial abundance of 7Li One of the essential inputs of the primordial nucleosynthesis calculation is the reaction rates of the
Chapter 2 The effect of the enhanced 8Li (a, n) 11 B reaction rate on primordial abundance of 7Li One of the essential inputs of the primordial nucleosynthesis calculation is the reaction rates of the
Hans-Herbert Fischer and Klaus Thiel
 A simulation tool for the calculation of NIEL in arbitrary materials using GEANT4 - Some new results - Hans-Herbert Fischer and Klaus Thiel Nuclear Chemistry Dept. University of Köln, FRG ESA GSP 2005
A simulation tool for the calculation of NIEL in arbitrary materials using GEANT4 - Some new results - Hans-Herbert Fischer and Klaus Thiel Nuclear Chemistry Dept. University of Köln, FRG ESA GSP 2005
S1. X-ray photoelectron spectroscopy (XPS) survey spectrum of
 Site-selective local fluorination of graphene induced by focused ion beam irradiation Hu Li 1, Lakshya Daukiya 2, Soumyajyoti Haldar 3, Andreas Lindblad 4, Biplab Sanyal 3, Olle Eriksson 3, Dominique Aubel
Site-selective local fluorination of graphene induced by focused ion beam irradiation Hu Li 1, Lakshya Daukiya 2, Soumyajyoti Haldar 3, Andreas Lindblad 4, Biplab Sanyal 3, Olle Eriksson 3, Dominique Aubel
Performance of MAX phase Ti 3 SiC 2 under the irradiation of He/H :
 Performance of MAX phase Ti 3 SiC 2 under the irradiation of He/H : Elaboration from DFT Yuexia Wang Institute of Modern Physics Fudan University Hefei-2016 Materials Issues Neutron flux (14MeV, 0.5-0.8
Performance of MAX phase Ti 3 SiC 2 under the irradiation of He/H : Elaboration from DFT Yuexia Wang Institute of Modern Physics Fudan University Hefei-2016 Materials Issues Neutron flux (14MeV, 0.5-0.8
sustainable nuclear energy
 Marcoule in service of > Atalante sustainable nuclear energy ATALANTE at the heart of international nuclear research Global energy demand will more than double in the next 40 years. Competitive, with minimal
Marcoule in service of > Atalante sustainable nuclear energy ATALANTE at the heart of international nuclear research Global energy demand will more than double in the next 40 years. Competitive, with minimal
Lecture 8 Chemical/Electronic Structure of Glass
 Lecture 8 Chemical/Electronic Structure of Glass Syllabus Topic 6. Electronic spectroscopy studies of glass structure Fundamentals and Applications of X-ray Photoelectron Spectroscopy (XPS) a.k.a. Electron
Lecture 8 Chemical/Electronic Structure of Glass Syllabus Topic 6. Electronic spectroscopy studies of glass structure Fundamentals and Applications of X-ray Photoelectron Spectroscopy (XPS) a.k.a. Electron
Set the initial conditions r i. Update neighborlist. Get new forces F i
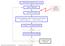 Set the initial conditions r i ( t 0 ), v i ( t 0 ) Update neighborlist Potential models ionic compounds Get new forces F i ( r i ) Solve the equations of motion numerically over time step Δt : r i ( t
Set the initial conditions r i ( t 0 ), v i ( t 0 ) Update neighborlist Potential models ionic compounds Get new forces F i ( r i ) Solve the equations of motion numerically over time step Δt : r i ( t
The number of protons in the nucleus is known as the atomic number Z, and determines the chemical properties of the element.
 I. NUCLEAR PHYSICS I.1 Atomic Nucleus Very briefly, an atom is formed by a nucleus made up of nucleons (neutrons and protons) and electrons in external orbits. The number of electrons and protons is equal
I. NUCLEAR PHYSICS I.1 Atomic Nucleus Very briefly, an atom is formed by a nucleus made up of nucleons (neutrons and protons) and electrons in external orbits. The number of electrons and protons is equal
AP Chemistry Chapter 7: Bonding
 AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
Chemistry. Atomic and Molecular Structure
 Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
Chapter 12: Structures & Properties of Ceramics
 Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Structures of ceramic materials: How do they differ from those of metals? Point defects: How are they different from those in metals?
Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Structures of ceramic materials: How do they differ from those of metals? Point defects: How are they different from those in metals?
Requirements for Prospective Teachers General Science 11.1g Distinguish between physical and chemical change and provide examples of each
 General Chemistry 001 A B C - Syllabus Addendum for Prospective Teachers Silberberg, M. S. (2006). Chemistry: The molecular nature of matter and change Fourth Edition Chapter Ch 1-Keys to the study of
General Chemistry 001 A B C - Syllabus Addendum for Prospective Teachers Silberberg, M. S. (2006). Chemistry: The molecular nature of matter and change Fourth Edition Chapter Ch 1-Keys to the study of
