Chemistry 14C Winter 2017 Final Exam Part A Page 1
|
|
|
- Matilda West
- 5 years ago
- Views:
Transcription
1 Chemistry 14C Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. The active site of an enzyme is a cleft or hole in the enzyme's structure where the enzyme's chemical reactions take place. The active site is lines with amino acid side chains. Imagine an active site containing these standard amino acid side chains. ote the one-letter abbreviations (C, T, S, and L) that will be used for questions 1 13 on this exam. C 2 S C 2 C 2 C 2 C 2 C 2 2 Cysteine (C) Tyrosine (T) Serine (S) Lysine (L) 1. (2) Complete this statement by adding no more than ten words: A standard amino acid is an amino acid that... C 2 S 2. (2) Complete this drawing of cysteine: 3. (2) Complete this statement by adding no more than ten words: Cysteine is unique, or one of only two among the twenty standard amino acids because cysteine (6) Answer each question by writing one amino acid letter (C, T, S, or L) in each answer blank. In case of a tie write two or more letters. If none of the amino acid choices are correct, write '' (for none). (a) Which amino acid has the most signals in its C-MR spectrum? (b) Which amino acid has the most deshielded carbon atom in its C-MR spectrum? (c) Which amino acid fits the following C-MR spectrum: 175 ppm (singlet), 55 ppm (doublet), 40 ppm (triplet), 31 ppm (triplet), 27 ppm (triplet), and 22 ppm (triplet). Answer: 5. (5) For each part of this question write 'Y' (for yes) in the blank if the listed property can be proven (measured directly) by x-ray crystallography, or '' (for no) if it cannot be proven or measured directly. (a) Cysteine's S bond length: (b) Whether or not cysteine can form a hydrogen bond (c) Cysteine's S C bond angle: (d) If tyrosine is R or S: (e) If tyrosine has resonance: Page 1 score =
2 Chemistry 14C Winter 2017 Final Exam Part A Page 2 It was found that the enzyme mentioned on page 1 binds to phenol using noncovalent attractive forces. Phenol 6. (5) For each amino acid listed below, write all of the noncovalent attractive forces that might occur between that amino acid's side chain and phenol. If no noncovalent attractive forces can occur write 'none'. (a) Cysteine: (b) Tyrosine: 7. (6) For each statement below write one amino acid side chain letter (C, T, S, or L) in each blank, or '' of the answer is none. If you write '' you are done with that part of the question. If you write an amino acid letter complete the statement by adding no more than fifteen words of explanation. The explanations must be different for each part. Be precise and specific. If more than one amino acid applies, it does not matter which one you pick. (a) Amino acid cannot be a hydrogen bond donor because this amino acid... (b) Amino acid cannot be a hydrogen bond acceptor because this amino acid (4) Rank the water solubility of the four amino acids by writing one amino acid letter (C, T, S, or L) in each blank. Most soluble in water: Least soluble in water: The chemical reaction that occurs on the bound phenol might involve protonation by an amino acid side chain so predicting the relative acidity of these side chains can provide useful information. 9. (6) We learned in lecture of the various aspects of molecular structure that control acidity. For each structural factor listed below, write the letter (C, T, S, or L) of one amino acid side chain whose acidity is most enhanced or most increased by this factor. If more than one amino acid's acidity is enhanced, write just one letter. Resonance: Atomic radius: Electronegativity: 10. (4) Write the names of two structural features not mentioned in question 9 that might also enhance the acidity of a any molecule (not necessarily just the four amino acids mentioned on the first page). 11. (4) ow rank the acidity of the four enzyme amino acid side chains by writing one amino acid letter (C, T, S, or L) in each blank. In case of a tie write just one letter in each blank. Most acidic: Least acidic: 12. (4) The pk a of water is In the answer blank write the letter(s) (C, T, S, or L) of all amino acid side chain(s) whose pk a is distinctly less than the pk a of water: Page 2 score =
3 Chemistry 14C Winter 2017 Final Exam Part A Page (6) Write one or more amino acid side chain letter(s) (C, T, S, or L) in each blank. as a hydrophobic side chain: as a basic side chain: Fentanyl is an opioid analgesic (pain reliever) but often results in addiction. It is believed that changing the way the opioid binds to the receptor may reduce or eliminate its potential for addiction. The extent to which the opioid is protonated (i.e., its basicity) plays a role in binding. With this in mind, fentanyl analog FEPP was tested, and found that it binds less strongly (and therefore has less potential for abuse). Recall that Ph = phenyl = a benzene ring with one substituent (C 6 5 X). F F PhC 2 C 2 PhC 2 C 2 PhC 2 C 2 Fentanyl (F) FEPP () Protonated FEPP 14. (2) In the blank write the letter (F or ) of the molecule that is the weakest base: 15. (4) Binding to the opioid receptor occurs via various noncovalent interactions. Write the name(s) of all noncovalent molecular forces that might allow protonated FEPP to bind to the receptor, but are absent in FEPP. Lorenzo's oil has been used to treat adrenoleukodystrophy, a genetic disease. The oil gained widespread publicity due to the 1992 film Lorenzo's il. The oil consists of about 20% of the lipid shown below. C 3 (C 2 ) 7 C=C(C 2 ) 11 (C 2 ) 11 C=C(C 2 ) 7 C 3 (C 2 ) 11 C=C(C 2 ) 7 C 3 Lorenzo's oil lipid 16. (2) By writing no more than twenty words, provide a precise yet concise definition of lipid. ote this definition has two essential parts. 17. (2) In lecture we learned of seven general lipid categories. To which of these categories does the Lorenzo's oil lipid belong? 18. (6) Complete each statement below by writing the name of one of the seven general lipid categories in the blank, and then add no more than ten words of explanation. (a) The Lorenzo's oil lipid is not an example of a because the Lorenzo's oil lipid... (b) The Lorenzo's oil lipid is not an example of a because the Lorenzo's oil lipid... Page 3 score =
4 Chemistry 14C Winter 2017 Final Exam Part A Page (2) Complete this statement by writing C (for cis), T (for trans), or X (for cannot determine) in the blank. The alkenes present in the Lorenzo's oil lipid are most likely. 20. (2) Another lipid component of Lorenzo's oil contains 57 carbons. Write in the blank the letter of least likely number of oxygen atoms in the structure:. Answer choices: (a) zero oxygens; (b) six oxygens; (c) 57 oxygens. 21. (8) For each set of molecules, write the number of the molecule with the highest boiling point in the answer box. Choice 1 Choice 2 Choice 3 Choice 4 Answer Box (a) LiF ClF C 3 F F 2 (b) F 2 FCl FBr FI (c) C 4 C 3 C 3 C 2 C 2 C 2 (d) F 2 Cl 2 Br 2 I (8) For each set of molecules, write the number of the most acidic molecule in the answer box. Choice 1 Choice 2 Choice 3 Choice 4 Answer Box (a) C 3 (b) F Cl Br I (c) F Cl Br 2 (d) 2 F 3 C 4 Page 4 score =
5 Chemistry 14C Winter 2017 Final Exam Part A Page (2) In the answer blank write the letter of the most acidic, boxed hydrogen atom. Answer: (a) (b) (c) (d) 3 C 2 C C 3 3 C Questions 24 and 25 refer to this DA nucleobase: (4) In each blank write the letter of the best answer from among the given answer choices. This nucleobase is part of. Answer choices: A, C, G, T, or U. This nucleobase is a. Answer choices: (a) purine, or (b) pyrimidine This nucleobase is. Answer choices (a) aromatic, or (b) not aromatic 25. (2) Complete each sentence by writing a number in the blank: This nucleobase forms a Watson-Crick base pair involving hydrogen bonds. Page 5 score =
Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1
 Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1 1. (2) Write the letter of the structure that best fits the following 13 C-NMR spectrum: 51 ppm (doublet), 44 ppm (triplet), 27 ppm (quartet), 26
Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1 1. (2) Write the letter of the structure that best fits the following 13 C-NMR spectrum: 51 ppm (doublet), 44 ppm (triplet), 27 ppm (quartet), 26
Chemistry 14C Winter 2016 Final Exam Part A Page 1
 hemistry 14 Winter 2016 Final Exam Part A Page 1 1. (6) n the blank after each set of 13 -MR data write the letter of the molecule that is the closest fit. f none of the molecules fit, write none of these.
hemistry 14 Winter 2016 Final Exam Part A Page 1 1. (6) n the blank after each set of 13 -MR data write the letter of the molecule that is the closest fit. f none of the molecules fit, write none of these.
Chemistry 14C Spring 2016 Final Exam Part B Page 1
 hemistry 14 Spring 2016 Final Exam Part B Page 1 In lecture we discussed the possibility that the first cells may have been formed in boiling mud puddles, which have been shown (in the lab) to produce
hemistry 14 Spring 2016 Final Exam Part B Page 1 In lecture we discussed the possibility that the first cells may have been formed in boiling mud puddles, which have been shown (in the lab) to produce
Chemistry 14C Fall 2015 Final Exam Part B Page 1
 Chemistry 14C Fall 2015 Final Exam Part B Page 1 Uric acid is a normal metabolic product derived from purine nucleosides. Gout is a painful arthritic condition in which excess uric acid precipitates as
Chemistry 14C Fall 2015 Final Exam Part B Page 1 Uric acid is a normal metabolic product derived from purine nucleosides. Gout is a painful arthritic condition in which excess uric acid precipitates as
Chemistry 14C Spring 2016 Final Exam Part B Solutions Page 1
 hemistry 14 Spring 2016 Final Exam Part B Solutions Page 1 Statistics: igh score, average and low score will be posted on the course web site after exam grading is complete. A note about exam keys: The
hemistry 14 Spring 2016 Final Exam Part B Solutions Page 1 Statistics: igh score, average and low score will be posted on the course web site after exam grading is complete. A note about exam keys: The
Chemistry 14C Spring 2018 Final Exam Part A Page 1
 hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
Chem 14C Lecture 2 Spring 2017 Final Part B Solutions Page 1
 hem 14 Lecture 2 Spring 2017 Final Part B Solutions Page 1 Statistics: igh score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than
hem 14 Lecture 2 Spring 2017 Final Part B Solutions Page 1 Statistics: igh score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than
Organic Chemistry II (CHE ) Examination I February 11, Name (Print legibly): Key. Student ID#:
 rganic hemistry II (HE 232-001) Examination I February 11, 2009 Name (Print legibly): Key (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
rganic hemistry II (HE 232-001) Examination I February 11, 2009 Name (Print legibly): Key (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
Chemistry 14D Fall 2017 Final Exam Part A Page 1
 Chemistry 14D Fall 2017 Final Exam Part A Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer.
Chemistry 14D Fall 2017 Final Exam Part A Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer.
Chem 14C Lecture 1 Spring 2016 Exam 2 Solutions Page 1
 Chem 14C Lecture 1 Spring 2016 Exam 2 Solutions Page 1 Statistics: High score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than
Chem 14C Lecture 1 Spring 2016 Exam 2 Solutions Page 1 Statistics: High score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than
Chemistry 14D Winter 2010 Exam 2 Page 1
 Chemistry 14D Winter 2010 Exam 2 Page 1 1. (2) Circle the best statement of Markovnikov s rule. (a) When X adds to an alkene, the hydrogen of X becomes bonded to the alkene carbon that bears the least
Chemistry 14D Winter 2010 Exam 2 Page 1 1. (2) Circle the best statement of Markovnikov s rule. (a) When X adds to an alkene, the hydrogen of X becomes bonded to the alkene carbon that bears the least
Chemistry 14C Summer 2017 Second Midterm Exam Page 1
 Chemistry 14C Summer 2017 Second Midterm Exam Page 1 Begin this exam by gently removing the last two pages of this exam. Nothing o n these pages will be graded. They will be discarded before grading. Please
Chemistry 14C Summer 2017 Second Midterm Exam Page 1 Begin this exam by gently removing the last two pages of this exam. Nothing o n these pages will be graded. They will be discarded before grading. Please
Chapter 02 - Polar Covalent Bonds; Acids and Bases
 Exhibit 2-1 Give the corresponding letter of the term that best matches the given definition. a. Brønsted-Lowry Acid f. Ionic Bond b. Brønsted-Lowry Base g. Covalent Bond c. Lewis Acid h. Polar-Covalent
Exhibit 2-1 Give the corresponding letter of the term that best matches the given definition. a. Brønsted-Lowry Acid f. Ionic Bond b. Brønsted-Lowry Base g. Covalent Bond c. Lewis Acid h. Polar-Covalent
NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY
 NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY A STUDENT SHOULD BE ABLE TO: 1. Identify and explain the processes involved in proton ( 1 H) and carbon-13 ( 13 C) nuclear magnetic resonance
NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY A STUDENT SHOULD BE ABLE TO: 1. Identify and explain the processes involved in proton ( 1 H) and carbon-13 ( 13 C) nuclear magnetic resonance
Chem 2320 Exam 1. January 30, (Please print)
 Chem 2320 Exam 1 January 30, 2006 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple Choice ( /20) Score /60 II /15 III /25 Total score /100 I. Multiple choice questions. (3 points each).
Chem 2320 Exam 1 January 30, 2006 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple Choice ( /20) Score /60 II /15 III /25 Total score /100 I. Multiple choice questions. (3 points each).
Chapter 02 - Polar Covalent Bonds; Acids and Bases. Exhibit 2-1
 Exhibit 2-1 Organic Chemistry 9th Edition McMurry TEST BANK Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurry-test-bank/ Organic Chemistry 9th Edition McMurry
Exhibit 2-1 Organic Chemistry 9th Edition McMurry TEST BANK Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurry-test-bank/ Organic Chemistry 9th Edition McMurry
CHEM 4170 Problem Set #1
 CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
CHEM 4170 Problem Set #1 0. Work problems 1-7 at the end of Chapter ne and problems 1, 3, 4, 5, 8, 10, 12, 17, 18, 19, 22, 24, and 25 at the end of Chapter Two and problem 1 at the end of Chapter Three
Chem 213 Final 2012 Detailed Solution Key for Structures A H
 Chem 213 Final 2012 Detailed Solution Key for Structures A H COMPOUND A on Exam Version A (B on Exam Version B) C 8 H 6 Cl 2 O 2 DBE = 5 (aromatic + 1) IR: 1808 cm 1 suggests an acid chloride since we
Chem 213 Final 2012 Detailed Solution Key for Structures A H COMPOUND A on Exam Version A (B on Exam Version B) C 8 H 6 Cl 2 O 2 DBE = 5 (aromatic + 1) IR: 1808 cm 1 suggests an acid chloride since we
1. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = 116
 Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
Practice Midterm Exam 200 points total 75 minutes Multiple Choice (3 pts each 30 pts total) Mark your answers in the space to the left:
 MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
Paper 12: Organic Spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Exam III. Please read through each question carefully, and make sure you provide all of the requested information.
 09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
Chemistry 14D Winter 2018 Final Part B Page 1
 Chemistry 14D Winter 2018 Final Part B Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. Transition
Chemistry 14D Winter 2018 Final Part B Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. Transition
Chemistry 14D Winter 2017 Final Exam Part A Page 1
 Chemistry 14D Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. All reactants and solvents written above
Chemistry 14D Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. All reactants and solvents written above
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
CHAPTER 2: RESONANCE THEORY
 CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Due in class on Thursday Sept. 8 th
 Problem Set #1 Chem 391 Due in class on Thursday Sept. 8 th ame Solutions A note: To help me read quickly, I structure the problem set like an exam. I have made small spaces for answers where I d like
Problem Set #1 Chem 391 Due in class on Thursday Sept. 8 th ame Solutions A note: To help me read quickly, I structure the problem set like an exam. I have made small spaces for answers where I d like
REACTIONS OF AROMATIC COMPOUNDS
 A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
CHEM 213 FALL 2018 MIDTERM EXAM 2 - VERSION A
 CEM 213 FALL 2018 MIDTERM EXAM 2 - VERSIN A Answer multiple choice questions on the green computer sheet provided with a PENCIL. Be sure to encode both your NAME and Registration Number (V#). You will
CEM 213 FALL 2018 MIDTERM EXAM 2 - VERSIN A Answer multiple choice questions on the green computer sheet provided with a PENCIL. Be sure to encode both your NAME and Registration Number (V#). You will
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 9, 2002 ANSWERS
 INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
Periodic Table. 8/3/2006 MEDC 501 Fall
 Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
Chemistry 14C Winter 2017 Exam 2 Solutions Page 1
 Chemistry 14C Winter 2017 Exam 2 Solutions Page 1 Statistics: High score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than one
Chemistry 14C Winter 2017 Exam 2 Solutions Page 1 Statistics: High score, average, and low score will be posted on the course web site after exam grading is complete. Some questions have more than one
AMINES. 4. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
Organic Chemistry II (CHE ) Examination I March 1, Name (Print legibly): _KEY _. Student ID#: _
 Organic Chemistry II (CHE 232-002) Examination I March 1, 2007 Name (Print legibly): _KEY _ (last) (first) Student ID#: _ PLEASE observe the following: You are allowed to have scratch paper (provided by
Organic Chemistry II (CHE 232-002) Examination I March 1, 2007 Name (Print legibly): _KEY _ (last) (first) Student ID#: _ PLEASE observe the following: You are allowed to have scratch paper (provided by
Organic Chemistry II* (CHE ) Examination I March 1, Name (Print legibly): KEY. Student ID#:
 Organic Chemistry II* (CHE 232-002) Examination I March 1, 2007 Name (Print legibly): KEY (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
Organic Chemistry II* (CHE 232-002) Examination I March 1, 2007 Name (Print legibly): KEY (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
10. Amines (text )
 2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
Answers to Assignment #5
 Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
MULTIPLE CHOICE 2 points each
 Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
The Final Learning Experience
 Chemistry 416 Spectroscopy Fall Semester 1997 Dr. Rainer Glaser The Final Learning Experience Monday, December 15, 1997 3:00-5:00 pm Name: Answer Key Maximum Question 1 (Combination I) 20 Question 2 (Combination
Chemistry 416 Spectroscopy Fall Semester 1997 Dr. Rainer Glaser The Final Learning Experience Monday, December 15, 1997 3:00-5:00 pm Name: Answer Key Maximum Question 1 (Combination I) 20 Question 2 (Combination
BI/CH421 Biochemistry I Exam 1 09/29/2014
 Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1
 Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
CHEM 2430 Organic Chemistry I Fall Instructor: Paul Bracher. Final Examination
 CHEM 2430 Organic Chemistry I Fall 2015 Final Exam Solutions Key Page 1 of 10 CHEM 2430 Organic Chemistry I Fall 2015 Instructor: Paul Bracher Final Examination Friday, December 11 th, 2015 12:00 1:50
CHEM 2430 Organic Chemistry I Fall 2015 Final Exam Solutions Key Page 1 of 10 CHEM 2430 Organic Chemistry I Fall 2015 Instructor: Paul Bracher Final Examination Friday, December 11 th, 2015 12:00 1:50
CHM 233 : Fall 2018 Quiz #9 - Answer Key
 HM 233 : Fall 2018 Quiz #9 - Answer Key Question 1 M25c How many different signals would you expect to see in a proton-decoupled carbon nmr spectrum of the following compound? A 3 B 4 6 D 8 3 1 2 carbons
HM 233 : Fall 2018 Quiz #9 - Answer Key Question 1 M25c How many different signals would you expect to see in a proton-decoupled carbon nmr spectrum of the following compound? A 3 B 4 6 D 8 3 1 2 carbons
Proton NMR. Four Questions
 Proton NMR Four Questions How many signals? Equivalence Where on spectrum? Chemical Shift How big? Integration Shape? Splitting (coupling) 1 Proton NMR Shifts Basic Correlation Chart How many 1 H signals?
Proton NMR Four Questions How many signals? Equivalence Where on spectrum? Chemical Shift How big? Integration Shape? Splitting (coupling) 1 Proton NMR Shifts Basic Correlation Chart How many 1 H signals?
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Acids and Bases. Acids and Bases
 BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
4. NMR spectra. Interpreting NMR spectra. Low-resolution NMR spectra. There are two kinds: Low-resolution NMR spectra. High-resolution NMR spectra
 1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
C. Correct! The abbreviation Ar stands for an aromatic ring, sometimes called an aryl ring.
 Organic Chemistry - Problem Drill 05: Drawing Organic Structures No. 1 of 10 1. What does the abbreviation Ar stand for? (A) Acetyl group (B) Benzyl group (C) Aromatic or Aryl group (D) Benzoyl group (E)
Organic Chemistry - Problem Drill 05: Drawing Organic Structures No. 1 of 10 1. What does the abbreviation Ar stand for? (A) Acetyl group (B) Benzyl group (C) Aromatic or Aryl group (D) Benzoyl group (E)
Hour Examination # 1
 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
CHEM 2410 Organic Chemistry 1 Fall 2017 Exam # 1 Problem Booklet Page 1 of 11 CHEM 2410 Organic Chemistry 1 Fall 2017 Exam Booklet No. Instructors: Paul Bracher & Erin Whitteck Hour Examination # 1 Wednesday,
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
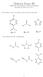 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
NH 2. Biochemistry I, Fall Term Sept 9, Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter
 Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Chemistry 1110 Exam 4 Study Guide
 Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
CHEM- 457: Inorganic Chemistry
 CHEM- 457: Inorganic Chemistry Midterm I March 13 th, 2014 NAME This exam is comprised of six questions and is ten pages in length. Please be sure that you have a complete exam and place your name on each
CHEM- 457: Inorganic Chemistry Midterm I March 13 th, 2014 NAME This exam is comprised of six questions and is ten pages in length. Please be sure that you have a complete exam and place your name on each
HW #3: 14.26, 14.28, 14.30, 14.32, 14.36, 14.42, 14.46, 14.52, 14.56, Alcohols, Ethers and Thiols
 Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chemistry 131 Lecture 8: Alcohols, Ethers and Sulfur Analogs: Structure, Nomenclature, Physical Properties, and Chemical Reactivity Chapter 14 in McMurry, Ballantine, et. al. 7 th edition HW #3: 14.26,
Chapter 15 Lecture Outline
 Organic Chemistry, First Edition Janice Gorzynski Smith University of Hawaii Chapter 5 Lecture Outline Introduction to NMR Two common types of NMR spectroscopy are used to characterize organic structure:
Organic Chemistry, First Edition Janice Gorzynski Smith University of Hawaii Chapter 5 Lecture Outline Introduction to NMR Two common types of NMR spectroscopy are used to characterize organic structure:
1. Which compound would you expect to have the lowest boiling point? A) NH 2 B) NH 2
 MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Chemistry 3351: Organic Chemistry Thursday: Sept. 7:00pm 9:00/1 st Exam. Name: (please print)
 Chemistry 3351: Organic Chemistry Thursday: Sept. 23 @ 7:00pm 9:00/1 st Exam 1 Name: (please print) Page Possible Points Score 2 9 3 9 4 8 5 10 6 10 7 4 8 9 9 10 10 10 11 10 12 10 13 10 TOTAL 109 2 1.
Chemistry 3351: Organic Chemistry Thursday: Sept. 23 @ 7:00pm 9:00/1 st Exam 1 Name: (please print) Page Possible Points Score 2 9 3 9 4 8 5 10 6 10 7 4 8 9 9 10 10 10 11 10 12 10 13 10 TOTAL 109 2 1.
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Paper 12: Organic Spectroscopy
 Subject hemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 34: ombined problem on UV, IR, 1 H NMR, 13 NMR and Mass- Part 6 HE_P12_M34 TABLE OF ONTENTS 1. Learning
Subject hemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 34: ombined problem on UV, IR, 1 H NMR, 13 NMR and Mass- Part 6 HE_P12_M34 TABLE OF ONTENTS 1. Learning
Organic Chemistry I (CHE ) Examination I S ep t ember 2 9, KEY Name (PRINT LEGIBLY):
 Organic hemistry I (E 2 3 0-001 ) Examination I S ep t ember 2 9, 2 0 0 4 KEY Name (PRINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings
Organic hemistry I (E 2 3 0-001 ) Examination I S ep t ember 2 9, 2 0 0 4 KEY Name (PRINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings
A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D. III > IV > I > II
 Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
Chapter 2 Lecture Outline
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 2 Lecture Outline Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 2 Lecture Outline Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
CHEMISTRY 216 WINTER TERM 2007 END OF TERM EXAM. Time Allowed 2 hours
 EMISTRY 216 WITER TERM 2007 ED F TERM EXAM Time Allowed 2 hours ame KEY GSI ame ID umber Lab Section Write legibly. Illegible or messy answers will not be graded. Read these instructions carefully. In
EMISTRY 216 WITER TERM 2007 ED F TERM EXAM Time Allowed 2 hours ame KEY GSI ame ID umber Lab Section Write legibly. Illegible or messy answers will not be graded. Read these instructions carefully. In
CHEM311 FALL 2005 Practice Exam #3
 EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Midterm Exam 1. Chem 3B, Fall 2016 Thursday, September 29, :00 9:00 pm. Name. Student ID
 Midterm Exam 1 Chem 3B, Fall 2016 Thursday, September 29, 2016 7:00 9:00 pm ame Student ID You have 120 minutes to complete this exam. Please provide all answers in the space provided. Work drawn in the
Midterm Exam 1 Chem 3B, Fall 2016 Thursday, September 29, 2016 7:00 9:00 pm ame Student ID You have 120 minutes to complete this exam. Please provide all answers in the space provided. Work drawn in the
Christopher M. Hadad Chemistry 253N Spring Final Exam. Name (PRINT) ANSWER KEY I have neither given nor received aid on this exam (SIGN)
 Christopher M. adad Chemistry 253 Spring 2002 Final Exam ame (PRIT) ASWER KEY I have neither given nor received aid on this exam (SIG) There are 8 pages and 300 points on this exam. Please read each question
Christopher M. adad Chemistry 253 Spring 2002 Final Exam ame (PRIT) ASWER KEY I have neither given nor received aid on this exam (SIG) There are 8 pages and 300 points on this exam. Please read each question
Chapter 9. Nuclear Magnetic Resonance. Ch. 9-1
 Chapter 9 Nuclear Magnetic Resonance Ch. 9-1 1. Introduction Classic methods for organic structure determination Boiling point Refractive index Solubility tests Functional group tests Derivative preparation
Chapter 9 Nuclear Magnetic Resonance Ch. 9-1 1. Introduction Classic methods for organic structure determination Boiling point Refractive index Solubility tests Functional group tests Derivative preparation
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
17.24 To name the compounds use the directions from Answer 17.3.
 Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Chemistry /002 Exam 1 Version Green. The Periodic Table
 Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
Name: Last First MI Chemistry 233-001/002 Exam 1 Version Green Fall 2018 Dr. J. sbourn Instructions: The first 13 questions of this exam should be answered on the provided Scantron. You must use a pencil
SAMPLE FINAL 3150:110
 SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
IR, MS, UV, NMR SPECTROSCOPY
 CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
University of York. BA, BSc, and MSc Degree Examinations Department : BIOLOGY. Title of Exam: Biochemical reaction mechanisms
 Examination Candidate Number: Desk Number: University of York BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Biochemical reaction mechanisms Time Allowed: 1 hour Marking
Examination Candidate Number: Desk Number: University of York BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Biochemical reaction mechanisms Time Allowed: 1 hour Marking
Acids and Bases: Molecular Structure and Acidity
 Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
ORGANIC CHEMISTRY. Classification of organic compounds
 ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
Organic Chemistry I Exam 3 Fall 2001 November 30, Which of the following compounds corresponds to the spectral data given below?
 . Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
. Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
iclicker Question #12A - before lecture Which of the following dotted lines shows a possible hydrogen bond? (A) (B) (C) H
 Bio 111 andout for hemistry 4 This handout contains: 1. Today s ilicker Questions 2. The handout for today s lecture. 3. Summary of Basic hemistry Lectures ilicker Question #12A - before lecture Which
Bio 111 andout for hemistry 4 This handout contains: 1. Today s ilicker Questions 2. The handout for today s lecture. 3. Summary of Basic hemistry Lectures ilicker Question #12A - before lecture Which
4) Chapter 1 includes heredity (i.e. DNA and genes) as well as evolution. Discuss the connection between heredity and evolution?
 Name- Chapters 1-5 Questions 1) Life is easy to recognize but difficult to define. The dictionary defines life as the state or quality that distinguishes living beings or organisms from dead ones and from
Name- Chapters 1-5 Questions 1) Life is easy to recognize but difficult to define. The dictionary defines life as the state or quality that distinguishes living beings or organisms from dead ones and from
ORGANIC - BROWN 8E CH NUCLEAR MAGNETIC RESONANCE.
 !! www.clutchprep.com CONCEPT: 1 H NUCLEAR MAGNETIC RESONANCE- GENERAL FEATURES 1 H (Proton) NMR is a powerful instrumental method that identifies protons in slightly different electronic environments
!! www.clutchprep.com CONCEPT: 1 H NUCLEAR MAGNETIC RESONANCE- GENERAL FEATURES 1 H (Proton) NMR is a powerful instrumental method that identifies protons in slightly different electronic environments
