Chemistry 14D Fall 2017 Final Exam Part A Page 1
|
|
|
- Cecil Smith
- 5 years ago
- Views:
Transcription
1 Chemistry 14D Fall 2017 Final Exam Part A Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. K to use B/B notation on any mechanism in this exam (except question 17). eroin is an opiate analgesic whose tragic addiction epidemic is well known. eroin is produced from morphine, which is extracted from the poppy flower. Interestingly, heroin was sold over-the-counter as an analgesic in the decades around N 2 6 N Morphine eroin 1. (2) In the space below write the name of the one functional group present in heroin but absent in morphine. 2. (2) Write one letter in the blank. Combined with a mild base (B) reactant is an efficient way to convert morphine into heroin. (a) (b) (c) (d) 3. (6) Using the molecule you selected in question 2 plus mild base (B), write a mechanism for acetylation of the morphine phenolic group: Ar Question 2 choice B Ar 4. (2) In each answer blank write 'T' (for true) or 'F' (for false). The mechanism I wrote in question 3 is an example of......the Fischer esterification reaction:...the Chichibabin reaction: 5. (2) Morphine can also be converted into heroin according to the following equilibrium: Ar Morphine Ar S S eroin The equilibrium favors. Complete the statement by writing 'M' (for morphine; K eq < 1), '' (for heroin; K eq > 1), or 'E' (if K eq = 1) in the blank above. Page 1 score =
2 Chemistry 14D Fall 2017 Final Exam Part A Page 2 ne reason for the strongly addictive nature of heroin (especially when injected) is rapid hydrolysis to morphine in the brain. The questions on this exam page concern this hydrolysis reaction. 6. (10) Write a mechanism for this hydrolysis reaction: Ar 3 2 Ar 7. (6) efer to the heroin structure on page 1. Write a number (6, 2, or 0 if the rates are equal) in the blank. If you write 0 you are done with this question. If you write 2 or 6 in the blank complete the statement by adding no more than ten words of explanation. ydrolysis of heroin group is faster because this group (10) Various analogs of heroin have been made in order to study their drug properties. For each change in the heroin structure shown, write "F" if the change causes hydrolysis to be faster, or "S" if the hydrolysis is slower. Complete each explanation by adding no more than fifteen words in each case. Use a very different explanation in each case. (a) Ar changed to Ar This change makes the hydrolysis because this new structure... CF 3 (b) Ar changed to Ar This change makes the hydrolysis because this new structure... Page 2 score =
3 Chemistry 14D Fall 2017 Final Exam Part A Page 3 9. (9) Synthesis of other heroin analogs might be attempted using the reactions shown below. For each reaction, write two organic products. (These are formed in equal amounts. int: think about the mechanisms.) If no reaction occurs write N in the boxes. Note that the portion of the structure abbreviated as 'Ar' does not change in these reactions. rganic product #1 rganic product #2 (a) Ar 1. C 2 MgBr 2. 3 (b) Ar NaB 4 (c) Ar N 3 To increase its water solubility and aid in excretion, morphine (abbreviated here as M ) is metabolically converted into its glucuronide by coupling with glucuronic acid. Glucuronic acid is derived from glucose. Questions 10 and 11 refer to this reaction scheme: Question 10(a) C 2 Enzyme Glucose Glucuronic acid Morphine glucuronide 10. (4) For each carbon in the scheme above, write "" if this carbon is oxidized in this conversion, "" if it is reduced, "N" if neither oxidation nor reduction occurs, or "B" if oxidation and reduction both occur simultaneously. Glucose glucuronic acid: Question 10(b) C Enzyme M Glucuronic acid morphine glucuronide: C M 11. (8) Write a mechanism for the simplified coupling of morphine (M ) with glucuronic acid to produce morphine glucuronide (shown at the right). The enzyme (Enz) is both an acid and a base. Enzyme M M Page 3 score =
4 Chemistry 14D Fall 2017 Final Exam Part A Page (3) Another problem with the current heroin epidemic is that heroin is being mixed with fentanyl, a very powerful and short-acting opioid analgesic. A dose of as little as 2 milligrams of fentanyl can be fatal. Complete the following reaction for synthesis of a fentanyl precursor molecule by writing a reasonable reactant in the reactant box. K to use 'Ph' where appropriate for this question. LiAl 4 N N eactant Fentanyl precursor The remaining exam questions are not directly related to heroin, morphine, or fentanyl. 13. (3) For equilibrium A, K eq ~ 1. For equilibrium B, K eq << Equilibrium A (K eq ~ 1) Equilibrium B (K eq << 1) Complete this explanation by adding no more than fifteen words in each answer space: Equilibrium A has K eq ~ 1 but equilibrium B has K eq << 1 because equilibrium A......whereas equilibrium B (4) In protein synthesis, the carboxylic acid end of one amino acid cannot be coupled with the amine end of another amino acid to produce a new peptide bond: 2 N X N Instead, the carboxylic acid must be converted into the corresponding phosphate to make the protein: P P protein 2 N Complete this statement by adding no more than fifteen words: The to phosphate conversion is necessary because... Page 4 score =
5 Chemistry 14D Fall 2017 Final Exam Part A Page (3) Write the names of three carbonyl-containing functional groups that have resonance delocalization over no less than three atoms. Questions 16 and 17 concern molecule C: Molecule C 16. (2) Write the name of all carbonyl-containing functional group in molecule C. 17. (9) Write the names of the three common carbonyl group fates, as discussed in lecture. Illustrate each using molecule C and water as the only reactants. Include all curved arrows, but do not go beyond one mechanism step. Do not use B/B for this problem. If the fate can occur in more than one way show only the most likely pathway. If the fate named is not likely to occur, write "not likely" instead of illustrating the fate. Name of carbonyl group fate # 1: Illustration: 2 Name of carbonyl group fate # 2: Illustration: 2 Name of carbonyl group fate # 3: Illustration: 2 Page 5 score =
6 Chemistry 14D Fall 2017 Final Exam Part A Page (3) Assign pk a values to the indicated protons by writing numbers below the arrows. pk a choices: 9, 19, and 21. Each pk a value will be used exactly once. SCoA is an abbreviation for sulfur bonded to a large biological group whose exact structure is irrelevant to the question. SCoA SCoA 19. (6) Write a reasonable mechanism for the following reaction. The enzyme (Enz) is both a moderate acid and a moderate base. If any molecules involved have resonance show only the most significant resonance contributor for that molecule. Enz SCoA SCoA SCoA 20. (2) Write exactly two or three words in the blank. Be specific! The reaction of the previous question is an example of a(n) reaction. 21. (2) Write a clear example of an aldol reaction using the given starting material. Include all reactants and products, but do not include a mechanism. 22. (2) In the blank after each term or concept below write the number of one question from this exam, such as 36(x), which contains an example of that term. If no exam question includes this term or concept, write '0'. rganometallic compound: Nucleophilic carbonyl substitution without a tetrahedral intermediate: Page 6 score =
Chemistry 14D Winter 2017 Final Exam Part A Page 1
 Chemistry 14D Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. All reactants and solvents written above
Chemistry 14D Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. All reactants and solvents written above
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chemistry 14C Winter 2017 Final Exam Part A Page 1
 Chemistry 14C Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. The active site of an enzyme is a cleft
Chemistry 14C Winter 2017 Final Exam Part A Page 1 Please use the backs of the exam pages for scratch space. Please do not use the exam margins for this purpose. The active site of an enzyme is a cleft
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1
 Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
Chem 312 SS05 Page 1 of 6 Kantorowski. 17 August 2005 Name:
 Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Exam III 17 August 2005 Name:
 Chem 312 SS05 Page 1 of 6 Kantorowski Exam III 17 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 312 SS05 Page 1 of 6 Kantorowski Exam III 17 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Organic Chemistry FINAL EXAM A (250 points)
 UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
Chemistry 14D Lecture 2 Fall 2016 Exam 2 Page 1
 Chemistry 14D Lecture 2 Fall 2016 Exam 2 Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer.
Chemistry 14D Lecture 2 Fall 2016 Exam 2 Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer.
Chem 263 Nov 28, Reactions of Carboxylic Acids and Derivatives: Strong Nucleophiles
 Chem 263 ov 28, 2013 eactions of Carboxylic Acids and Derivatives: Strong ucleophiles The strong nucleophiles (u: - ) that we have learned in this course are either hydride anion ( - ) or alkyl anion (
Chem 263 ov 28, 2013 eactions of Carboxylic Acids and Derivatives: Strong ucleophiles The strong nucleophiles (u: - ) that we have learned in this course are either hydride anion ( - ) or alkyl anion (
Enolates, Enols, and Enamines Part 3
 Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
CHEM Organic Chemistry with Biological Applications EXAM 1A (250 points)
 UCSC, Binder Name Student ID # Section Day/Time CEM 109 rganic Chemistry with Biological Applications EXAM 1A (250 points) D NT BEGIN TE EXAM R TURN TE PAGE UNTIL INSTRUCTED T D S. In the meantime, please
UCSC, Binder Name Student ID # Section Day/Time CEM 109 rganic Chemistry with Biological Applications EXAM 1A (250 points) D NT BEGIN TE EXAM R TURN TE PAGE UNTIL INSTRUCTED T D S. In the meantime, please
Partial Periodic Table
 EMITY 3331, pring 2005 Professor Walba Final Exam, May 3 U onor ode Pledge: n my honor, as a University of olorado at oulder tudent, I have neither given nor received unauthorized assistance. scores: 1)
EMITY 3331, pring 2005 Professor Walba Final Exam, May 3 U onor ode Pledge: n my honor, as a University of olorado at oulder tudent, I have neither given nor received unauthorized assistance. scores: 1)
Chemistry 14D Winter 2018 Final Part B Page 1
 Chemistry 14D Winter 2018 Final Part B Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. Transition
Chemistry 14D Winter 2018 Final Part B Page 1 K to use "Ph" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. Transition
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHEMISTRY 252 Exam pts. Section 703 Grand Rapids 10 August 2006
 ame PID CEMISTRY 5 Exam 100 pts. Section 703 Grand Rapids 10 August 006 Make sure you have all 8 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of this
ame PID CEMISTRY 5 Exam 100 pts. Section 703 Grand Rapids 10 August 006 Make sure you have all 8 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of this
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
UCSC, Binder. CHEM 8B Organic Chemistry II FINAL EXAM (300 points)
 UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Chemistry 234 Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
H 3 C. biotin dependent O O. C O + Pi 2 + ADP 3 + H H 2 C C SCoA malonyl-scoa
 1 EXAM F SCIETIFIC CULTUE CEMISTY BLEM 1: BISYTESIS F FATTY ACIDS The biosynthesis of fatty acids proceeds by lengthening of a hydrocarbon chain by condensation of two-carbon units derived from acetyl-coenzyme
1 EXAM F SCIETIFIC CULTUE CEMISTY BLEM 1: BISYTESIS F FATTY ACIDS The biosynthesis of fatty acids proceeds by lengthening of a hydrocarbon chain by condensation of two-carbon units derived from acetyl-coenzyme
CHEM 234, Spring 2015 PRINTED FIRST NAME. Final Exam ASU ID or Posting ID. Ian R. Gould PRINTED LAST NAME
 CEM 234, Spring 2015 PRINTED FIRST NAME PRINTED LAST NAME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) 1 /12 2 /20 3 /25 4 /12 5 /20 6 /64
CEM 234, Spring 2015 PRINTED FIRST NAME PRINTED LAST NAME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) 1 /12 2 /20 3 /25 4 /12 5 /20 6 /64
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
CHEM 241 (Davis) Organic Chemistry II Final Exam Friday December 14, NAME (Last, First) DISCUSSION SECTION #
 CEM 241 (Davis) rganic Chemistry II Final Exam Friday December 14, 2007 1 NAME (Last, First) DISCUSSIN SECTIN # Initial of last name Instructions Please fill in your name in the space above and on the
CEM 241 (Davis) rganic Chemistry II Final Exam Friday December 14, 2007 1 NAME (Last, First) DISCUSSIN SECTIN # Initial of last name Instructions Please fill in your name in the space above and on the
Review Activity Module 1: Biological Chemistry
 Review Activity Module 1: Biological Chemistry Laroche: The picture above is of a molecule calle MC1R. Based on what you ve learned so far about the various biological macromolecules, what kind of macromolecule
Review Activity Module 1: Biological Chemistry Laroche: The picture above is of a molecule calle MC1R. Based on what you ve learned so far about the various biological macromolecules, what kind of macromolecule
Midterm Exam 2. Chem 3B, Fall 2016 Thursday, Nov. 3, :00 9:00 pm. Name
 Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
Chemistry 14C Spring 2018 Final Exam Part A Page 1
 hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
California State Polytechnic University, Pomona. Exam Points 1. Nomenclature (1) 25
 hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
2. Which of the following are nucleophiles and which are electrophiles?
 Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Compounds Part 1: Ionic Cpds - Formula Units & Nomenclature (29:15) Video Tutorial Lecture Notes
 Exam 1 Video Tutorials and Activities beginning of lecture for exam 1. The materials need to be organized according to the TOC for FULL credit. Refer to the Video/Activity grading rubric. Exam 1 is based
Exam 1 Video Tutorials and Activities beginning of lecture for exam 1. The materials need to be organized according to the TOC for FULL credit. Refer to the Video/Activity grading rubric. Exam 1 is based
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
CHAPTER 2: RESONANCE THEORY
 CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES
 A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Acids and Bases: Molecular Structure and Acidity
 Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Henderson - Hasselbalch equation
 Structure & Properties of Water Bent geometry, O- bond length of 0.958Å Can form ydrogen bonds Comprehensive Exam eview 12/03/2009 enderson - asselbalch equation From [ ][ ] K = [ ] The 6 step approach
Structure & Properties of Water Bent geometry, O- bond length of 0.958Å Can form ydrogen bonds Comprehensive Exam eview 12/03/2009 enderson - asselbalch equation From [ ][ ] K = [ ] The 6 step approach
TA Section Day/Time. Organic Chemistry EXAM 1A (200 points)
 UCSC, Binder ame TA Section Day/Time rganic Chemistry EXAM 1A (200 points) D T WRITE TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. Page 1 (40) Page 2
UCSC, Binder ame TA Section Day/Time rganic Chemistry EXAM 1A (200 points) D T WRITE TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. Page 1 (40) Page 2
Chem 332, Exam 4. Spring Provide reagents for the following transformations (2 pts each) NaOH , KOH. 4) H2/Pd NO 2. 1) AlCl 3 O 2) H 2 NNH 2
 AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
7.014 Quiz I Handout
 7.014 Quiz I andout Quiz I announcements: Quiz I: Friday, February 27 12:05 12:55 Walker Gym, rd floor (room 5040) **This will be a closed book exam** Quiz Review Session: Wednesday, February 25 7:00 9:00
7.014 Quiz I andout Quiz I announcements: Quiz I: Friday, February 27 12:05 12:55 Walker Gym, rd floor (room 5040) **This will be a closed book exam** Quiz Review Session: Wednesday, February 25 7:00 9:00
Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1
 Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1 1. (2) Write the letter of the structure that best fits the following 13 C-NMR spectrum: 51 ppm (doublet), 44 ppm (triplet), 27 ppm (quartet), 26
Chem 14C Lecture 1 Spring 2017 Final Exam Part B Page 1 1. (2) Write the letter of the structure that best fits the following 13 C-NMR spectrum: 51 ppm (doublet), 44 ppm (triplet), 27 ppm (quartet), 26
Chemical Kinetics. Goal. Objectives
 4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 10. Biochemical Transformations II. Phosphoryl transfer and the kinetics and thermodynamics of energy currency in the cell: ATP and GTP.
Score: Homework Problem Set 6 Iverson CH320N Due Friday, March 10. NAME (Print): Chemistry 320N Dr. Brent Iverson 6th Homework March 3, 2017
 omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
Unit 7 Part I: Introductions to Biochemistry
 Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19-Mar-14 Averett 1 Chemical Reactions Chemical Reactions Process by which one set of chemicals is changed into another
Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19-Mar-14 Averett 1 Chemical Reactions Chemical Reactions Process by which one set of chemicals is changed into another
O O O CH 2 O 7. 2 = C=O hydration H B. 6 = reverse aldol H O. 9b = acetal formation add alcohol (step 2)
 1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008
 First Three Letters of Last Name NAME Network ID CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
First Three Letters of Last Name NAME Network ID CHEMISTRY 332 SUMMER 08 EXAM I June 26-28, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed, electronic,
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Exam II (100 points total) November 5, 2012
 Chemistry 271, Section 22xx Your Name: Prof. Jason Kahn University of Maryland, College Park Your SID #: General Chemistry and Energetics Your Section #: (+1 point) Exam II (100 points total) November
Chemistry 271, Section 22xx Your Name: Prof. Jason Kahn University of Maryland, College Park Your SID #: General Chemistry and Energetics Your Section #: (+1 point) Exam II (100 points total) November
Energy Transformation and Metabolism (Outline)
 Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
CHAPTER 15 Metabolism: Basic Concepts and Design
 CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
CHAPTER 15 Metabolism: Basic Concepts and Design Chapter 15 An overview of Metabolism Metabolism is the sum of cellular reactions - Metabolism the entire network of chemical reactions carried out by living
G. GENERAL ACID-BASE CATALYSIS
 G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Under strongly acidic conditions at ph = 1 every functional group in phosphoserine that can pick up a proton, does.
 1. (48 pts) a. L-Phosphoserine is a modified amino acid that is generated in proteins by phosphorylation of serine residues. The amino acid side chain has two acidic protons, which exhibit different pk
1. (48 pts) a. L-Phosphoserine is a modified amino acid that is generated in proteins by phosphorylation of serine residues. The amino acid side chain has two acidic protons, which exhibit different pk
UCSC, Binder. CHEM 8B Organic Chemistry II EXAM 2A, Winter 2018 (200 points)
 UCSC, Binder Name CEM 8B rganic Chemistry II EXAM 2A, Winter 2018 (200 points) Use your knowledge of organic chemistry conventions to answer the questions in the proper manner. Be sure to read all questions
UCSC, Binder Name CEM 8B rganic Chemistry II EXAM 2A, Winter 2018 (200 points) Use your knowledge of organic chemistry conventions to answer the questions in the proper manner. Be sure to read all questions
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
Subject Overview Curriculum pathway
 Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Lecture 18. Oxidation and Reduction. Oxidation. Reduction O CH 4 CH 3 OH H C H. Chemistry 328N
 Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Energy in Chemical and Biochemical Reactions
 Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Energy in Chemical and Biochemical Reactions Reaction Progress Diagram for Exothermic Reaction Reactants activated complex Products ENERGY A + B Reactants E a C + D Products Δ rxn Reaction coordinate The
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
Chemistry 14C Fall 2015 Final Exam Part B Page 1
 Chemistry 14C Fall 2015 Final Exam Part B Page 1 Uric acid is a normal metabolic product derived from purine nucleosides. Gout is a painful arthritic condition in which excess uric acid precipitates as
Chemistry 14C Fall 2015 Final Exam Part B Page 1 Uric acid is a normal metabolic product derived from purine nucleosides. Gout is a painful arthritic condition in which excess uric acid precipitates as
CHE 331 Molecular Science II
 CHE 331 Molecular Science II Exam 2 Form 1 Wednesday March 9, 2016 8:45 PM 10:15 PM 1. Write your nine digit University ID number in the space provided and then bubble in each of the nine digits. 2. Print
CHE 331 Molecular Science II Exam 2 Form 1 Wednesday March 9, 2016 8:45 PM 10:15 PM 1. Write your nine digit University ID number in the space provided and then bubble in each of the nine digits. 2. Print
Organic Chemistry FINAL EXAM A (250 points)
 UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
California State Polytechnic University, Pomona 1
 alifornia State Polytechnic University, Pomona 1 hem 2010 Final Fall, 2018 Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 30 2. 3D structure and resonance 20 3.
alifornia State Polytechnic University, Pomona 1 hem 2010 Final Fall, 2018 Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 30 2. 3D structure and resonance 20 3.
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
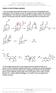 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
ENV SCI 22 GROUP QUIZ WEEK 2
 ENV SCI 22 GROUP QUIZ WEEK 2 ph OF ACIDS AND BASES 1) A decrease of one unit in the ph scale above represents a tenfold increase in the hydrogen ion concentration of a solution. For example, a solution
ENV SCI 22 GROUP QUIZ WEEK 2 ph OF ACIDS AND BASES 1) A decrease of one unit in the ph scale above represents a tenfold increase in the hydrogen ion concentration of a solution. For example, a solution
Introduction to Life Science. BSC 1005 Fall 2011 Homework 1! Connect Due Date: 9/18/ :59PM. Multiple Choice Portion
 Introduction to Life Science BSC 1005 Fall 2011 Homework 1 Connect Due Date: 9/18/2011 11:59PM Instructions Complete this homework assignment as the material is covered in class. You may refer to any of
Introduction to Life Science BSC 1005 Fall 2011 Homework 1 Connect Due Date: 9/18/2011 11:59PM Instructions Complete this homework assignment as the material is covered in class. You may refer to any of
Final Exam Key. Chem. 310N, Spring 2008, Professor Krische. Last Name: First Name:
 Chem. 310, pring 2008, Professor Krische Final Exam Key Last ame: First ame: Problem 1. (30 points) 2. (12 points) 3. (10 points) 4. (14 points) 5. (15 points) 6. (10 points) 7. (24 points) 8. (20 points)
Chem. 310, pring 2008, Professor Krische Final Exam Key Last ame: First ame: Problem 1. (30 points) 2. (12 points) 3. (10 points) 4. (14 points) 5. (15 points) 6. (10 points) 7. (24 points) 8. (20 points)
Unit 1: Chemistry of Life Guided Reading Questions (80 pts total)
 Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Chapter 1 Exploring Life Unit 1: Chemistry of Life Guided Reading Questions
Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Chapter 1 Exploring Life Unit 1: Chemistry of Life Guided Reading Questions
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Structure and Reactivity: Prerequired Knowledge
 Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Name Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #2 - March 12, 2001
 Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #2 - March 12, 2001 INSTRUTINS This examination has two parts. Part I is in multiple choice format and the answers should
Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #2 - March 12, 2001 INSTRUTINS This examination has two parts. Part I is in multiple choice format and the answers should
I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (OL 2007, 9, 385). OCH OCH 3
 ame Page 1 F.08.215FEP1 I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (L 2007, 9, 85). a l B. Provide the step-wise, curved arrow mechanism for the following (JAS 2002,
ame Page 1 F.08.215FEP1 I. (42 points) A. Provide the step-wise, curved arrow mechanism for the following (L 2007, 9, 85). a l B. Provide the step-wise, curved arrow mechanism for the following (JAS 2002,
MgBr 17 H 16 N 2 O 3 HO N. no partial. 4 each; can be either order -2 if steps unnumbered 1) CH 3 OH/TsOH. 1) NaH 2) CH 3 I
 I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
