H 3 C. biotin dependent O O. C O + Pi 2 + ADP 3 + H H 2 C C SCoA malonyl-scoa
|
|
|
- Rosaline Henry
- 5 years ago
- Views:
Transcription
1 1 EXAM F SCIETIFIC CULTUE CEMISTY BLEM 1: BISYTESIS F FATTY ACIDS The biosynthesis of fatty acids proceeds by lengthening of a hydrocarbon chain by condensation of two-carbon units derived from acetyl-coenzyme A (noted acetyl-scoa). n 3 C C SCoA acetyl-scoa 3 C C 2 C 2 C n-1 + n CoAS This multi step process requires initially the biosynthesis of an intermediate, the malonyl-scoa, obtained through the reaction of the hydrogenocarbonate ion C 3 with acetyl-scoa. This reaction is coupled to the hydrolysis of AT into AD and the phosphate ion (i). In addition this reaction necessitates the intervention of biotin, a molecule that takes care of the «C 2» part, in order to allow the carboxylation of acetyl-scoa as shown in equation (1): (1) C 3 C SCoA acetyl-scoa biotin dependent + + AT 4 enzyme C + i 2 + AD 3 + C 3 2 C C SCoA malonyl-scoa The first part of this problem deals with the study of the biosynthesis of malonyl-scoa, and then in a second part the transformation sequences resulting in the formation of the hydrocarbon chain of fatty acids will be examined. 1.1 The bioavailability of carbon dioxide depends on its hydration state and the consecutive proton exchange reactions. Carbon dioxide gas, C 2(g), is initially dissolved in water (dissolved C 2 is noted without index) and then hydrated to form dihydrogenocarbonate 2 C 3 which is in turn involved in the acid-base reactions. The rate constants and thermodynamic values given below were measured in water at 300 K. k h = 0,03 s -1 : apparent rate constant of the hydration k d = 30 s -1 : rate constant of the dehydration pk a1 = 3,8 pk a2 = 10, Which form(s) of C 2 hydrated or not is/are predominant at p 7? At neutral p, the dissolution of C 2 in water occurs according to mechanism (a).
2 a) Given that the rate constant of the reaction between the ion C - 3 and a proton 3 + has a value of approximately mol -1 Ls -1, justify the application of a steady state approximation to 2 C 3. b) Deduce the expression for the rate of disappearance of C 2 dissolved in water according to mechanism (a) as a function of k h, k d, K a1 and the concentrations of the different species Given that the standard enthalpy associated to the acid-base reaction corresponding to the constant K a1 is equal to r 1 = 12 kj.mol -1, to which extent is the reaction at equilibrium changed when the temperature is raised? Justify your answer. 2 At p > 8, another mechanism (b) is in competition with the mechanism (a) for the formation of the hydrogenocarbonate ion: The following values are given at 300 K: k b = 8500 mol -1.L.s -1 k b = 2 x 10-4 s Write the rate expression of the disappearance of C 2 dissolved in water according to mechanism (b) Compare the reaction rates of the addition of - to C 2 at p = 8 and of 2 to C 2. ow can you interpret this result? In a biological environment the hydration reaction of dissolved C 2 is catalyzed by the enzyme carbonic anhydrase. This enzyme has a Zn 2+ ion as a metallic cofactor which is coordinated to three imidazole residues of histidine. The active site is schematized below showing the principal protagonists of the reaction: four imidazole residues of histidine (is), three water molecules, and the zinc ion. is is is Zn II is C 2
3 1.1.6 What is the effect of the Zn(II) cation on the acidity of the coordinated water molecule? Explain What is the role of the hydrogen bonding network represented in this scheme? 3 Biotin is a bicyclic compound, as shown below: (C1) (C2) S (C3) Biotine Indicate the absolute configurations of the numbered carbon atoms (C1), (C2) and (C3). Justify your answer Is biotin a chiral molecule? 1.2 C 3 is a very poor electrophile. Therefore, it is necessary to activate this ion in order to carboxylate biotin. C 3 is activated by phosphorylation by AT which results in the formation of a mixed anhydride carboxyphosphate. The biosynthesis of this mixed anhydride proceeds according to the concerted mechanism shown below, with the participation of a Mg 2+ ion: Glu(1) 2 2 Mg(II) Glu(2) Glu(3) Ad Glu(1) Mixed anhydride carboxyphosphate 2 Glu(2) 2 Glu(3) Mg(II) Ad The arrows translate a S2 type nucleophilic attack on the terminal phosphorous atom of AT. Which orbital of this phosphorus atom accepts the electrons of the nucleophile? What is the role of the glutamate residue Glu(1) in this reaction? Explain What is the role of the Mg 2+ ion in this reaction? Explain. The mixed anhydride formed in the reaction of the hydrogenocarbonate ion C 3 with AT transfers then its C 2 group to biotin in order to form the carboxybiotine as shown in the equation below: S 2 S Carboxybiotine
4 During this C 2 group transfer reaction one nitrogen atom of biotin is deprotonated. The pka value of urea in water is > Urea In the active site of the enzyme biotin is activated through the presence of an arginine residue among others as shown below: Arg S Which interactions are possible between the arginine residue and biotin? As a consequence of these interactions, what is the effect on the pka of the first deprotonation of biotin? 1.3 The biosynthesis of fatty acids is catalyzed by an enzymatic complex, the fatty acid synthase. The inhibition of this enzyme may have an important role in a multitude of therapies and in consequence there exists a major interest in the synthesis of new inhibitors. ne of these potential inhibitors is shown below: The synthesis of this type of inhibitor is presented below: Step 1 Step 2 Step 3 1) t-buli rotecting group (G) 2) C 1) t-buli 2)Bu 3 SnCl G Bu 3 Sn G Step 4 Step 5 (h 3 ) 4 d - G deprotection ArCl Ar Ar G Step 6 xidant Ar For the steps 1 and 2, specify briefly the role of each reagent ropose a role played by the palladium complex in step ropose an oxidant for the step 6.
5 5 EXAM F SCIETIFIC CULTUE CEMISTY BLEM 2: This exercise deals with the following synthetic pathway: Which base would you adopt to deprotonate the diene 1? Justify your answer Draw the mesomeric forms of the anion resulting from the reaction of the diene 1 with an appropriate base Draw the structure of the compound ropose a mechanism accounting for the transformation 1 3. Justify your choice A compound 3 which is a regioisomer of 3 can form under the reaction conditions. Draw the structure of this compound and indicate which one among 3 and 3 is at the most stable According to your opinion, is it better to work at low or high temperature to preferentially obtain 3? Justify your answer Draw in Cram representation the diol 4 which is required to get the compound ropose a mechanism accounting for the conversion of the compound 3 into the compound ow many chiral centers are contained in the compound 5? ow many stereoisomers of the compound 5 are obtained? Justify your answer.
6 2.3.1 What are the partial charges born by the nitrogen and bromine atoms in the BS molecule? Deduce the reactivity, either electrophilic or nucleophilic, of the bromine atom in this molecule From the answer to the preceding question, draw the intermediate, which is obtained after reaction of the double bond of the compound 5 with BS ropose a mechanism to form the compound 6 from the preceding intermediate Draw the Lewis representation of the borane molecule B 3 (boron atomic number: Z=5) Borane gives a complex with one tetrahydrofurane molecule. Draw its structure and propose an explanation to account for its formation Borane adds onto the double bond of the compound 6 and an alcohol function is introduced in the resulting product at the location of the boron atom during the oxidation step by hydrogen peroxide. ropose a structure for the product Briefly explain the nature of the reactions involved in the transformation of the compound 7 into the product Draw the structure of compound Justify the regioselectivity observed during the reaction with the 9 derivative. 2.6 ropose a reagent to obtain the compound 11. 6
7 7 EXAM F SCIETIFIC CULTUE CEMISTY BLEM 3: Menadione Q is a quinone that is the biological precursor of vitamine K. It can cause very important hemolytical problems for people with a deficiency in glucose-6-phosphate dehydrogenase. This phenomenon is partially inhibited by coumarins. menadione Q 4-hydroxy coumarin Complete the Lewis structure of the two molecules and indicate the hydrogen atoms and all non bonding electron pairs What are the necessary conditions for a molecule to be aromatic? Are menadione and 4- hydroxy coumarin aromatic? Justify your answer. Menadione Q can undergo two successive reduction reactions (a) and (b). The standard redox potentials of the two associated couples, (Q/Q - ) and (Q - /Q 2- ), are given below: (a) Q + e - Q - E (a) = -0,84V (b) Q - + e - Q 2- E (b) = -1,34V Write the Lewis structure of the semiquinonic radical Q - and the hydroquinone Q 2- and indicate the hydrogen atoms, the bonding and non-bonding electron pairs, as well as the charges Write the reaction that is thermodynamically spontaneous between the couples (Q/Q - ) and (Q - /Q 2- ) under standard conditions. Justify your answer Indicate the role of the semiquinonic radical Q - in the couples (Q/Q - ) and (Q - /Q 2- ) in the reaction that you wrote for the preceding question. The hydroquinone Q 2- may be protonated according to the successive equations (c) and (d) below: (c) Q Q K (c) = (d) Q Q K (d) = Calculate the pka values of the acid-base couples Q - /Q 2- and Q 2 /Q Given that the p in biological systems is about 7, as which predominant form exists the hydroquinone (Q 2-, Q - ou Q 2 ) at this p? 3.2 The derivatives of coumarin are compounds that have anticoagulant properties. The echmann reaction allows the access to a large number of compounds with a similar structure. ence, the
8 treatment of 1,3-diol benzene (or resorcinol) A with ethyl 3-oxobutanone (or ethyl acetylacetate) B in the presence of a strong acid results in the formation the 7-hydroxy-4- methylcoumarin C: 8 This synthesis can be decomposed into several steps and the first step is shown below: Justify the regioselectivity of the reaction of B on the aromatic cycle of A Identify the most electrophilic carbon atom in compound B. Justify your answer ropose a mechanism for the formation of the compound D in acidic medium ow many stereoisomers has compound D? Justify your answer. The dehydration step (D E) is followed by an isomerization (E F) as shown below: During the isomerization step E into F a carbocation is formed in acidic medium. Identify this carbocation and justify its exclusive formation compared to the formation of other potential carbocations Indicate the stereochemical relationship that exists between E and F. The last step is the cyclization of F in order to form 7-hydroxy-4- methylcoumarin C.
9 3.2.7 Write the reaction mechanism of the formation of C. 9
Theophylline (TH), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma.
 1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
EXAM OF SCIENTIFIC CULTURE MAJOR CHEMISTRY. CO 2 hydrogenation
 EXAM OF SCIETIFIC CULTURE MAJOR CHEMISTRY CO 2 hydrogenation One possibility to limit CO 2 imprint on the global warming is to reduce CO 2 to more usable forms such as hydrocarbons. These can serve as
EXAM OF SCIETIFIC CULTURE MAJOR CHEMISTRY CO 2 hydrogenation One possibility to limit CO 2 imprint on the global warming is to reduce CO 2 to more usable forms such as hydrocarbons. These can serve as
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
Topics in the November 2014 Exam Paper for CHEM1102
 November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
It s the amino acids!
 Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Name: CHEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!)
 ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
5. Reactions of Alkenes (text )
 2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
Catalysis. Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica
 Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
2. Which of the following are nucleophiles and which are electrophiles?
 Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Chapter 15: Enyzmatic Catalysis
 Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Hour Examination # 1
 CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
CHE 331 Molecular Science II
 CHE 331 Molecular Science II Exam 2 Form 1 Wednesday March 9, 2016 8:45 PM 10:15 PM 1. Write your nine digit University ID number in the space provided and then bubble in each of the nine digits. 2. Print
CHE 331 Molecular Science II Exam 2 Form 1 Wednesday March 9, 2016 8:45 PM 10:15 PM 1. Write your nine digit University ID number in the space provided and then bubble in each of the nine digits. 2. Print
ChemWiki BioWiki GeoWiki StatWiki PhysWiki MathWiki SolarWiki
 Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 8 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge Roadmap 8 In this chapter you will learn how Enzymes use
4 Examples of enzymes
 Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Lecture 12. Metalloproteins - II
 Lecture 12 Metalloproteins - II Metalloenzymes Metalloproteins with one labile coordination site around the metal centre are known as metalloenzyme. As with all enzymes, the shape of the active site is
Lecture 12 Metalloproteins - II Metalloenzymes Metalloproteins with one labile coordination site around the metal centre are known as metalloenzyme. As with all enzymes, the shape of the active site is
MODULE CODE: CHEM10003 ORGANIC CHEMISTRY 4 EXAM
 School of Science and Sport Physical Sciences, Chemistry Paisley Campus Session 2014-15 Trimester 2 MDULE CDE: CHEM10003 RGANIC CHEMISTRY 4 EXAM Date: 15th May 2015 Time: 10.00am 12.00pm Attempt THREE
School of Science and Sport Physical Sciences, Chemistry Paisley Campus Session 2014-15 Trimester 2 MDULE CDE: CHEM10003 RGANIC CHEMISTRY 4 EXAM Date: 15th May 2015 Time: 10.00am 12.00pm Attempt THREE
2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. (g) + O 2
 2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. N 2 (g) + 2 (g) 2N(g) equation 1.1 Under normal atmospheric conditions, a further reaction occurs
2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. N 2 (g) + 2 (g) 2N(g) equation 1.1 Under normal atmospheric conditions, a further reaction occurs
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
Page (Extra space) (4) Benzene can be converted into amine U by the two-step synthesis shown below.
 Q1. The hydrocarbons benzene and cyclohexene are both unsaturated compounds. Benzene normally undergoes substitution reactions, but cyclohexene normally undergoes addition reactions. (a) The molecule cyclohexatriene
Q1. The hydrocarbons benzene and cyclohexene are both unsaturated compounds. Benzene normally undergoes substitution reactions, but cyclohexene normally undergoes addition reactions. (a) The molecule cyclohexatriene
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
ORGANIC - BROWN 8E CH ALKENES AND REACTIONS OF ALKENES
 !! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
!! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
13.3A: The general mechanism for an aldol reaction
 Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry
 Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
CHERRY HILL TUITION OCR (SALTERS) CHEMISTRY A2 PAPER Answer all the questions. O, is formed in the soil by denitrifying bacteria. ...
 2 Answer all the questions. 1 itrous oxide gas, 2, is formed in the soil by denitrifying bacteria. (a) Give the systematic name for nitrous oxide. ne model of the bonding in nitrous oxide includes a dative
2 Answer all the questions. 1 itrous oxide gas, 2, is formed in the soil by denitrifying bacteria. (a) Give the systematic name for nitrous oxide. ne model of the bonding in nitrous oxide includes a dative
4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive charges:
 Mechanism Steps: Answer Key 1. Alcohols are nucleophilic and like to grab protons,, to form water molecules: 2. 3. 4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive
Mechanism Steps: Answer Key 1. Alcohols are nucleophilic and like to grab protons,, to form water molecules: 2. 3. 4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive
Lecture 14 (10/18/17) Lecture 14 (10/18/17)
 Lecture 14 (10/18/17) Reading: Ch6; 190-191, 194-195, 197-198 Problems: Ch6 (text); 7, 24 Ch6 (study guide-facts); 4, 13 NEXT Reading: Ch6; 198-203 Ch6; Box 6-1 Problems: Ch6 (text); 8, 9, 10, 11, 12,
Lecture 14 (10/18/17) Reading: Ch6; 190-191, 194-195, 197-198 Problems: Ch6 (text); 7, 24 Ch6 (study guide-facts); 4, 13 NEXT Reading: Ch6; 198-203 Ch6; Box 6-1 Problems: Ch6 (text); 8, 9, 10, 11, 12,
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
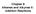 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems
 Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Alcohols: Contain a hydroxy group( OH) bonded to an sp 2 or sp 3 hybridized
 Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
Ethers. Chapter 14: Ethers, Epoxides, & Sulfides. General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
 Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
1. Addition of HBr to alkenes
 eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
p Bonds as Electrophiles
 Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
ORGANIC - CLUTCH CH ADDITION REACTIONS.
 !! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
!! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
Discussion Section (Day, Time): TF:
 ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
(a) (i) Use these data to show that benzene is 152 kj mol 1 more stable than the hypothetical compound cyclohexa 1,3,5 triene
 Q1.Equations for the hydrogenation of cyclohexene and of benzene, together with the enthalpies of hydrogenation, are shown. (a) (i) Use these data to show that benzene is 152 kj mol 1 more stable than
Q1.Equations for the hydrogenation of cyclohexene and of benzene, together with the enthalpies of hydrogenation, are shown. (a) (i) Use these data to show that benzene is 152 kj mol 1 more stable than
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
CATEDRA DE BIOCHIMIE ȘI BIOCHIMIE CLINICA INDICAȚIE METODICA FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6
 FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6 Analizată și aprobată la ședința catedrei din, proces verbal nr șeful catedrei de Biochimie și Biochimie Clinică, conferențiar universitar, doctor habilitat
FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6 Analizată și aprobată la ședința catedrei din, proces verbal nr șeful catedrei de Biochimie și Biochimie Clinică, conferențiar universitar, doctor habilitat
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
FIVE MEMBERED AROMATIC HETEROCYCLES
 FIVE MEMBERED AROMATIC HETEROCYCLES 63 Electrophilic aromatic substitution reaction of five membered aromatic heterocycles X = O, S or NH a-substitution b-substitution The Substitution is regioselective
FIVE MEMBERED AROMATIC HETEROCYCLES 63 Electrophilic aromatic substitution reaction of five membered aromatic heterocycles X = O, S or NH a-substitution b-substitution The Substitution is regioselective
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
3.2.9 Alkenes. Addition Reactions. 271 minutes. 268 marks. Page 1 of 35
 ..9 Alkenes Addition Reactions 71 minutes 68 marks Page 1 of 5 Q1. Propene reacts with bromine by a mechanism known as electrophilic addition. (a) Explain what is meant by the term electrophile and by
..9 Alkenes Addition Reactions 71 minutes 68 marks Page 1 of 5 Q1. Propene reacts with bromine by a mechanism known as electrophilic addition. (a) Explain what is meant by the term electrophile and by
Lecture 21 Cations, Anions and Hydrolysis in Water:
 2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
Chemistry Problem Set #9 Due on Thursday 11/15/18 in class.
 Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
Problem Points Score GSI I 30 II 21 III 28 IV 30 V 31 Total 140
 hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
hemistry 0 First ame Third Examination Last ame.5 h; 0 points please print clearly Dr. Kathleen olta ignature UM ID # PRIT TE FIRT LETTER F YUR LAT AME ERE: Problem Points core GI I 0 II III 8 IV 0 V Total
COURSE UNIT DESCRIPTION. Type of the course unit. Mode of delivery Period of delivery Language of instruction Face to face Autumn English
 Course unit title Organic Chemistry I Lecturer(s) Dr. Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery
Course unit title Organic Chemistry I Lecturer(s) Dr. Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Section Practice Exam II Solutions
 Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions.
 Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
