Chemistry Chapter 13
|
|
|
- Annabelle Lynch
- 5 years ago
- Views:
Transcription
1 Chemistry 2100 Chapter 13
2 Discovering Aromatics C C C C C C C 2 3 C C C C C C 3 De war 1867 Ladenburg 1878 C 6 6 Br 2 / Fe (-Br) C 6 5 Br Br 2 / Fe (-Br) C 6 4 Br 2 C 3 C 3 C 2 C 2 C Å 1.33 Å 1.39 Å
3 Aromatics
4 Resonance
5 Resonance
6 Resonance
7 Resonance
8 Resonance
9 Resonance
10 Resonance
11 Resonance
12 Resonance
13 Graphene
14 Carbon Nanotubes
15 Nomenclature for Arenes Many common names retained PA Naphthalene Anthracene Phenanthrene Benzo[a]pyrene
16 Nomenclature Monosubstituted alkylbenzenes are named as derivatives of benzene; for example, ethylbenzene. The IUPAC system retains certain common names for several of the simpler monosubstituted alkylbenzenes. C 2 C 3 C 3 C=C 2 Ethylbenzene Toluene Styrene
17 Nomenclature The common names for these monosubstituted benzenes are also retained O OC 3 N 2 O C- O C-O Phenol Anisole Aniline Benzaldehyde Benzoic acid Phenyl group (C or Ph-): The substituent group derived by removal of an from benzene. C Phenyl group 1-Phenylcyclohexene 4-Phenyl-1-butene
18 Nomenclature When two substituents occur on a benzene ring, three isomers are possible; they may be located by: numbering the atoms of the ring or using the locators ortho (o), meta (m), and para (p). 1 COO Br 2 1 C C 2 C Bromobenzoic acid (o-bromobenzoic acid) C 3 1,3-Dimethylbenzene (m-xylene) 3 1 Cl 1-Chloro-4-ethylbenzene (p-chloroethylbenzene) 2
19 Aromatic Reactions
20 Reactions of Benzene By far the most characteristic reaction of aromatic compounds is substitution at a ring carbon. This reaction is called aromatic substitution. Some groups that can be introduced directly on the ring are the halogens, the nitro (-NO 2 ) group, and the sulfonic acid (- SO 3 ) group. alogenation: FeCl + Cl 3 2 Cl + Cl Benzene Chlorobenzene 20!
21 Phenols The functional group of a phenol is a hydroxyl ( -O) group bonded to a benzene ring. Name substituted phenols either as derivatives of phenol or by common names. O O O O O O Phenol 3-Methylphenol (m-cresol) 1,2-Benzenediol (Catechol) O 1,3-Benzenediol (Resorcinol) O 1,4-Benzenediol (ydroquinone) 21!
22 Noteworthy Phenols O Cl O O Cl C 3 O eugenol O Cl Cl C 2 Cl Cl O hexachlorop hene hexylresorc inol O O O O O CON 2 O Cl O C 3 N(C3 ) 2 O O tetrahydrocannabinol aur eomycin
23 Phenol Acidity Acidity: O C 3 C O > > O C 3 O acids > thiols ~ phenols > water ~ alcohols Comparative acidities of 0.1 M aqueous solutions of representative acids A Cl OAc PhO EtS EtO O K a % ionized [ 3 O + ], M p ~ ~100 ~ acids > phenols ~ thiols > water ~ alcohols
24 Phenols as Antioxidants Autoxidation C 2 C=C-C Section of a fatty acid hydrocarbon chain light or heat C 2 C=C-C A carbon radical O-O C 2 C=C-C + O-O C 2 C=C-C Oxygen is a diradical A hydroperoxy radical O-O C 2 C=C-C + C 2 C=C-C Section of a new fatty acid hydrocarbon chain O-O- C 2 C=C-C -C 2 A hy droperoxide + C 2 C=C-C A new carbon radical
25 Natural Phenolic Antioxidants C 3 C C 3 O 3 C 3 O C 3 C 3 C 3 C 3 Vitamin E (α-tocopherol) ( α toc opherol) O O Resveratrol O
26 Man-made Phenolic Antioxidants O O O C 3 BT OC 3 BA O C OC 3 me thyl par aben
27 Phenols as Weapons?
28 COO C 2 C N 2 p henyla lanine
29 COO C 2 C N 2 p henyla lanine
30 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine
31 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] O COO C 2 C N 2 O L-DOPA
32 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] O COO C 2 C N 2 O L-DOPA
33 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] COO O C 2 C 2 N 2 O C 2 C N 2 -CO 2 O dopamine O L-DOPA
34 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] COO O C 2 C 2 N 2 O C 2 C N 2 -CO 2 O dopamine O L-DOPA [O] O O C C 2 N 2 O nor epinephrine
35 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] COO O C 2 C 2 N 2 O C 2 C N 2 -CO 2 O dopamine O L-DOPA [O] O O O C C 2 N 2 "C 3 " O C C 2 N C 3 O nor epinephrine O epinephrine
36 COO C 2 C N 2 [O] COO C 2 C N 2 p henyla lanine O tyrosine [O] COO O C 2 C 2 N 2 O C 2 C N 2 -CO 2 O dopamine O L-DOPA [O] O O O C C 2 N 2 "C 3 " O C C 2 N C 3 O nor epinephrine O epinephrine
37 C C N "phenethylamine"
38 O C C N C 3 C 3 ephedrine (ephedra) / pseudoephedrine
39 O C C N C 3 C 3 ephedrine (ephedra) / pseudoephedrine
40 C C N 2 C 3 C 3 methylenedioxymethamphetamine / MDMA / Ecstacy
41 O C2 C N C 3 O C 3 methylenedioxymethamphetamine / MDMA / Ecstacy
42 O C2 C N C 3 O C 3 methylenedioxymethamphetamine / MDMA / Ecstacy
43 O C2 C N C 3 O C 3 methylenedioxymethamphetamine / MDMA / Ecstacy
44 Organic Food Dye: Carminic Acid O O O O COO O O O carminic ac id O
45 Aromatic eterocycles O C N 2 N N N C 3 niacin (B 3 ) / nicotinamide nicotine N N 2 Cl N C 2 S C 3 C 2 C 2 O ()C 3 N O N C 3 C 3 N O N N thiamine (B 1 ) C 3 caffeine (theobromine)
46 eterocycles store life s information () C 3 O Sugar N N O (U) T = A
47 () C 3 O N N Sugar N N O N N N Sugar (U) T = A
BRCC CHM 102 Class Notes Chapter 13 Page 1 of 6
 BRCC CHM 102 ass Notes Chapter 13 Page 1 of 6 Chapter 13 Benzene and Its Derivatives aliphatic hydrocarbons include alkanes, alkenes, and alkynes aromatic hydrocarbons compounds that contain one or more
BRCC CHM 102 ass Notes Chapter 13 Page 1 of 6 Chapter 13 Benzene and Its Derivatives aliphatic hydrocarbons include alkanes, alkenes, and alkynes aromatic hydrocarbons compounds that contain one or more
BENZENE AND AROMATIC COMPOUNDS
 BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
Naming Aromatic Compounds (Benzene as the Parent)
 aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
Seminar_3. 1. Substituded derivatives of benzene and their nomenclature
 1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
1. Substituded derivatives of benzene and their nomenclature 2. Reactions of arenes. Electrophilic aromatic substitutions 3. Activating substituents. Orientation in the aromatic ring Seminar_3 TEST - Aromatic
Chapter 21. Phenols and Aryl Halides. Nucleophilic Aromatic Substitution. Ch. 21-1
 Chapter 21 Phenols and Aryl alides Nucleophilic Aromatic Substitution Ch. 21-1 1. Structure and Nomenclature of Phenols Phenol 1-Naphthol (α-naphthol) 9-Phenanthrol Ch. 21-2 1A. Nomenclature of Phenols
Chapter 21 Phenols and Aryl alides Nucleophilic Aromatic Substitution Ch. 21-1 1. Structure and Nomenclature of Phenols Phenol 1-Naphthol (α-naphthol) 9-Phenanthrol Ch. 21-2 1A. Nomenclature of Phenols
Chapter 16 Aromatic Compounds. Discovery of Benzene
 Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C: ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C: ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 8 Dr Ali El-Agamey Nomenclature of Benzene Derivatives Nomenclature of Benzene Derivatives To name a benzene ring with one substituent, name
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 8 Dr Ali El-Agamey Nomenclature of Benzene Derivatives Nomenclature of Benzene Derivatives To name a benzene ring with one substituent, name
Chemistry B11 Chapters Alkanes, Alkenes, Alkynes and Benzene
 Chapters 10-11 Alkanes, Alkenes, Alkynes and Benzene Organic compounds: organic chemistry is the chemistry of carbon and only a few other elements-chiefly, hydrogen, oxygen, nitrogen, sulfur, halogens,
Chapters 10-11 Alkanes, Alkenes, Alkynes and Benzene Organic compounds: organic chemistry is the chemistry of carbon and only a few other elements-chiefly, hydrogen, oxygen, nitrogen, sulfur, halogens,
Phenols, Ethers, and Organic Sulfur Compound
 Phenols, Ethers, and rganic Sulfur Compound Phenols - Structure General Structure - A hydroxy () group attached directly to an aromatic ring: Phenol α-naphthol β-naphthol Note: C2 is not a phenol. Phenols
Phenols, Ethers, and rganic Sulfur Compound Phenols - Structure General Structure - A hydroxy () group attached directly to an aromatic ring: Phenol α-naphthol β-naphthol Note: C2 is not a phenol. Phenols
Lecture 10. More Aromatics. February 18, Chemistry 328N
 Lecture 10 More Aromatics February 18, 2016 eterocyclic Aromatic Compounds N N O S Pyridine Pyrrole Furan Thiophene ückel and Pyridine ückel and Pyrrole uckel and Furan Recognizing Aromatic Compounds Be
Lecture 10 More Aromatics February 18, 2016 eterocyclic Aromatic Compounds N N O S Pyridine Pyrrole Furan Thiophene ückel and Pyridine ückel and Pyrrole uckel and Furan Recognizing Aromatic Compounds Be
Chapter 5. Aromatic Compounds
 Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chemistry 204: Benzene and Aromaticity
 Chemistry 204: Benzene and Aromaticity Structure of and Bonding in Benzene benzene, C 6 H 6, was first isolated in 1825 (Michael Faraday), but it was not until more than 100 years later that an adequate
Chemistry 204: Benzene and Aromaticity Structure of and Bonding in Benzene benzene, C 6 H 6, was first isolated in 1825 (Michael Faraday), but it was not until more than 100 years later that an adequate
Chapter 4: Aromatic Compounds. Bitter almonds are the source of the aromatic compound benzaldehyde
 Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
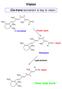 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Electrophilic Aromatic Substitution (Aromatic compounds) Ar-H = aromatic compound 1. Nitration Ar-H + HNO 3, H 2 SO 4 Ar-NO 2 + H 2 O 2.
 Electrophilic Aromatic Substitution (Aromatic compounds) Ar- = aromatic compound 1. Nitration Ar- + NO 3, 2 SO 4 Ar- + 2 O 2. Sulfonation Ar- + 2 SO 4, SO 3 Ar-SO 3 + 2 O 3. alogenation Ar- + X 2, Fe Ar-X
Electrophilic Aromatic Substitution (Aromatic compounds) Ar- = aromatic compound 1. Nitration Ar- + NO 3, 2 SO 4 Ar- + 2 O 2. Sulfonation Ar- + 2 SO 4, SO 3 Ar-SO 3 + 2 O 3. alogenation Ar- + X 2, Fe Ar-X
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
Chapter 09 Benzene and Its Derivatives
 Chapter 09 Benzene and Its Derivatives Benzene First isolated in 1825 from whale oil by Michael Faraday Unsaturated hydrocarbon but did not have the typical reactivity of alkenes or alkynes. CM 240: Fall
Chapter 09 Benzene and Its Derivatives Benzene First isolated in 1825 from whale oil by Michael Faraday Unsaturated hydrocarbon but did not have the typical reactivity of alkenes or alkynes. CM 240: Fall
CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION A B C D E
 CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION 1. Consider carefully the mechanism of the following electrophilic aromatic substitution reaction and indicate which of
CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION 1. Consider carefully the mechanism of the following electrophilic aromatic substitution reaction and indicate which of
Chapter 17 Reactions of Aromatic Compounds
 rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
Arenes. i) It is cyclic, planar, and all atoms must have at least one unhybridized p orbital.
 Arenes An arene is a molecule that meets the following criteria: i) It is cyclic, planar, and all atoms must have at least one unhybridized p orbital. ii) It contains at least 2, usually carbon-carbon,
Arenes An arene is a molecule that meets the following criteria: i) It is cyclic, planar, and all atoms must have at least one unhybridized p orbital. ii) It contains at least 2, usually carbon-carbon,
CH223. Objectives. Domino effect. The secret. Energy consideration O. Kay Sandberg, Welcome Ph.D. Organic chemistry: What? C students (at best) Fred
 Kay Sandberg, Welcome Ph.D. This lecture s objectives: bjectives ) Review 0, basic concepts ) Introduce arenes & aromaticity Get started on the right foot! Strategy for success Domino effect Understanding
Kay Sandberg, Welcome Ph.D. This lecture s objectives: bjectives ) Review 0, basic concepts ) Introduce arenes & aromaticity Get started on the right foot! Strategy for success Domino effect Understanding
Alkenes, Alkynes and Aromatic
 BIO-ORGANI EMISTRY (Organic hemistry for Biology Students) (SQBS 1603) Alkenes, Alkynes and Aromatic Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. hem.)(utm), MSc (hem)(utm), PhD (hem)(utm), A.M.I. Senior
BIO-ORGANI EMISTRY (Organic hemistry for Biology Students) (SQBS 1603) Alkenes, Alkynes and Aromatic Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. hem.)(utm), MSc (hem)(utm), PhD (hem)(utm), A.M.I. Senior
2. Which of the following is NOT an electrophile in an electrophilic aromatic substitution reaction? A) NO 2
 Name: CHEM 226 Practice Quiz 3 Chapter 4-Aromatic Compounds and Chapter 7- Alcohols, Phenols and Thiols Attempt all questions showing your answers and work clearly for full and partial credits 1. Which
Name: CHEM 226 Practice Quiz 3 Chapter 4-Aromatic Compounds and Chapter 7- Alcohols, Phenols and Thiols Attempt all questions showing your answers and work clearly for full and partial credits 1. Which
Benzenes & Aromatic Compounds
 Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
William H. Brown & Christopher S. Foote
 William. Brown & Christopher S. Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, arcourt Brace & Company, 6277 Sea arbor Drive, Orlando,
William. Brown & Christopher S. Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, arcourt Brace & Company, 6277 Sea arbor Drive, Orlando,
Chemical Reactions of Unsaturated Compounds
 hemical Reactions of Unsaturated ompounds hemical Reactions of Alkenes hemical Reactions of Alkenes Structure of the Double Bond in Alkenes The two components of the double bond are pictured the same,
hemical Reactions of Unsaturated ompounds hemical Reactions of Alkenes hemical Reactions of Alkenes Structure of the Double Bond in Alkenes The two components of the double bond are pictured the same,
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
Reactions of Aromatic Compounds
 2-1 Reactions of Aromatic Compounds 15.1 2-2 lectrophilic Aromatic Substitution Reactions Aromatic hydrocarbons (= arenes) undergo a substitution reaction with electrophiles: + catalyst + xample: omination
2-1 Reactions of Aromatic Compounds 15.1 2-2 lectrophilic Aromatic Substitution Reactions Aromatic hydrocarbons (= arenes) undergo a substitution reaction with electrophiles: + catalyst + xample: omination
Benzene and aromaticity
 aromaticity The word "benzene" derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 15th century
aromaticity The word "benzene" derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 15th century
Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Reactivity of Benzene
 hapter 16 hemistry of Benzene: Electrophilic Aromatic Substitution Reactivity of Benzene - stabilization due to aromaticity makes benzene significantly less reactive than isolated alkenes 2 no reaction
hapter 16 hemistry of Benzene: Electrophilic Aromatic Substitution Reactivity of Benzene - stabilization due to aromaticity makes benzene significantly less reactive than isolated alkenes 2 no reaction
15.10 Effect of Substituents on Reactivity and Orientation
 15.10 ffect of Substituents on Reactivity and Orientation Z NO 3 2 SO 4 Z Z Z + + o- p- m- Z O Me CN o(%) 40 59 30 6 17 p(%) 60 37 69
15.10 ffect of Substituents on Reactivity and Orientation Z NO 3 2 SO 4 Z Z Z + + o- p- m- Z O Me CN o(%) 40 59 30 6 17 p(%) 60 37 69
IUPAC Nomenclature Chem12A, Organic Chemistry I
 IUPAC Nomenclature ChemA, rganic Chemistry I IUPAC PEFIXES Prefix Substituent Group Number of Carbons meth- methyl eth- ethyl prop- propyl but- butyl pent- pentyl hex- hexyl hept- heptyl 7 oct- octyl 8
IUPAC Nomenclature ChemA, rganic Chemistry I IUPAC PEFIXES Prefix Substituent Group Number of Carbons meth- methyl eth- ethyl prop- propyl but- butyl pent- pentyl hex- hexyl hept- heptyl 7 oct- octyl 8
4. AROMATIC COMPOUNDS
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
 . 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
. 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings.
 766 hapter 16 Electrophilic Attack on Derivatives of enzene Problems 30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain
766 hapter 16 Electrophilic Attack on Derivatives of enzene Problems 30. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain
Lecture 24 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 24 Organic Chemistry 1 Professor Duncan Wardrop April 6, 2010 1 Which shorthand orbital diagram best represents the LUMO of a dienophile in a Diels-Alder
CEM 232 Organic Chemistry I at Chicago Lecture 24 Organic Chemistry 1 Professor Duncan Wardrop April 6, 2010 1 Which shorthand orbital diagram best represents the LUMO of a dienophile in a Diels-Alder
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution
 Lecture 12 Electrophilic Aromatic Substitution E E February 22, 2018 Electrophilic Aromatic Substitution Electrophilic aromatic substitution: a reaction in which a hydrogen atom on an aromatic ring is
Lecture 12 Electrophilic Aromatic Substitution E E February 22, 2018 Electrophilic Aromatic Substitution Electrophilic aromatic substitution: a reaction in which a hydrogen atom on an aromatic ring is
Frost Circles a Great Trick
 Aromatics Frost Circles a Great Trick Inscribe a polygon of the same number of sides as the ring to be examined such that one of the vertices is at the bottom of the ring The relative energies of the MOs
Aromatics Frost Circles a Great Trick Inscribe a polygon of the same number of sides as the ring to be examined such that one of the vertices is at the bottom of the ring The relative energies of the MOs
Fundamentals of Organic Chemistry
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
08. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16
 08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
MOLECULER MODELS/ISOMERS ORGANIC STRUCTURES AND NAMING
 REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
Benzene and Its Derivatives
 9 Benzene and Its Derivatives Peppers of the capsicum family. ot peppers contain significant amounts of the chemical capsaicin, which is used for medicinal purposes as well as for tantalizing taste buds
9 Benzene and Its Derivatives Peppers of the capsicum family. ot peppers contain significant amounts of the chemical capsaicin, which is used for medicinal purposes as well as for tantalizing taste buds
Chapter 13: Unsaturated Hydrocarbons
 Chemistry 121(01) Winter 2009 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@chem.latech.edu Office: 311 Carson Taylor all ; Phone:
Chemistry 121(01) Winter 2009 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@chem.latech.edu Office: 311 Carson Taylor all ; Phone:
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
NAME PER DATE DUE ACTIVE LEARNING IN CHEMISTRY EDUCATION CHAPTER 27 INTRODUCTION TO ORGANIC COMPOUNDS. (Part 3) , A.J.
 NAME PER DATE DUE ACTIVE LEARNING IN CEMISTRY EDUCATION CAPTER 27 INTRODUCTION TO ORGANIC COMPOUNDS (Part 3) 27-1 1997, A.J. Girondi NOTICE O RIGTS All rights reserved. No part of this document may be
NAME PER DATE DUE ACTIVE LEARNING IN CEMISTRY EDUCATION CAPTER 27 INTRODUCTION TO ORGANIC COMPOUNDS (Part 3) 27-1 1997, A.J. Girondi NOTICE O RIGTS All rights reserved. No part of this document may be
Benzene. Named benzene by Eilhardt Mitscherlich in < Molecular formula: C 6 H 6. < Representative of the aromatic hydrocarbon family:
 Benzene < Discovered in 1825 by Michael Faraday: e called it bicarburet of hydrogen. Named benzene by Eilhardt Mitscherlich in 1833. < Molecular formula: C 6 6 < Representative of the aromatic hydrocarbon
Benzene < Discovered in 1825 by Michael Faraday: e called it bicarburet of hydrogen. Named benzene by Eilhardt Mitscherlich in 1833. < Molecular formula: C 6 6 < Representative of the aromatic hydrocarbon
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or
 Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
H 2 SO 4 Ar-NO 2 + H2O
 Phenyl group: Shorthand for phenyl: Ph, C 6 5,. An aryl group is an aromatic group: phenyl, substituted phenyl, or other aromatic group. Shorthand: Ar Generalized electrophilic aromatic substitution: E
Phenyl group: Shorthand for phenyl: Ph, C 6 5,. An aryl group is an aromatic group: phenyl, substituted phenyl, or other aromatic group. Shorthand: Ar Generalized electrophilic aromatic substitution: E
Nitration of (Trifluoromethyl( Trifluoromethyl)benzene CF 3 HNO 3 + +
 Effect on Rate Rate and Regioselectivity in Electrophilic Aromatic Substitution A substituent already present on the ring affects both the rate and regioselectivity of electrophilic aromatic substitution.
Effect on Rate Rate and Regioselectivity in Electrophilic Aromatic Substitution A substituent already present on the ring affects both the rate and regioselectivity of electrophilic aromatic substitution.
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
2 ethane CH 3 CH 3. 3 propane CH 3 CH 2 CH 3
 #100 Notes Unit 12: Introduction to Organic and Biochemistry Ch. Organic/ Biochemistry I. Alkanes, C n H 2n+2 (saturated hydrocarbons: no C=C or C C) *always 4 bonds on carbon # Carbons parent chain name
#100 Notes Unit 12: Introduction to Organic and Biochemistry Ch. Organic/ Biochemistry I. Alkanes, C n H 2n+2 (saturated hydrocarbons: no C=C or C C) *always 4 bonds on carbon # Carbons parent chain name
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
NBS, CCl 4 heat A B C D
 1. What is(are) the expected product(s) of the following reaction? 2 C=CC( ) 2 NBS, CCl 4 heat A B C D 1) only B 2) only C 3) A and C 4) B and D 2. Which of the following is the 1,4-addition product in
1. What is(are) the expected product(s) of the following reaction? 2 C=CC( ) 2 NBS, CCl 4 heat A B C D 1) only B 2) only C 3) A and C 4) B and D 2. Which of the following is the 1,4-addition product in
Chapter 15 Benzene and Aromaticity
 Chapter 15 Benzene and Aromaticity Aromatic Compounds Aromatic Originally used to describe fragrant substances Refers to a class of compounds that meets Hückel criteria for aromaticity 2 Aromatic Compounds
Chapter 15 Benzene and Aromaticity Aromatic Compounds Aromatic Originally used to describe fragrant substances Refers to a class of compounds that meets Hückel criteria for aromaticity 2 Aromatic Compounds
March 08 Dr. Abdullah Saleh
 March 08 Dr. Abdullah Saleh 1 Effects of Substituents on Reactivity and Orientation The nature of groups already on an aromatic ring affect both the reactivity and orientation of future substitution Activating
March 08 Dr. Abdullah Saleh 1 Effects of Substituents on Reactivity and Orientation The nature of groups already on an aromatic ring affect both the reactivity and orientation of future substitution Activating
Organic Chemistry II A KEY February 24, 2011
 rganic Chemistry II A KEY ebruary 24, 2011 Exam 1: VERI A 1. Which of the following reagent(s) (or set of reagents) could react with atorvastatin (LIPITR) as starting material? D or E Atvorvastatin (LIPITR)
rganic Chemistry II A KEY ebruary 24, 2011 Exam 1: VERI A 1. Which of the following reagent(s) (or set of reagents) could react with atorvastatin (LIPITR) as starting material? D or E Atvorvastatin (LIPITR)
Benzene and Aromatic Compounds
 1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
Unsaturated hydrocarbons. Chapter 13
 Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
Reading Skill Practice
 system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
ORGANIC - BROWN 8E CH. 22- REACTIONS OF BENZENE AND ITS DERIVATIVES
 !! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
!! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
More EAS. Lecture 12. Di- and Polysubstitution CH 3 + H + H HNO 2 NO 2. February 25, /25/16 OCH 3 OCH OCH. o-nitro-anisole (31%) Anisole
 Lecture 12 More EAS February 25, 2016 Di- and Polysubstitution O O OC OC 3 3 NO 3 2 SO 4 Anisole o-nitro-anisole (31%) m-nitro-anisole (2%) p-nitro-anisole (67%) l -O is ortho-para directing and activating
Lecture 12 More EAS February 25, 2016 Di- and Polysubstitution O O OC OC 3 3 NO 3 2 SO 4 Anisole o-nitro-anisole (31%) m-nitro-anisole (2%) p-nitro-anisole (67%) l -O is ortho-para directing and activating
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
REACTIONS OF AROMATIC COMPOUNDS
 A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
Chapter 17: Reactions of Aromatic Compounds
 1 Chapter 17: Reactions of Aromatic Compounds I. Introduction to Electrophilic Aromatic Substitution (EAS) A. General Mechanism II. Reactions of Electrophilic Aromatic Substitution A. Halogenation (E =
1 Chapter 17: Reactions of Aromatic Compounds I. Introduction to Electrophilic Aromatic Substitution (EAS) A. General Mechanism II. Reactions of Electrophilic Aromatic Substitution A. Halogenation (E =
Chapter 19: Benzene and Aromatic Substitution Reactions [Sections: 18.2, 18.6; ]
![Chapter 19: Benzene and Aromatic Substitution Reactions [Sections: 18.2, 18.6; ] Chapter 19: Benzene and Aromatic Substitution Reactions [Sections: 18.2, 18.6; ]](/thumbs/96/128021536.jpg) Chapter 19: Benzene and Aromatic Substitution eactions [Sections: 18.2, 18.6; 19.1-19.12] omenclature of Substituted Benzenes i. Monosubstituted Benzenes C 2 C 3 ii. Disubstituted Benzenes X X X Y Y Y
Chapter 19: Benzene and Aromatic Substitution eactions [Sections: 18.2, 18.6; 19.1-19.12] omenclature of Substituted Benzenes i. Monosubstituted Benzenes C 2 C 3 ii. Disubstituted Benzenes X X X Y Y Y
NAME PER DATEDUE ACTIVE LEARNING IN CHEMISTRY EDUCATION CHAPTER 27 INTRODUCTION TO ORGANIC COMPOUNDS. (Part 3) A.J.
 ACTIVE LEARNING IN CEMISTRY EDUCATION (Part 3) COMPOUNDS TO ORGANIC INTRODUCTION CAPTER 27 NAME PER DATEDUE 27-1 @1997k A.J. Giroridi SECTiON 27.1 Aromatic Compounds An important class of carbon compounds
ACTIVE LEARNING IN CEMISTRY EDUCATION (Part 3) COMPOUNDS TO ORGANIC INTRODUCTION CAPTER 27 NAME PER DATEDUE 27-1 @1997k A.J. Giroridi SECTiON 27.1 Aromatic Compounds An important class of carbon compounds
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
Examples of Substituted Benzenes
 Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Chem 261 Dec 6, 2017
 209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
Introduction to Organic Chemistry. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
Organic Chemistry. February 18, 2014
 Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Circle those of the following structures which you would expect to show aromaticity. N + -
 Sample Exam Solution CEM 263, Section B2 1 (6 pts) Aromaticity Circle those of the following structures which you would expect to show aromaticity. - N - N 2. (12 pts) Structure and Nomenclature Draw structures
Sample Exam Solution CEM 263, Section B2 1 (6 pts) Aromaticity Circle those of the following structures which you would expect to show aromaticity. - N - N 2. (12 pts) Structure and Nomenclature Draw structures
3 free radical is most stable. Q.5. A + Cl 2 hv monochloro product To maximize the yield of monochloro product in the above reaction? Cl 2 must be add
 Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
Functional Groups. Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity.
 Functional Groups Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity. Organic halides: a hydrogen is replaced by a halogen fluoro-,
Functional Groups Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity. Organic halides: a hydrogen is replaced by a halogen fluoro-,
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Lecture 10. More Aromatics. February 15, Chemistry 328N
 Lecture 10 More Aromatics February 15, 2018 ückel's Rule for Aromaticity To be Aromatic a compound must : 1. be Cyclic 2. have one P orbital on each atom in the ring 3. be planar or nearly so to give orbital
Lecture 10 More Aromatics February 15, 2018 ückel's Rule for Aromaticity To be Aromatic a compound must : 1. be Cyclic 2. have one P orbital on each atom in the ring 3. be planar or nearly so to give orbital
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives
 C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
11/26/ Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds
 9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
Chapter 16: Aromatic Compounds
 Chamras Chemistry 106 Lecture otes xamination 2 Materials Chapter 16: Aromatic Compounds Benzene, the Most Commonly Known Aromatic Compound: The aromatic nature of benzene stabilizes it 36 kcal.mol 1.
Chamras Chemistry 106 Lecture otes xamination 2 Materials Chapter 16: Aromatic Compounds Benzene, the Most Commonly Known Aromatic Compound: The aromatic nature of benzene stabilizes it 36 kcal.mol 1.
Organic Chemistry. Saturated Hydrocarbons: The Alkanes. ethane H C C H CH 3 CH 3
 rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
TOPIC 2. REACTIONS OF AROMATIC COMPOUNDS (Chapters 15, parts of 20, and 21)
 L TPIC 2. REACTINS F ARMATIC CMPUNDS (Chapters 15, parts of 20, and 21) Add e.g. of SNAr, replace aniline example, turn BT into and example L TPIC 2. TC PAIN KILLERS BJECTIVES 1. Describe the reactions
L TPIC 2. REACTINS F ARMATIC CMPUNDS (Chapters 15, parts of 20, and 21) Add e.g. of SNAr, replace aniline example, turn BT into and example L TPIC 2. TC PAIN KILLERS BJECTIVES 1. Describe the reactions
