Net Ionic Equation Worksheet
|
|
|
- Hubert Bond
- 5 years ago
- Views:
Transcription
1 Honors Chemistry Net Ionic Equation Worksheet Name Period READ THIS: When two solutions of ionic compounds are mixed, a solid may form. This type of reaction is called a precipitation reaction, and the solid produced in the reaction is known as the precipitate. You can predict whether a precipitate will form using a list of solubility rules such as those found in the table below. When a combination of ions is described as insoluble, a precipitate forms. There are three types of equations that are commonly written to describe a precipitation reaction. The molecular equation shows each of the substances in the reaction as compounds with physical states written next to the chemical formulas. The complete ionic equation shows each of the aqueous compounds as separate ions. Insoluble substances are not separated and these have the symbol (s) written next to them. Water is also not separated and it has a (l) written next to it. Notice that there are ions that are present on both sides of the reaction arrow > that is, they do not react. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. The remaining equation is known as the net ionic equation. For example: The reaction of potassium chloride and lead II nitrate 2KCl (aq) + Pb(NO 3) 2 (aq) -> 2KNO 3 (aq) + PbCl 2 (s) 2K + (aq) + 2Cl - (aq) + Pb 2+ (aq) + 2NO 3 (aq) -> 2K + (aq) + 2NO3 (aq) + PbCl 2 (s) 2Cl - (aq) + Pb 2+ (aq) -> PbCl 2 (s) Directions: Write balanced molecular, ionic, and net ionic equations for each of the following reactions. Assume all reactions occur in aqueous solution. Include states of matter in your balanced equation. 1. Sodium chloride and lead II nitrate 2. Sodium carbonate and Iron II chloride
2 3. Magnesium hydroxide and hydrochloric acid 4. Potassium chromate and calcium chloride 5. Ammonium phosphate and zinc nitrate 6. Lithium hydroxide and barium chloride
3 7. Sodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water 8. Magnesium nitrate and sodium chromate 9. Iron III chloride and magnesium metal 10. Barium Bromide and sodium sulfate
4 11. Silver nitrate and magnesium iodide 12. Ammonium chromate and aluminum perchlorate 13. Nickel nitrate and sodium hydroxide
5 14. Hydrobromic acid (HBr) and lead II perchlorate 15. Potassium fluoride and magnesium nitrate 16. Sodium phosphate and nickel II perchlorate 17. Copper II chloride and silver acetate
6 Net Ionic Equation Worksheet - answers 1. 2NaCl(aq) + Pb(NO3)2(aq) PbCl2(s) + 2NaNO3(aq) Ionic Equation: 2Na + (aq) + 2Cl - (aq) + Pb 2+ (aq) + 2NO3 - (aq) PbCl2(s) + 2Na + (aq) + 2NO3 - (aq) 2Cl - (aq) + Pb 2+ (aq) PbCl2(s) 2. Na2CO3(aq) + FeCl2(aq) FeCO3(s) + 2NaCl(aq) Ionic Equation: 2Na + (aq) + CO3 2- (aq) + Fe 2+ (aq) + 2Cl - (aq) FeCO3(s) + 2Na + (aq) + 2 Cl - (aq) CO3 2- (aq) + Fe 2+ (aq) FeCO3(s) 3. Mg(OH)2(aq) + 2HCl(aq) MgCl2(aq) + 2 H2O(l) Ionic Equation: Mg 2+ (aq) + 2 OH - (aq) + 2 H + (aq) + 2 Cl - (aq) Mg 2+ (aq) + 2 Cl - (aq) + 2 H2O(l) 2 OH - (aq) + 2 H + (aq) 2 H2O(l) (your final answer would be: OH - (aq) + H + (aq) H2O(l) ) 4. K2CrO4 (aq) + CaCl2 (aq) 2 KCl(aq) + CaCrO4 (aq) Ionic Equation: 2 K + (aq) + CrO4 2- (aq) + Ca 2+ (aq) + 2Cl - (aq) 2 K + (aq) + 2 Cl - (aq) + Ca 2+ + CrO4 2- N/A, all spectator ions 5. 2 (NH4)3PO4(aq) + 3 Zn(NO3)2(aq) 6 NH4NO3(aq) + Zn3(PO4)2(s) Ionic Equation: 6 NH4 + (aq) + 2 PO4 3- (aq) + 3 Zn 2+ (aq) + 6 NO3 - (aq) 6 NH4 + (aq) + 6NO3 - (aq) + Zn3(PO4)2(s) 2 PO4 3- (aq) + 3 Zn 2+ (aq) Zn3(PO4)2(s) 6. 2 LiOH(aq) + BaCl2(aq) 2 LiCl(aq) + Ba(OH)2(aq) Ionic Equation: 2 Li + (aq) + 2 OH - (aq) + Ba 2+ (aq) + 2 Cl - (aq) 2 Li + (aq) + 2 Cl - (aq) + Ba OH - N/A, all spectator ions 7. Na2CO3(aq) + 2 HCl(aq) 2 NaCl(aq) + CO2(g) + H2O(l) Ionic Equation: 2 Na + (aq) + CO3 2- (aq) + 2 H + (aq) + 2 Cl - (aq) 2 Na + (aq) + 2 Cl - (aq) + CO2(g) + H2O(l) CO3 2- (aq) + 2 H + (aq) CO2(g) + H2O(l) 8. Mg(NO3)2(aq) + Na2CrO4(aq) 2NaNO3(aq) + MgCrO4(s) Ionic Equation: Mg 2+ (aq) + 2 NO3 - (aq) + 2 Na + (aq) + CrO4 2- (aq) 2 Na + (aq) + 2 NO3 - (aq) + MgCrO4(s) Mg 2+ (aq) + CrO4 2- (aq) MgCrO4(s)
7 9. 2 FeCl3(aq) + 3 Mg(s) 3 MgCl2(aq) + 2 Fe(s) Ionic Equation: 2 Fe 3+ (aq) + 6 Cl - (aq) + 3 Mg(s) 3 Mg 2+ (aq) + 6 Cl - (aq) + 2 Fe(s) 2 Fe 3+ (aq) + 3 Mg(s) 3 Mg 2+ (aq) + 2 Fe(s) 10. BaBr2(aq) + Na2SO4(aq) BaSO4(s) + 2 NaBr(aq) Ionic Equation: Ba 2+ (aq) + 2 Br - (aq) + 2 Na + (aq) + SO4 2- (aq) BaSO4(s) + 2 Na + (aq) + 2 Br - (aq) Ba 2+ (aq) + SO4 2- (aq) BaSO4(s) AgNO3(aq) + MgI2(aq) 2 AgI(s) + Mg(NO3)2(aq) Ionic Equation: 2 Ag + (aq) + 2 NO3 - (aq) + Mg 2+ (aq) + 2 I - (aq) 2 AgI(s) + Mg 2+ (aq) + 2 NO3 - (aq) 2 Ag + (aq) + 2 I - (aq) 2 AgI(s) (your final answer would be: Ag + (aq) + I - (aq) AgI(s) ) (NH4)2CrO4(aq) + 2 Al(ClO4)3(aq) Al2(CrO4)3(s) + 6 NH4ClO4(aq) Ionic Equation: 6 NH4 + (aq) + 3 CrO4 2- (aq) + 2 Al 3+ (aq) + 6 ClO4 - (aq) 6 NH4 + (aq) + 6 ClO4 - (aq) + Al2(CrO4)3(s) 3 C2O4 2- (aq) + 2 Al 3+ (aq) Al2(CrO4)3(s) 13. Ni(NO3)2(aq) + 2 NaOH(aq) Ni(OH)2(s) + 2 NaNO3(aq) Ionic Equation: Ni 2+ (aq) + 2 NO3 - (aq) + 2 Na + (aq) + 2 OH - (aq) Ni(OH)2(s) + 2 Na + (aq) + NO3 - (aq) Ni 2+ (aq) + 2 OH - (aq) Ni(OH)2(s) HBr(aq) + Pb(ClO4)2(aq) 2 HClO4(aq) + PbBr2(s) Ionic Equation: 2 H + (aq) + 2 Br - (aq) + Pb 2+ (aq) + 2ClO4 - (aq) 2H + (aq) + 2 ClO4 - (aq) + PbBr2(s) 2 Br - (aq) + Pb 2+ (aq) PbBr2(s) KF(aq) + Mg(NO3)2(aq) 2 KNO3(aq) + MgF2(s) Ionic Equation: 2 K + (aq) + 2 F - (aq) + Mg 2+ (aq) + 2NO3 - (aq) 2 K + (aq) + 2 NO3 - (aq) + MgF2(s) 2 F - (aq) + Mg 2+ (aq) MgF2(s) Na3PO4(aq) + 3 Ni(ClO4)2(aq) 6 NaClO4(aq) + Ni3(PO4)2(s) Ionic Equation: 6 Na + (aq) + 2 PO4 3- (aq) + 3 Ni 2+ (aq) + 6 ClO4 - (aq) 6 Na + (aq) + 6 ClO4 - (aq) + Ni3(PO4)2(s) 2 PO4 3- (aq) + 3 Ni 2+ (aq) Ni3(PO4)2(s) 17. CuCl2(aq) + 2 AgC2H3O2(aq) Cu(C2H3O2)2(aq) + 2 AgCl(s) Ionic Equation: Cu 2+ (aq) + 2 Cl - (aq) + 2 Ag + (aq) + 2 C2H3O2 - (aq) Cu 2+ (aq) + 2 C2H3O2 - (aq) + 2 AgCl(s) Cl - (aq) + Ag + (aq) AgCl(s)
6. Classify each of the following substances as a strong electrolyte or a nonelectrolyte: a. CH3OH b. BaCl2 c. KF d. H2SO4 e. KOH f.
 Chapter 4B Practice Problems 1. Balance the following equations: a. NaBH4(s) + H2O(l) NaBO2(aq) + H2(g) b. Mg(N3)2(s) + H2O(l) Mg(OH)2(aq) + HN3(aq) c. NaCl(aq) + SO3(g) + H2O(l) Na2SO4(aq) + HCl(aq) d.
Chapter 4B Practice Problems 1. Balance the following equations: a. NaBH4(s) + H2O(l) NaBO2(aq) + H2(g) b. Mg(N3)2(s) + H2O(l) Mg(OH)2(aq) + HN3(aq) c. NaCl(aq) + SO3(g) + H2O(l) Na2SO4(aq) + HCl(aq) d.
insoluble partial very soluble (< 0.1 g/100ml) solubility (> 1 g/100ml) Factors Affecting Solubility in Water
 Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
Net Ionic Equations. Making Sense of Chemical Reactions
 Making Sense of Chemical Reactions Now that you have mastered writing balanced chemical equations it is time to take a deeper look at what is really taking place chemically in each reaction. There are
Making Sense of Chemical Reactions Now that you have mastered writing balanced chemical equations it is time to take a deeper look at what is really taking place chemically in each reaction. There are
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) When the following equation is balanced, the coefficients are. 1) NH3 (g) + O2 (g) NO2
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) When the following equation is balanced, the coefficients are. 1) NH3 (g) + O2 (g) NO2
CHE 105 FA17 Exam 2. How many moles of beryllium are in 15.0 grams of Be?
 CHE 105 FA17 Exam 2 Your Name: Your ID: Question #: 1 How many moles of beryllium are in 150 grams of Be? A 66 B 13515 C 901 D 0601 Question #: 2 Vanillin, C8H8O3, is the molecule responsible for the vanilla
CHE 105 FA17 Exam 2 Your Name: Your ID: Question #: 1 How many moles of beryllium are in 150 grams of Be? A 66 B 13515 C 901 D 0601 Question #: 2 Vanillin, C8H8O3, is the molecule responsible for the vanilla
Chapter 8 Chemical Reactions
 Chemistry/ PEP Name: Date: Chapter 8 Chemical Reactions Chapter 8: 1 7, 9 18, 20, 21, 24 26, 29 31, 46, 55, 69 Practice Problems 1. Write a skeleton equation for each chemical reaction. Include the appropriate
Chemistry/ PEP Name: Date: Chapter 8 Chemical Reactions Chapter 8: 1 7, 9 18, 20, 21, 24 26, 29 31, 46, 55, 69 Practice Problems 1. Write a skeleton equation for each chemical reaction. Include the appropriate
What is one of the spectator ions (with correct coefficient)? A)
 Chem 101 Exam Fall 01 Section 001 1. Based on the solubility rules Mg (PO 4 ) is A) soluble B) insoluble. An aqueous solution of potassium sulfate is allowed to react with an aqueous solution of What is
Chem 101 Exam Fall 01 Section 001 1. Based on the solubility rules Mg (PO 4 ) is A) soluble B) insoluble. An aqueous solution of potassium sulfate is allowed to react with an aqueous solution of What is
Aqueous Reactions. The products are just the cation-anion pairs reversed, or the outies (A and Y joined) and the innies (B and X joined).
 Aqueous Reactions Defining Aqueous Reactions Aqueous reactions are reactions that take place in water. To understand them, it is important to understand how compounds behave in water. Some compounds are
Aqueous Reactions Defining Aqueous Reactions Aqueous reactions are reactions that take place in water. To understand them, it is important to understand how compounds behave in water. Some compounds are
CHM 130LL: Double Replacement Reactions
 CHM 130LL: Double Replacement Reactions One of the main purposes of chemistry is to transform one set of chemicals (the reactants) into another set of chemicals (the products) via a chemical reaction:
CHM 130LL: Double Replacement Reactions One of the main purposes of chemistry is to transform one set of chemicals (the reactants) into another set of chemicals (the products) via a chemical reaction:
CHEMICAL REACTIONS. The process by which one or more substances are changed into one or more different substances
 CHEMICAL REACTIONS The process by which one or more substances are changed into one or more different substances Equations Reactions are represented by a chemical equation Reactants Products Must have
CHEMICAL REACTIONS The process by which one or more substances are changed into one or more different substances Equations Reactions are represented by a chemical equation Reactants Products Must have
5. Pb(IO 3) BaCO 3 8. (NH 4) 2SO 3
 Chemistry 11 Solution Chemistry II Name: Date: Block: 1. Ions in Solutions 2. Solubility Table 3. Separating Ions Ions in Solutions Ionization Equation - Represents the salt breaking apart into ions. Practice:
Chemistry 11 Solution Chemistry II Name: Date: Block: 1. Ions in Solutions 2. Solubility Table 3. Separating Ions Ions in Solutions Ionization Equation - Represents the salt breaking apart into ions. Practice:
temperature change a) On heating, solid calcium carbonate yields solid calcium oxide and gaseous carbon dioxide. 4Li(s) + O2(g) 2Li2O(s)
 CHEMISTRY WKST CH. 7 & 8 REVIEW (REACTIONS & EQUATIONS) NAME: JMS 1) What are the 5 evidences that a chemical reaction took place? precipitate forms color change gas released temperature change 2) Consider
CHEMISTRY WKST CH. 7 & 8 REVIEW (REACTIONS & EQUATIONS) NAME: JMS 1) What are the 5 evidences that a chemical reaction took place? precipitate forms color change gas released temperature change 2) Consider
Solubility Rules and Net Ionic Equations
 Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
Chemical Reactions CHAPTER Reactions and Equations
 CHAPTER 9 Chemical Reactions 9.1 Reactions and Equations The process by which atoms of one or more substances are rearranged to form different substances is called a chemical reaction. There are a number
CHAPTER 9 Chemical Reactions 9.1 Reactions and Equations The process by which atoms of one or more substances are rearranged to form different substances is called a chemical reaction. There are a number
Honors Unit 4 Homework Packet
 1 Honors Homework Packet Reactions in Aqueous Solutions Part I: Aqueous Solns. Part II: Acid/Base Chemistry Part III: Redox Reactions Name: 2 Molarity of Solutions (pg. 2 & 3) Directions: Solve each of
1 Honors Homework Packet Reactions in Aqueous Solutions Part I: Aqueous Solns. Part II: Acid/Base Chemistry Part III: Redox Reactions Name: 2 Molarity of Solutions (pg. 2 & 3) Directions: Solve each of
Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67)
 Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67) I. Elecrolytes a. Soluble substances b. Insoluble substances c. Electrolytes d. Non-Electrolytes e. Ions and electrical conductivity f. Strong and
Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67) I. Elecrolytes a. Soluble substances b. Insoluble substances c. Electrolytes d. Non-Electrolytes e. Ions and electrical conductivity f. Strong and
EXPERIMENT A5: TYPES OF REACTIONS. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT A5: TYPES OF REACTIONS Upon completion of this lab, the student will be able to: 1) Examine different types of chemical reactions. 2) Express chemical equations in molecular,
1 Learning Outcomes EXPERIMENT A5: TYPES OF REACTIONS Upon completion of this lab, the student will be able to: 1) Examine different types of chemical reactions. 2) Express chemical equations in molecular,
EXPERIMENT #7 Double Replacement Reactions
 OBJECTIVES: EXPERIMENT #7 Double Replacement Reactions To determine if a chemical reaction occurs when pairs of reactants are mixed To recognize electrolytes, non-electrolytes, strong and weak acids, and
OBJECTIVES: EXPERIMENT #7 Double Replacement Reactions To determine if a chemical reaction occurs when pairs of reactants are mixed To recognize electrolytes, non-electrolytes, strong and weak acids, and
1. Hydrochloric acid is mixed with aqueous sodium bicarbonate Molecular Equation
 NAME Hr Chapter 4 Aqueous Reactions and Solution Chemistry Practice A (Part 1 = Obj. 1-3) (Part 2 = Obj. 4-6) Objective 1: Electrolytes, Acids, and Bases a. Indicate whether each of the following is strong,
NAME Hr Chapter 4 Aqueous Reactions and Solution Chemistry Practice A (Part 1 = Obj. 1-3) (Part 2 = Obj. 4-6) Objective 1: Electrolytes, Acids, and Bases a. Indicate whether each of the following is strong,
Precipitation Reactions
 Precipitation Reactions Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions. 1) reactions between aqueous solutions of ionic compounds 2) produce
Precipitation Reactions Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions. 1) reactions between aqueous solutions of ionic compounds 2) produce
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON
 Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
EXAM 3 CHEM 1310 WS09 Key Version #2
 EXAM 3 CHEM 1310 WS09 Key Version #2 1. (p. 116) Select the correct name and chemical formula for the precipitate that forms when the following reactants are mixed. CuCl 2 (aq) + Na 2 CO 3 (aq) A. copper(ii)
EXAM 3 CHEM 1310 WS09 Key Version #2 1. (p. 116) Select the correct name and chemical formula for the precipitate that forms when the following reactants are mixed. CuCl 2 (aq) + Na 2 CO 3 (aq) A. copper(ii)
Macroscopic, particle and symbolic representations of aqueous reactions
 Macroscopic, particle and symbolic representations of aqueous reactions Name: DS: Learning Objective: After completing this activity, you should be able to understand the difference between macroscopic,
Macroscopic, particle and symbolic representations of aqueous reactions Name: DS: Learning Objective: After completing this activity, you should be able to understand the difference between macroscopic,
You try: 2) HC 7H 6O 2 3) N 2O 5. 5) HClO 4. 7) Rb 2C 2O 4 8) H 3PO 4 9) AgI 10) Sr(OH) 2. What kind of compound is it? NON ELECTROLYTE (NE)
 Solubility: Solubility is the measure of how much of a solute will dissolve in a solvent. In general chemistry, we usually talk about water as the solvent, so we are talking about what compounds will dissolve
Solubility: Solubility is the measure of how much of a solute will dissolve in a solvent. In general chemistry, we usually talk about water as the solvent, so we are talking about what compounds will dissolve
Solubility & Net Ionic review
 Solubility & Net Ionic review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following statements is/are correct? 1. All ionic compounds
Solubility & Net Ionic review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following statements is/are correct? 1. All ionic compounds
5072 CHEMISTRY (NEW PAPERS WITH SPA) BASIC TECHNIQUES 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) BASIC TECHNIQUES
 5072 CHEMISTRY (NEW PAPERS WITH SPA) BASIC TECHNIQUES 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) BASIC TECHNIQUES LEARNING OUTCOMES a) Be able to write formulae of simple compounds b) Be able to write
5072 CHEMISTRY (NEW PAPERS WITH SPA) BASIC TECHNIQUES 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) BASIC TECHNIQUES LEARNING OUTCOMES a) Be able to write formulae of simple compounds b) Be able to write
Electrodeposition. - Worksheet - Envisioning Chemistry. 1. Write half reactions for the following processes under electrical current.
 Electrodeposition 1. Write half reactions for the following processes under electrical current. (1). Formation of copper from copper (II) ion Example: Cu 2+ + 2e --> Cu (2). Formation of tin from tin ion
Electrodeposition 1. Write half reactions for the following processes under electrical current. (1). Formation of copper from copper (II) ion Example: Cu 2+ + 2e --> Cu (2). Formation of tin from tin ion
Net Ionic Reactions. The reaction between strong acids and strong bases is one example:
 Net Ionic Reactions Model 1 Net Ionic Reactions. Net ionic reactions are frequently used when strong electrolytes react in solution to form nonelectrolytes or weak electrolytes. These equations let you
Net Ionic Reactions Model 1 Net Ionic Reactions. Net ionic reactions are frequently used when strong electrolytes react in solution to form nonelectrolytes or weak electrolytes. These equations let you
CHEMICAL REACTIONS. Introduction. Chemical Equations
 CHEMICAL REACTIONS Chemistry I Chapter 7 1 Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O 2 (g) ---> 2 Al 2 O 3 (s) The
CHEMICAL REACTIONS Chemistry I Chapter 7 1 Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O 2 (g) ---> 2 Al 2 O 3 (s) The
Chemical reactions describe processes involving chemical change
 1.1 Chemical Reactions 1.2 Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into
1.1 Chemical Reactions 1.2 Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into
CHEM 200/202. Professor Jing Gu Office: EIS-210. All s are to be sent to:
 CHEM 200/202 Professor Jing Gu Office: EIS-210 All emails are to be sent to: chem200@mail.sdsu.edu My office hours will be held in GMCS-212 on Monday from 9 am to 11 am or by appointment. ANNOUNCEMENTS
CHEM 200/202 Professor Jing Gu Office: EIS-210 All emails are to be sent to: chem200@mail.sdsu.edu My office hours will be held in GMCS-212 on Monday from 9 am to 11 am or by appointment. ANNOUNCEMENTS
Chapter 4 Aqueous Reactions and Solution Stoichiometry
 Chapter 4 Aqueous Reactions and Solution Stoichiometry 4.1 General Properties of Aqueous Solutions What is a solution? How do you identify the following two? Solvent. Solute(s). Dissociation. What is it?
Chapter 4 Aqueous Reactions and Solution Stoichiometry 4.1 General Properties of Aqueous Solutions What is a solution? How do you identify the following two? Solvent. Solute(s). Dissociation. What is it?
EXPERIMENT 10: Precipitation Reactions
 EXPERIMENT 10: Precipitation Reactions Metathesis Reactions in Aqueous Solutions (Double Displacement Reactions) Purpose a) Identify the ions present in various aqueous solutions. b) Systematically combine
EXPERIMENT 10: Precipitation Reactions Metathesis Reactions in Aqueous Solutions (Double Displacement Reactions) Purpose a) Identify the ions present in various aqueous solutions. b) Systematically combine
26. N 2 + H 2 NH N 2 + O 2 N 2 O 28. CO 2 + H 2 O C 6 H 12 O 6 + O SiCl 4 + H 2 O H 4 SiO 4 + HCl 30. H 3 PO 4 H 4 P 2 O 7 + H 2 O
 Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
AP Chemistry Honors Unit Chemistry #4 2 Unit 3. Types of Chemical Reactions & Solution Stoichiometry
 HO AP Chemistry Honors Unit Chemistry #4 2 Unit 3 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to:! Predict to some extent whether a substance
HO AP Chemistry Honors Unit Chemistry #4 2 Unit 3 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to:! Predict to some extent whether a substance
Last Lecture. K 2 SO 4 (aq) + Ba(NO 3 ) 2 (aq) AgNO 3 (aq) + KCl(aq) NaNO 3 (aq) + KCl(aq) What will happen when these are mixed together?
 Announcements Precipitation lab write-up due tomorrow at the start of discussion Text HW due tomorrow in discussion Lon-capa HW #4 Type 1 due Monday, Oct 15 th at 7:00pm Lon-capa HW #4 Type 2 due Wednesday,
Announcements Precipitation lab write-up due tomorrow at the start of discussion Text HW due tomorrow in discussion Lon-capa HW #4 Type 1 due Monday, Oct 15 th at 7:00pm Lon-capa HW #4 Type 2 due Wednesday,
Exam 3. Objectives: Nomenclature
 Exam 3 Objectives: o Nomenclature m-nm, m(vos)-nm, nm-nm o Evidence for Chemical Reactions o Writing Chemical Equations o Balancing Chemical Equations o Classifying Chemical Reactions o Combination Reactions
Exam 3 Objectives: o Nomenclature m-nm, m(vos)-nm, nm-nm o Evidence for Chemical Reactions o Writing Chemical Equations o Balancing Chemical Equations o Classifying Chemical Reactions o Combination Reactions
Reaction Classes. Precipitation Reactions
 Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
Chapter 6. Chemical Reactions. Sodium reacts violently with bromine to form sodium bromide.
 Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS
 UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
Honors Chemistry - Unit 5 Chapter 8 Chemical Equations Quiz on Diatomic Molecules: Tues., Nov. 15th Test Date: Fri., Nov. 26th
 Honors Chemistry - Unit 5 Chapter 8 Chemical Equations Quiz on Diatomic Molecules: Tues., Nov. 15th Test Date: Fri., Nov. 26th VOCABULARY Assignment Use the Two-column Notes format/strategy to study/complete
Honors Chemistry - Unit 5 Chapter 8 Chemical Equations Quiz on Diatomic Molecules: Tues., Nov. 15th Test Date: Fri., Nov. 26th VOCABULARY Assignment Use the Two-column Notes format/strategy to study/complete
TYPES OF CHEMICAL REACTIONS
 TYPES OF CHEMICAL REACTIONS Precipitation Reactions Compounds Soluble Ionic Compounds 1. Group 1A cations and NH 4 + 2. Nitrates (NO 3 ) Acetates (CH 3 COO ) Chlorates (ClO 3 ) Perchlorates (ClO 4 ) Solubility
TYPES OF CHEMICAL REACTIONS Precipitation Reactions Compounds Soluble Ionic Compounds 1. Group 1A cations and NH 4 + 2. Nitrates (NO 3 ) Acetates (CH 3 COO ) Chlorates (ClO 3 ) Perchlorates (ClO 4 ) Solubility
UNIT 12: Solutions Lesson 3: Table F Solubility Guidelines
 Name: Period: Date: General Chemistry KIPP NYC College Prep UNIT 12: Solutions Lesson 3: Table F Solubility Guidelines By the end of today, you will have an answer to: How do we determine if a substance
Name: Period: Date: General Chemistry KIPP NYC College Prep UNIT 12: Solutions Lesson 3: Table F Solubility Guidelines By the end of today, you will have an answer to: How do we determine if a substance
Solution Stoichiometry
 Chapter 8 Solution Stoichiometry Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the
Chapter 8 Solution Stoichiometry Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the
Chemical Reactions and Equations
 Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Reactions in Aqueous Solutions
 Chapter 4 Reactions in Aqueous Solutions Some typical kinds of chemical reactions: 1. Precipitation reactions: the formation of a salt of lower solubility causes the precipitation to occur. precipr 2.
Chapter 4 Reactions in Aqueous Solutions Some typical kinds of chemical reactions: 1. Precipitation reactions: the formation of a salt of lower solubility causes the precipitation to occur. precipr 2.
Unit 8 Chemical Reactions- Funsheets
 Part A- Balancing Equations and Types of Reactions Balance AND identify the following reactions: Unit 8 Chemical Reactions- Funsheets 1) Mg + Zn(NO 3) 2 Zn Mg(NO 3) 2 2) Ba + AgNO 3 Ag + Ba(NO 3) 2 3)
Part A- Balancing Equations and Types of Reactions Balance AND identify the following reactions: Unit 8 Chemical Reactions- Funsheets 1) Mg + Zn(NO 3) 2 Zn Mg(NO 3) 2 2) Ba + AgNO 3 Ag + Ba(NO 3) 2 3)
Chemical Equations and Chemical Reactions
 Chemical Equations Chemical Equations and Chemical Reactions Chemical equations are concise representations of chemical reactions. Chemical Equations Symbols Used in Chemical Equations The formulas of
Chemical Equations Chemical Equations and Chemical Reactions Chemical equations are concise representations of chemical reactions. Chemical Equations Symbols Used in Chemical Equations The formulas of
NET IONIC REACTIONS in AQUEOUS SOLUTIONS AB + CD AD + CB
 NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
Chapter 5 Classification and Balancing of Chemical Reactions
 Chapter 5 Classification and Balancing of Chemical Reactions 5.1 Chemical Equations Chemical equations describe chemical reactions. - As words: hydrogen plus oxygen combine to form water - As a chemical
Chapter 5 Classification and Balancing of Chemical Reactions 5.1 Chemical Equations Chemical equations describe chemical reactions. - As words: hydrogen plus oxygen combine to form water - As a chemical
Name Date Class CHEMICAL REACTIONS. SECTION 11.1 DESCRIBING CHEMICAL REACTIONS (pages )
 Name Date Class 11 CHEMICAL REACTIONS SECTION 11.1 DESCRIBING CHEMICAL REACTIONS (pages 321 329) This section explains how to write equations describing chemical reactions using appropriate symbols. It
Name Date Class 11 CHEMICAL REACTIONS SECTION 11.1 DESCRIBING CHEMICAL REACTIONS (pages 321 329) This section explains how to write equations describing chemical reactions using appropriate symbols. It
Chapter 4. The Major Classes of Chemical Reactions 4-1
 Chapter 4 The Major Classes of Chemical Reactions 4-1 The Major Classes of Chemical Reactions 4.1 The Role of Water as a Solvent 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions
Chapter 4 The Major Classes of Chemical Reactions 4-1 The Major Classes of Chemical Reactions 4.1 The Role of Water as a Solvent 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions
Solubility Equilibria. Dissolving a salt... Chem 30S Review Solubility Rules. Solubility Equilibrium: Dissociation = Crystalization
 Chem 30S Review Solubility Rules Solubility Equilibria Salts are generally more soluble in HOT water(gases are more soluble in COLD water) Alkali Metal salts are very soluble in water. NaCl, KOH, Li 3
Chem 30S Review Solubility Rules Solubility Equilibria Salts are generally more soluble in HOT water(gases are more soluble in COLD water) Alkali Metal salts are very soluble in water. NaCl, KOH, Li 3
Honors Chemistry - Unit 5
 Honors Chemistry - Unit 5 Chapter 8 Chemical Equations Unit 5 Packet - Page 1 of 18 Quiz on Diatomic Molecules: Tues., Nov. 9 th Vocab Assignment Due: Tues., Nov. 9 th Problem Set Due: Wed., Nov. 17th
Honors Chemistry - Unit 5 Chapter 8 Chemical Equations Unit 5 Packet - Page 1 of 18 Quiz on Diatomic Molecules: Tues., Nov. 9 th Vocab Assignment Due: Tues., Nov. 9 th Problem Set Due: Wed., Nov. 17th
Check Your Solution The net ionic equation is balanced, including the charges on the ions.
 Ba 2+ (aq) + 2PO (aq) Ba (PO ) 2 (s) 2. Practice Problem (page 10) Write the net ionic equation for this reaction: Na 2 SO (aq) + Sr(OH) 2 (aq) SrSO (s) + NaOH(aq) You need to write the net ionic equation
Ba 2+ (aq) + 2PO (aq) Ba (PO ) 2 (s) 2. Practice Problem (page 10) Write the net ionic equation for this reaction: Na 2 SO (aq) + Sr(OH) 2 (aq) SrSO (s) + NaOH(aq) You need to write the net ionic equation
What Do You Think? Investigate GOALS
 Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Honors Chemistry - Unit 7 Chapter 11 Chemical Reactions
 Honors Chemistry - Unit 7 Chapter 11 Chemical Reactions Vocab Assignment Due: Unit 7 Packet - Page 1 of 15 UT Quest(s): Quiz on Diatomic Molecules & Balancing: Prediction Quiz : Test Date: VOCABULARY Assignment
Honors Chemistry - Unit 7 Chapter 11 Chemical Reactions Vocab Assignment Due: Unit 7 Packet - Page 1 of 15 UT Quest(s): Quiz on Diatomic Molecules & Balancing: Prediction Quiz : Test Date: VOCABULARY Assignment
The Copper Cycle. HCl(aq) H + (aq) + Cl (aq) HCl(aq) + H 2 O(l) H 3 O + (aq) + Cl (aq)
 The Copper Cycle Introduction Many aspects of our lives involve chemical reactions from the batteries that power our cars and cell phones to the thousands of processes occurring within our bodies. We cannot
The Copper Cycle Introduction Many aspects of our lives involve chemical reactions from the batteries that power our cars and cell phones to the thousands of processes occurring within our bodies. We cannot
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Chapter 8 Prep Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. General Solubility Guidelines 1. Most sodium, potassium, and ammonium compounds
Chapter 8 Prep Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. General Solubility Guidelines 1. Most sodium, potassium, and ammonium compounds
Chapter 4 Suggested end-of-chapter problems with solutions
 Chapter 4 Suggested end-of-chapter problems with solutions a. 5.6 g NaHCO 1 mol NaHCO 84.01 g NaHCO = 6.69 10 mol NaHCO M = 6.69 10 mol 50.0 m 1000 m = 0.677 M NaHCO b. 0.1846 g K Cr O 7 1 mol K 94.0 g
Chapter 4 Suggested end-of-chapter problems with solutions a. 5.6 g NaHCO 1 mol NaHCO 84.01 g NaHCO = 6.69 10 mol NaHCO M = 6.69 10 mol 50.0 m 1000 m = 0.677 M NaHCO b. 0.1846 g K Cr O 7 1 mol K 94.0 g
4.2. Double Displacement Reactions. Characteristics of Double Displacement Reactions SECTION. Key Terms
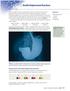 SECTION 4.2 Double Displacement Reactions X rays are highly useful for creating images of bones in the human body, but they generally do not show soft tissues clearly. To help doctors diagnose conditions
SECTION 4.2 Double Displacement Reactions X rays are highly useful for creating images of bones in the human body, but they generally do not show soft tissues clearly. To help doctors diagnose conditions
11.3 Reactions in Aqueous Solution. Chapter 11 Chemical Reactions Reactions in Aqueous Solution
 Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
Chapter 9. Vocabulary Ch Kick Off Activity. Objectives. Interpreting Formulas. Interpreting Formulas
 Chapter 9 Chemical Vocabulary Ch. 9.1 Chemical reaction Reactant Product Word Equation Skeleton Equation Chemical equation Coefficient 1 2 Objectives Write chemical equations to describe chemical reactions
Chapter 9 Chemical Vocabulary Ch. 9.1 Chemical reaction Reactant Product Word Equation Skeleton Equation Chemical equation Coefficient 1 2 Objectives Write chemical equations to describe chemical reactions
Precipitation Reactions
 Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions reactions between aqueous solutions of ionic compounds produce an ionic compound that is insoluble
Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions reactions between aqueous solutions of ionic compounds produce an ionic compound that is insoluble
SOLUTIONS - CHAPTER 9 Problems
 SOLUTIONS - CHAPTER 9 Problems NOTE: Exam 3 will be on Tuesday, November 22 nd, from 8:30pm to 10:30pm. Room assignments for the exam are the same as for the first two exams. The exam will cover the following
SOLUTIONS - CHAPTER 9 Problems NOTE: Exam 3 will be on Tuesday, November 22 nd, from 8:30pm to 10:30pm. Room assignments for the exam are the same as for the first two exams. The exam will cover the following
Chemistry 12 Solubility Equilibrium I. Name: Date: Block: 1. Solutions Vocab & Calculations 2. Predicting Solubility 3.
 Chemistry 12 Solubility Equilibrium I Name: Date: Block: 1. Solutions Vocab & Calculations 2. Predicting Solubility 3. Writing Equations Solutions Vocab & Calculations What is a solution? A homogenous
Chemistry 12 Solubility Equilibrium I Name: Date: Block: 1. Solutions Vocab & Calculations 2. Predicting Solubility 3. Writing Equations Solutions Vocab & Calculations What is a solution? A homogenous
Reactions in Aqueous Solutions Chang & Goldsby modified by Dr. Hahn
 Reactions in Aqueous Solutions Chang & Goldsby modified by Dr. Hahn Chapter 4 Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of
Reactions in Aqueous Solutions Chang & Goldsby modified by Dr. Hahn Chapter 4 Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of
Definition: the process by which one or more substances are rearranged to form different substances. Another name for a chemical change.
 Chemical Reactions I. What is a chemical reaction? Definition: the process by which one or more substances are rearranged to form different substances. Another name for a chemical change. A. How can you
Chemical Reactions I. What is a chemical reaction? Definition: the process by which one or more substances are rearranged to form different substances. Another name for a chemical change. A. How can you
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS
 UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
Balancing Equations Notes
 . Unit 6 Chemical Equations and Reactions What is a Chemical Equation? A Chemical Equation is a written representation of the process that occurs in a chemical reaction. A chemical equation is written
. Unit 6 Chemical Equations and Reactions What is a Chemical Equation? A Chemical Equation is a written representation of the process that occurs in a chemical reaction. A chemical equation is written
Chapter 4: Stoichiometry of Chemical Reactions. 4.1 Writing and Balancing Chemical Equations
 Chapter 4: Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations A chemical equation represents or symbolizes a chemical reaction. o Substances are represents by their chemical
Chapter 4: Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations A chemical equation represents or symbolizes a chemical reaction. o Substances are represents by their chemical
Topic 8: Chemical Reactions Chemical Equations & Reactions
 Topic 8: Chemical Reactions Chemical Equations & Reactions (Chapter 8 in Modern Chemistry) Describing Chemical Reactions A chemical reaction is the process by which one or more substances are changed into
Topic 8: Chemical Reactions Chemical Equations & Reactions (Chapter 8 in Modern Chemistry) Describing Chemical Reactions A chemical reaction is the process by which one or more substances are changed into
The solvent is the dissolving agent -- i.e., the most abundant component of the solution
 SOLUTIONS Definitions A solution is a system in which one or more substances are homogeneously mixed or dissolved in another substance homogeneous mixture -- uniform appearance -- similar properties throughout
SOLUTIONS Definitions A solution is a system in which one or more substances are homogeneously mixed or dissolved in another substance homogeneous mixture -- uniform appearance -- similar properties throughout
AP Chemistry Unit #4. Types of Chemical Reactions & Solution Stoichiometry
 AP Chemistry Unit #4 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to: Predict to some extent whether a substance will be a strong electrolyte,
AP Chemistry Unit #4 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to: Predict to some extent whether a substance will be a strong electrolyte,
IONIC CHARGES. Chemistry 51 Review
 IONIC CHARGES The ionic charge of an ion is dependent on the number of electrons lost or gained to attain a noble gas configuration. For most main group elements, the ionic charges can be determined from
IONIC CHARGES The ionic charge of an ion is dependent on the number of electrons lost or gained to attain a noble gas configuration. For most main group elements, the ionic charges can be determined from
Which of the following answers is correct and has the correct number of significant figures?
 Avogadro s Number, N A = 6.022 10 23 1. [7 points] Carry out the following mathematical operation: 6.06 10 3 + 1.1 10 2 Which of the following answers is correct and has the correct number of significant
Avogadro s Number, N A = 6.022 10 23 1. [7 points] Carry out the following mathematical operation: 6.06 10 3 + 1.1 10 2 Which of the following answers is correct and has the correct number of significant
Part 01 - Notes: Reactions & Classification
 Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Reactions in aqueous solutions Redox reactions
 Reactions in aqueous solutions Redox reactions Redox reactions In precipitation reactions, cations and anions come together to form an insoluble ionic compound. In neutralization reactions, H + ions and
Reactions in aqueous solutions Redox reactions Redox reactions In precipitation reactions, cations and anions come together to form an insoluble ionic compound. In neutralization reactions, H + ions and
CHEMISTRY - CLUTCH CH.4 - CHEMICAL QUANTITIES & AQUEOUS REACTIONS
 !! www.clutchprep.com CONCEPT: MOLARITY Molarity (M) can serve as the connection between the interconversion of to and vice versa. For example, a 5.8 M NaCl solution really means per. ( Molarity = MolesSolute
!! www.clutchprep.com CONCEPT: MOLARITY Molarity (M) can serve as the connection between the interconversion of to and vice versa. For example, a 5.8 M NaCl solution really means per. ( Molarity = MolesSolute
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION
 TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds.
 Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds. Forming a bond makes an atom more stable, so atoms form as many bonds are they are able to. Bonds are made using
Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds. Forming a bond makes an atom more stable, so atoms form as many bonds are they are able to. Bonds are made using
Balancing Equations Notes
 . Unit 9 Chemical Equations and Reactions What is a Chemical Equation? A is a written representation of the process that occurs in a chemical reaction. A chemical equation is written with the (starting
. Unit 9 Chemical Equations and Reactions What is a Chemical Equation? A is a written representation of the process that occurs in a chemical reaction. A chemical equation is written with the (starting
CHEMISTRY - ZUMDAHL 2E CH.6 - TYPES OF CHEMICAL REACTIONS AND SOLUTION STOICHIOMETRY
 !! www.clutchprep.com CONCEPT: MOLARITY Molarity (M) can serve as the connection between the interconversion of to and vice versa. For example, a 5.8 M NaCl solution really means per. ( Molarity = MolesSolute
!! www.clutchprep.com CONCEPT: MOLARITY Molarity (M) can serve as the connection between the interconversion of to and vice versa. For example, a 5.8 M NaCl solution really means per. ( Molarity = MolesSolute
Fe(s) + O2(g) Chapter 11 Chemical Reactions. Chemical Equations. Fe + O2. January 26, What is a chemical reaction?
 Chapter 11 Chemical Reactions What is a chemical reaction? Chemical Reaction: process by which one or more substances are changed into one or more different substances. Indications of a chemical reaction
Chapter 11 Chemical Reactions What is a chemical reaction? Chemical Reaction: process by which one or more substances are changed into one or more different substances. Indications of a chemical reaction
Chemistry: A Molecular Approach (Tro) Chapter 4. Chemical Quantities and Aqueous Reactions
 Chemistry: A Molecular Approach (Tro) Chapter 4 Chemical Quantities and Aqueous Reactions 1) According to the following balanced reaction, how many moles of NO are formed from 8.44 moles of NO 2 if there
Chemistry: A Molecular Approach (Tro) Chapter 4 Chemical Quantities and Aqueous Reactions 1) According to the following balanced reaction, how many moles of NO are formed from 8.44 moles of NO 2 if there
SOLUBILITY REVIEW QUESTIONS
 Solubility Problem Set 1 SOLUBILITY REVIEW QUESTIONS 1. What is the solubility of calcium sulphate in M, g/l, and g/100 ml? 2. What is the solubility of silver chromate? In a saturated solution of silver
Solubility Problem Set 1 SOLUBILITY REVIEW QUESTIONS 1. What is the solubility of calcium sulphate in M, g/l, and g/100 ml? 2. What is the solubility of silver chromate? In a saturated solution of silver
NCEA Chemistry 2.2 Identify Ions AS 91162
 NCEA Chemistry 2.2 Identify Ions AS 91162 What is this NCEA Achievement Standard? When a student achieves a standard, they gain a number of credits. Students must achieve a certain number of credits to
NCEA Chemistry 2.2 Identify Ions AS 91162 What is this NCEA Achievement Standard? When a student achieves a standard, they gain a number of credits. Students must achieve a certain number of credits to
Reactions in Aqueous Solutions
 Copyright 2004 by houghton Mifflin Company. Reactions in Aqueous Solutions Chapter 7 All rights reserved. 1 7.1 Predicting if a Rxn Will Occur When chemicals are mixed and one of these driving forces can
Copyright 2004 by houghton Mifflin Company. Reactions in Aqueous Solutions Chapter 7 All rights reserved. 1 7.1 Predicting if a Rxn Will Occur When chemicals are mixed and one of these driving forces can
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction:
 Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
1) What is the volume of a tank that can hold Kg of methanol whose density is 0.788g/cm 3?
 1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
Double Displacement (Exchange or Metathesis) Reactions Practicum
 Double Displacement (Exchange or Metathesis) Reactions Practicum Part I: Instructions: Write the molecular, complete ionic and net ionic equations for every one of the following reactions. If a reaction
Double Displacement (Exchange or Metathesis) Reactions Practicum Part I: Instructions: Write the molecular, complete ionic and net ionic equations for every one of the following reactions. If a reaction
Balancing Equations Notes
 . Unit 9 Chemical Equations and Reactions What is a Chemical Equation? A Chemical Equation is a written representation of the process that occurs in a chemical reaction. A chemical equation is written
. Unit 9 Chemical Equations and Reactions What is a Chemical Equation? A Chemical Equation is a written representation of the process that occurs in a chemical reaction. A chemical equation is written
Chemical Reactions. Ch. 11 Chemical Reactions. Chemical Reactions. Chemical Reactions
 Chemical Reactions Ch. 11 Chemical Reactions when a substance changes identity Reactants - original Products - resulting law of conservation of mass total mass of reactants = total mass of products In
Chemical Reactions Ch. 11 Chemical Reactions when a substance changes identity Reactants - original Products - resulting law of conservation of mass total mass of reactants = total mass of products In
Barium nitrate and sodium carbonate. What Is Given? Reactants: barium nitrate and sodium carbonate Type of reaction: double displacement
 12. Practice Problem (page 175) Barium nitrate and sodium carbonate Determine the products that form when barium nitrate and sodium carbonate Reactants: barium nitrate and sodium carbonate Barium nitrate:
12. Practice Problem (page 175) Barium nitrate and sodium carbonate Determine the products that form when barium nitrate and sodium carbonate Reactants: barium nitrate and sodium carbonate Barium nitrate:
Reactions in Aqueous Solutions
 Reactions in Aqueous Solutions 1 Chapter 4 General Properties of Aqueous Solutions (4.1) Precipitation Reactions (4.2) Acid-Base Reactions (4.3) Oxidation-Reduction Reactions (4.4) Concentration of Solutions
Reactions in Aqueous Solutions 1 Chapter 4 General Properties of Aqueous Solutions (4.1) Precipitation Reactions (4.2) Acid-Base Reactions (4.3) Oxidation-Reduction Reactions (4.4) Concentration of Solutions
A reaction in which a solid forms is called a precipitation reaction. Solid = precipitate
 Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
3. Which of the following compounds is soluble? The solubility rules are listed on page 8.
 1. Classify the following reaction. Sb 2 O 3 + 3 Fe 2 Sb + 3 FeO a) Combination reaction b) Decomposition reaction c) Neutralization reaction d) Single-replacement reaction e) Double-replacement reaction
1. Classify the following reaction. Sb 2 O 3 + 3 Fe 2 Sb + 3 FeO a) Combination reaction b) Decomposition reaction c) Neutralization reaction d) Single-replacement reaction e) Double-replacement reaction
CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY
 Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY Day Plans
Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY Day Plans
Reaction Writing Sheet #1 Key
 Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
