Synthesis of Biodegradable and Biocompostable Polyesters
|
|
|
- Augusta Sparks
- 5 years ago
- Views:
Transcription
1 Synthesis of Biodegradable and Biocompostable Polyesters Joanna Chajecka, Prof. Doctor João Carlos Moura Bordado Abstract The main objective of this work was to synthesize linear saturated polyester polyols by polyesterification reaction from dicarboxylic acids and diols. The process conditions were optimized by the temperature profile and also point of vacuum distillation. During the experimental production of each polyester polyol synthesis the process, parameters were controlled. A special attention was paid to the determination of the convention procedures such as determination of acid and hydroxyl value, as well as viscosity. Different dicarboxylic acids with a number of various glycols were used as raw materials to give distinct molecular structures with specific features. The Properties of the products, their application and processing methods depend both on composition and structure of the polyesters and on the composition and structure of the compounds with which they react to produce final products. The scale up of the manufacture was made at laboratory scale of 1 or 2 liters production reactors. To obtain extended and higher average molecular weight diphenylmethane disocyanate (MDI) was additionally added polyester polyols. For characterization of the product colour determination were carried out. Confirmation of a successfully conducted process of polyesterification was obtained by FTIR-ATR spectroscopy. (Infrared Fourier Transform Attenuated Total Reflectance), as well as viscosity determination. To evaluate preliminarily the biodegradation of polyester polyols, the hydrolytic stability test performed, due well know statement that biodegradation will start with hydrolysis, and this one would require water absorption. Keywords: polyester polyol, dimer acid, prepolymers, biodegradability. 1. Introduction Polyurethanes are versatile polymers with a wide range of properties and the broadest range of industrial applications. Polyurethanes are used in almost all fields of life and economy. Depending on the composition of raw materials and reaction conditions on the composition of the polymer can include ether groups, ester, urethane, urea, allophanate, and others.[1-2] The type of reaction is used to synthesise macromolecular compounds, by reaction of diisocyanate (or polyisocyanate which is a molecule with 2 or more isocyanate functional groups, R-(N=C=O)n 2) and an oligomeric polyol (which is molecule with 2 or more hydroxyl functional groups, R'-(OH)n 2). They are processed, both by classical methods and energy-efficient reactive injection molding. Reactions occur in the presence of catalyst, diamines, additives blocking agents[2]. 1
2 Polyols used in the reaction are either polyethers or polyesters. Group of materials representing a reactive intermediate between monomeric isocyanates and polyurethane polymers are called prepolymers.[3] A prepolymer is the reaction product of a diisocyanates (rigid component) with an oligo-polyol (flexible component) at the molecular ratio [diisocyanate]/[oh group] lower then 2:1. [4] Polyesters polyol are esterification products of glycol and a dicarboxylic acid or acid derivatives. [5-8] 1.1. Materials and methods The raw materials for polyester polyols production were commercial products. Acids used are Adipic acid (AD), Succinic acid (AS) and also Maleic anhydride (MA) all them were provided by Chimica. For synthesis besides acids, diols and triols were used. Diols provide commercially such as: 1,4-Butanediol (BD), Diethylene glycol (DEG), Isosurbide (ISOR), Monopropylene glycol (MPG), Neopenthyl glycol (NPG). Glycerol, Trimethylopropane (TMP) and Glycerol propoxylate were used as triols. The catalyst (Titanium (IV) butoxide) Excess of diols was equal to 10%. Reflux of water was continued during whole synthesis process, while in the beginning was very rapid when reaction reached second stage was barely visible. Before second stage and after large number of water is eliminated and catalyst ought to be used. The pressure in second stage should be decrease to Pa. Controlled parameters The evolution of the polyesterification reaction is monitored by measuring the quantity of water distilled and by the determination of acid used was a commercial product as well provided by Fluka (Sigma-Aldrich) Synthesis of polyester polyol Polyester polyols are formed in the polycondensation between dicarboxylic acids and glycols reaction by direct esterification. Reversible equillibrium reaction occurs, the equillibrium being shifted to the formation of polyester polyols by continuous elimination of water from the reaction system.[9] The polyesterification was carried out in a round bottom flask fitted with a stirrer, a thermometer, a nitrogen sparge, a thermocouple and a reflux condenser. The reactions were operated under inert atmosphere. The temperature of reaction should be slowly in steps, continuously raised up until reaches 220ºC which is the highest possible temperature of reaction. A mixture of the acids and the glycols (mixture) in the stoichiometric ratio is n mol of diacid for (n+1) mol of diol which eguation 1 shows. Eq(1) number, hydroxyl number and viscosity. Those parameters should be calculated and checked during the whole reaction process, while determination of colour, density and FITR-ATR should be monitored after finishing the reaction. According to ASTM-D570 Standard Test Method for water Absorption of Plastic methods were used. 2
3 2. Experimental Results and discussion 2.1. Acid number, hydroxyl number and Molecular Weight The acid value, hydroxyl value and MW were very strictly controlled during the whole synthesis process. Hydroxyl and acid numbers decomposition of the diols, although maximum temperature is higher than this performed during synthesis. For determination of colour The Gardner Colour scale as specified in ASTM D1544 was apply. are indirectly proportional to polyesters molecular weight (mention the equation number). Through duration of the polycondensation reaction, the acid and the hydroxyl values decreased until the desired values are obtained. For acid value calculated by titration with O.1 N alcoholic solution of 2.3. Viscosity Given the fact that the viscosity increases logarithmically with molecular weight, it is clear why the control of viscosity is so important in the processing of polymers. ICI cone plate viscometer equipment was used during KOH less than five units (mg KOH/g polyol) viscosity determination with temperature was desire for OH value enough to achieve range between C.[10] high MW. [4] The final results of all synthesise for this three parameters are shown in Table FTIR-ATR Polyester polyols formation was confirmed by FTIR-ATR. Each sample posses two spectra, 2.2. Colour If considering the colour parameter in the case of the most commercially attractive products, then the lighter ones should be on the first place with low MW. Although, darker colours are still acceptable for production point of view. Taking this in account the purpose of this work was to obtain polyester with high molecular weight and in a solid state, which also will be easy to undergo through treatment process. Unfortunately, during the synthesis process correlation between the duration of the process and its color was not found. Dark colour of one is the spectrum of polyester polyol (blue colour), and the second one is spectrum of polyester polyol with MDI chain extender. Between polyesters polyols based on the same pair dicarboxylic acid/diol, no significance change was observed. In order to evaluate the structural differences between polyester polyols and polyester polyols with MDI, the spectrums of elements are compared. It can be notice that the more relevant peaks presents in each one of the spectra showed, occurs for the same wavelengths. [11] samples might be explained by the thermal 3
4 Figure 1. Transmission ART-FTIR of Sample no.1 Starting identification of spectrum from the left side it can be notice that in the region cam -1 stretching band of OH can be find (Figure 1). The bands are broadened and sometimes may pass unnoticed, it may happened because of low intensity comparing to other peaks. It was expected that the presence of polyester polyol terminal OH group will be confirmed through FTIR-ART spectrum. However, the purpose of this determination was not quantitative, the fact that for all polyester polyols which were studied it is observed low intensivity of OH peak, can be explain by the reality that the ratio between the terminal hydroxyl groups and the other group which are parts of polyester polyol chain is rather significant. [12-14] The peaks corresponding to the C-H stretching (on spectrums respectively 2930 and 2850 cm - 1 ), are not significant, but easy to noticed. The sharp intense band located at around 1700 cm- 1, which corresponds to the saturated carboxylic acids. In case of sample presented on Figure 1 it can be observed that free carbonyl group is in the same position 1724 cm -1. It should be emphasized that the presence of the mentioned peak testified can be confirmation of accomplished the esterification process. However in case of samples where MDI was added, according to the literature the free carbonyl group should appear at 1690 cm -1, that corresponds to urea, but in performed determination it was not found. At lower wavenumber, significant peaks which are presented around 1140cm -1 (1135 cm -1 ) may be attributed of C-O linkage Resistance hydrolysis and water absorption The first requirement for hydrolysis is water absorption. one of the properties of polyester polyols is greater resistance to hydrolysis, with 4
5 dicarboxylic acids. In order to assess the impact on resistance to hydrolysis, short at temperature 95 0 C during 2 or 5 hours and long at temperature 24 0 C for 15 days water absorption were conducted on several samples, those with the highest MW and most solid state, results are listed in Table 1. Considering long time (15 days) water absorption the polyester with the highest percentage gain of weight and this one with the lower can be easy marked. The first remark to make after the analysis of Table 2 is that one prepolymer polyol sample has clearly the highest value of water absorption (sample 2, the same one as in case of long time water absorption). But it need to be mention that this sample during the test melt slightly and hence change its previous shape. For two of samples the most significant weight increase happened during first two hours of immersion. Those samples also possess the highest MW from those that were determined during the test. This is entirely reasonable since through immersion in 95ºC water two phenomena may take place. First one is water absorption with successive swelling and weight increase. The second one is hydrolysis of chemical bonds with subsequent loss of small molecules to the water and weight decrease. Table 1 Weight gain of the samples after short and long time water absorption test. Number of formulation Short time water absorption (temp C) 2 hours Long time water absorption (temp C) 5 hours 15 days 1. +MDI MDI MDI MDI
6 Table 2 Final results of acid value and hydroxyl value determination, number of days reaction occurred, colour of product and amount of water distilled during process Numberof formulation (sinthesis grade) Capacity of reaktor [l] V AN [mg KOH/g polyol] V OH [mg KOH/g polyol] MW [g/mol] nº of days (approx.) Colour [Gardner] Reflux of water [ml] Appearance 1. (AD+AS+BD+DEG) Solid state 2. (AD+AS+BD+DEG+glycerol) Solid state 3. (AD+AS+BD+DEG+glycerol) very viscous liquid 4. (AD+AS+BD+ISOR+TMP) gel 5. (AD+AS+BD+TMP) gel 6. (AD+AS+MPG+TMP) viscous liquid 7. (AD+AS+BD+ISOR+glycerol) viscous liqiud 8. (AD+AS+BD+ISOR) gel 9. (AD+AS+BD+ISOR+glycerol perop) very viscous liqiud 10. (AD+MA+BD+glycerol perop) solid 11. (AD+AS+MA+BD+glycerol perop) viscous liqiud 12. (AD+AS+BD+glycerol perop) solid 13. (AD+AS+DEG+NPG) solid 14. (AD+AS+DEG+glycerol perop) very viscous liquid 15. (AD+AS+DEG+glycerol perop) viscous liquid 6
7 3. Conclusions The experimental part of this work involved synthesis of saturated polyester polyols with dimer acids, which can be used as a building blocks for the polyester macromolecular structure. Different molecular structure were achieved, however main goal of this thesis was to obtain saturated polyester polyols with high molecular weight. Target has been attained fully only once. However, strong and stiff polyester polyols with low acid value and fairly low hydroxyl value and high viscosity were obtained. Hardness of products was obtained by the use of an chain extender which also increase the performance at elevated temperatures. The role of chain extenders is very important in the enhancement of certain polyurethane properties, together with MDI they increase the hard segment content. usual which confers them a highly hydrophobic character to the polyester backbone. List of Abbreviations and Symbols V OH [mg KOH / g sample] - the hydroxyl value of the sample, V AN [mg KOH / g sample] -acid value of the sample, V olblank [L] - the volume of KOH solution used in the titration of the blank, V ol [L] - the volume of KOH solution used in the titration of the sample, 0.5 [eq/l] - the normality of the KOH solution (hydroxyl value), f- functionality, the number of OH groups/mol, 56,10- [g/mol] MW of KOH, MW- Molecular Weight, 0.1 [eq/l] is the normality of the KOH solution (acid value), m [g] is the weight of the sample. Confirmation of successfully conducted process of polyesterification was confirmed via FTIR-ATR spectroscopy. All spectra which have been obtained shows satisfactory results. On the other hand in case of samples with MDI free carbonyl group corresponds to urea group was not found. Nevertheless, additionally transmission test should be performed to confirm fully structure of product. Biodegradable behavior is last feature of the important characteristics that the present work intends to prove. The use of dimer acids in the polyester backbone structure is a way of improving the hydrolysis resistance of such prepolymers because dimeracid-based polyesters contain a higher content of methylene groups per repeating ester unit than Bibliography 1. Berins, M.L., Plastics Engineering Handbook of the Society of the Plastics Industry, 5/e, Chapman and Hall, New York, Bayer, O., Polyurethanes. Modern Plastics, Vilar, Walter Dias; Chemistry and Technology of Polyurethanes; 3rd edition, Mihail Ionescu, Rapra Technology Limited Chemistry and Technology of Polyols for Polyurethanes, Pielichowski J., Puszyński A. Technologia Tworzyw sztucznych WNT Warszawa, Olczyk W. Poliuretany WNT Warszawa, Wirpsza Z. Poliuretany WNT Warszawa, Dyson R. W., Specialty Polymers Blackie USA: Chapman and Hall, New York, Randall R., S.L., ed. The Huntsman polyurethanes book., John Wiley & Sons, Ltd., Colour Grading according to the Gardner Colour Scale (ASTM D1544)Lovibond Gardner Comparator 3000, AF r/af228gardner.pdf last accessed in 2011/07/ Baschetti, M.; Piccinini, E.; Barbari, T. A.; Sarti, G. C.; Quantitative Analysis of Polymer Dilation during Sorption Using FTIR-ATR Spectroscopy, Macromolecules;
8 12. Elabd Y.A., Baschetti M., Barbari T.A., Timeresolved Fourier transform infrared/attenuated total reflection spectroscopy for the measurement of molecular diffusion in polymers Journal of Polymer Science Part B: Polymer Physics 41, 22, 2003, Sarpeshkar A., e.a., Polyurethanes with improved water resistance. Polyurethanes Expo 98, Cray Valley Products for Urethane Elastomers, Cray Valley Technical Bulletin No. 1560, Cray Valley Co., Pytela, J., M. Sufcak, J. Cermak, and J. G. Drobny Novel Isocyanate Prepolymers Based on Polybutadiene Diols for Composite Binders and Cast Elastomers,
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector
 A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
The present chapter deals with synthesis of Polyurethane acrylate Oligomers.
 The present chapter deals with synthesis of Polyurethane acrylate Oligomers. 2.1 Principle reactions in preparation of UV-curable polyurethane acrylates oligomers If polymer chain carries -NCO group as
The present chapter deals with synthesis of Polyurethane acrylate Oligomers. 2.1 Principle reactions in preparation of UV-curable polyurethane acrylates oligomers If polymer chain carries -NCO group as
race to the Second Edition Preface to the First Edition 'kno\t'ledgements Contents
 Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
Physical and Mechanical Properties of Polymers
 MATE 453/MSE 553 Physical and Mechanical Properties of Polymers Guided Lecture Notes for Fall 2012 Prof. Michael Kessler Department of Materials Science and Engineering Iowa State University PHYSICAL AND
MATE 453/MSE 553 Physical and Mechanical Properties of Polymers Guided Lecture Notes for Fall 2012 Prof. Michael Kessler Department of Materials Science and Engineering Iowa State University PHYSICAL AND
Rheological Characterization of Medical Thermoplastic Polyurethanes
 Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
1.1 Basic Polymer Chemistry. 1.2 Polymer Nomenclature. 1.3 Polymer Synthesis. 1.4 Chain Growth Polymerization. Polymer =
 1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
Infrared Spectroscopy
 Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Chain extension and branching of poly(l-lactic acid) produced by reaction with a DGEBA-based epoxy resin
 express Polymer Letters Vol.1, No.11 (2007) 734 739 Available online at www.expresspolymlett.com DOI: 10.3144/expresspolymlett.2007.101 Chain extension and branching of poly(l-lactic acid) produced by
express Polymer Letters Vol.1, No.11 (2007) 734 739 Available online at www.expresspolymlett.com DOI: 10.3144/expresspolymlett.2007.101 Chain extension and branching of poly(l-lactic acid) produced by
POLYVINYL ALCOHOL. SYNONYMS Vinyl alcohol polymer, PVOH, INS No DEFINITION DESCRIPTION FUNCTIONAL USES CHARACTERISTICS
 POLYVINYL ALCOHOL New specifications prepared at the 61 st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI of 50 mg/kg bw was established at 61 st JECFA (2003). SYNONYMS Vinyl alcohol polymer,
POLYVINYL ALCOHOL New specifications prepared at the 61 st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI of 50 mg/kg bw was established at 61 st JECFA (2003). SYNONYMS Vinyl alcohol polymer,
Polymer Molecular Weight
 Chapter 3 Polymer Molecular Weight 3.1 Introduction Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids
Chapter 3 Polymer Molecular Weight 3.1 Introduction Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids
Liquid Polybutadienes and Derivatives
 Liquid Polybutadienes and Derivatives Coatings & Colorants Product Range Our polyoils and derivatives are stereospecific, lowviscosity and unsaponifiable liquid polybutadienes having a high 1.4-cis double
Liquid Polybutadienes and Derivatives Coatings & Colorants Product Range Our polyoils and derivatives are stereospecific, lowviscosity and unsaponifiable liquid polybutadienes having a high 1.4-cis double
Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes
 The University of Akron From the SelectedWorks of Joseph P. Kennedy June 15, 1985 Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes Joseph P. Kennedy, University of Akron
The University of Akron From the SelectedWorks of Joseph P. Kennedy June 15, 1985 Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes Joseph P. Kennedy, University of Akron
Flexible Packaging Adhesives The Basics. Larry Jopko Rohm and Haas Company. Abstract
 Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Effect of Aliphatic Diisocyanate and Aromatic Diisocyanate on the Properties of Castor Oil-Based Polyurethanes
 IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 11, Issue 7 Ver. III (July. 2018), PP 52-59 www.iosrjournals.org Effect of Aliphatic Diisocyanate and Aromatic Diisocyanate on the
IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 11, Issue 7 Ver. III (July. 2018), PP 52-59 www.iosrjournals.org Effect of Aliphatic Diisocyanate and Aromatic Diisocyanate on the
COMPARATIVE STUDIES ON CHEMICAL RECYCLING (DEPOLYMERIZATION) OF POLYURETHANE SCRAP MATERIAL
 Int. J. Chem. Sci.: 8(2), 21, 17-12 COMPARATIVE STUDIES ON CHEMICAL RECYCLING (DEPOLYMERIZATION) OF POLYURETHANE SCRAP MATERIAL VISHWANATH S. ZOPE * and SUMIT V. JADHAV a Department of Chemistry, M. J.
Int. J. Chem. Sci.: 8(2), 21, 17-12 COMPARATIVE STUDIES ON CHEMICAL RECYCLING (DEPOLYMERIZATION) OF POLYURETHANE SCRAP MATERIAL VISHWANATH S. ZOPE * and SUMIT V. JADHAV a Department of Chemistry, M. J.
Study on Military Polyurethane Adhesive
 Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
Polymerization Modeling
 www.optience.com Polymerization Modeling Objective: Modeling Condensation Polymerization by using Functional Groups In this example, we develop a kinetic model for condensation polymerization that tracks
www.optience.com Polymerization Modeling Objective: Modeling Condensation Polymerization by using Functional Groups In this example, we develop a kinetic model for condensation polymerization that tracks
Introduction to Polyurethane!
 Introduction to Polyurethane! Chemistry and Structure-Property Relationships 05/07/16 Monomers and Polymers Monomer (mono one; mer part): Small molecules Polymer Cons8tutes many monomers. Polymeriza8on
Introduction to Polyurethane! Chemistry and Structure-Property Relationships 05/07/16 Monomers and Polymers Monomer (mono one; mer part): Small molecules Polymer Cons8tutes many monomers. Polymeriza8on
The Effects of Organotin Catalysts on Hydrolytic Condensation of
 Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON
 POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
ORGANIC - BRUICE 8E CH MASS SPECT AND INFRARED SPECTROSCOPY
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state
 2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
1 Answer. 2 Answer A B C D
 216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur. Lecture - 4 Step-growth Polymerization
 Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
Synthesis and Characterisation of Novel Crosslinked Biopolyurethane from Soyabean oil as ecofriendly Biodegradable Material
 Synthesis and Characterisation of Novel Crosslinked Biopolyurethane from Soyabean oil as ecofriendly Biodegradable Material 1 M.L.Ginju, 2 Dr. S. Begila David 1 Research Scholar, Department of Chemistry
Synthesis and Characterisation of Novel Crosslinked Biopolyurethane from Soyabean oil as ecofriendly Biodegradable Material 1 M.L.Ginju, 2 Dr. S. Begila David 1 Research Scholar, Department of Chemistry
1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation. Fuel cell. Infrared spectroscopy
 1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation Fuel cell Infrared spectroscopy Reduction of dichromate(vi) ions 2 Propanal, H 3 H 2 HO, and propanone,
1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation Fuel cell Infrared spectroscopy Reduction of dichromate(vi) ions 2 Propanal, H 3 H 2 HO, and propanone,
Page 2. Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? alcohols C nh 2n+2O. aldehydes C nh 2n+1O
 Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Segmented Copolymers. Segmented Polyurethanes (Prof. Hammond s thesis)
 0.569 Synthesis of Polymers Prof. Paula ammond Lecture 0: Introduction to Radical Polymerization Segmented opolymers Segmented Polyurethanes (Prof. ammond s thesis) soft segment ends in groups - oligomer
0.569 Synthesis of Polymers Prof. Paula ammond Lecture 0: Introduction to Radical Polymerization Segmented opolymers Segmented Polyurethanes (Prof. ammond s thesis) soft segment ends in groups - oligomer
elimination of H 2 O
 1 L, M, N and P are straight-chain organic compounds containing C, H and O only. The flowchart shows reactions involving these compounds. L elimination of H 2 O N polymer P M r ~ 10,000 H + /Cr 2 O 7 2
1 L, M, N and P are straight-chain organic compounds containing C, H and O only. The flowchart shows reactions involving these compounds. L elimination of H 2 O N polymer P M r ~ 10,000 H + /Cr 2 O 7 2
SYNTHESIS OF AMPHIPHILIC PEG-PCL-PEG TRIBLOCK COPOLYMERS
 Journal of the University S. Petrova, of Chemical S. Miloshev, Technology R. Mateva, and Metallurgy, I. Iliev 43, 2, 2008, 199-204 SYNTHESIS OF AMPHIPHILIC PEG-PCL-PEG TRIBLOCK COPOLYMERS S. Petrova, S.
Journal of the University S. Petrova, of Chemical S. Miloshev, Technology R. Mateva, and Metallurgy, I. Iliev 43, 2, 2008, 199-204 SYNTHESIS OF AMPHIPHILIC PEG-PCL-PEG TRIBLOCK COPOLYMERS S. Petrova, S.
Polyethylenterephthalat - PET
 Polyethylenterephthalat - PET PET = Poly(oxy-1,2-ethanediyloxycarbonyl-1,4-phenylenecarbonyl) Patents concerning PET-Synthesis go back to the 40th of the 20 th century Research on esterification and transesterification
Polyethylenterephthalat - PET PET = Poly(oxy-1,2-ethanediyloxycarbonyl-1,4-phenylenecarbonyl) Patents concerning PET-Synthesis go back to the 40th of the 20 th century Research on esterification and transesterification
Poly(ether-ester) Multiblock Copolymers Based on Poly(oxymethylene-alt-oxyalkylene) Glycols
 Macromolecular Research, Vol. 10, No. 4, pp 230-235 (2002) Poly(ether-ester) Multiblock Copolymers Based on Poly(oxymethylene-alt-oxyalkylene) Glycols Jin Bong Kim*, Jae Hwan Chun, Dong Hee Kim, Yun Hee
Macromolecular Research, Vol. 10, No. 4, pp 230-235 (2002) Poly(ether-ester) Multiblock Copolymers Based on Poly(oxymethylene-alt-oxyalkylene) Glycols Jin Bong Kim*, Jae Hwan Chun, Dong Hee Kim, Yun Hee
CH 3 CH 2 CH 2 CH 2 OH
 1 Alcohols are used in the industrial production of many organic compounds. (a) Complete the flowchart below to show the organic product formed in each of the reactions of butan-1-ol. K 2 Cr 2 O 7 (aq)
1 Alcohols are used in the industrial production of many organic compounds. (a) Complete the flowchart below to show the organic product formed in each of the reactions of butan-1-ol. K 2 Cr 2 O 7 (aq)
... [1] Draw another structural isomer of these two alcohols.
![... [1] Draw another structural isomer of these two alcohols. ... [1] Draw another structural isomer of these two alcohols.](/thumbs/75/72508533.jpg) 1 This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol (a) Which alcohol is an example of a tertiary alcohol?... [1]
1 This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol (a) Which alcohol is an example of a tertiary alcohol?... [1]
(a) Name the alcohol and catalyst which would be used to make X. (2)
 1 The chemical X is an ester with formula CH 3 COOC(CH 3 ) 3 which occurs in raspberries and pears. It can be prepared in the laboratory by refluxing ethanoic acid with an alcohol in the presence of a
1 The chemical X is an ester with formula CH 3 COOC(CH 3 ) 3 which occurs in raspberries and pears. It can be prepared in the laboratory by refluxing ethanoic acid with an alcohol in the presence of a
Surface modification of Microfibrillated Cellulose films by Gas-Phase Esterification: Improvement of Barrier Properties.
 Surface modification of Microfibrillated Cellulose films by Gas-Phase Esterification: Improvement of Barrier Properties. G. Rodionova*, M. Lenes**, Ø. Eriksen**, B. H. Hoff*, Ø. W. Gregersen* * Norwegian
Surface modification of Microfibrillated Cellulose films by Gas-Phase Esterification: Improvement of Barrier Properties. G. Rodionova*, M. Lenes**, Ø. Eriksen**, B. H. Hoff*, Ø. W. Gregersen* * Norwegian
Alcohols. Ethanol Production. 182 minutes. 181 marks. Page 1 of 25
 3..10 Alcohols Ethanol Production 18 minutes 181 marks Page 1 of 5 Q1. Ethanol is produced commercially by fermentation of aqueous glucose, C 6 H 1 O 6 State two conditions, other than temperature, which
3..10 Alcohols Ethanol Production 18 minutes 181 marks Page 1 of 5 Q1. Ethanol is produced commercially by fermentation of aqueous glucose, C 6 H 1 O 6 State two conditions, other than temperature, which
Supporting Information for Polybenzimidazolium Salts: A New Class of. Anion-Conducting Polymer
 Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
FOAMING AND STAIN REMOVING LAUNDRY LIQUID DETERGENTS BASED ON NOVEL POLYMER AND SORBITOL
 Int. J. Chem. Sci.: 12(3), 24, 933-940 ISSN 0972-768X www.sadgurupublications.com FOAMING AND STAIN REMOVING LAUNDRY LIQUID DETERGENTS BASED ON NOVEL POLYMER AND SORBITOL A. G. DESHMUKH * and B. B. GOGTE
Int. J. Chem. Sci.: 12(3), 24, 933-940 ISSN 0972-768X www.sadgurupublications.com FOAMING AND STAIN REMOVING LAUNDRY LIQUID DETERGENTS BASED ON NOVEL POLYMER AND SORBITOL A. G. DESHMUKH * and B. B. GOGTE
SYNTHESIS OF POTASSIUM-S-1,2- DIHYDROXYPROPYLSULPHONATE AND ITS INCORPORATION TO SYNTHESISE POLYESTER IONOMER
 SYNTHESIS OF POTASSIUM-S-1,2- DIHYDROXYPROPYLSULPHONATE AND ITS INCORPORATION TO SYNTHESISE POLYESTER IONOMER Pinku Sarma 1 P.G. Student, Department of Chemistry, Gauhati University, G uwahati, India 1
SYNTHESIS OF POTASSIUM-S-1,2- DIHYDROXYPROPYLSULPHONATE AND ITS INCORPORATION TO SYNTHESISE POLYESTER IONOMER Pinku Sarma 1 P.G. Student, Department of Chemistry, Gauhati University, G uwahati, India 1
Orientational behavior of liquid-crystalline polymers with amide groups
 Advances in Materials 2014; 3(6): 89-93 Published online January 06, 2015 (http://www.sciencepublishinggroup.com/j/am) doi: 10.11648/j.am.20140306.14 ISSN: 2327-2503 (Print); ISSN: 2327-252X (Online) Orientational
Advances in Materials 2014; 3(6): 89-93 Published online January 06, 2015 (http://www.sciencepublishinggroup.com/j/am) doi: 10.11648/j.am.20140306.14 ISSN: 2327-2503 (Print); ISSN: 2327-252X (Online) Orientational
Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION Historical Perspective Some of the earliest useful polymeric
 Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid)
 Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Research on the Properties of Rigid Polyurethane Foam with Heteroaromatic Polyol Hong GUO a, Qun GAO b, *, Chun-Fa OUYANG c
 International Conference on Material Science and Application (ICMSA 2015) Research on the Properties of Rigid Polyurethane Foam with Heteroaromatic Polyol Hong GUO a, Qun GAO b, *, Chun-Fa OUYANG c School
International Conference on Material Science and Application (ICMSA 2015) Research on the Properties of Rigid Polyurethane Foam with Heteroaromatic Polyol Hong GUO a, Qun GAO b, *, Chun-Fa OUYANG c School
Electronic Supplementary Information for New Journal of Chemistry
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation
 Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation Adam Al-Mulla* Chemical Engineering Department, Kuwait University, P.O. Box 5969, 36 Safat, Kuwait, ABSTRACT Thermotropic
Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation Adam Al-Mulla* Chemical Engineering Department, Kuwait University, P.O. Box 5969, 36 Safat, Kuwait, ABSTRACT Thermotropic
Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters
 Iranian Polymer Journal ' Volume 5 Number 4 (29961 IO26-I265196 Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters Mehdi Barikani and Mohammad Barmar Polymer
Iranian Polymer Journal ' Volume 5 Number 4 (29961 IO26-I265196 Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters Mehdi Barikani and Mohammad Barmar Polymer
Preparation of Series Schiff Bases and Studying of their Liquid Crystalline Behavior
 Preparation of Series Schiff Bases and Studying of their Liquid Crystalline Behavior Dr. Kareem Jaber 1 1 Assistant Professor, Department of Chemistry, Faculty of Science. Email: karee2000@hotmail.com
Preparation of Series Schiff Bases and Studying of their Liquid Crystalline Behavior Dr. Kareem Jaber 1 1 Assistant Professor, Department of Chemistry, Faculty of Science. Email: karee2000@hotmail.com
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis
 SWST 2015 International Convention Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis Xiaomei Liu Department
SWST 2015 International Convention Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis Xiaomei Liu Department
ORGANIC - BROWN 8E CH INFRARED SPECTROSCOPY.
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
CHEMISTRY (SALTERS) Chemistry of Materials. OXFORD CAMBRIDGE AND RSA EXAMINATIONS Advanced GCE
 XFRD CAMBRIDGE AND RSA EXAMINATINS Advanced GCE CHEMISTRY (SALTERS) Chemistry of Materials 2849 Monday 19 JUNE 2006 Afternoon 1 hour 30 minutes Candidates answer on the question paper. Additional materials:
XFRD CAMBRIDGE AND RSA EXAMINATINS Advanced GCE CHEMISTRY (SALTERS) Chemistry of Materials 2849 Monday 19 JUNE 2006 Afternoon 1 hour 30 minutes Candidates answer on the question paper. Additional materials:
Citation for published version (APA): Verhoeven, V. W. A. (2006). The reactive extrusion of thermoplastic polyurethane s.n.
 University of Groningen The reactive extrusion of thermoplastic polyurethane Verhoeven, Vincent Wilhelmus Andreas IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
University of Groningen The reactive extrusion of thermoplastic polyurethane Verhoeven, Vincent Wilhelmus Andreas IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
Studies on Furan Polymer Concrete
 Studies on Furan Polymer Concrete Rajesh Katiyar 1, Shobhit Shukla 2 1Associate Professor, Department of Chemical engineering, H.B.T.U., Kanpur-208002, India 2Research Scholar, Department of Chemical engineering
Studies on Furan Polymer Concrete Rajesh Katiyar 1, Shobhit Shukla 2 1Associate Professor, Department of Chemical engineering, H.B.T.U., Kanpur-208002, India 2Research Scholar, Department of Chemical engineering
TitleSynthesis of Itaconic Acid from Eth. Author(s) Kunichika, Sango; Oka, Shinzaburo;
 TitleSynthesis of Itaconic Acid from Eth Author(s) Kunichika, Sango; Oka, Shinzaburo; Citation Bulletin of the Institute for Chemi University (1966), 44(3): 221-225 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76124
TitleSynthesis of Itaconic Acid from Eth Author(s) Kunichika, Sango; Oka, Shinzaburo; Citation Bulletin of the Institute for Chemi University (1966), 44(3): 221-225 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76124
One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting from RAFT polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Waterborne UV Curable Polyurethane Dispersions
 Waterborne Curable Polyurethane Dispersions Bo Lv, Zhongqun Xue Jiangsu Sanmu Group, Yixing 214258, Jiangsu, China Abstract Two high molecular weight polyurethane dispersions (PUDs) were synthesized by
Waterborne Curable Polyurethane Dispersions Bo Lv, Zhongqun Xue Jiangsu Sanmu Group, Yixing 214258, Jiangsu, China Abstract Two high molecular weight polyurethane dispersions (PUDs) were synthesized by
9. Hydroboration-Oxidation of Alkenes
 9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS
 SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
Biodiesel Fundamentals for High School Chemistry Classes. Laboratory 4: Chemical Equilibrium in Biodiesel
 Laboratory 4: Chemical Equilibrium in Biodiesel Production Topics Covered Forward chemical reactions vs. reverse reactions Chemical reactions in equilibrium Ways to stimulate a reaction to proceed towards
Laboratory 4: Chemical Equilibrium in Biodiesel Production Topics Covered Forward chemical reactions vs. reverse reactions Chemical reactions in equilibrium Ways to stimulate a reaction to proceed towards
Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs
 Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs Application note Academic Author Frank Higgins and Alan Rein Agilent Technologies Danbury, CT, USA Introduction
Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs Application note Academic Author Frank Higgins and Alan Rein Agilent Technologies Danbury, CT, USA Introduction
Suggest TWO aspects to show approach II is considered to be a greener method than using approach I.
 1. (a) Answer the following short questions Nowadays, products manufactured from industrial process emphasize on a green approach. An organic compound is manufactured by two different approaches as shown
1. (a) Answer the following short questions Nowadays, products manufactured from industrial process emphasize on a green approach. An organic compound is manufactured by two different approaches as shown
Operating Characteristics of Diene-Urethane Elastomers on the base of NISSOG 3000 Oligodienediol
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (5): Pg. 2363-2370 Operating Characteristics
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (5): Pg. 2363-2370 Operating Characteristics
Poly(N-substituted urethane)s with different molecular weights of polyethylene glycol
 Iranian Polymer Journal 14 (9), 005, 815-81 Synthesis and Characterization of N-Polyethylene Glycol Monomethyl Ether Substituted Polyurethane S. Mohammad Seyed Mohaghegh 1, Mehdi Barikani *, and Ali Akbar
Iranian Polymer Journal 14 (9), 005, 815-81 Synthesis and Characterization of N-Polyethylene Glycol Monomethyl Ether Substituted Polyurethane S. Mohammad Seyed Mohaghegh 1, Mehdi Barikani *, and Ali Akbar
Alkoxylates Promoting superior performance
 Our range of versatile liquid polyols Promote superior end-product performance in radiation curing Enhance properties of polyurethane elastomers and foams Tailor ideal additives for a wide variety of applications
Our range of versatile liquid polyols Promote superior end-product performance in radiation curing Enhance properties of polyurethane elastomers and foams Tailor ideal additives for a wide variety of applications
Polymers are high molecular mass macromolecules composed of repeating structural
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
POLYMERS Mega molecules Out of Small Ones
 Back Recommended grades level(s) 11-12 Time Duration: - 50 minutes POLYMERS Mega molecules Out of Small Ones Objective(s): The learner will produce a linear and a cross-linked polyester. The learner will
Back Recommended grades level(s) 11-12 Time Duration: - 50 minutes POLYMERS Mega molecules Out of Small Ones Objective(s): The learner will produce a linear and a cross-linked polyester. The learner will
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
OPTICAL PLASTICS FROM ACRYLATES & CINNAMATES; INCORPORATION OF BARIUM FOR IMPROVED PROPERTIES
 IJRRAS 12 (1) July 12 www.arpapress.com/volumes/vol12issue1/ijrras_12_1_11.pdf OPTICAL PLASTICS FROM ACRYLATES & CINNAMATES; INCORPORATION OF BARIUM FOR IMPROVED PROPERTIES Gunjan Suri, Pranshu Chhabra
IJRRAS 12 (1) July 12 www.arpapress.com/volumes/vol12issue1/ijrras_12_1_11.pdf OPTICAL PLASTICS FROM ACRYLATES & CINNAMATES; INCORPORATION OF BARIUM FOR IMPROVED PROPERTIES Gunjan Suri, Pranshu Chhabra
MALEIC ANHYDRIDE TREATMENT OF SOFTWOOD EFFECT ON WOOD STRUCTURE AND PROPERTIES
 CELLULOSE CHEMISTRY AND TECHNOLOGY MALEIC ANHYDRIDE TREATMENT OF SOFTWOOD EFFECT ON WOOD STRUCTURE AND PROPERTIES CARMEN-ALICE TEACĂ, RUXANDA BODÎRLĂU and IULIANA SPIRIDON Petru Poni Institute of Macromolecular
CELLULOSE CHEMISTRY AND TECHNOLOGY MALEIC ANHYDRIDE TREATMENT OF SOFTWOOD EFFECT ON WOOD STRUCTURE AND PROPERTIES CARMEN-ALICE TEACĂ, RUXANDA BODÎRLĂU and IULIANA SPIRIDON Petru Poni Institute of Macromolecular
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Chapter 4 Acrylic Polyurethane Emulsion Polymers
 Chapter 4 Acrylic Polyurethane Emulsion Polymers In this chapter, isophorone diisocyanate (IPDI), a cycloaliphatic diisocyanate, has been selected to react with the different types of hydroxyl acrylic
Chapter 4 Acrylic Polyurethane Emulsion Polymers In this chapter, isophorone diisocyanate (IPDI), a cycloaliphatic diisocyanate, has been selected to react with the different types of hydroxyl acrylic
Catalisi e stabilizzazione di schiume PIR: recenti sviluppi
 Catalisi e stabilizzazione di schiume PIR: recenti sviluppi Milano, 25 Maggio 2017 Jobst Grimminger Andrea Stefani Introduction With high energy costs, increasing importance is being placed on insulation
Catalisi e stabilizzazione di schiume PIR: recenti sviluppi Milano, 25 Maggio 2017 Jobst Grimminger Andrea Stefani Introduction With high energy costs, increasing importance is being placed on insulation
Properties of Polyurethane Elastomers Obtained with Various Chain Extenders
 Properties of Polyurethane Elastomers Obtained with Various Chain Extenders STEFAN OPREA*, VERONICA OPREA 1 1 P. Poni Institute of Macromolecular Chemistry, 41 A Aleea Grigore Ghica Drive, 700487, Iasi,Romania
Properties of Polyurethane Elastomers Obtained with Various Chain Extenders STEFAN OPREA*, VERONICA OPREA 1 1 P. Poni Institute of Macromolecular Chemistry, 41 A Aleea Grigore Ghica Drive, 700487, Iasi,Romania
# Ans Workings / Remarks
 # Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
# Ans Workings / Remarks 1 B Atomic mass and temperature affects the rate of diffusion of gas. The lower the atomic mass, the lighter the substance. The higher the temperature, the higher the rate of collision
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/334/6058/965/dc1 Supporting Online Material for Silica-Like Malleable Materials from Permanent Organic Networks Damien Montarnal, Mathieu Capelot, François Tournilhac,
www.sciencemag.org/cgi/content/full/334/6058/965/dc1 Supporting Online Material for Silica-Like Malleable Materials from Permanent Organic Networks Damien Montarnal, Mathieu Capelot, François Tournilhac,
SUPPORTING INFORMATION. Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder. Click Reaction; A New Class of Coating Material
 SUPPORTING INFORMATION Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder Click Reaction; A New Class of Coating Material Prasanta Kumar Behera, Prantik Mondal, Nikhil K. Singha*
SUPPORTING INFORMATION Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder Click Reaction; A New Class of Coating Material Prasanta Kumar Behera, Prantik Mondal, Nikhil K. Singha*
Synthesis and characterization of polyurethane microspheres
 Pure & Appl. Chern., Vol. 70, No. 6, pp. 12951299,1998, Printed in Great Britain. Q 1998 IUPAC Synthesis and characterization of polyurethane microspheres L.S. Ramanathan, P. G. Shukla And S. Sivaram Division
Pure & Appl. Chern., Vol. 70, No. 6, pp. 12951299,1998, Printed in Great Britain. Q 1998 IUPAC Synthesis and characterization of polyurethane microspheres L.S. Ramanathan, P. G. Shukla And S. Sivaram Division
Synthesis of isobutyl acetate
 Practical course in organic chemistry (OCP 1) Spring semester 2008 529-0230-00L Synthesis of isobutyl acetate (cherry, raspberry, strawberry) Janosch Ehrenmann BSc Biotechnology D CHAB, ETH Zurich Zurich,
Practical course in organic chemistry (OCP 1) Spring semester 2008 529-0230-00L Synthesis of isobutyl acetate (cherry, raspberry, strawberry) Janosch Ehrenmann BSc Biotechnology D CHAB, ETH Zurich Zurich,
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Product Bulletin. SMA Multi-Functional Resins
 Product Bulletin SMA Multi-Functional Resins TABLE OF CONTENTS INTRODUCTION... 4 CRAY VALLEY S STYRENE MALEIC ANHYDRIDE RESINS......5-6 PROPERTIES OF STYRENE MALEIC ANHYDRIDE RESINS... 7-11 REACTIONS
Product Bulletin SMA Multi-Functional Resins TABLE OF CONTENTS INTRODUCTION... 4 CRAY VALLEY S STYRENE MALEIC ANHYDRIDE RESINS......5-6 PROPERTIES OF STYRENE MALEIC ANHYDRIDE RESINS... 7-11 REACTIONS
Synthesis and properties of aqueous polyurethane dispersions: Influence of molecular weight of polyethylene glycol
 Korean J. Chem. Eng., 30(12), 2259-2263 (2013) DOI: 10.1007/s11814-013-0166-9 INVITED REVIEW PAPER pissn: 0256-1115 eissn: 1975-7220 Synthesis and properties of aqueous polyurethane dispersions: Influence
Korean J. Chem. Eng., 30(12), 2259-2263 (2013) DOI: 10.1007/s11814-013-0166-9 INVITED REVIEW PAPER pissn: 0256-1115 eissn: 1975-7220 Synthesis and properties of aqueous polyurethane dispersions: Influence
3.2.8 Haloalkanes. Nucleophilic Substitution. 267 minutes. 264 marks. Page 1 of 36
 3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
CHAPTER IV HOFMANN REARRANGEMENT IN CROSSLINKED POLYMERIC MATRICES
 CHAPTER IV HOFMANN REARRANGEMENT IN CROSSLINKED POLYMERIC MATRICES The Hofmann degradation reaction has been used as a synthetic route for the preparation of amines 180-187 Tanaka and Senju reported the
CHAPTER IV HOFMANN REARRANGEMENT IN CROSSLINKED POLYMERIC MATRICES The Hofmann degradation reaction has been used as a synthetic route for the preparation of amines 180-187 Tanaka and Senju reported the
Supporting Information. Copolymers of Tetrahydrofuran and Epoxidized Vegetable Oils: Application to Elastomeric Polyurethanes
 Supporting Information Copolymers of Tetrahydrofuran and Epoxidized Vegetable Oils: Application to Elastomeric Polyurethanes Andrew J Clark,* Seng Soi Hoong Department of Chemistry, University of Warwick,
Supporting Information Copolymers of Tetrahydrofuran and Epoxidized Vegetable Oils: Application to Elastomeric Polyurethanes Andrew J Clark,* Seng Soi Hoong Department of Chemistry, University of Warwick,
Chemistry 216. First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh. Lab section. GSI name. Name Please print.
 Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Chemistry 216 First Exam (March 16, 2010) (1 hr 15 min, 80 points) Dr. Kyoung Moo Koh Lab section GSI name Name Please print Signature Student ID# I 8 II 10 III 6 IV 12 V 12 VI 10 VII 14 VIII 8 Total 80
Tolonate X FLO 100. Bio-based & low viscosity aliphatic isocyanate polymer
 Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
The functionality of a monomer is the number of binding sites that is/are present in that monomer.
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Fomrez catalyst UL-50
 Technical Data Sheet Fomrez catalyst UL-50 Description Momentive Performance Materials offers the Fomrez line of tin catalysts. This line of world-class organometallic tin(iv) catalysts is one of the broadest
Technical Data Sheet Fomrez catalyst UL-50 Description Momentive Performance Materials offers the Fomrez line of tin catalysts. This line of world-class organometallic tin(iv) catalysts is one of the broadest
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER
 SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
F G C G H H C B B A A DD E E
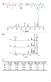 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION Catalysis is an important process to improve the production of chemicals. This phenomenon can be employed in a chemical reaction that is favored thermodynamically but is very slow
1 CHAPTER 1 INTRODUCTION Catalysis is an important process to improve the production of chemicals. This phenomenon can be employed in a chemical reaction that is favored thermodynamically but is very slow
DIELS-ALDER REACTION OF 1,3-BUTADIENE AND MALEIC ANHYDRIDE TO PRODUCE 4-CYCLOHEXENE-CIS-1,2-DICARBOXYLIC ACID. Douglas G. Balmer. (T.A.
 DIELS-ALDER REACTIN F,3-BUTADIENE AND MALEIC ANYDRIDE T PRDUCE 4-CYCLEXENE-CIS-,2-DICARBXYLIC ACID Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted August 2007 Balmer Introduction: The purpose of
DIELS-ALDER REACTIN F,3-BUTADIENE AND MALEIC ANYDRIDE T PRDUCE 4-CYCLEXENE-CIS-,2-DICARBXYLIC ACID Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted August 2007 Balmer Introduction: The purpose of
Organic Chemistry. Alkanes are hydrocarbons in which the carbon atoms are joined by single covalent bonds.
 Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
Fourier Transform Infrared Spectrophotometry Studies of Chromium Trioxide-Phthalic Acid Complexes
 DOI:10.7598/cst2016.1260 Chemical Science Transactions ISSN:2278-3458 2016, 5(3), 770-774 RESEARCH ARTICLE Fourier Transform Infrared Spectrophotometry Studies of Chromium Trioxide-Phthalic Acid Complexes
DOI:10.7598/cst2016.1260 Chemical Science Transactions ISSN:2278-3458 2016, 5(3), 770-774 RESEARCH ARTICLE Fourier Transform Infrared Spectrophotometry Studies of Chromium Trioxide-Phthalic Acid Complexes
COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download)
 COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download) Dr. Debasis Samanta Senior Scientist & AcSIR Assistant Professor Polymer Science & Technology Department., CSIR-CLRI,
COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download) Dr. Debasis Samanta Senior Scientist & AcSIR Assistant Professor Polymer Science & Technology Department., CSIR-CLRI,
