K.C. Nicolaou Publications
|
|
|
- Imogene Stone
- 5 years ago
- Views:
Transcription
1 K.C. Nicolaou Publications 1. Diastereomeric Monocyclic Diallenes. The Synthesis of Cyclododeca- 3,4,9,10-tetraene-1,7-dione, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, J. Chem. Soc., Chem. Commun. 1970, Synthesis of Cyclotetradeca-3,4,5,10,11,12-hexaene-1,8-dione, a Monocyclic Dicumulenedione, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, J. Chem. Soc., Chem. Commun. 1971, Diastereomeric Monocyclic Diallenes. The Stereochemistry of Formation of Racemic and Meso-3,4,9,10-Cyclododecatetraene-1,7-dione, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, Tetrahedron Lett. 1972, Diastereomeric Monocyclic Diallenes. The Synthesis and Properties of the Diastereomeric 3,4,9,10-Cyclodecatetraene-1,7-diones and 3,4,10,11- Cyclotetradecatetraene-1,8-diones, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, J. Am. Chem. Soc. 1973, 95, Monocyclic Allenes. The Synthesis of 3,8,9-Cycloundecatriene-1,6-dione and 12-Oxabicyclo[7.2.1]dodeca-5,6,9,11-tetraene-3-one, a Furanophane Containing an Allene Group, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, J. Org. Chem. 1973, 38, The Synthesis of 3,4,5,10,11,12-Cyclotetradecahexaene-1,8-dione, a Monocyclic Dicumulene dione, K.C. Nicolaou, P.J. Garratt, F. Sondheimer, J. Org. Chem. 1973, 38, Additions of Chlorosulfonyl Isocyanate and Sulfenyl Halides to Benzvalene, K.C. Nicolaou, Thomas J. Katz, J. Am. Chem. Soc. 1974, 96, A Short Synthetic Route to Prostaglandins Utilizing Position-Selective Epoxide Opening by the Vinyl Gilman Reagent, E.J. Corey, K.C. Nicolaou, D.J. Beames, Tetrahedron Lett. 1974, An Efficient and Mild Lactonization Method for the Synthesis of Macrolides, E.J. Corey, K.C. Nicolaou, J. Am. Chem. Soc. 1974, 96, Synthesis of Novel Macrocyclic Lactones in the Prostaglandin and Polyether Antibiotic Series, E.J. Corey, K.C. Nicolaou, L.S. Melvin, J. Am. Chem. Soc. 1975, 97, Synthesis of Brefeldin A, Carpaine, Vertaline, and Erythronolide B from Non-Macro-cyclic Precursors, E.J. Corey, K.C. Nicolaou, L.S. Melvin, J. Am. Chem. Soc. 1975, 97, Total Synthesis of (±)-Vermiculine, E.J. Corey, K.C. Nicolaou, T. Toru, J. Am. Chem. Soc. 1975, 97, Superoxide Ion as a Synthetically Useful Oxygen Nucleophile, E.J. Corey, K.C. Nicolaou, M. Shibasaki, Y. Machida, C.S. Shiner, Tetrahedron Lett. 1975, A Useful Method for the Conversion of Azides to Amines, E.J. Corey, K.C. Nicolaou, M. Shibasaki, Y. Machida, C.S. Shiner, Synthesis 1975, Synthesis and Biological Properties of a 9,11-Azo-Prostanoid. A Highly Active Biochemical Mimic of Prostaglandin Endoperoxides. E.J. Corey, K.C.
2 Nicolaou, Y. Machida, C.L. Malmsten, B. Samuelsson, Proc. Natl. Acad. Sci. U. S. A. 1975, 72, Simple Stereospecific Routes to 9-epi-Prostaglandin F 2 (PGF 2 ) and 11-epi- Prostaglandin F 2, E.J. Corey, K.C. Nicolaou, M. Shibasaki, J. Chem. Soc., Chem. Commun. 1975, A Simple, Stereocontrolled Total Synthesis of a Biologically Active Analog of Prostaglandin Endoperoxides (PGH 2, PGG 2 ), E.J. Corey, M. Shibasaki, K.C. Nicolaou, C.L. Malmsten, B. Samuelsson, Tetrahedron Lett. 1976, Stimulation of Renin Release from Rabbit Renal Cortex by Arachidonic Acid and Prostaglandin Endoperoxides, P.C. Weber, C. Larsson, E. Anggard, M. Hamberg, E.J. Corey, K.C. Nicolaou, B. Samuelsson, Circulation Res. 1976, 39, A Translactonization Route to Macrocyclic Lactones, E.J. Corey, D.J. Brunelle, K.C. Nicolaou, J. Am. Chem. Soc. 1977, 99, Synthesis of Macrolides, K.C. Nicolaou, Tetrahedron 1977, 33, Phenylselenolactonization. An Extremely Mild and Synthetically Useful Cyclization Process, K.C. Nicolaou, Zenon Lysenko, J. Am. Chem. Soc. 1977, 99, Phenylsulfenylactonization: An Easy and Synthetically Useful Lactonization Procedure, K.C. Nicolaou, Zenon Lysenko, J. Chem. Soc., Chem. Commun. 1977, The Use of PhSeCl in the Synthesis of Cyclic Ethers, K.C. Nicolaou, Zenon Lysenko, Tetrahedron Lett. 1977, Synthesis of (4E)-Deoxy-6,9-Epoxy-4-PGF 1, a Prostacyclin (PGX) Isomer, K.C. Nicolaou, W.E. Barnette, J. Chem. Soc., Chem. Commun. 1977, Simple Efficient Synthesis of Prostacyclin (PGI 2 ), K.C. Nicolaou, W.E. Barnette, G.P. Gasic, R.L. Magolda, W.J. Sipio, J. Chem. Soc., Chem. Commun. 1977, ,9-Thiaprostacyclin. A Stable and Potent Biological Analog of Prostacyclin (PGI 2 ), K.C. Nicolaou, W.E. Barnette, G.P. Gasic, R.L. Magolda, J. Am. Chem. Soc. 1977, 99, Total Synthesis of Erythromycins (Part III). Stereoselective Routes to Intermediates Corresponding to C(1) to C(9) and C(10) to C(13) Fragments of Erythronolide B, E.J. Corey, E.J. Trybulski, L.J. Melvin, Jr., K.C. Nicolaou, J.A. Secrist, R. Lett, P.W. Sheldrake, J.R. Falck, D.J. Brunelle, M.F. Haslanger, S. Kim, S. Yoo, J. Am. Chem. Soc. 1978, 100, Total Synthesis of Erythromycins (Part IV). Total Synthesis of Erythromolide B, E.J. Corey, S. Kim, S. Yoo, K.C. Nicolaou, L.J. Melvin, Jr., D.J. Brunelle, J.R. Falck, E.J. Trybulski, R. Lett, P.W. Sheldrake, J. Am. Chem. Soc. 1978, 100, Synthesis and Biological Properties of Prostaglandin Endoperoxides, Thromboxanes and Prostacyclins, K.C. Nicolaou, G.P. Gasic, W.E. Barnette, Angew. Chem. Int. Ed. Engl. 1978, 17,
3 30. Organoselenium-Induced Ring Closures. Sulfur-Containing Prostacyclins. Stereoselctive Synthesis of Cyclic, α,β-unsaturated Sulfoxides, Sulfones and Sulfides and Synthesis of 6,9-Sulfoxa-5(E)-and 5(Z)-Prostacyclin, and 6,9- Sulfo-5(E)- and 5(Z)-Prostacyclin, 6,9-Sulfo-6α- and 6β-4(E)-Isoprostacyclin and 6,9-Thiaprostacyclin, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, J. Am. Chem. Soc. 1978, 100, Synthesis of Sulfur-Containing Prostacyclin PGI 1 Analogues. 6,9-Epithio- PGI 1, its S-Oxide Stereoisomers and its SS-Dioxide, K.C. Nicolaou, R.L. Magolda, W.E. Barnette, J. Chem. Soc., Chem. Commun. 1978, Total Synthesis of 14-Fluoroprostaglandin F 2α and 14-Fluoroprostacyclin, P.A. Grieco, Y. Yokoyama, K.C. Nicolaou, W.E. Barnette, J.B. Smith, M. Ogletree, A.M. Lefer, Chem. Lett. 1978, Synthesis and Biological Properties of 12-Fluoroprostacyclin, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, P.A. Grieco, W. Owens, C.-L. J. Wang, J.B. Smith, M. Ogletree, A.M. Lefer, Prostaglandins 1978, 16, Synthesis of Prostaglandin H 2 (PGH 2 ) and Prostacyclin (PGI 2 ) Analogs: Tetrathia-PGH 2 and PGI 2 -Ketal Methyl Ester, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, Prostaglandins and Medicine 1978, 1, Total Synthesis of Carboprostacyclin, a Stable and Biologically Active Analog of Prostacyclin (PGI 2 ), K.C. Nicolaou, W.J. Sipio, R.L. Magolda, S. Seitz, W.E. Barnette, J. Chem. Soc., Chem. Commun. 1978, Prostacyclin: A Potentially Valuable Agent for Preserving Myocardial Tissue in Acute Myocardial Ischemia, A.M. Lefer, M.L. Ogletree, J.B. Smith, M.J. Silver, G.P. Gasic, W.E. Barnette, K.C. Nicolaou, Science 1978, 200, Prostaglandin I 2 as a Potentiator of Acute Inflammation in Rats, K. Komoriya, J. Ohmori, A. Azuma, S. Kurozumi, Y. Hashimoto, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, Prostaglandins 1978, 15, Cardiac and Renal Prostaglandin I 2 : Biosynthesis and Biological Effects in Isolated Perfused Rabbit Tissues, P. Needleman, S.D. Bronson, A. Wyche, K.C. Nicolaou, M. Sivakoff, J. Clin. Invest. 1978, 61, Effects of Prostacyclin on Perfusion Pressure, Electrical Activity Rate and Force of Contraction in Isolated Rat and Rabbit Hearts, M. Karmazyn, D.F. Horrobin, M.S. Manku, S.C. Cunnane, R.A. Karmali, A.I. Ally, R.O. Morgan, K.C. Nicolaou, Life Sci. 1978, 22, Antibodies which Antagonize the Effects of Prostacyclin, J.B. Smith, M. Ogletree, A.M. Lefer, K.C. Nicolaou, Nature 1978, 274, Prostaglandins E 1, E 2 and I 2 : Evidence for Three District Actions in Vascular Smooth Muscle, M.S. Manku, D.F. Horrobin, S.C. Cunnane, A.I. Ally, M. Karmazyn, R.A. Karmali, R.O. Morgan, K.C. Nicolaou, W.E. Barnette, Biochem. Biophys. Res. Commun. 1978, 83, Prostaglandin I 2 Has More Potent Hypotensive Properties than Prostaglandin E 2 in the Normal Spontaneously Hypertensive Rat, C.R. Pace-Asciak, M.C. Carrera, K.C. Nicolaou, Prostaglandins 1978, 15, Enhanced Formation of PGI 2, a Potent Hypotensive Substance by Aortic Rings and Homogenates of the Spontaneously Hypertensive Rat, C.R. Pace-
4 Asciak, M.C. Carrera, G. Rangaraj, K.C. Nicolaou, Prostaglandins 1978, 15, A Triple Test for Screening Biological Activity of Prostacyclin Analogs, R.J. Gryglewski, K.C. Nicolaou, Experientia 1978, 34, Studies of the Mechanism Involved in the Fate of Prostacyclin (PGI 2 ) and 6- Keto-PGF 2α in the Pulmonary Circulation, H.J. Hawkins, J.B. Smith, K.C. Nicolaou, T.E. Eling, Prostaglandins 1978, 16, Synthesis and Chemistry of Prostacyclin (PGI 2 ), K.C. Nicolaou, W.E. Barnette, R.L. Magolda, J. Chem. Res. (M) 1979, Synthesis and Biological Properties of Prostacyclins and Prostaglandin Endoperoxide Analogs, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, in Chemistry, Biochemistry and Pharmacological Properties of Prostanoids, S.M. Roberts and F. Scheinmann, Eds., Pergamon Press, New York, 1979, pp ,9-Pyridazaprostacyclin and Derivatives. The First "Aromatic" Prostacyclins, K.C. Nicolaou, R.L. Magolda, W.E. Barnette, J. Am. Chem. Soc. 1979, 101, Reversal of the Phenylseleno- and Phenylsulpheno-cyclizations. Synthesis of Olefins from Phenylseleno-lactones, Phenylseleno-ethers and α- Hydroxyselenides, K.C. Nicolaou, W.J. Sipio, D.A. Claremon, R.L. Magolda, J. Chem. Soc., Chem. Commun. 1979, Reaction of Phenylselenenyl Chloride with Enol Ethers and 1,2-Ethanediol Acetals. Synthesis of α-phenylseleno-aldehydes and α-phenylseleno-acetals, K.C. Nicolaou, R.L. Magolda, W.J. Sipio, Synthesis 1979, Phenylseleno- and Phenylsulfenolactonizations. Two, Highly Efficient and Synthetically Useful Cyclization Procedures, K.C. Nicolaou, S. Seitz, W.J. Sipio, J.F. Blount, J. Am. Chem. Soc. 1979, 101, Organoselenium-Induced Ring Closures. Cyclization of Dienes with "PhSeOH" to Form Cyclic Ethers, R.M. Scarborough, Jr., A.B. Smith, III, W.E. Barnette, K.C. Nicolaou, J. Org. Chem. 1979, 44, N-Phenylselenophthalimide (N-PSP) and N-Phenylselenosuccinimide (N- PSS). Two Versatile Carriers of the Phenylseleno Group. Oxyselenation of Olefins and a Selenium-Based Macrolide Synthesis, K.C. Nicolaou, D.A. Claremon, W.E. Barnette, S.P. Seitz, J. Am. Chem. Soc. 1979, 101, Synthesis and Biological Properties of Pinane-Thromboxane A 2 (PTA 2 ). A Selective Inhibitor of Coronary Artery Constriction, Platelet Aggregation and Thromboxane Formation, K.C. Nicolaou, R.L. Magolda, J.B. Smith, D. Aharony, E.F. Smith, A.M. Lefer, Proc. Natl. Acad. Sci. U. S. A. 1979, 76, Synthesis of 16-Membered Ring Macrolide Antibiotics I. Stereoselective Construction of the "Right Wing" of the Carbomycins and Leucomycins from D-Glucose, K.C. Nicolaou, M.R. Pavia, S.P. Seitz, Tetrahedron Lett. 1979,
5 56. Synthesis of Macrocycles by Intramolecular Ketophosphonate Reactions. Stereoselective Construction of the "Left Wing" of Carbomycin B and a Synthesis of dl-muscone from Oleic Acid, K.C. Nicolaou, S.P. Seitz, M.R. Pavia, N. Petasis, J. Org. Chem. 1979, 44, Total Synthesis of Oestra-1,3,5(10)-trien-17-one, K.C. Nicolaou, W.E. Barnette, J. Chem. Soc., Chem. Commun. 1979, Studies on the Protective Effect of Prostacyclin on Acute Myocardial Ischemia, M.L. Ogletree, A.M. Lefer, J.B. Smith, K.C. Nicolaou, Eur. J. Pharmacol. 1979, 56, Effect of 6,9-Thia Prostacyclin I 2 (A Stable PGI 2 Analogue) on Passive Cutaneous Anaphylaxis (PCA) in Rats, K. Komoriya, H. Ohmori, A. Azuma, S. Kurozumi, Y. Hashimoto, K.C. Nicolaou, Jap. J. Pharmacol. 1979, 29, Prostacyclin, Thromboxanes and The Arachidonic Acid Cascade, K.C. Nicolaou, J.B. Smith, Annual Reports in Medicinal Chemistry, Academic Press, New York, Vol. 14, Ch. 17, 1979, pp Selective Binding Site for 3 H-Prostaglandin I 2 on Platelets, A.M. Single, J.B. Smith, M.J. Silver, K.C. Nicolaou, D. Ahern, J. Clin. Invest. 1979, 63, Prostaglandin Synthesis by Fetal Rat Bone In Vitro: Evidence for a Role of Prostacyclin, L.G. Raisz, J.Y. Vanderhoek, H.A. Simmons, B.E. Kream, K.C. Nicolaou, Prostaglandins 1979, 17, Effects of PGI 2 on Platelet Aggregation Induced by Various Stimulants, A. Ohtsu, K. Hoshina, S. Kurozumi, Y. Hashimoto, T. Noguchi, K.C. Nicolaou, Acta. Haem. Jap. 1979, 42, Effects of Prostactyclin and Albumin on Platelet Loss During In Vitro Simulation of Extracorporeal Circulation, V.P. Addonizio, Jr., E.J. Macarak, K.C. Nicolaou, L.H. Edmunds, R.W. Colman, Blood 1979, 53, Protective Effects of a Novel Thromboxane Analog in Lethal Traumatic Shock, A.M. Lefer, H. Araki, J.B. Smith, K.C. Nicolaou, R.L. Magolda, Prostaglandins and Medicine 1979, 3, Circulatory and Platelet Actions of 6,9-Thiaprostacyclin in the Cat, A.M. Lefer, G.J. Trachte, J.B. Smith, W.E. Barnette, K.C. Nicolaou, Life Sci. 1979, 25, Phenylselenoetherification. A Highly Efficient Cyclization Process for the Synthesis of O- and S-Heterocycles, K.C. Nicolaou, R.L. Magolda, W.J. Sipio, W.E. Barnette, Z. Lysenko, M.M. Joullié, J. Am. Chem. Soc. 1980, 102, A Remarkably Simple, Highly Efficient and Stereoselective Synthesis of Steroids and Other Polycyclic Systems. Total Synthesis of Estra-1,3,5(10)- trien-17-one via Intramolecular Capture of o-quinodimethanes Generated by Cheletropic Elimination of SO 2, K.C. Nicolaou, W.E. Barnette, P. Ma, J. Org. Chem. 1980, 45, Carbocyclic Thromboxane A 2 (CTA 2 ), K.C. Nicolaou, R.L. Magolda, D.A. Claremon, J. Am. Chem. Soc. 1980, 102,
6 70. Prostaglandin Endoperoxides, Thromboxanes and Prostacyclins. Prostanoids with Important Biological Properties and Significant Chemical Interest, K.C. Nicolaou, N.A. Petasis, Chimika Chronika, General Ed. 1980, 45, (Review in Greek). 71. Total Synthesis of (±)-Zoapatanol, K.C. Nicolaou, D.A. Claremon, W.E. Barnette, J. Am. Chem. Soc. 1980, 102, Synthesis and Biological Properties of Prostacyclins, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, in Prostaglandins in Cardiovascular and Renal Function, Spectrum Publications, Inc., New York, 1980, pp Prostacyclin Has Effects on Proximal and Distal Tubular Function in the Dog, H.-G. Gullner, K.C. Nicolaou, F.C. Bartter, Prostaglandins and Medicine 1980, 6, Dissociation of Vasoconstrictor and Platelet Aggregatory Activities of Thromboxanes by Carbocyclic Thromboxane A 2 (CTA 2 ), a New Stable Analog of Thromboxane A 2 (TA 2 ), A.M. Lefer, E.F. Smith, III, H. Araki, J.B. Smith, D. Aharony, D.A. Claremon, R.L. Magolda, K.C. Nicolaou, Proc. Natl. Acad. Sci. U. S. A. 1980, 77, In Vivo Release and Turnover of Secreted Platelet Antiheparin Proteins in Rhesus Monkey (M. Mulatta), J. Musial, S. Niewiarowski, L.H. Edmunds, Jr., V.P. Addonizio, Jr., K.C. Nicolaou, R.W. Colman, Blood 1980, 56, Effect of Prostacyclin (PGI 2 ) on Renal Function and Renin Secretion in Hypophysectomized Dogs, H.-G. Gullner, K.C. Nicolaou, F.C. Bartter, G. Kelly, Nephron 1980, 25, PGI 2 -Specific Antibodies Administered In Vivo Suggest Against a Role for Endogenous PGI 2 as a Circulating Vasodepressor Hormone in the Normotensive and Spontaneously Hypertensive Rat, C.R. Pace-Asciak, M.C. Carrara, L. Levine, K.C. Nicolaou, Prostaglandins 1980, 20, Preservation of the Ischemic Myocardium by Pinane Thromboxane A 2, K. Schror, E.F. Smith, III. M. Bickerton, J.B. Smith, R.L. Magolda, K.C. Nicolaou, A.M. Lefer, Am. J. Physiol. 1980, 238, H87 H Organoselenium-Based Synthesis of Oxygen-Containing Prostacyclins, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, J. Am. Chem. Soc. 1981, 103, Organoselenium-Based Synthesis of Sulfur-Containing Prostacyclins, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, J. Am. Chem. Soc. 1981, 103, Synthesis of 16-Membered Ring Macrolide Antibiotics 3. Carbomycin B and Leucomycin A 3 : Retrosynthetic Studies, K.C. Nicolaou, S.P. Seitz, M.R. Pavia, J. Am. Chem. Soc. 1981, 103, Synthesis of 16-Membered Ring Macrolide Antibiotics 4. Carbomycin B and Leucomycin A 3 : Synthesis of Key Intermediate, K.C. Nicolaou, M.R. Pavia, S.P. Seitz, J. Am. Chem. Soc. 1981, 103, Ionophore Antibiotic X-14547A. Degradation Studies and Stereoselective Construction of the "Left Wing" (C 11 -C 25 Fragment) by an Intramolecular
7 Diels Alder Reaction, K.C. Nicolaou, R.L. Magolda, J. Org. Chem. 1981, 46, Synthesis of 5Z- and 5E-6,9-Thiaprostacyclins, K.C. Nicolaou, W.E. Barnette, R.L. Magolda, J. Am. Chem. Soc. 1981, 103, Total Synthesis of Ionophore Antibiotic X-14547A. 1. Enantioselective Synthesis of the Tetrahydropyran and Tetrahydroindan Building Blocks, K.C. Nicolaou, D.P. Papahatjis, D.A. Claremon, R.E. Dolle, III, J. Am. Chem. Soc. 1981, 103, Total Synthesis of Ionophore Antibiotic X-14547A. 2. Coupling of the Tetrahydropyran and Tetrahydroindan Systems and Construction of the Butadienyl and Ketopyrrole Moieties, K.C. Nicolaou, D.A. Claremon, D.P. Papahatjis, R.L. Magolda, J. Am. Chem. Soc. 1981, 103, N-Phenylselenophthalimide. A Useful Reagent for the Facile Transformation of (1) Carboxylic Acids into Either Selenol Esters or Amides and (2) Alcohols into Alkyl Phenyl Selenides, P.A. Grieco, J.Y. Jaw, D.A. Claremon, K.C. Nicolaou, J. Org. Chem. 1981, 46, An Expeditious and Efficient Entry into the Aphidicolin and Related Natural Products Ring Skeleton, K.C. Nicolaou, R. Zipkin, Angew. Chem. Int. Ed. Engl. 1981, 20, A Mild Method for the Synthesis of 2-Ketopyrroles from Carboxylic Acids, K.C. Nicolaou, D.A. Claremon, D.P. Papahatjis, Tetrahedron Lett. 1981, 22, ,6-Methanoleukotriene A 4. A Stable and Biologically Active Analogue of Leukotriene A 4. K.C. Nicolaou, N.A. Petasis, S.P. Seitz, J. Chem. Soc., Chem. Commun. 1981, Spasmogenic Effects of the Anti-Fertility Agent Zoapatanol, K.B. Smith, E.F. Smith, III, A.M. Lefer, K.C. Nicolaou, Life Sci. 1981, 28, Mechanism of Coronary Vasoconstriction Induced by Carbocyclic Thromboxane A 2, E.F. Smith, A.M. Lefer, K.C. Nicolaou, Am. J. Physiol. 1981, 240, H493 H Carbocyclic Thromboxane A 2 : Aggrevation of Myocardial Ischemia by a New Synthetic Thromboxane A 2 Analog, E.F. Smith, III, A.M. Lefer, D. Aharony, J.B. Smith, R.L. Magolda, D.A. Claremon, K.C. Nicolaou, Prostaglandins 1981, 21, Organoselenium-Induced Cyclizations in Organic Synthesis, K.C. Nicolaou, Tetrahedron Symp. in Print 1981, 37, Agonist-Specific Desensitization of Prostaglandin-Stimulated Cyclic AMP Accumulation in Palatal Mesenchymal Cells, R.M. Greene, L.A. Verbruggen, M.R. Lloyd, K.C. Nicolaou, J. Craniofacial Genet. Dev. Biol. 1981, 1, Thromboxane Synthetase Inhibitors Differentially Antagonize Thromboxane Receptors in Vascular Smooth Muscle, E.F. Smith, III, A.M. Lefer, J.B. Smith, K.C. Nicolaou, Naunyn-Schmiedeberg's Archives of Pharmacology 1981, 318,
8 97. Cardiovascular Actions of Two Thromboxane A 2 Analogs, A.M. Lefer, J.B. Smith, K.C. Nicolaou, in Adv. in Physiol. Sci., Cardiovascular Physiology: Micro Circulatory and Capillary Exchange, A.G.B. Kovach, J. Hamar, L. Szabo, Eds., Pergamon Press, London, Vol. 7, 1981, pp Synthesis of Stable Thromboxane A 2 Analogs: Pinane Thromboxane A 2 Analogs: Pinane Thromboxane A 2 (PTA 2 ) and Carbocyclic Thromboxane A 2 (CTA 2 ), K.C. Nicolaou, R.L. Magolda, in Methods in Enzymology, W.E.M. Lands and W.L. Smith, Eds., Academic Press, New York, Vol. 86, 1982, pp Anti-Thromboxane A 2 Action of Pinane Thromboxane A 2 Derivatives, D.M. Roth, A.M. Lefer, J.B. Smith, K.C. Nicolaou, Prostaglandins, Leukotrienes and Medicine 1982, 9, Carbohydrates in Organic Synthesis. Synthesis of 16-Membered Ring Macrolide Antibiotics. 5. Total Synthesis of O-Mycinosyl Tylonolide: Synthesis of Key Intermediates, K.C. Nicolaou, M.R. Pavia, S.P. Seitz, J. Am. Chem. Soc. 1982, 104, Carbohydrates in Organic Synthesis. Synthesis of 16-Membered Ring Macrolide Antibiotics. 6. Total Synthesis of O-Mycinosyl Tylonolide: Coupling of Key Intermediate and Macrocyclization, K.C. Nicolaou, S.P. Seitz, M.R. Pavia, J. Am. Chem. Soc. 1982, 104, The Endiandric Acid Cascade. Electrocyclizations in Organic Synthesis. 1. Stepwise, Stereocontrolled Total Synthesis of Endiandric Acids A and B, K.C. Nicolaou, N.A. Petasis, R.E. Zipkin, J. Uenishi, J. Am. Chem. Soc. 1982, 104, The Endiandric Acid Cascade. Electrocyclizations in Organic Synthesis. 2. Stepwise, Stereocontrolled Total Synthesis of Endiandric Acids C-G, K.C. Nicolaou, N.A. Petasis, J. Uenishi, R.E. Zipkin, J. Am. Chem. Soc. 1982, 104, The Endiandric Acid Cascade. Electrocyclizations in Organic Synthesis. 3. "Biomimetic" Approach to Endiandric Acids A-G. Synthesis of Precursors, K.C. Nicolaou, R.E. Zipkin, N.A. Petasis, J. Am. Chem. Soc. 1982, 104, The Endiandric Acid Cascade. Electrocyclizations in Organic Synthesis. 4. "Biomimetic" Approach to Endiandric Acids A-G. Total Synthesis and Thermal Studies, K.C. Nicolaou, N.A. Petasis, R.E. Zipkin, J. Am. Chem. Soc. 1982, 104, Selective Inhibition of 5-Lipoxygenase by 5,6-Methanoleukotriene A 4, A Stable Analogue of Leukotriene A 4, Y. Koshihara, S.-I. Murota, N.A. Petasis, K.C. Nicolaou, FEBS Lett. 1982, 143, Stereocontrolled Synthesis of 1,3,5...2n+1 Polyols, K.C. Nicolaou, J. Uenishi, J. Chem. Soc., Chem. Commun. 1982, Chemistry and Pharmacology of a Series of New Thromboxane Analogs, K.C. Nicolaou, J.B. Smith, A.M. Lefer, Drugs Fut. 1982, VII ,6-Methano Leukotriene A 4 : A Potent Specific Inhibitor for 5-Lipoxygenase, Y. Koshihara, S. Murota, K.C. Nicolaou, in Advances in Prostaglandin,
9 Thromboxane and Leukotriene Research, B. Samuelsson, R. Paoletti, P.W. Ramwell, Eds., Raven Press, New York, Vol. 11, 1983, pp A Mild and General Method for the Synthesis of O-Glycosides, K.C. Nicolaou, S.P. Seitz, D.P. Papahatjis, J. Am. Chem. Soc. 1983, 105, Responsiveness of Platelets and Coronary Arteries from Different Species to Synthetic Thromboxane and Prostaglandin Endoperoxide Analogs, S.E. Burke, A.M. Lefer, K.C. Nicolaou, G.M. Smith, J.B. Smith, Br. J. Pharm. 1983, 78, Ethanoarachidonic Acids. A New Class of Arachidonic Acid Cascade Modulators 1. Monoethanocompounds, K.C. Nicolaou, N.A. Petasis, W.S. Li, T. Ladduwahetty, J.L. Randall, S.E. Webber, P.E. Hernandez, J. Org. Chem. 1983, 48, Ethanoarachidonic Acids. A New Class of Arachidonic Acid Cascade Modulators 2. Polyethanocompounds, K.C. Nicolaou, P.E. Hernandez, T. Ladduwahetty, J.L. Randall, S.E. Webber, W.S. Li, N. A. Petasis, J. Org. Chem. 1983, 48, Pericyclic Reactions in Organic Synthesis and Biosynthesis. Synthetic Adventures with Endiandric Acids A-G, K.C. Nicolaou, N.A. Petasis, in Strategies and Tactics of Organic Synthesis, T. Lindberg, Ed., Academic Press, New York, Ch. 6, 1984, pp Total Synthesis of the Potent Mutagen (S)-3-(1,3,5,7,9-Dodecapentanyloxy)- 1,2-propanediol, K.C. Nicolaou, R. Zipkin, D. Tanner, J. Chem. Soc., Chem. Commun. 1984, Synthesis of 7,13-Bridged Arachidonic Acid Analogs, K.C. Nicolaou, S. Webber, J. Chem. Soc., Chem. Commun. 1984, A General and Stereocontrolled Total Synthesis of Leukotriene B 4 and Analogs, K.C. Nicolaou, R.E. Zipkin, R.E. Dolle, B.D. Harris, J. Am. Chem. Soc. 1984, 106, A Practical Synthesis of Oligosaccharides. Partial Synthesis of Avermectin B 1a, K.C. Nicolaou, R.E. Dolle, D.P. Papahatjis, J.L. Randall, J. Am. Chem. Soc. 1984, 106, Reactions of 2,3-Epoxyhalides. Synthesis of Optically Active Allylic Alcohols and Homoallylic Epoxides, K.C. Nicolaou, M.E. Duggan, T. Ladduwahetty, Tetrahedron Lett. 1984, 25, Reactions of Hydroxyl Groups with Tosylchloride-Dimethylaminopyridine System. Direct Synthesis of Chlorides from Hydroxycompounds, C.K. Hwang, W.S. Li, K.C. Nicolaou, Tetrahedron Lett. 1984, 25, Reactions of Glycosyl Fluorides. Synthesis of C-Glycosides, K.C. Nicolaou, R.E. Dolle, A. Chucholowski, J.L. Randall, J. Chem. Soc., Chem. Commun. 1984, Reactions of Glycosyl Fluorides. Synthesis of O-, S- and N-Glycosides, K.C. Nicolaou, A. Chucholowski, R.E. Dolle, J.L. Randall, J. Chem. Soc., Chem. Commun. 1984,
10 123. Total Synthesis of 5(S), 15(S)-Dihydroxy-6,13-trans-8,11-cis-eicosatetraenoic Acid (5,15-DiHETE), and 8(S), 15(S)-Dihydroxy-5,11-cis-9,13-transeicosatetraenoic Acid (8,15-DiHETE): Two Novel Metabolites of Arachidonic Acid, K.C. Nicolaou, S.E. Webber, J. Am. Chem. Soc. 1984, 106, Total Synthesis of Elfamycins Aurodox and Efrotomycin. 1. Strategy and Construction of Key Intermediates, R.E. Dolle, K.C. Nicolaou, J. Am. Chem. Soc. 1985, 107, Total Synthesis of Elfamycins Aurodox and Efrotomycin. 2. Coupling of Key Intermediates and Completion of the Synthesis, R.E. Dolle, K.C. Nicolaou, J. Am. Chem. Soc. 1985, 107, A General Strategy for the Total Synthesis of the Presumed Lipoxin Structures, K.C. Nicolaou, S.E. Webber, J. Chem. Soc., Chem. Commun. 1985, An Intramolecular Diels Alder Approach to Forskolin, K.C. Nicolaou, W.S. Li, J. Chem. Soc., Chem. Commun. 1985, Total Synthesis of Ionophore Antibiotic X-14547A, K.C. Nicolaou, D.P. Papahatjis, D.A. Claremon, R.L. Magolda, R.E. Dolle, J. Org. Chem. 1985, 50, Modulation of Leukotriene Synthesis and Actions by Synthetic Derivatives of Arachidonic Acid, J.B. Smith, D.M. Roth, A.M. Lefer, N.A. Petasis, K.C. Nicolaou, Prostaglandins 1985, 29, Modern Synthetic Technology and Total Synthesis of Bioactive Molecules, K.C. Nicolaou, N.A. Petasis, R.E. Dolle, III, W.S. Li, D.P. Papahatjis, J. Uenishi, R.E. Zipkin, in New Methods in Drug Research, A. Makriyannis, Ed., J.R. Prous Science, Barcelona, Ch. 11, 1985, pp Carbohydrate-Based Syntheses of the Goldinolactone and the Tetrahydrofuran Fragments of Aurodox and Efrotomycin, R.E. Dolle, K.C. Nicolaou, J. Chem. Soc., Chem. Commun. 1985, New Synthetic Technology and Total Synthesis. The Elfamycins, K.C. Nicolaou, Chem. Br. 1985, Activation of 6-Endo over 5-Exo Epoxide Openings. Ring Selective Formation of Tetrahydropyran Systems and Stereocontrolled Synthesis of the ABC Ring Framework of Brevetoxin B, K.C. Nicolaou, M.E. Duggan, C.-K. Hwang, P.K. Somers, J. Chem. Soc., Chem. Commun. 1985, Stereospecific Synthesis of Rhynchosporosides: A Family of Fungal Metabolites Causing Scald Disease in Barley and Other Grasses, K.C. Nicolaou, J.L. Randall, G.T. Furst, J. Am. Chem. Soc. 1985, 107, N-Phenylselenophthalimide (NPSP): A Valuable Selenenylating Agent, K.C. Nicolaou, N.A. Petasis, D.A. Claremon, Tetrahedron 1985, 41, Stereocontrolled Total Synthesis of (5Z, 8Z, 11Z, 13E)(15S)-15- Hydroxyeicosa-5,8,11,13-tetraenoic Acid (15S-HETE) and Analogues, K.C. Nicolaou, T. Ladduwahetty, E.M. Elisseou, J. Chem. Soc., Chem. Commun. 1985,
11 137. Stereocontrolled Total Synthesis of Lipoxins A, K.C. Nicolaou, C.A. Veale, S.E. Webber, H. Katerinopoulos, J. Am. Chem. Soc. 1985, 107, Total Synthesis of 20-Hydroxy- and 20-Carboxy-Leukotrienes B 4, K.C. Nicolaou, Y.S. Chung, P.E. Hernandez, I.M. Taffer, R.E. Zipkin, Tetrahedron Lett. 1986, 27, Retrosynthetic and Synthetic Chemistry of Amphotericin B. Synthesis of C 1 - C 20 and C 21 -C 38 Fragments and Construction of the 38-Membered Macrocycle, K.C. Nicolaou, T.K. Chakraborty, R.A. Daines, N.S. Simpkins, J. Chem Soc., Chem. Commun. 1986, Stereospecific 1,2-Migrations in Carbohydrates. Stereocontrolled Synthesis of α- and β-2-deoxyglycosides, K.C. Nicolaou, T. Ladduwahetty, J.L. Randall, A. Chucholowski, J. Am. Chem. Soc. 1986, 108, New Synthetic Technology for the Construction of Oxocenes, K.C. Nicolaou, M.E. Duggan, C.-K. Hwang, J. Am. Chem. Soc. 1986, 108, A General Strategy for the Synthesis of Monohydroxy-eicosatetraenoic Acids: Total Synthesis of 5(S)-Hydroxy-6(E),8,11,14(Z)-eicosatetraenoic Acid (5- HETE) and 12(S)-Hydroxy-5,8,14(Z),10(E)-eicosatetraenoic Acid (12-HETE), K.C. Nicolaou, T. Ladduwahetty, I.M. Taffer, R.E. Zipkin, Synthesis 1986, Organic Chemistry Today and Tomorrow, K.C. Nicolaou, N.A. Petasis, Chimika Chronika, General Ed. 1986, 51, (Review in Greek) Stereocontrolled Total Synthesis of Lipoxins B, K.C. Nicolaou, S.E. Webber, Synthesis 1986, Coronary Vasoactivity of Four 7,13-Bridged Analogs of Arachidonic Acid, A. Yanagisawa, M. Brezinshi, A.M. Lefer, S.E. Webber, K.C. Nicolaou, Prostaglandins, Leukotrienes and Medicine 1986, 23, Stereospecific Synthesis of 1,1-Dialkylglycosides, K.C. Nicolaou, C.-K. Hwang, M.E. Duggan, J. Chem. Soc., Chem. Commun. 1986, Synthesis and Vascular Actions of an Arachidonic Acid Ethylene-Diamino- Triethyl-Ester (AA-EDTA) Derivative, A. Yanagisawa, A.M. Lefer, M.E. Elisseou, K.C. Nicolaou, Prostaglandins 1986, 31, Lipoxin A: Stereochemistry and Biosynthesis, C.N. Serhan, K.C. Nicolaou, S.E. Webber, C.A. Veale, S.-E. Dahlen, T.J. Puustinen, B. Samuelsson, J. Biol. Chem. 1986, 261, Bridging of Macrocycles to Bicycles. New Synthetic Technology for the Construction of Oxopolycyclic Systems, K.C. Nicolaou, C.-K. Hwang, M.E. Duggan, K. Bal Reddy, B.E. Marron, D.G. McGarry, J. Am. Chem. Soc. 1986, 108, Evidence for a 5(6)-Epoxy-Tetraene Intermediate in the Biosynthesis of Lipoxins in Human Leukocytes: Conversion into Lipoxin by Cytosolic Epoxide Hydrolase, T.J. Puustinen, W.E. Webber, K.C. Nicolaou, J. Haeggstrom, C.N. Serhan, B. Samuelsson, FEBS Lett. 1986, 207, Stereoselective Total Synthesis of the Presumed Biosynthetic Precursor to Lipoxins A and B: Methyl (7E, 9E, 11Z, 13E, 5S, 6S, 15S)-5,6-Epoxy-15-
12 hydroxyicosa-7,9,11,13-tetraenoate and Its 5,6-Methano Analogue, K.C. Nicolaou, S.E. Webber, J. Chem. Soc., Chem. Commun. 1986, Synthesis of Eicosanoids, K.C. Nicolaou, N.A. Petasis, in Handbook of Eicosanoids, Prostaglandins and Related Lipids, Part B (Chemical and Biochemical Aspects), A.L. Willis, Ed., CRC Press, Boca Raton, Florida, 1987, pp Organoselenium-Based Ring Closure Reactions, K.C. Nicolaou, N.A. Petasis, D.A. Claremon, in Organoselenium Chemistry, D. Liotta, Ed., John Wiley & Sons, New York, 1987, pp Lipoxins A Induced Inhibition of Human Natural Killer Cell Cytotoxicity: Studies on Stereospecificity of Inhibition and Mode of Action, U. Ramstedt, C.N. Serhan, K.C. Nicolaou, S.E. Webber, H. Wigzell, B. Samuelsson, J. Immun. 1987, 138, Stereocontrolled Construction of Key Building Blocks for the Total Synthesis of Amphoteronolide B and Amphotericin B, K.C. Nicolaou, R.A. Daines, J. Uenishi, W.S. Li, D.P. Papahatjis, T.K. Chakraborty, J. Am. Chem. Soc. 1987, 109, Total Synthesis of Amphoteronolide B, K.C. Nicolaou, R.A. Daines, T.K. Chakraborty, J. Am. Chem. Soc. 1987, 109, Total Synthesis of Amphotericin B, K.C. Nicolaou, R.A. Daines, T.K. Chakraborty, Y. Ogawa, J. Am. Chem. Soc. 1987, 109, Amphoteronolide B Methylester. Novel Oxidative Deglycosidation of Amphotericin B, K.C. Nicolaou, T.K. Chakraborty, R.A. Daines, Y. Ogawa, J. Chem. Soc., Chem. Commun. 1987, Nucleophilic Additions to Thionolactones. New Synthetic Technology for the Construction of Medium and Large Ring Ethers, K.C. Nicolaou, D.G. McGarry, C.A. Veale, P.K. Somers, J. Am. Chem. Soc. 1987, 109, Thermal Cycloadditions of Dimethyl Acetylenedicarboxylate with Cyclic Enolethers. An Entry into Medium Size Oxocyclic Systems, K.C. Nicolaou, C.-K. Hwang, M.E. Duggan, K. Bal Reddy, Tetrahedron Lett. 1987, 28, Stereochemistry and Biosynthesis of Lipoxins, C.N. Serhan, K.C. Nicolaou, S.E. Webber, C.A. Veale, J. Haeggstrom, T.J. Puustinen, B. Samuelsson, Adv. Prost. Thromb. & Leukot. Res., B. Samuelsson, R. Paoletti, P.W. Ramwell, Eds., Raven Press, New York, Vol. 17A, 1987, pp The Effects of Lipoxin A and Lipoxin B on Functional Responses of Human Granulocytes, J. Palmblad, H. Gyllenhammar, B. Ringertz, C.N. Serhan, B. Samuelsson, K.C. Nicolaou, Biochem. Biophys. Res. Commun. 1987, 145, The Action of Lipoxin-A on Glomerular Microcirculatory Dynamics in The Rat, K.F. Badr, C.N. Serhan, K.C. Nicolaou, B. Samuelsson, Biochem. Biophys. Res. Commun. 1987, 145, Dithiatopazine. The First Stable 1,2-Dithietane, K.C. Nicolaou, C.-K. Hwang, M.E. Duggan, P.J. Carroll, J. Am. Chem. Soc. 1987, 109,
13 165. Stereocontrolled Total Synthesis of Lipoxins A 5 and B 5, K.C. Nicolaou, S.E. Webber, J. Ramphal, Y. Abe, Angew. Chem. Int. Ed. Engl. 1987, 26, The Total Synthesis of the Lipoxins and Related Compounds, S.E. Webber, C.A. Veale, K.C. Nicolaou, in Advances in Experimental Medicine and Biology, P.Y. Wong and C.N. Serhan, Eds., Proc. FASEB Symp. on Lipoxins: Biosynthesis and Pharmacology, Plenum Press, New York, Vol. 229, 1987, Synthetic Eicosanoids, K.C. Nicolaou, N.A. Petasis, in Biology and Chemistry of Prostaglandins and Related Eicosanoids, P.B. Curtis-Prior, Ed., Churchill Livingstone, Edinburgh, 1988, pp Preparation and Reactions of Glycosyl Fluorides, J.L. Randall, K.C. Nicolaou, in Fluorinated Carbohydrates, Chemical and Biochemical Aspects, ACS Symposium Series, N.F. Taylor, Ed., Vol. 374, 1988, Ch. 2, pp Chemistry of Amphotericin B. Degradation Studies and Preparation of Amphoteronolide B, K.C. Nicolaou, T.K. Chakraborty, Y. Ogawa, R.A. Daines, N.S. Simpkins, G.T. Furst, J. Am. Chem. Soc. 1988, 110, Total Synthesis of Amphoteronolide B and Amphotericin B. 1. Strategy and Stereocontrolled Construction of Key Building Blocks, K.C. Nicolaou, R.A. Daines, J. Uenishi, W.S. Li, D.P. Papahatjis, T.K. Chakraborty, J. Am. Chem. Soc. 1988, 110, Total Synthesis of Amphoteronolide B and Amphotericin B. 2. Total Synthesis of Amphoteronolide B, K.C. Nicolaou, R.A. Daines, T.K. Chakraborty, J. Am. Chem. Soc. 1988, 110, Total Synthesis of Amphotericin B. 3. The Final Stages, K.C. Nicolaou, R.A. Daines, Y. Ogawa, T.K. Chakraborty, J. Am. Chem. Soc. 1988, 110, Cyclic Conjugated Enediynes Related to Calicheamicins and Esperamicins: Calculations, Synthesis and Properties, K.C. Nicolaou, G. Zuccarello, Y. Ogawa, E.J. Schweiger, T. Kumazawa, J. Am. Chem. Soc. 1988, 110, Methyl 4-Hydroxy-5-iodo-2,3-dimethoxy-6-methylbenzoate: The Aromatic Fragment of Calichemicin γ 1 αi. Synthesis, X-Ray Crystallographic Analysis, and Properties, K.C. Nicolaou, T. Ebata, N.A. Stylianides, R.D. Groneberg, P.J. Carroll, Angew. Chem. Int. Ed. Engl. 1988, 27, Novel Chemistry of Dithiatopazine, K.C. Nicolaou, C.-K. Hwang, S. DeFrees, N.A. Stylianides, J. Am. Chem. Soc. 1988, 110, Actions of Lipoxin A 4 and Related Compounds in Smooth Muscle Preparations and on the Microcirculation in vivo, S.E. Dahlen, L. Franzen, J. Raud, C.N. Serhan, P. Westlund, E. Wikstrom, T. Bjorck, H. Matsuda, S.E. Webber, C.A. Veale, T. Puustinen, J. Haeggstrom, K.C. Nicolaou, B. Samuelsson, in Lipoxins: Biosynthesis, Chemistry and Biological Activities, P.Y.-K. Wong and C.N. Serhan, Eds., Plenum Press, New York, 1988, pp
14 177. DNA Cleavage by a Synthetic Mimic of the Calicheamicin-Esperamicin Class of Antibiotics, K.C. Nicolaou, Y. Ogawa, G. Zuccarello, H. Kataoka, J. Am. Chem. Soc. 1988, 110, A Practical and Enantioselective Synthesis of Glycosphingolipids and Related Compounds. Total Synthesis of Globotriaosylceramide (Gb 3 ), K.C. Nicolaou, T. Caulfield, H. Kataoka, T. Kumazawa, J. Am. Chem. Soc. 1988, 110, A Photolytic Entry into Oxepane Systems, K.C. Nicolaou, C.-K. Hwang, D.A. Nugiel, Angew. Chem. Int. Ed. Engl. 1988, 27, Lipoxins A 4 and B 4 : Comparison of Eicosanoids Having Bronchoconstrictor and Vasodilator Actions but Lacking Platelet Aggregatory Activity, A.M. Lefer, G.L. Stahl, D.J. Lefer, M.E. Brezinski, K.C. Nicolaou, C.A. Veale, Y. Abe, J.B. Smith, Proc. Natl. Acad. Sci. U. S. A. 1988, 85, Differential Effects of 20-Trifluoromethyl Leukotriene B 4 on Human Neutrophil Functions, B.S. Tsai, R.H. Keith, D. Villani-Price, R.A. Haack, R.F. Bauer, R. Leonard, Y. Abe, K.C. Nicolaou, Prostaglandins 1989, 37, Identification of a Novel 7-cis-11-trans-lipoxin A 4 Generated by Human Neutrophils: Total Synthesis, Spasmogenic Activities, and Comparison with Other Geometric Isomers of Lipoxin A 4 and B 4, K.C. Nicolaou, B.E. Marron, C.A. Veale, S.E. Webber, S.E. Dahlen, B. Samuelsson, Biochim. Biophys. Acta 1989, 1003, Cyclizations of Hydroxydithioketals. New Synthetic Technology for the Construction of Oxocenes and Related Medium Ring Systems, K.C. Nicolaou, C.V.C. Prasad, C.-K. Hwang, M.E. Duggan, C.A. Veale, J. Am. Chem. Soc. 1989, 111, Activation of 6-Endo over 5-Exo Hydroxy Epoxide Openings. Stereo- and Ringselective Synthesis of Tetrahydrofuran and Tetrahydropyran Systems, K.C. Nicolaou, C.V.C. Prasad, P.K. Somers, C.-K. Hwang, J. Am. Chem. Soc. 1989, 111, Activation of 7-Endo over 6-Exo Epoxide Openings. Synthesis of Oxepane and Tetrahydropyran Systems, K.C. Nicolaou, C.V.C. Prasad, P.K. Somers, C.-K. Hwang, J. Am. Chem. Soc. 1989, 111, Pharmacologic Profile of Lipoxins A 5 and B 5 : New Biologically Active Eicosanoids, G.L. Stahl, P. Tsao, A.M. Lefer, J.Y. Ramphal, K.C. Nicolaou, Eur. J. Pharmacol. 1989, 163, A Synthetic Route to Forskolin, K.C. Nicolaou, S. Kubota, W.S. Li, J. Chem. Soc., Chem. Commun. 1989, Synthesis and Stereochemical Assignment of the C 1 -C 10 Fragment of Nystatin A 1, K.C. Nicolaou, K.H. Ahn, Tetrahedron Lett. 1989, 30, Stereocontrolled Synthesis of the Sex Pheromone of the Green Stink Bug, K.C. Nicolaou, B.E. Marron, Synthesis 1989, 7, Synthetic Studies on the Dioxepane Region of Brevetoxin B. New Synthetic Technology for the Construction of Oxepanes and Synthesis of a Model for
15 the CDEF Ring Skeleton of Brevetoxin B, K.C. Nicolaou, C.-K. Hwang, D.A. Nugiel, J. Am. Chem. Soc. 1989, 111, Stereocontrolled Total Synthesis of (5S,6R)-, (5S,6R)-, (5S,6R)- and (5S,6R)- (7E,9E,11Z,14Z)-5,6-Dihydroxy-7,9,11,14-icosatetraenoic Acid (5,6- DiHETE) Methyl Esters, K.C. Nicolaou, J.Y. Ramphal, J.M. Palazon, R.A. Spanevello, Angew. Chem. Int. Ed. Engl. 1989, 28, Synthesis of the ABC Ring System of Brevetoxin B, K.C. Nicolaou, M.E. Duggan, C.-K. Hwang, J. Am. Chem. Soc. 1989, 111, Synthesis of the FG Ring System of Brevetoxin B, K.C. Nicolaou, M.E. Duggan, C.-K. Hwang, J. Am. Chem. Soc. 1989, 111, Synthesis of the Brevetoxin B IJK Ring System, K.C. Nicolaou, C.-K. Hwang, M.E. Duggan, J. Am. Chem. Soc. 1989, 111, A New Class of DNA-Cleaving Molecules. ph-dependent DNA Cleavage by Propargylic and Allenic Sulfones, K.C. Nicolaou, G. Skokotas, P. Maligres, G. Zuccarello, E.J. Schweiger, K. Toshima, S. Wendeborn, Angew. Chem. Int. Ed. Engl. 1989, 28, Pheromone Blends of Green Stink Bugs and Possible Parasitoid Selection, J.R. Aldrich, W.R. Lusby, B.E. Marron, K.C. Nicolaou, M.P. Hoffmann, L.T. Wilson, Naturwissenschaften 1989, 76, Synthesis of 19,19,20,20,20-Pentadeuterated Lipoxin A 4 Methyl Ester and 19,19,20,20,20-Pentadeuterated Arachidonic Acid and Demonstration of Their Use in the Quantitative Detection of Naturally Occurring Eicosanoids, B.E. Marron, R.A. Spanevello, M.E. Elisseou, C.N. Serhan, K.C. Nicolaou, J. Org. Chem. 1989, 54, Total Synthesis of Novel Geometric Isomers of Lipoxins A 4 and Lipoxin B 4, K.C. Nicolaou, B.E. Marron, C.A. Veale, S.E. Webber, C.N. Serhan, J. Org. Chem. 1989, 54, Stereocontrolled Total Synthesis of Methyl 12(S)-Hydroxy-5Z,8E,10Eheptadecatrienoate, K.C. Nicolaou, N.A. Stylianides, J.Y. Ramphal, J. Chem. Soc., Perkin Trans , Lipoxin A 4 Causes Generation of Thromboxane A 2 in the Guinea-Pig Lung, E. Wikstrom, P. Westlund, K.C. Nicolaou, S.-E. Dahlen, Agents and Actions 1989, 26, Pharmacodynamics of Lipoxin A 4 in Airway Smooth Muscle, S.-E. Dahlen, C.A. Veale, S.E. Webber, B.E. Marron, K.C. Nicolaou, C.N. Serhan, Agents and Actions 1989, 26, Lipoxin Stimulate Prostacyclic Generation by Human Endothelial Cells, M.E. Brezinski, M.A. Gimbrone, Jr., K.C. Nicolaou, C.N. Serhan, FEBS Lett. 1989, 245, Stereocontrolled Total Synthesis of (5Z,8Z,10E,14Z)-12-Hydroxy-5,8,10,14- icosatetraenoic Acid [12(R)-HETE], K.C. Nicolaou, J. Ramphal, Y. Abe, Synthesis 1989, 12,
16 204. Lipoxin A 4 Inhibits Leukotriene B 4 -Induced Inflammation in the Hamster Cheek Pouch, P. Hedqvist, U. Palmertz, J. Haeggstrom, K.C. Nicolaou, S.-E. Dahlen, Acta Physiol. Scand. 1989, 137, Platelet-Activiting Factor PAF Antagonistic Actions of Two New Analogs of PAF, P.S. Tsao, G.A. Stahl, A.M. Lefer, J.M. Salvino, S. Pietranico, K.C. Nicolaou, J. Lipid Mediators 1989, 1, Aspects of Eicosanoids in Inflammation, P. Hedqvist, S.E. Dahlen, J. Raud, L. Lindbom, A. Thuresson-Klein, K.C. Nicolaou, NATO ASI Ser. A 1989, 177, Ca2+ Mobilization with Leukotriene A 4 and Epoxytetraenes in Human Neutrophils, F.W. Luscinskas, K.C. Nicolaou, S.E. Webber, C.A. Veale, M.A. Gimbrone, Jr., C.N. Serhan, Biochem. Pharmacol. 1990, 39, Dithiatopazine and Related Systems. Synthesis, Chemistry, X-Ray Crystallographic Analysis and Calculations, K.C. Nicolaou, S.A. DeFrees, C.- K. Hwang, N. Stylianides, P.J. Carrol, J.P. Snyder, J. Am. Chem. Soc. 1990, 112, Bridging of Macrodithionolactones to Bicyclic Systems. Synthesis of Oxapolycyclic Frameworks. K.C. Nicolaou, C.-K. Hwang, B.E. Marron, S.A. DeFrees, E.A. Couladouros, Y. Abe, P.J. Carrol, J. Snyder, J. Am. Chem. Soc. 1990, 112, Identification of Lipoxin A 4 and Its Relationship to the Sulfidopeptide Leukotrienes C 4 D 4 and E 4 in the Bronchoalveolar Lavage Fluids Obtained from Patients with Selected Pulmonary Diseases, T.H. Lee, A.E.G. Crea, V. Grant, B.W. Spur, B.E. Marron, K.C. Nicolaou, E. Reardon, M. Brezinski, C.N. Serhan, Am. Rev. Respir. Dis. 1990, 141, Total Synthesis of Globotriaosylceramide (Gb 3 ) and Lysoglobotriaosylceramide (LysoGb 3 ), K.C. Nicolaou, T.J. Caulfield, H. Kataoka, Carbohydr. Res. 1990, 202, Total Synthesis of the Tumor-Associated Lex Family of Glycosphingolipids, K.C. Nicolaou, T.J. Caulfield, H. Kataoka, N.A. Stylianides, J. Am. Chem. Soc. 1990, 112, New Synthetic Strategies for the Construction of Medium Size Ethers. Stereocontrolled Synthesis of the BCD Ring Framework of Brevetoxin A, K.C. Nicolaou, D.G. McGarry, P.K. Somers, J. Am. Chem. Soc. 1990, 112, Novel Strategy for the Construction of the Oligosaccharide Fragment of Calicheamicin γ 1 αi. Synthesis of the ABC Skeleton, K.C. Nicolaou, R.D. Groneberg, J. Am. Chem. Soc. 1990, 112, Synthesis of Medium-Sized Ring Ethers from Thionolactones. Applications to Polyether Synthesis, K.C. Nicolaou, D.G. McGarry, P.K. Somers, B.H. Kim, W.W. Ogilvie, G. Yiannikouros, C.V.C. Prasad, C.A. Veale, R.R. Hark, J. Am. Chem. Soc. 1990, 112, Synthesis of the CD and E Ring Systems of the Calicheamicin γ 1 I Oligosaccharide, K.C. Nicolaou, R.D. Groneberg, N.A. Stylianides, T. Miyazaki, J. Chem. Soc., Chem. Commun. 1990, 18,
17 217. Stereocontrolled Second Generation Syntheses of the ABC and FG Ring Systems of Brevetoxin B, K.C. Nicolaou, D.A. Nugiel, E. Couladouros, C.-K. Hwang, Tetrahedron 1990, 46, Golfomycin A: A Novel Designer Molecule with DNA-Cleaving Properties and Antitumor Activity, K.C. Nicolaou, G. Skokotas, S. Furuya, H. Suemune, D. Nicolaou, Angew. Chem. Int. Ed. Engl. 1990, 29, Novel Stereocontrolled Construction of the Nonacene Ring System of Brevetoxin A, K.C. Nicolaou, C.V.C. Prasad, W.W. Ogilvie, J. Am. Chem. Soc. 1990, 112, Evaluation of Synthetic Sphingosine, Lysosphingolipids and Glycosphingolipids as Inhibitors of Functional Responses of Human Neutrophils, S. Fiore, K.C. Nicolaou, T. Caulfield, H. Kataoka, C.N. Serhan, Biochem. J. 1990, 266, Peptides: Chemistry, Structure and Biology, K.C. Nicolaou, J.M. Salvino, K. Raynor, S. Pietranico, T. Reisine, R.M. Freidinger, R. Hirschmann, Proc. 11 th Am. Pept. Symp., J.E. Rivier, G.R. Marshall, Eds., Escom, Leichen, 1990, pp DNA-Cleavage and Antitumor Activity of Designed Molecules with Conjugated Phosphine-allene-ene-yne Functionalities, K.C. Nicolaou, P. Maligres, J. Shin, E. de Leon, D. Rideout, J. Am. Chem. Soc. 1990, 112, Total Synthesis of the Oligosaccharide Fragment of Calicheamicin γ 1 I, K.C. Nicolaou, R.D. Groneberg, T. Miyazaki, N.A. Stylianides, T.J. Schultze, W. Stahl, J. Am. Chem. Soc. 1990, 112, Total Synthesis of Amphoteronolide B and Amphotericin B, K.C. Nicolaou, W.W. Ogilvie, ChemTracts 1990, 3, Synthesis of Dynemicin A Models, K.C. Nicolaou, C.-K. Hwang, A.L. Smith, S.V. Wendeborn, J. Am. Chem. Soc. 1990, 112, Synthesis of Novel Oligosaccharides, K.C. Nicolaou, T.J. Caulfield, R.D. Groneberg, Pure Appl. Chem. 1991, 63, Lipoxins and Related Eicosanoids: Biosynthesis, Chemical Synthesis and Biological Properties, K.C. Nicolaou, J.Y. Ramphal, N.A. Petasis, C.N. Serhan, Angew. Chem. Int. Ed. Engl. 1991, 30, Selectivity and Mechanism of Action of Novel DNA Cleaving Agents, K.C. Nicolaou, S. Wendeborn, K. Isshiki, N. Zein, G. Ellestad, Angew. Chem. Int. Ed. Engl. 1991, 30, Synthesis and Chemistry of Dynemicin A Models, K.C. Nicolaou, A.L. Smith, S.V. Wendeborn, C.K. Hwang, J. Am. Chem. Soc. 1991, 113, Chemical Synthesis of Glycosphingolipids, K.C. Nicolaou, ChemTracts 1991, 4, Novel Strategies for the Construction of Complex Polycyclic Ether Frameworks. Stereocontrolled Synthesis of the FGHIJ Ring System of Brevetoxin A, K.C. Nicolaou, C.A. Veale, C.-K. Hwang, J. Hutchinson,
18 C.V.C. Prasad, W.W. Ogilvie, Angew. Chem. Int. Ed. Engl. 1991, 30, Synthetic Strategy for the Coupling of the Calicheamicin Oligosaccharide with Aglycons. Synthesis of Dynemicin A Calicheamicin Hybrid Structures, K.C. Nicolaou, E.P. Schreiner, W. Stahl, Angew. Chem. Int. Ed. Engl. 1991, 30, Stereocontrolled Synthesis of Sialyl Le x, the Oligosaccharide Binding Ligand to ELAM-1, K.C. Nicolaou, C.W. Hummel, N.J. Bockovich, C.-H. Wong, J. Chem. Soc., Chem. Commun. 1991, Enediyne Compounds Equipped with Acid-, Base-, and Photo-Sensitive Triggering Devices. Simulation of the Dynemicin A Cascade, K.C. Nicolaou, W.-M. Dai, S. Wendeborn, A.L. Smith, Angew. Chem. Int. Ed. Engl. 1991, 30, Synthesis of Novel Oligosaccharides Based on Glycosyltransferases: β1,4galactosyltransferase, C-H. Wong, T. Krach, C. Gautheron-Le Narvor, Y. Ichikawa, G.L. Look, K.C. Nicolaou, Tetrahedron Lett. 1991, 32, Chemistry and Biology of the Enediyne Anticancer Antibiotics, K.C. Nicolaou, W.-M. Dai, Angew. Chem. Int. Ed. Engl. 1991, 30, Novel Enediynes Equipped with Triggering and Detection Devices. Isolation of cis-diol Models of the Dynemicin A Cascade, K.C. Nicolaou, Y.-P. Hong, S.-C. Tsay, W.-M. Dai, J. Am. Chem. Soc. 1991, 113, Total Synthesis of Calicheamicin Dynemicin Hybrid Molecules, K.C. Nicolaou, E.P. Schreiner, Y. Iwabuchi, T. Suzuki, Angew. Chem. Int. Ed. Engl. 1992, 31, Cationization of Organometallo Carbonyl Compounds Through Fast Ion Bombardment, G. Siuzdak, S.V. Wendeborn, K.C. Nicolaou, Int'nl J. Mass Spectr. & Ion Proc. 1992, 112, Designed Enediynes. A New Class of DNA-Cleaving Molecules with Potent and Selective Anticancer Activity, K.C. Nicolaou, W.-M. Dai, S.-C. Tsay, V.A. Estevez, W. Wrasidlo, Science 1992, 256, Total Synthesis of Sialyl Dimeric Le x, K.C. Nicolaou, C.W. Hummel, Y. Iwabuchi, J. Am. Chem. Soc. 1992, 114, Enantioselective Total Synthesis of (+)-Calicheamicinone, A.L. Smith, C.-K. Hwang, E. Pitsinos, G.R. Scarlato, K.C. Nicolaou, J. Am. Chem. Soc. 1992, 114, Total Synthesis of the Carbohydrate Fragments of Esperamicin A 1, K.C. Nicolaou, D. Clark, Angew. Chem. Int. Ed. Engl. 1992, 31, Design, Synthesis and Study of Simple Monocyclic Conjugated Enediynes. The 10-Membered Ring Enediyne Moiety of the Enediyne Anticancer Antibiotics, K.C. Nicolaou G. Zuccarello, C. Reimer, V.A. Estevez, W.-M. Dai, J. Am. Chem. Soc. 1992, 114, Molecular Design and Chemical Synthesis of Potent Enediynes 1. Dynemicin Model Systems Equipped with N-Tethered Triggering Devices, K.C.
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F)
 CPC - C07B - 2017.08 C07B GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) General methods for the preparation
CPC - C07B - 2017.08 C07B GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) General methods for the preparation
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
ORGANIC CHEMISTRY. Fifth Edition. Stanley H. Pine
 ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
Classics in Total Synthesis
 Classics in Total Synthesis K. C. Nicolaou and E. J. Sorensen Targets, Strategies, Methods With a Foreword by E. J. Corey VCH Weinheim New York Basel Cambridge Tokyo Chapter 1 Introduction: Constructing
Classics in Total Synthesis K. C. Nicolaou and E. J. Sorensen Targets, Strategies, Methods With a Foreword by E. J. Corey VCH Weinheim New York Basel Cambridge Tokyo Chapter 1 Introduction: Constructing
Pericyclic Reactions 6 Lectures Year 3 Handout 2 Michaelmas 2017
 Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
Perspectives in total synthesis: a personal account
 Tetrahedron 59 (2003) 6683 6738 Perspectives in total synthesis: a personal account K. C. Nicolaou a,b, * a Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute,
Tetrahedron 59 (2003) 6683 6738 Perspectives in total synthesis: a personal account K. C. Nicolaou a,b, * a Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute,
Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
 Pergamon Tetrahedron Letters 41 (2000) 9719 9723 TETRAHEDRON LETTERS Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
Pergamon Tetrahedron Letters 41 (2000) 9719 9723 TETRAHEDRON LETTERS Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
Amphoteric Molecules < Chemistry of Andrei K. Yudin > Hyung Min Chi 17 JUNE 2014
 Amphoteric Molecules < Chemistry of Andrei K. Yudin > Hyung Min Chi 17 JUNE 2014 1 Amphoteric molecules Amphoteric? Greek word amphoteros (both of two) Amphoterism in acid/base chemistry Amino acids (thermodynatic
Amphoteric Molecules < Chemistry of Andrei K. Yudin > Hyung Min Chi 17 JUNE 2014 1 Amphoteric molecules Amphoteric? Greek word amphoteros (both of two) Amphoterism in acid/base chemistry Amino acids (thermodynatic
Synthesis of Abyssomicin C. Marie-Caroline Cordonnier Litterature Review 23/01/2009
 Synthesis of Abyssomicin C Marie-Caroline Cordonnier Litterature Review 23/01/2009 Isolation Isolated in 2004 from the actinomycete Verrucosispora strain collected from a sediment at a depth of 289m in
Synthesis of Abyssomicin C Marie-Caroline Cordonnier Litterature Review 23/01/2009 Isolation Isolated in 2004 from the actinomycete Verrucosispora strain collected from a sediment at a depth of 289m in
Suggested solutions for Chapter 34
 s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr.
 April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid
 Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
KJM 3200 Required Reading (Pensum), Fall 2016
 KJM 3200 Required Reading (Pensum), Fall 2016 John McMurry: Organic Chemistry 8 nd ed. or Paula Y. Bruice, Organic Chemistry 7 nd ed. as specified below, as specified below. Lise-Lotte Gundersen KJM 3200.
KJM 3200 Required Reading (Pensum), Fall 2016 John McMurry: Organic Chemistry 8 nd ed. or Paula Y. Bruice, Organic Chemistry 7 nd ed. as specified below, as specified below. Lise-Lotte Gundersen KJM 3200.
Keywords: amination ate complexes carbomagnesiation carbometalation Grignard reagents homocoupling magnesates magnesium compounds metalation
 VII Abstracts 2010 p1 7.6.5.6 Aryl Grignard Reagents H. Yorimitsu Halogen magnesium exchange between aryl iodides or bromides and an isopropylmagnesium chloride lithium chloride complex or lithium trialkylmagnesates
VII Abstracts 2010 p1 7.6.5.6 Aryl Grignard Reagents H. Yorimitsu Halogen magnesium exchange between aryl iodides or bromides and an isopropylmagnesium chloride lithium chloride complex or lithium trialkylmagnesates
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008
 PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
Advanced Studies in Organic Chemistry
 Advanced Studies in Organic Chemistry Organic Chemistry of Drug Compounds 783614S 20 After this course the student is familiar with basic principles and techniques of medicinal chemistry, tactics and tools
Advanced Studies in Organic Chemistry Organic Chemistry of Drug Compounds 783614S 20 After this course the student is familiar with basic principles and techniques of medicinal chemistry, tactics and tools
Theophylline (TH), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma.
 1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
Total Synthesis of (+)-Cytosporolide A via a Biomimetic
 Literature report Total Synthesis of (+)-Cytosporolide A via a Biomimetic etero-diels Alder Reaction Reporter: Zhang-Pei Chen Checker: Shu-Bo u Date: 29/12/2015 Takao, K. et al. Takao, K. et al. J. Am.
Literature report Total Synthesis of (+)-Cytosporolide A via a Biomimetic etero-diels Alder Reaction Reporter: Zhang-Pei Chen Checker: Shu-Bo u Date: 29/12/2015 Takao, K. et al. Takao, K. et al. J. Am.
Modern Organic Synthesis an Introduction
 Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Page 1 of 9. Sessional Examination (November 2017) Max Marks: 20 Date: Time: One Hour. Model Answers
 Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras
 Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #08 Pericyclic Reactions -Cycloaddition Reactions
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #08 Pericyclic Reactions -Cycloaddition Reactions
Steroids. Prostaglandins
 Steroids Cholesterol Progesterone Cortisone PGF 1 C 2 C 2 Prostaglandins C 2 PGE 1 PGA 1 C 2 Six-membered Rings Five-membered Rings Stereocontrol in Cyclic Systems Acyclic Diastereoselection Biomimetic
Steroids Cholesterol Progesterone Cortisone PGF 1 C 2 C 2 Prostaglandins C 2 PGE 1 PGA 1 C 2 Six-membered Rings Five-membered Rings Stereocontrol in Cyclic Systems Acyclic Diastereoselection Biomimetic
Suggested solutions for Chapter 27
 uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
I. Introduction. II. Retrosynthetic Analysis. Andrew Baggett. Liu lab
 Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
Synthesis of Resorcinylic Macrolides
 Synthesis of Resorcinylic Macrolides X H H H H Cl X= Radicicol (1) X= CH2 Cycloproparadicicol (2) Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, ASAP Danishefsky, S. J. rg. Lett. 2004, 6, 413-416 Danishefsky,
Synthesis of Resorcinylic Macrolides X H H H H Cl X= Radicicol (1) X= CH2 Cycloproparadicicol (2) Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, ASAP Danishefsky, S. J. rg. Lett. 2004, 6, 413-416 Danishefsky,
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
water methanol dimethyl ether Ether can only act as a hydrogen bond acceptor H-bond acceptor O R
 Chapter 14: Ethers and Epoxides; Thiols and Sulfides 14.1 Introduction to Ethers An ether group is an oxygen atom that is bonded to two carbons. The ether carbons can be part of alkyl, aryl, or vinyl groups.
Chapter 14: Ethers and Epoxides; Thiols and Sulfides 14.1 Introduction to Ethers An ether group is an oxygen atom that is bonded to two carbons. The ether carbons can be part of alkyl, aryl, or vinyl groups.
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
ORGANIC CHEMISTRY. Wiley STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ROBERT G. JOHNSON JON ANTILLA ELEVENTH EDITION. University of South Florida
 STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
ROADMAP FOR REACTIONS Chapter 6
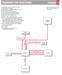 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
به نام خدا. Organic Synthesis 1 سنتز مواد آلی. Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran
 به نام خدا Organic Synthesis 1 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic
به نام خدا Organic Synthesis 1 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic
Dienes & Polyenes: An overview and two key reactions (Ch )
 Dienes & Polyenes: An overview and two key reactions (h. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes,
Dienes & Polyenes: An overview and two key reactions (h. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes,
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Organic Chemistry of Drug Degradation
 Organic Chemistry of Drug Degradation Li New Jersey Email: minli88@yahoo.com Publishing Contents Chapter 1 Introduction 1 1.1 Drug Impurities, and the Importance of Understanding Drug Chemistry 1 Characteristics
Organic Chemistry of Drug Degradation Li New Jersey Email: minli88@yahoo.com Publishing Contents Chapter 1 Introduction 1 1.1 Drug Impurities, and the Importance of Understanding Drug Chemistry 1 Characteristics
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
The Legends of the Star Tetrodotoxin
 The Legends of the Star Tetrodotoxin Advisors: Prof. YANG Prof. CHEN Prof. TANG Reporter: Haixin YU History Puffer Fish Delicious food in Japan fugu Poisonous For Japanese and Egyptians Millennia For Europeans
The Legends of the Star Tetrodotoxin Advisors: Prof. YANG Prof. CHEN Prof. TANG Reporter: Haixin YU History Puffer Fish Delicious food in Japan fugu Poisonous For Japanese and Egyptians Millennia For Europeans
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
روشهای 1 سنتز مواد آلی
 به نام خدا Advanced Organic Chemistry روشهای 1 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir References: 1. Carey, F. A.; Sundberg,
به نام خدا Advanced Organic Chemistry روشهای 1 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir References: 1. Carey, F. A.; Sundberg,
COURSE UNIT DESCRIPTION. Type of the course unit. Mode of delivery Period of delivery Language of instruction Face to face Autumn English
 Course unit title Organic Chemistry I Lecturer(s) Dr. Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery
Course unit title Organic Chemistry I Lecturer(s) Dr. Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Olefin-Forming Reactions I
 C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Current Literature. Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8
 Current Literature Development of ighly Potent and Selective Steroidal Inhibitors and Degraders of CDK8 ACS Med. Chem. Lett. 2018, ASAP Rational Drug Development simplification Cortistatin A 16-30 steps;
Current Literature Development of ighly Potent and Selective Steroidal Inhibitors and Degraders of CDK8 ACS Med. Chem. Lett. 2018, ASAP Rational Drug Development simplification Cortistatin A 16-30 steps;
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Pericyclic reactions
 Pericyclic reactions In pericyclic reactions the breaking and making of bonds occur simultaneously by the way of a single cyclic transition state (concerted reaction). There are no intermediates formed
Pericyclic reactions In pericyclic reactions the breaking and making of bonds occur simultaneously by the way of a single cyclic transition state (concerted reaction). There are no intermediates formed
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chem 263 Sept 22, Beta-carotene (depicted below) is the orange-red colour in carrots. β-carotene
 hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C. Claude Spino et al., J. Org. Chem. 2013, 78,
 Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
به نام خدا روشهای سنتز مواد آلی
 به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry.
 Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Dual enantioselective control by heterocycles of (S)-indoline derivatives*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Chapter 5B. Functional Group Transformations: The Chemistry. Related Reactions
 Chapter 5B Functional Group Transformations: The Chemistry of fcarbon-carbon C b π-bonds B d and Related Reactions Oxymercuation-Demercuration Markovnikov hydration of a double bond 1 Mechanism Comparision
Chapter 5B Functional Group Transformations: The Chemistry of fcarbon-carbon C b π-bonds B d and Related Reactions Oxymercuation-Demercuration Markovnikov hydration of a double bond 1 Mechanism Comparision
Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
 Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
(agmatine, AGM) 1994 Li. (alpha 22adrenergic re2 ceptor, 2 2AR) (imidazoline receptor, IR) (0 4 ) Krebs2Henseleit ( K2H)
 , 1999 6, 51 (3), 321 326 321 Acta Physiologica Sinica 3 (, 050017), (agmatine, AGM) : (1) AGM ; (2), AGM (1 mmol/ L),, ; (3) L2NAME (015 mmol/ L), AGM (1 mmol/ L) ; (4) (imidazoline receptor, IR) 2 2
, 1999 6, 51 (3), 321 326 321 Acta Physiologica Sinica 3 (, 050017), (agmatine, AGM) : (1) AGM ; (2), AGM (1 mmol/ L),, ; (3) L2NAME (015 mmol/ L), AGM (1 mmol/ L) ; (4) (imidazoline receptor, IR) 2 2
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations
 Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY
 STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3
 Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3 COURSE DESCRIPTION: This course will present a brief overview of Nuclear Chemistry. The major portion of the semester will
Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3 COURSE DESCRIPTION: This course will present a brief overview of Nuclear Chemistry. The major portion of the semester will
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
Stereodivergent Catalysis. Aragorn Laverny SED Group Meeting July
 Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
C h a p t e r N i n e: Addition Reactions of Alkenes
 C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3
 Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3 COURSE DESCRIPTION: This course emphasizes introductory Organic Chemistry and Biochemistry. The examination
Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3 COURSE DESCRIPTION: This course emphasizes introductory Organic Chemistry and Biochemistry. The examination
Units-1 & 2: Pericyclic Reactions
 Units-1 & 2: Pericyclic Reactions Section-A: Each question carries 5 marks. 1. Write the Molecular orbitals of ethylene and explain number of nodes, symmetry properties of molecular orbitals. 2. Write
Units-1 & 2: Pericyclic Reactions Section-A: Each question carries 5 marks. 1. Write the Molecular orbitals of ethylene and explain number of nodes, symmetry properties of molecular orbitals. 2. Write
Homework for Chapter 17 Chem 2320
 Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Enantioselective Borylations. David Kornfilt Denmark Group Meeting Sept. 14 th 2010
 Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Non-Enzymatic Enantioselective Polyene Cyclizations. Adam Hill Chem 535 May, 2 nd 2013
 Non-Enzymatic Enantioselective Polyene Cyclizations Adam Hill Chem 535 May, 2 nd 2013 Enantioselective Polyene Cyclization (?) General Method to Rapidly Build Molecular Complexity (+) Exquisite Stereo-
Non-Enzymatic Enantioselective Polyene Cyclizations Adam Hill Chem 535 May, 2 nd 2013 Enantioselective Polyene Cyclization (?) General Method to Rapidly Build Molecular Complexity (+) Exquisite Stereo-
Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization
 Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization Denmark, S.E. and Collins, W.R. rg. Lett. 2007, 9, 3801-3804. C 2 H + Se Lewis Base CH 2 Cl 2 Se Presented
Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization Denmark, S.E. and Collins, W.R. rg. Lett. 2007, 9, 3801-3804. C 2 H + Se Lewis Base CH 2 Cl 2 Se Presented
Renaud Group Exercise Set
 Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
COWLEY COLLEGE & Area Vocational Technical School
 COWLEY COLLEGE & Area Vocational Technical School COURSE PROCEDURE FOR ORGANIC CHEMISTRY II CHM 4251 5 Credit Hours Student Level: This course is open to students on the college level in the sophomore
COWLEY COLLEGE & Area Vocational Technical School COURSE PROCEDURE FOR ORGANIC CHEMISTRY II CHM 4251 5 Credit Hours Student Level: This course is open to students on the college level in the sophomore
7 Steroids and sugars
 7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Biology - Summer Work - Chapter 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Organic chemistry is a science based on the study of
AP Biology - Summer Work - Chapter 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Organic chemistry is a science based on the study of
Some questions and answers that we will get out of this example synthesis:
 UTLINE 535 LECTURE 3 (2004) Page 22 The Synthesis of Cubane I am showing you this synthesis because it is elegant and exemplifies many different concepts. We can use it to talk in context about the philosophical
UTLINE 535 LECTURE 3 (2004) Page 22 The Synthesis of Cubane I am showing you this synthesis because it is elegant and exemplifies many different concepts. We can use it to talk in context about the philosophical
