Z-Protected Glutamic Acid-Based Biodegradable Thermoplastic and Thermosetting Polyesters: Synthesis and Characterization
|
|
|
- Brandon Goodwin
- 6 years ago
- Views:
Transcription
1 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Biomaterials Chemical and Biomolecular Engineering Research and Publications May 1999 Z-Protected Glutamic Acid-Based Biodegradable Thermoplastic and Thermosetting Polyesters: Synthesis and Characterization R M. Tadros Pine Bluff Arsenal, Pine Bluff, Arkansas Hossein Noureddini Department of Chemical Engineering, University of Nebraska-Lincoln, hnouredd@unlnotes.unl.edu Delmar C. Timm Department of Chemical Engineering, University of Nebraska-Lincoln, dtimm@unlserve.unl.edu Follow this and additional works at: Part of the Biomaterials Commons Tadros, R M.; Noureddini, Hossein; and Timm, Delmar C., "Z-Protected Glutamic Acid-Based Biodegradable Thermoplastic and Thermosetting Polyesters: Synthesis and Characterization" (1999). Papers in Biomaterials This Article is brought to you for free and open access by the Chemical and Biomolecular Engineering Research and Publications at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Biomaterials by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln.
2 Z-Protected Glutamic Acid-Based Biodegradable Thermoplastic and Thermosetting Polyesters: Synthesis and Characterization R. M. TADROS, H. NOUREDDINI, D. C. TIMM "This article is a preprint of an article published in Journal of Applied Polymer Science, Vol. 73, Issue 6, (1999). Published Online: 25 May 1999 Copyright 1999 John Wiley & Sons, Inc.CCC /99/ This article is available at the publishers site:
3 ABSTRACT: Biodegradable polymers were formed from N-benzyloxycarbonyl-L-glutamic acid with the comonomers ethylene glycol, diglycidyl ether of 1,4-butanediol, and diglycidyl ether of bisphenol A. The three polymers were a linear and a rosslinked heterochain polyester and a crosslinked polyester that contained aromatic units within its network chains. The thermoplastic resin and the soluble fractions for the thermosetting resins were characterized by gel permeation chromatography. Conversions for carboxylic acid were determined by titrations. A quality, 22,000 molecular weight thermoplastic resin was formed. The two thermosets were cured past their gel points. Gelation analysis revealed that the relative rate constants for the sequential oxirane/ acid and alcohol/acid reactions were distinct. With diglycidyl ether of 1,4- butanediol, the ratio of the respective rate constants was 3; with diglycidyl ether of bisphenol A, the ratio approached 200. The resins hydrolyzed to monomers in the presence of lipase, but in the presence of a mixed microbial culture, only the first two resins decayed to biomass, respiratory gases, and water. The third resin was inert during the period of observation. Key words: amino-protected glutamic acid; biodegradable polymers; gelation; polymer networks; polyesters INTRODUCTION The development of biodegradable polymers using value-added, renewable agricultural resources was a prime motivation for this research. Annually, an enormous tonnage of plastics is sent to wastedisposal systems, such as landfills. Synthetic, organic polymers resist natural degradation due to variables that include a low surface area per unit mass, chemical structure, and high molecular weight. Degradation may be initiated through environmentally induced changes which transform the polymer into smaller segments. To be classified as biodegradable, these fragments should decay via microorganisms into biomass, respiratory gases, and water. In tertiary recycling, polymers decay to monomers.1 Potential uses of biodegradable polymers include controlled release formulations for drugs, the preparation of surgical implants, agricultural chemicals, and agricultural mulch. In this article, the synthesis and characterization of three quality polymers with distinct chain configurations is addressed. Companion articles discuss the results of biodegradation studies. Soybean protein and its derivatives were a focus of polymer research in the 1930s and 1940s. Today, proteins are used to form grafted, synthetic copolymers as well as protein-based biodegradable plastics.2 In general, mixed monomers reduce a plastic s physical properties. Therefore, instead of using complex protein molecules, glutamic acid was selected to produce resins with regular chain configurations. Glutamic acid is a major component of oilseed proteins, representing the order of 20% of their amino acid content. Several methods are available for separating glutamic acid from hydrolyzed amino acids.3 5 It is also produced by fermentation. In addition, poly(l-glutamic acid) is a biodegradable material6 and a block copolymer between poly(g-ethyl-llutamate) and polybutadiene is biocompatible as well as biodegradable.7 A review of the literature regarding biodegradability further directed the research toward the chemical design and synthesis of polyesters.8 Glutamic acid is an a-amino acid containing two carboxylic acids and one amino group. Heating glutamic acid with a diol can result in the formation of a cyclic amide, a five-member lactam. In addition, dipeptides easily cycle to sixmember diketopiperazines. These are major side reactions encountered in peptide synthesis.9,10 In our work, oligomeric-forming side reactions were observed to occur in abundance. Cyclization was controlled through protection of the a-amino group using benzyloxycarbonyl (Z) (see Fig. 1). Different amino-protecting groups were discussed by Jones.9 Since polyesterifications are acid-catalyzed reactions, the benzyloxycarbonyl group (ArOCH2OOOCOO) was selected due to its stability under acidic conditions.11 Comonomerstructures also appear in Figure 1. as an acid or as a base in an aqueous medium of different ph. The suitable ph for activating the acid form of the amino acid is lower than the
4 isoionic point for each ionization group. In acidic aqueous media, the amino acid is an active acid and thus directs a synthesis to ward forming polyesters with dioles. Pramanick and Rey12 reported the formation of a 4000 molecular weight polymer with ethylene glycol in an aqueous acidic medium. Since our goal was to synthesize higher molecular weight resins, we did not explore this avenue. Bulk polymerizations with protected amino acids were selected. For the second monomers, a diol and diepoxies were chosen. The diol yields a thermoplastic; diepoxies yield thermosets. An epoxy moiety initially opens during the polymerization, forming a hydroxyl group and an ester linkage. The hydroxyl site then couples with a second carboxyl group, forming a second ester bond and water. The functionality for a diepoxy monomer is four, and networks form (see Fig. 2). In this sketch, functionality is emphasized. The diepoxy branch node is represented by a tetrafunctional chain link. The amino acid is expressed by a bifunctional chain link. The order for reactions is labeled 1st and 2nd, arbitrarily, for discussion. On the upper right, an unreacted epoxy is represented by two unreacted branches; at the lower right, an unreacted alcohol is indicated by one unreacted branch. In the interior of the sketch, both the oxirane and formed alcohol have reacted. Variables appearing on the sketch will be discussed in the section on gelation. The Z-protecting group is not indicated but is pendent to the bifunctional chain links.
5 EXPERIMENTAL Materials Benzyloxycarbonyl-L-glutamic acid (ZGluOH) and L-glutamic acid (HGluOH) (Aldrich), ethylene glycol (EG; Fisher), diglycidyl ether of 1,4-butanediol (DGEB; Ciba Chemical), and diglycidyl ether of bisphenol A (DGEBA; Shell Chemical) were used as received. Tetrahydrofuran (THF; Quaker Oats) was the mobil phase during GPC fractionations. In studies designed to address the thermal stability of the Z protection, the monofunctional 1-nonanol (Aldrich) was reacted with ZGluOH. Fractionations used reversed-phase high-performance chromatography (HPLC). The mobil phase was a gradient of acetonitrile (Aldrich) and water. The catalyst was p-toluene sulfonic acid (PTS). Thermal Stability of the Amino Protection In discussing the thermal stability of urethanes, Stevens13 stated that a polycarbamate derived from aliphatic-based monomers can be melt-processed at 180 C, but warns that the higher temperatures needed for melt polymerization of resins formulated with aromatic diisocyanates tend to cause dissociation into alcohol and isocyanate or degradation into amines, olefins, and carbon dioxide. In our formulations, alcoholysis was of concern. To examine the stability of the Z-protecting group, a carbamate, the reaction between ZGluOH and the monofunctional alcohol 1-nonanol was studied using HPLC. Batch reactions with
6 formulations based on stoichiometry ratios of acids/alcohols at 170 C and atmospheric pressure were conducted. The condensation product water was allowed to escape from the reactor. If appreciable levels of alcoholysis occurred, esters of varying chemical composition would be observed through fractionations. In a 2-h interval, the formation of mono- and diesters was readily observed. Similar compounds formed in the presence of alcoholysis were not observed. Therefore, we concluded that the major polymerization site would be the carboxylic acid moiety. A Waters chromatograph was used with a m- bondapak C18 column, mm. The gradient of 40 90% acetonitrile/water was conducted over 1 h. An ultraviolet detector at 280 nm was used. The reaction was catalyzed with PTS. Polymerization Procedures Thermoplastic Resin In an initial polymerization, HGluOH/EG/PTS was melt-mixed with a molar ratio of 1/1/0.01. The polymerization proceeded by amidization/esterification reactions. Water was stripped under a vacuum (400 mmhg) from a batch reactor to shift the chemical equilibrium toward higher conversions. 1,14 A slow stream of inert gas (N2) was bubbled through the molten mix to assist with moisture removal and to limit resin oxidation. The reaction temperature was maintained within1 C of the set point, 170 C. This procedure was then modified by melt-mixing ZGluOH/EG/PTS with a stoichiometric ratio of 1/1.08/0.01. In latter stages of the cure, excess ethylene glycol was stripped from the reactor during transesterification reactions.1 Isothermal cure temperatures were 110 and 170 C. Samples (.0.1 g) were analyzed through end-group analysis and gel permeation chromatography (GPC).15,16 The reactor apparatus consisted of a 100-cc round, Pyrex glass flask. A glass tube about 10 cm long was used to admit a stream of nitrogen to bubble through the molten resin. Another tube was used for removing samples. A type-k thermocouple allowed temperatures to be observed. The apparatus was heated in a temperature-regulated liquid bath. Thermoset Resins ZGluOH/diepoxy/PTS polyesters were prepared by melt-mixing the molar ratios of 1.0/1.0/0.02. To limit the extent of crosslinking, the acid group was selected as the limiting reactive site. The polymer mix was initially placed into a 50-mL forcedair, electric oven. The chosen, set-point temperatures ranged between 110 and 130 C, 60.5 C. After 15 min of preheating, approximately 5 ml of the molten mixture was poured into each 10-mL glass tube. The tubes were placed in the oven at the same initial temperature. At different time intervals, a polymerization sample was withdrawn and thermally quenched. The oven was blanketed with nitrogen.
7 Characterization Gel Permeation Chromatography A Waters GPC was equipped with five Ultrastyragel columns of , 500, and Å and a differential refractometer. The mobil THF phase was at 0.5 ml/min. Fractionations were at ambient temperature. For miscible materials, 75 mg was dissolved in 50 ml of THF and 0.5 ml of this solution was injected. For thermosets, between 0.2 and 0.3 g was placed with 20 ml of THF in a cylindrical, thick-walled brass container and extracted at a temperature in excess of the specimen s glass transition temperature (Tg). The insoluble gel fraction was dried at 70 C under a vacuum and weighed. The soluble sol fraction was diluted to approximately 75 mg in 50 ml of THF for analysis. The average molecular weight of the oligomeric fraction was compared to a polystyrene semilogarithmic correlation of the standard s molecular weight as a function of its peak elution volume. End-Group Analysis On average, a linear polymeric chain has a single carboxylic group. The carboxylic groups in the resin were titrated against KOH using phenolphthalein as an indicator. The polymer samples were dissolved in 50 ml of chloroform. The KOH was dissolved in methyl alcohol to form a 0.01N KOH solution. A clear, sharp, color change occurred at the end point. To measure conversion, a similar procedure was
8 followed for the thermosets. Samples were initially finely ground and allowed to swell in the solvent before titration. Titrations were conducted over an interval of time under nitrogen until the end point became stable for at least 24 h. DATA ANALYSES Linear Resins Reaction Dynamics For the HGluOH/EG/PTS resin, samples collected at different times were screened by GPC. Samples cured for 2 and 9 h indicated a minimal change in molecular weight (see Fig. 3). Molecular weights were close to that for the monomers. Cyclic structures likely formed.9,10 ZGluOH/EG/PTS was polymerized under the same conditions. With the protected amine group, molecular weight advanced. A quality resin formed. In Figure 4, shifts in chromatograms reveal the development of polymeric materials with advancing cure time.
9
10 End-Group Analysis The conversion of acid sites r may be expressed in terms of the cumulative molar concentration of molecules within the resin: The molar concentration of all molecules that contain i monomeric links is Pi. If i is even, the molecule contains one unreacted carboxylic group and one unreacted alcohol group. When i is odd, half of these molecules contain two acid sites or two alcohol moieties. Subject to a balanced stoichiometry, their molar concentrations are equal. On average, each molecule of degree of polymerization i contains one acid group. The number-average molecular weight is where the average molecular weight of the chain link is MW. The data tabulated in Tables I and II summarize the end-group dynamics, the calculated extents of reaction [eq. (1)], and the resins numberaverage molecular weights [eq. (2)] for cures at 110 and 170 C as functions of time. After 16 h at 170 C, a quality polymer with a molecular weight of 22,000 had formed. Polymerization dynamics did not yield expected second-order responses.17 Conversion data correlate as a semilogarithmic function of time
11 (see Fig. 5), with correlation coefficients of 0.97 at 110 C and 0.94 at 170 C. Several factors could contribute to a pseudo-, first-order reaction. Initially, reaction rate constants were considered. Flory17 observed that rate constants for esterification reactions are dependent on the number of atoms separating carboxyl groups when these atoms are few in number. For the current resin, only three carbon atoms separate the functional groups. Due to electron shielding and steric factors, the first reacting chemical moiety could experience a distinct rate constant.18 A second consideration is attributed to rate constants becoming conversion-dependent. At higher conversions, reaction rates, change for example, due to vitrification19 and to changing dielectric constants.20 Finally, chemical equilibrium was addressed. Physical operating conditions can affect observations. In our research, the concentration of the condensation product C was not controlled or observed. But with increasing reaction times and diminishing rates of formation, its concentration could have varied.
12 To illustrate the consequences of a variable concentration C, equilibrium reactions Pi 1 Pj º Pi1j 1 C are addressed. Stockmayer21 and Blatz and Tobolsky22 showed that the most probable distribution is appropriate for both equilibrium and for kinetically controlled, irreversible reactions. The equilibrium population of molecules may be expressed as a function of monomer concentration P1, the ratio of the equilibrium constant and the by-product concentration K/C, and conversion p eq : If equilibrium reactions between oligomers are initially considered, the resulting relationship also fits the above, arbitrary bimolecular reaction. The relationships MWn / MW =5 SiPi / S Pi = 1/(1 - KP 1 /C) and eq. (2) were utilized in deriving the second result above. One can see that if the ratio K/C varied during an experiment experimental observations will deviate from the most probable distribution. We believe this could be a major contributor to our observations. Network Resins With epoxy monomers, the two oxiranes are separated by a sufficient number of atoms so that their reactivities are independent of oxirane reaction states. This has been confirmed with DGEBA.18 For mathematical simplicity23 25 and due to limited data, the chemical reactivities of the two acid groups on ZGluOH were also assumed to be independent of their reaction states. Flory s data indicate that rate constants gradually change with the number of carbon atoms separating the functional groups. As will be discussed, our results indicate substantial changes. Our analysis was constrained to distinct reactivities for oxiranes and alcohols only. Derivations implicitly incorporate vitrification constraints on kinetic constants as reported by Cole19: This function of conversion indicates that the reaction constant K(r) is nearly constant at K0 for r, rc, but rapidly decreases at high conversions. The constant a is a data-fitting parameter. Cole assumed reactions to be second-order and irreversible. Crosslinked Polyesters ZGluOH/BDE/PTS produced a ductile resin at room temperature with soluble sol and insoluble gel fractions. The chromatograms appearing in Figure 6 show an increasing molecular weight with time of cure at 110 C for a resin before its gel point. Resins cured at 130 C for 5 h resulted in a 12% gel fraction, and for 11.5 h, in a 20% gel fraction. The crosslinked aromatic polyester, ZGluOH/DGEBA/PTS, was cured at 110, 120, and 130 C. Chromatograms appearing in Figure 7 show a gradual increase in the molecular weight of the sol with time of cure at 110 C. Lower molecular weight materials in the elution time interval s diminish in concentration; higher molecular weight materials appear prior to 1000 s. The resin cured at 130 C for 5 h 40 µ yielded a 50% gel fraction. At ambient conditions, the material is hard and brittle. Reaction Kinetics
13 A combined kinetic and statistical model was used in the analyses of the thermosetting resins. Distinct rate constants kox and koh were assigned to reactions between an epoxy or oxirane and a carboxylic acid and between an alcohol and an acid. These rate constants were assumed to be independent of the degree of polymerization. In addition, the isothermal reactions were assumed to be void of intermolecular cyclization reactions. Previous work by Bokar and Gandhi,23 Gupta and Macosko,24 and Sarmoria and Miller25 addressed an amine-cured epoxy when rate constants for primary and secondary amines are distinct with a competing etherification of epoxy groups. Our description26 is a simplification, in that this minor competing reaction is neglected. DusUek et al.27 also considered these reactions and formulated a solution using probability-generating functions. Hydroxyl groups originate from the initial reaction between the carboxylic acid and oxirane moieties. Their reactions produce branch nodes and, ultimately, chain networks. The concentrations of the reactive groups (oxiranes, hydroxyls, and carboxylic acids) are represented by [OX], [OH], and [A]. The differential equation describing the molar concentration of oxiranes is D[OX]/dt = - k OX [A][OX]
14 The time t was transformed to the dimensionless time t by d T = k OX [A]dt The solution, subject to the initial condition [OX] 0, is [OX]=[OX] 0 exp(- T ) Therefore, the conversion of oxiranes equals Differentiation plus algebra yield a time-conversion transformation: The concentration of hydroxyl moieties was expressed in terms of a relative rate constant C, formulation parameter a, and conversion: For a balanced stoichiometry, a In our polymerizations, carboxylic acid sites were limiting, a 5 1. The differential equation describing the molar concentration of hydroxyl sites is
15 In the first rate term, the consumption of the hydroxyl groups by reactions with carboxylic groups is addressed. The second rate expression describes the formation of hydroxyls by reactions between carboxylic acids and oxiranes. The total number of hydroxyl sites formed at any time equals the number of oxiranes reacted [OX]0rOX. The solution incorporated an integration factor and is expressed in terms of the hydroxyl conversion The differential equation describing the molar concentration of carboxylic acids is The rate expressions describe the consumption of carboxylic groups by reactions with hydroxyl groups and oxiranes, respectively. The solution expressed in conversion ra is Expectation Theory Probability functions express the expectation that a reactive site is attached to chain segments of finite dimension, given all possible events. The sketch of a chain segment appearing in Figure 2 is referenced in the following derivation: Chain functionality is emphasized. The epoxy monomer is described as the tetrafunctional link and the bifunctional ZGluOH is described as the linear connector. An oxirane site on a branch node at a is considered. The probability that it is attached to chains of finite dimension looking out of the branch node is represented by P(FOX out). If the oxirane originally at a is unreacted (probability of 1 2 rox), the chain terminates. Alternately, the oxirane is reacted (probability of rox). The probability function looking into the ester bond formed (labeled 1st in Fig. 2) is indicated as P(FA in). The likelihood that the
16 formed hydroxyl is also part of a finite chain extension P(FOH out ) must also be addressed. Therefore, the expectation that a randomly selected oxirane leads to two finite chain segments at this branch point is The expectation that a hydroxyl site is connected to a finite chain extension P(FOH out) at b depends on its reaction state. The probability that this group is unreacted equals 1 2 roh. If it is reacted, the probability is roh. In Figure 2, this site is labeled b. Therefore, When looking into a bifunctional connecting link, the likelihood that this reacted site is part of a finite chain segment P(FA in) equals the probability P(FA out) that its second acid site is attached to finite structures: The reaction state of this second acid site is a function of three independent events: (1) The carboxylic acid may be unreacted (probability of 1 2 ra). (2) The acid group may have reacted with an oxirane. This event is labeled 1st at location c in Figure 2. The quantity arox equals the number of reacted oxiranes per carboxylic acids initially present. The fraction of reacted acid sites is ra. This yields the second term in eq. (9). (3) The acid site reacted with a hydroxyl group. This event is represented by point c, 2nd. The quantity arox also equals the number of hydroxyl sites that have formed at the time of observation, relative to the initial concentration of acid groups. The fraction of hydroxyl sites reacted is roh. This event contributes the last expression in the conditionalprobability function P(FA out):
17 When a branch node is entered from a reacted hydroxyl as at point c, 2nd, the likelihood that the exiting two chain segments are finite is labeled P(FOH in ). The probability that finite structures are attached at the second oxirane of the tetrafunctional link is P(F out OXA ). The probability of a finite chain extension at the reacted oxirane branch point is P(F A OH ) = P(F out OX ) P(F out A ) Alternately, a branch node is entered from a reacted acid site through the bond that formed when the oxirane reacted (see point c, 1st). The resulting branch point leads to conditional probability functions P(FOXout) at the second oxirane and P(FOH out ) at the formed hydroxyl. Point e may be referenced. For these independent events, Equations (6) (11) yield a cubic equation with roots P(FA out) = 1 and when p> p c, P(F out A ) < 1, and when p Xp c, P(F out A ) = 1, since all chains are finite. Therefore, eq. (12) may be evaluated for the critical conversion rc for gelation. For example, if C = 2.0 and P(F out A ) = 1, p A = 0.75 = p c. Recall that eqs. (4) and (5) correlate the conversion of hydroxyl and acid moieties as functions of oxirane conversion. Sol Fraction Relationships partitioning a monomeric link between the sol and gel are now derived. The mol fraction of branch nodes in the sol is labeled vox. The fraction of branch nodes in the gel equals 1 2 vox. For a chain link to be in the sol, each of its reactive sites must be part of finite chains. Therefore, the fraction of epoxy units including the monomer in the sol equals
18 The mol fraction of connecting links in the sol equals The mass fraction of the resin that is soluble is where the molecular weights of the epoxy monomer and ZGluOH are MWOX and MWA. Branch Node Distributions In the theory of rubber elasticity, the modulus of elasticity is a function of the number of elastically active junctions that contain three or more chain extensions to the gel. 28,29 Thermoset resins become elastomeric at temperatures exceeding their glass transition temperature Tg. For our resin systems, branch nodes evolve from the diepoxy monomers and are labeled n ROX,ROH. The initial index 0 X ROX 2 indicates the number of oxiranes reacted and the second index 0 X ROH ROX indicates the number of hydroxyls formed that have reacted. In conversion space, a series of first-order reactions associated with the formation and consumption of nodes yields their mol fractions: where the coefficient C ROX,ROH = 1 unless ROX and/or ROH = 1, then C ROX,ROH = 2. Kernels address the reaction states of the two functional groups, and, as appropriate, their powers equal the number of these sites reacted or unreacted. Subject to reaction constraints and normalization, the coefficient correlates permutations. Crosslinks Xm,0 that contribute to the modulus are nodes that contain a minimum of three chain
19 extensions to the gel or network. Such chains are considered to be infinite.28,29 The initial subscript is the number of reacted sites, and looking out of the node, the second subscript is the number of these sites attached to the network. Since each reacted site is attached to a bifunctional link, the probability that it extends to the gel is 1 2 P(FA out). The normalized molar concentration of elastically active nodes equals26: Analysis of Relative Rate Constant The conversion of carboxylic acid and the sol fraction for the crosslinked ZGluOH/BDE/PTS sample cured for h at 130 C was 76 and 80%, respectively. Subject to model constraints and assumptions, eqs. (6) (15) were solved with eqs. (4) and (5) to determine the value for the relative rate constant C. When C 5 3.0, the calculated sol mass fraction is 81%, and conversions rox and roh equal 0.47 and 0.60 when ra is The rate constant for carboxylic acid reactions with oxiranes is smaller than that with hy droxyl moieties; tetrafunctional chain segments have a preference for branching. The molecular theory of rubber elasticity28,29 predicts that a resin with more elastically active junctions will have a higher modulus at temperatures above Tg. The resin produced was rubbery, indicating a relatively low level of crosslinking. For the crosslinked ZGluOH/DGEBA/PTS polymer sample cured for 5 h 40min at 130 C, the average value for the acid s conversion was 68% and the sol fraction was For a solution with C 5 200, a sol fraction of 0.51 subject to conversions ra , roh , and rox was calculated. Not only did this resin contain a chain-stiffening, aromatic component within its chain links, but the conversion of hydroxyls to esters also was nearly complete. A high degree of branching and crosslinking occurred. The resin was brittle at ambient temperature. Crosslink Distributions To further characterize the network structure, the relative distribution of nodes that contribute to the modulus and other physical properties was calculated. Subject to experimental measurements of conversion and regression estimates of C, the fraction of tetrafunctional branch nodes with three or four extensions to the gel for the resin ZGluOH/BDE/PTS is X3, , X4, , and X4, , and for the resin ZGluOH/DGEBA/PTS, X3, , X4, , and X4, Although both resins contain a relatively large sol fraction due to an imbalanced stoichiometry and low extent of conversion, the results clearly demonstrate the increased level of network branching in the latter resin. The large value for the relative rate constant C results in a ratio of X4,4/X4, in the resin composed of aromatic chain links (compared to 0.1 for the first thermoset). These numbers show that when an oxirane reacts the formed hydroxyl group is converted to an ester bond at once, forming a branch point in the chain. This chemistry leads to network configurations. DISCUSSION
20 A major research objective was to produce quality thermoplastic and thermoset resins from an amino acid. The results of our studies indicate that the thermoplastic resin formulated from ZGluOH/EG/PTS had an average molecular weight of 22,000. Many commercial step-growth resins have similar average molecular weights. Analysis of the thermosetting resins identified their respective gel fractions at a final conversion. These data were incorporated into gelation theory to calculate the relative rate constants for reactions involving oxiranes and alcohols with carboxylic acids. Results indicate that the two rate constants are not equal and that their relative magnitudes are influenced by the chain structure. These observations are consistent with observations for amine-cured epoxies. Aliphatic or aromatic segments significantly alter the reactivities of primary and secondary amines.18 Furthermore, chemical analysis of chemical intermediates in a curing anhydride/epoxy resin20 revealed that the alcohol moiety is many times more reactive than is the acid group. Our results are also consistent with their observations. For resin systems described by Cole s model for diminishing reaction rates at higher conversions, the relative rate constant C incorporates these effects when the constants a for the oxirane and alcohol reactions are equal. The network chemical structure that contributes to the rubbery modulus of elasticity was estimated through branch-node distributions. The results reveal the effects of the conversion and the relative magnitudes for the distinct rate constants. In the ZGluOH/EG/PTS resin, branch points tend to be farther apart. The crosslink average molecular weight of elastically active strands is greater than that for the ZGluOH/ DGEBA/PTS resin. Increased chain flexibility caused by the chemical structure within the chains and the higher crosslink average molecular weight yield a ductile resin at room temperature. The increased chain stiffness caused by aromatic components within the chain links and the lower crosslink average molecular weight contributed to a brittle resin in the second case. In companion articles, the biodegradability of these three resins is reported. In summary, lipase hydrolized all three resins to the monomers, but in the presence of a mixed microbial culture, only the resins ZGluOH/EG/PTS and ZGluOH/BDE/ PTS were reduced to biomass and respiratory gases. Financial support from the Nebraska Soybean Development, Utilization and Marketing Board, the Center for Materials Research and Analysis, and NRI funds for Reaction Engineering Strategies to Produce Valueadded Chemicals from Agricultural Feedstocks, University of Nebraska-Lincoln, is appreciated. REFERENCES 1. Fried, J. R. Polymer Science and Technology; Prentice-Hall: Englewood Cliffs, NJ, 1995; p Paetau, I.; Chen, C.-Z.; Jane, J.-L. Ind Eng Chem Res 1994, 33, Dechow, F. J. Separation and Purification Techniques in Biotechnology; Noyes: Park Ridge, NJ, 1989; pp 268, 273, Fox, S. W.; Foster, J. F. Introduction to Protein Chemistry; Wiley: New York, Lee, K.; Hong, J. Transport of Amino Acids Through Ultrafiltration Membranes in the Presence of an Electric Field; Lee, K.; Hong, J., Ed.; AICHE: New York, 1992; Bull. 290, p Anderson, J. M.; Gibbons, D. F.; Martin, R. L.; Hiltner, A.; Woods, R. J Biomed Mater Res Symp 1974, 5, Sato, H.; Noishiki, Y.; Chen, G.; Hayashi, T.; Nakajima, A. Novel A-B-A Block Copolymer Consisting of Poly(g-ethyl L-glutamate) (A) and Polybutadiene (B) as Biomaterial; Kyoto University: Kyoto, Japan, 1984; #07929, p Lenz, R. W.; Kin, Y. B.; Fuller, R. C. J Bioact Compat Polym 1991, 6, Jones, J. Amino Acid and Peptide Synthesis; Oxford: Oxford, UK, 1992; pp 14, 23, 45.
21 10. Cyclic Polymers; Semlyen, J. A., Ed.; Elsevier: London, Albert, A. Heterocyclic Chemistry, 2nd ed.; Athlone: London, Pramanick, D.; Rey, T. T. Polym Bull 1987, 18, Stevens, M. P. Polymer Chemistry: An Introduction, 2nd ed.; Oxford University: Oxford, UK, 1990; pp Carothers, W. H. Chem Rev 1931, 8, Nielsen, J. A.; Chen, S. J.; Timm, D. C. Macromolecules 1993, 26, Tadros, R.; Timm, D. C. Macromolecules 1995, 28, Flory, P. J. Principles of Polymer Chemistry; Cornell University: Ithaca, NY, Rozenberg, B. A. In Advances in Polymer Science, Epoxy Resins and Composites; DusUek, K., Ed.; Springer-Verlag: Berlin, 1986; Vol. 78, p Cole, K. C. Macromolecules 1991, 24, 3093, Antoon, M. K.; Koenig, J. L. J Polym Sci 1981, 19, Stockmayer, W. H. J Polym Sci 1952, 9, Blatz, P. J.; Tobolsky, A. V. J Phys Chem 1945, 49, Bokare, U. M.; Gandhi, K. S. J Polym Sci Polym Phys Ed 1980, 18, Gupta, A. M.; Macosko, C. W. J Polym Sci Part B Polym Phys 1990, 28, Sarmoria, C.; Miller, D. R. Macromolecules 1991, 24, Robbins, D. J.; Timm, D. C. Macromolecules in press. 27. DusUek, K.; Ilavsky, M.; Lun a k, S. J Polym Sci Polym Symp 1975, 53, Dotson, N. A.; Galvan, R.; Laurence, R. L.; Tirrell, M. Polymerization Process Modeling; VCH: Cambridge, UK, Mark, J. E.; Erman, B. Rubberlike Elasticity: A Molecular Primer; Wiley: New York, 1988.
Deterministic Solutions for a Step-growth Polymerization
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 10-17-2003 Deterministic Solutions
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 10-17-2003 Deterministic Solutions
Lecture No. (1) Introduction of Polymers
 Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector
 A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
First Shell Substitution Effects: Diglyeidyl Ether of Bisphenol A cured with 4,4'- Diaminodiphenylmethane
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 4-1-1994 First Shell Substitution
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 4-1-1994 First Shell Substitution
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
A Technical Whitepaper Polymer Technology in the Coating Industry. By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA
 A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
Chapter 4. Results and Discussion. 4.1 Monomer Syntheses
 Chapter 4 Results and Discussion 4.1 Monomer Syntheses The syntheses of a family of novel, carbazole based methacrylate, dimethacrylate, and acrylate monomers, and subsequent purifications, were successful.
Chapter 4 Results and Discussion 4.1 Monomer Syntheses The syntheses of a family of novel, carbazole based methacrylate, dimethacrylate, and acrylate monomers, and subsequent purifications, were successful.
1.1 Basic Polymer Chemistry. 1.2 Polymer Nomenclature. 1.3 Polymer Synthesis. 1.4 Chain Growth Polymerization. Polymer =
 1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems
 Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems J. Baghdachi, Ph.D. Coatings Research Institute Eastern Michigan University (734) 487-3192 Freshpaint@aol.com jamil.baghdachi@emich.edu
Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems J. Baghdachi, Ph.D. Coatings Research Institute Eastern Michigan University (734) 487-3192 Freshpaint@aol.com jamil.baghdachi@emich.edu
Supporting Information for
 Supporting Information for Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets Mathieu Capelot, Damien Montarnal, François Tournilhac, Ludwik Leibler* I. Epoxy-acid and epoxy-anhydride
Supporting Information for Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets Mathieu Capelot, Damien Montarnal, François Tournilhac, Ludwik Leibler* I. Epoxy-acid and epoxy-anhydride
Periodic table with the elements associated with commercial polymers in color.
 Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state
 2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
(c) Dr. Payal B. Joshi
 Polymer (Greek: poly=many; mer=part) Made up of large molecules characterized by repeating units called monomers held together by covalent bonds Functionality To act as monomer, it must have at least two
Polymer (Greek: poly=many; mer=part) Made up of large molecules characterized by repeating units called monomers held together by covalent bonds Functionality To act as monomer, it must have at least two
Supporting Information
 Supporting Information Anion Conductive Triblock Copolymer Membranes with Flexible Multication Side Chain Chen Xiao Lin a,b, Hong Yue Wu a, Ling Li a, Xiu Qin Wang a, Qiu Gen Zhang a, Ai Mei Zhu a, Qing
Supporting Information Anion Conductive Triblock Copolymer Membranes with Flexible Multication Side Chain Chen Xiao Lin a,b, Hong Yue Wu a, Ling Li a, Xiu Qin Wang a, Qiu Gen Zhang a, Ai Mei Zhu a, Qing
MATERIALS SCIENCE POLYMERS
 POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
Chemically recyclable alternating copolymers with low polydispersity from
 Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Supporting Information
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Photochemical Regulation of a Redox-Active Olefin Polymerization
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Photochemical Regulation of a Redox-Active Olefin Polymerization
Combustion and thermal degradation of polymers
 Polymers and biomaterials - laboratory Combustion and thermal degradation of polymers Theoretical background dr Hanna Wilczura-Wachnik University of Warsaw Faculty of Chemistry Chemical Technology Division
Polymers and biomaterials - laboratory Combustion and thermal degradation of polymers Theoretical background dr Hanna Wilczura-Wachnik University of Warsaw Faculty of Chemistry Chemical Technology Division
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Polymer Bulletin 9 by Springer-Verlag 1980
 Polymer Bulletin 3, 391-398 (1980) Polymer Bulletin 9 by Springer-Verlag 1980 Homogeneous Epoxy-Acrylic Interpenetrating Polymer Networks: Preparation and Thermal Properties Alain Dubuisson, Dominique
Polymer Bulletin 3, 391-398 (1980) Polymer Bulletin 9 by Springer-Verlag 1980 Homogeneous Epoxy-Acrylic Interpenetrating Polymer Networks: Preparation and Thermal Properties Alain Dubuisson, Dominique
Chain extension and branching of poly(l-lactic acid) produced by reaction with a DGEBA-based epoxy resin
 express Polymer Letters Vol.1, No.11 (2007) 734 739 Available online at www.expresspolymlett.com DOI: 10.3144/expresspolymlett.2007.101 Chain extension and branching of poly(l-lactic acid) produced by
express Polymer Letters Vol.1, No.11 (2007) 734 739 Available online at www.expresspolymlett.com DOI: 10.3144/expresspolymlett.2007.101 Chain extension and branching of poly(l-lactic acid) produced by
Polymerization Modeling
 www.optience.com Polymerization Modeling Objective: Modeling Condensation Polymerization by using Functional Groups In this example, we develop a kinetic model for condensation polymerization that tracks
www.optience.com Polymerization Modeling Objective: Modeling Condensation Polymerization by using Functional Groups In this example, we develop a kinetic model for condensation polymerization that tracks
Personalised Learning Checklists AQA Chemistry Paper 2
 AQA Chemistry (8462) from 2016 Topics C4.6 The rate and extent of chemical change Calculate the rate of a chemical reaction over time, using either the quantity of reactant used or the quantity of product
AQA Chemistry (8462) from 2016 Topics C4.6 The rate and extent of chemical change Calculate the rate of a chemical reaction over time, using either the quantity of reactant used or the quantity of product
Size exclusion chromatography of branched polymers: Star and comb polymers
 Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties
 Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties The effect of resin molecular architecture on the small strain elastic constants of diamine-cured epoxy thermosets has been
Effect of Resin Molecular Architecture on Epoxy Thermoset Mechanical Properties The effect of resin molecular architecture on the small strain elastic constants of diamine-cured epoxy thermosets has been
Xiangxiong Chen, Mohd Yusuf Khan and Seok Kyun Noh* School of Chemical Engineering, Yeungnam University, Dae-dong, Gyeongsan,
 Electronic Supplementary Information For M Amount of Fe (III)-mediated ATR of MMA with hosphorus Containing Ligands in the Absence of Any Additives Xiangxiong Chen, Mohd Yusuf Khan and Seok Kyun Noh* School
Electronic Supplementary Information For M Amount of Fe (III)-mediated ATR of MMA with hosphorus Containing Ligands in the Absence of Any Additives Xiangxiong Chen, Mohd Yusuf Khan and Seok Kyun Noh* School
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Polymers are high molecular mass macromolecules composed of repeating structural
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
GCSE Chemistry. Module C7 Further Chemistry: What you should know. Name: Science Group: Teacher:
 GCSE Chemistry Module C7 Further Chemistry: What you should know Name: Science Group: Teacher: R.A.G. each of the statements to help focus your revision: R = Red: I don t know this A = Amber: I partly
GCSE Chemistry Module C7 Further Chemistry: What you should know Name: Science Group: Teacher: R.A.G. each of the statements to help focus your revision: R = Red: I don t know this A = Amber: I partly
Aziridine in Polymers: A Strategy to Functionalize Polymers by Ring- Opening Reaction of Aziridine
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling
 Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling Weirong Dang I, Masatoshi Kubouchi I, Shurou Yamamoto I, Hideki Sembokuya I, Kazuyoshi Arai and Ken Tsuda Department of Chemical Engineering,
Decomposition Mechanism of Epoxy Resin in Nitric Acid for Recycling Weirong Dang I, Masatoshi Kubouchi I, Shurou Yamamoto I, Hideki Sembokuya I, Kazuyoshi Arai and Ken Tsuda Department of Chemical Engineering,
TOPIC 7. Polymeric materials
 Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 7. Polymeric materials 1. Introduction Definition General characteristics Historic introduction Polymers: Examples 2.
Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 7. Polymeric materials 1. Introduction Definition General characteristics Historic introduction Polymers: Examples 2.
Chemical Recycling of Polyurethane Foam Waste Via Glycolysis
 Chemical Recycling of Polyurethane Foam Waste Via Glycolysis Alicia Aguado 1, Lidia Martínez 1, Alberto Moral 1, José Fermoso 1, Rubén Irusta* 1,2 1 CARTIF Centro Tecnológico, Environmental Division. Parque
Chemical Recycling of Polyurethane Foam Waste Via Glycolysis Alicia Aguado 1, Lidia Martínez 1, Alberto Moral 1, José Fermoso 1, Rubén Irusta* 1,2 1 CARTIF Centro Tecnológico, Environmental Division. Parque
POLYMER SCIENCE : lecture 1. Dr. Hanaa J. Alshimary Second class Poly. Eng. Dep. Introduction of Polymers Polymer poly mer Monomer Polymerization
 Introduction of Polymers Polymer - The word polymer is the Greek word : poly means many and mer means unit or parts, A Polymer is a large molecule that comprises repeating structural units joined by the
Introduction of Polymers Polymer - The word polymer is the Greek word : poly means many and mer means unit or parts, A Polymer is a large molecule that comprises repeating structural units joined by the
Lecture 4 Chapter 13 - Polymers. Functional Groups Condensation Rxns Free Radical Rxns
 Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Chemical Engineering Seminar Series
 Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics
 GPEC 24 Paper Abstract #52: Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics Author(s): V. Karayan, Clariant Masterbatches, and M. Villalobos,
GPEC 24 Paper Abstract #52: Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics Author(s): V. Karayan, Clariant Masterbatches, and M. Villalobos,
Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE
 Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE Introduction Gel permeation chromatography (GPC) and size exclusion chromatography
Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE Introduction Gel permeation chromatography (GPC) and size exclusion chromatography
Solubility of carbon dioxide in aqueous solutions of 2-amino-2-ethyl-1,3-propanediol
 Fluid Phase Equilibria 202 (2002) 359 366 Solubility of carbon dioxide in aqueous solutions of 2-amino-2-ethyl-1,3-propanediol Jung-Yeon Park a, Sang Jun Yoon a, Huen Lee a,, Ji-Ho Yoon b, Jae-Goo Shim
Fluid Phase Equilibria 202 (2002) 359 366 Solubility of carbon dioxide in aqueous solutions of 2-amino-2-ethyl-1,3-propanediol Jung-Yeon Park a, Sang Jun Yoon a, Huen Lee a,, Ji-Ho Yoon b, Jae-Goo Shim
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION
 SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION T. Ohishi, 1 K. Imato, 2 T. Kanehara, 2 A. Takahara, 1,2 and H. Otsuka 1,2 1 Institute for Materials
SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION T. Ohishi, 1 K. Imato, 2 T. Kanehara, 2 A. Takahara, 1,2 and H. Otsuka 1,2 1 Institute for Materials
Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries
 Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Supporting Information
 Supporting Information Facile polyisobutylene functionalization via thiol-ene Click chemistry Andrew J. D. Magenau, Justin W. Chan, Charles E. Hoyle, and Robson F. Storey School of Polymers and High Performance
Supporting Information Facile polyisobutylene functionalization via thiol-ene Click chemistry Andrew J. D. Magenau, Justin W. Chan, Charles E. Hoyle, and Robson F. Storey School of Polymers and High Performance
The functionality of a monomer is the number of binding sites that is/are present in that monomer.
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting from RAFT polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Figure 4.10 HPLC Chromatogram of the Carbazole-Phenoxy Based Methacrylate
 The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
Kinetic Study of the Effect of Catalyst in the Polycondensation of Lactic Acid to Produce Low Molecular Weight Polymers
 Kinetic Study of the Effect of Catalyst in the Polycondensation of Lactic Acid to Produce Low Molecular Weight Polymers Davide Moscatelli*, Simone Gelosa*, Harshe Yogesh and Giuseppe Storti * Dept. di
Kinetic Study of the Effect of Catalyst in the Polycondensation of Lactic Acid to Produce Low Molecular Weight Polymers Davide Moscatelli*, Simone Gelosa*, Harshe Yogesh and Giuseppe Storti * Dept. di
Due by 3:00 PM Tuesday, June 26 NO LATE PAPERS ACCEPTED!
 HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene
 Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Chapter 22 Hydrocarbon Compounds
 Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
An Introductions to Advanced GPC Solutions
 An Introductions to Advanced GPC Solutions Alan Brookes Sales Manager GPC Instruments EMEAI 9 th April 2014 Agilent GPC/SEC Solutions 1 Introduction to Polymers Polymers are long chain molecules produced
An Introductions to Advanced GPC Solutions Alan Brookes Sales Manager GPC Instruments EMEAI 9 th April 2014 Agilent GPC/SEC Solutions 1 Introduction to Polymers Polymers are long chain molecules produced
GPC/SEC An essential tool for polymer analysis
 GPC/SEC An essential tool for polymer analysis Ben MacCreath, PhD Product Manager GPC/SEC Instrumentation 26 th March 2013 Introduction to Polymers Where are they found? Polyolefins Engineering Polymers
GPC/SEC An essential tool for polymer analysis Ben MacCreath, PhD Product Manager GPC/SEC Instrumentation 26 th March 2013 Introduction to Polymers Where are they found? Polyolefins Engineering Polymers
Introduction to Polymerization Processes
 Introduction to Polymerization Processes Reference: Aspen Polymers: Unit Operations and Reaction Models, Aspen Technology, Inc., 2013. 1- Polymer Definition A polymer is a macromolecule made up of many
Introduction to Polymerization Processes Reference: Aspen Polymers: Unit Operations and Reaction Models, Aspen Technology, Inc., 2013. 1- Polymer Definition A polymer is a macromolecule made up of many
Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid)
 Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Le Lycee Mauricien. Proposed Syllabus Chemistry (5070) - Form 5
 Le Lycee Mauricien Proposed Syllabus 2017 Chemistry (5070) - Form 5 First Term 1. Metals Properties of metals - Physical properties of metals - Structure of alloys and uses Reactivity Series - Place metals
Le Lycee Mauricien Proposed Syllabus 2017 Chemistry (5070) - Form 5 First Term 1. Metals Properties of metals - Physical properties of metals - Structure of alloys and uses Reactivity Series - Place metals
The Characteristics of a Soln
 Goal 1 The Characteristics of a Soln Define the term solution, and, given a description of a substance, determine if it is a solution. The Characteristics of a Soln Solution (as used in chemistry) A homogenous
Goal 1 The Characteristics of a Soln Define the term solution, and, given a description of a substance, determine if it is a solution. The Characteristics of a Soln Solution (as used in chemistry) A homogenous
Supplementary Material for
 www.sciencemag.org/content/343/6173/873/suppl/dc1 Supplementary Material for Nonswellable Hydrogel Without Mechanical Hysteresis Hiroyuki Kamata, Yuki Akagi, Yuko Kayasuga-Kariya, Ung-il Chung, Takamasa
www.sciencemag.org/content/343/6173/873/suppl/dc1 Supplementary Material for Nonswellable Hydrogel Without Mechanical Hysteresis Hiroyuki Kamata, Yuki Akagi, Yuko Kayasuga-Kariya, Ung-il Chung, Takamasa
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Latest Developments in GPC Analysis of Adhesive and Sealant Polymers Mark Pothecary PhD Americas Product Manager Malvern Instruments
 Latest Developments in GPC Analysis of Adhesive and Sealant Polymers Mark Pothecary PhD Americas Product Manager Malvern Instruments Molecular weight The most fundamental molecular property that controls
Latest Developments in GPC Analysis of Adhesive and Sealant Polymers Mark Pothecary PhD Americas Product Manager Malvern Instruments Molecular weight The most fundamental molecular property that controls
Chapter 14. Molar Mass Distribution.
 Chapter 14. Molar Mass Distribution. Difficulty with M n and M w, etc. osome polymers are hard to describe from just M n, M w, etc. o Examples: Bimodal, multimodal, nonuniform, broad, etc. MWDs. oin early
Chapter 14. Molar Mass Distribution. Difficulty with M n and M w, etc. osome polymers are hard to describe from just M n, M w, etc. o Examples: Bimodal, multimodal, nonuniform, broad, etc. MWDs. oin early
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
Effect of Molecular Structure of Side Chain Polymers on "Click" Synthesis of Thermosensitive Molecular Brushes
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2017 Effect of Molecular Structure
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2017 Effect of Molecular Structure
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Chemistry (A-level)
 1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Electrophoresis (Chapter 27): Chemistry (A-level) Electrophoresis: the separation of charged particles by their different rates of movement in
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Electrophoresis (Chapter 27): Chemistry (A-level) Electrophoresis: the separation of charged particles by their different rates of movement in
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur. Lecture - 4 Step-growth Polymerization
 Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
Review Experiments Formation of Polymers Reduction of Vanillin
 Review Experiments Formation of Polymers What is a polymer? What is polymerization? What is the difference between an addition polymerization and a condensation polymerization? Which type of polymerization
Review Experiments Formation of Polymers What is a polymer? What is polymerization? What is the difference between an addition polymerization and a condensation polymerization? Which type of polymerization
Marine bio-inspired underwater contact adhesion
 Marine bio-inspired underwater contact adhesion Sean K. Clancy, Antonio Sodano, Dylan J. Cunningham, Sharon S. Huang, Piotr J. Zalicki, Seunghan Shin, * and B. Kollbe Ahn * Marine Science Institute, University
Marine bio-inspired underwater contact adhesion Sean K. Clancy, Antonio Sodano, Dylan J. Cunningham, Sharon S. Huang, Piotr J. Zalicki, Seunghan Shin, * and B. Kollbe Ahn * Marine Science Institute, University
Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using
 Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using an organocatalyst Mark T. Martello, Adam Burns, and Marc Hillmyer* *Department of Chemistry, University of Minnesota,
Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using an organocatalyst Mark T. Martello, Adam Burns, and Marc Hillmyer* *Department of Chemistry, University of Minnesota,
RAPID COMMUNICATION. Synthesis of Disyndiotactic Polylactide INTRODUCTION EXPERIMENTAL
 RAPID COMMUNICATION Synthesis of Disyndiotactic Polylactide M. BERO, P. DOBRZYŃSKI, J. KASPERCZYK Centre of Polymer Chemistry, Polish Academy of Sciences, 41-800 Zabrze, Poland, ul. M. Curie Sklodowskiej
RAPID COMMUNICATION Synthesis of Disyndiotactic Polylactide M. BERO, P. DOBRZYŃSKI, J. KASPERCZYK Centre of Polymer Chemistry, Polish Academy of Sciences, 41-800 Zabrze, Poland, ul. M. Curie Sklodowskiej
Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION Historical Perspective Some of the earliest useful polymeric
 Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Cellulose: chemical modification
 Cellulose: chemical modification CHEM-E2140 Eero Kontturi 3 rd November 2015 Outline (1) Chemical modification of cellulose motivation (2) Background: terminology, challenges (3) Esterification of cellulose
Cellulose: chemical modification CHEM-E2140 Eero Kontturi 3 rd November 2015 Outline (1) Chemical modification of cellulose motivation (2) Background: terminology, challenges (3) Esterification of cellulose
Malleable, Mechanically Strong, and Adaptive Elastomers. Enabled by Interfacial Exchangeable Bonds
 Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds Zhenghai Tang, Yingjun Liu, Baochun Guo,*, and Liqun Zhang*, Department of Polymer Materials and Engineering,
Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds Zhenghai Tang, Yingjun Liu, Baochun Guo,*, and Liqun Zhang*, Department of Polymer Materials and Engineering,
Sample Exam Solutions. Section A. The answers provided are suggestions only and do not represent directly or otherwise official VCAA answers.
 1 Sample Exam 1 2008 Solutions The answers provided are suggestions only and do not represent directly or otherwise official VAA answers. Section A Q1 n(agl) = 4.85 / (107.9+35.5) = 0.00338 mol n(kl) =
1 Sample Exam 1 2008 Solutions The answers provided are suggestions only and do not represent directly or otherwise official VAA answers. Section A Q1 n(agl) = 4.85 / (107.9+35.5) = 0.00338 mol n(kl) =
Polymers in Modified Asphalt Robert Q. Kluttz KRATON Polymers
 Polymers in Modified Asphalt Robert Q. Kluttz KRATON Polymers Polymers in Modified Asphalt Types of Polymers Compatibility of Polymers Effects of Polymers Analysis of polymers Recovery of PMA What Is a
Polymers in Modified Asphalt Robert Q. Kluttz KRATON Polymers Polymers in Modified Asphalt Types of Polymers Compatibility of Polymers Effects of Polymers Analysis of polymers Recovery of PMA What Is a
Final Exam Introduction to Polymers (each part, a,b,c,, is worth 2.2 points)
 168 Final Exam Introduction to Polymers (each part, a,b,c,, is worth 2.2 points) 1) Polymers are different than low-molecular weight oligomers. For example an oligomeric polyethylene is wax, oligomeric
168 Final Exam Introduction to Polymers (each part, a,b,c,, is worth 2.2 points) 1) Polymers are different than low-molecular weight oligomers. For example an oligomeric polyethylene is wax, oligomeric
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
SUPPLEMENTARY INFORMATION
 Methods: Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity Andrew J. Keefe and Shaoyi Jiang Materials: α-chymotrypsin from bovine pancreas
Methods: Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity Andrew J. Keefe and Shaoyi Jiang Materials: α-chymotrypsin from bovine pancreas
ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes
 ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes 1. Combustion Alkanes are very important fossil fuels. The combustion of alkanes is very exothermic and carbon dioxide and water are produced. General
ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes 1. Combustion Alkanes are very important fossil fuels. The combustion of alkanes is very exothermic and carbon dioxide and water are produced. General
Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth
 University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
F G C G H H C B B A A DD E E
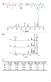 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Supporting Information
 Supporting Information Effect Of TiO 2 Nanoparticle Surface Functionalization On Protein Adsorption, Cellular Uptake and Cytotoxicity: The Attachment Of PEG Comb Polymers Using Catalytic Chain Transfer
Supporting Information Effect Of TiO 2 Nanoparticle Surface Functionalization On Protein Adsorption, Cellular Uptake and Cytotoxicity: The Attachment Of PEG Comb Polymers Using Catalytic Chain Transfer
Liquid Polybutadienes and Derivatives
 Liquid Polybutadienes and Derivatives Coatings & Colorants Product Range Our polyoils and derivatives are stereospecific, lowviscosity and unsaponifiable liquid polybutadienes having a high 1.4-cis double
Liquid Polybutadienes and Derivatives Coatings & Colorants Product Range Our polyoils and derivatives are stereospecific, lowviscosity and unsaponifiable liquid polybutadienes having a high 1.4-cis double
Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation
 Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation Adam Al-Mulla* Chemical Engineering Department, Kuwait University, P.O. Box 5969, 36 Safat, Kuwait, ABSTRACT Thermotropic
Novel Hydroquinone Diacetate based copolyesters: A detailed kinetic investigation Adam Al-Mulla* Chemical Engineering Department, Kuwait University, P.O. Box 5969, 36 Safat, Kuwait, ABSTRACT Thermotropic
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
GPC / SEC Theory and Understanding
 Dr. Jason S. Davies, Smithers Rapra, UK Gel permeation chromatography (GPC), also known as size exclusion chromatography (SEC) is a branch of liquid chromatography specifically concerned with characterisation
Dr. Jason S. Davies, Smithers Rapra, UK Gel permeation chromatography (GPC), also known as size exclusion chromatography (SEC) is a branch of liquid chromatography specifically concerned with characterisation
Innovative. Technologies. Chemie des Klebens Chemistry of Adhesives. Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013
 Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
The Effects of Organotin Catalysts on Hydrolytic Condensation of
 Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
Chapter 5. Ionic Polymerization. Anionic.
 Chapter 5. Ionic Polymerization. Anionic. Anionic Polymerization Dr. Houston S. Brown Lecturer of Chemistry UH-Downtown brownhs@uhd.edu What you should know: What is anionic polymerization? What is MWD,
Chapter 5. Ionic Polymerization. Anionic. Anionic Polymerization Dr. Houston S. Brown Lecturer of Chemistry UH-Downtown brownhs@uhd.edu What you should know: What is anionic polymerization? What is MWD,
Polymer Molecular Weight
 Chapter 3 Polymer Molecular Weight 3.1 Introduction Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids
Chapter 3 Polymer Molecular Weight 3.1 Introduction Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids
Paper Atomic structure and the periodic table
 Paper 1 4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes Use the names and symbols of the first 20 elements in
Paper 1 4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes Use the names and symbols of the first 20 elements in
Sol-Gel Methods. Hydrolysis Condensation Gelation Ageing Drying Densification
 Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
Polymer analysis by GPC-SEC. Technical Note. Introduction
 Polymer analysis by GPC-SEC Technical Note Introduction Gel Permeation Chromatography (GPC), also referred to as Size Exclusion Chromatography (SEC) is a mode of liquid chromatography in which the components
Polymer analysis by GPC-SEC Technical Note Introduction Gel Permeation Chromatography (GPC), also referred to as Size Exclusion Chromatography (SEC) is a mode of liquid chromatography in which the components
Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate
 1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst
 Reac Kinet Mech at (211) 14:9 15 DI 1.17/s11144-11-337-9 Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst Maria
Reac Kinet Mech at (211) 14:9 15 DI 1.17/s11144-11-337-9 Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst Maria
Asia Flow Chemistry System
 Asia Flow Chemistry System Flow Chemistry Continuous Flow Synthesis of Carboxylic Acids from Grignard Reagents with Carbon Dioxide Gas MAR-000289 Version: 1.1 Issue date: 11/05/2016 Author: Andrew Mansfield
Asia Flow Chemistry System Flow Chemistry Continuous Flow Synthesis of Carboxylic Acids from Grignard Reagents with Carbon Dioxide Gas MAR-000289 Version: 1.1 Issue date: 11/05/2016 Author: Andrew Mansfield
Synthesis of Arborescent Polybutadiene. Ala Alturk and Mario Gauthier, IPR Symposium, University of Waterloo, N2L 3G1 Canada
 Synthesis of Arborescent Polybutadiene Ala Alturk and Mario Gauthier, IPR Symposium, University of Waterloo, N2L 3G1 anada Arborescent polymers are characterized by a tree-like architecture and a high
Synthesis of Arborescent Polybutadiene Ala Alturk and Mario Gauthier, IPR Symposium, University of Waterloo, N2L 3G1 anada Arborescent polymers are characterized by a tree-like architecture and a high
Techniques useful in biodegradation tracking and biodegradable polymers characterization
 Techniques useful in biodegradation tracking and biodegradable polymers characterization Version 1 Wanda Sikorska and Henryk Janeczek 1 Knowledge on biodegradable polymers structures is essential for the
Techniques useful in biodegradation tracking and biodegradable polymers characterization Version 1 Wanda Sikorska and Henryk Janeczek 1 Knowledge on biodegradable polymers structures is essential for the
Supporting Information. Competitive Interactions of π-π Junctions and their Role on Microphase Separation of Chiral Block Copolymers
 Supporting Information Competitive Interactions of π-π Junctions and their Role on Microphase Separation of Chiral Block Copolymers Tao Wen, Jing-Yu Lee, Ming-Chia Li, Jing-Cherng Tsai and Rong-Ming Ho
Supporting Information Competitive Interactions of π-π Junctions and their Role on Microphase Separation of Chiral Block Copolymers Tao Wen, Jing-Yu Lee, Ming-Chia Li, Jing-Cherng Tsai and Rong-Ming Ho
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
