Ready; Catalysis Conjugate Addition
|
|
|
- Chloe Hutchinson
- 5 years ago
- Views:
Transcription
1 eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based systems b. h-based systems 3. Catalytic addition of heteroatoms Topics not covered: enolate conjugate additions, Friedel-Crafts-type conjugate additions
2 eady; Catalysis Conjugate Addition: origin 1,4 addition reactions involving copper eview: rg. xns, 199, 41, st report: Kharasch, JACS, 1941, 308 "Factors Determining the Course and Mechanisms of Grignard eactions" C C 3 3 C 3 MgBr additive 3 C 3 C C C 3 C 3 3 C 3 C 3 C 3 C 3 C 3 C 3 C 3 C 3 additive none CuCl (1 mol%) an unusual example of the catalytic reaction predating the stoichiometric one classes of copper reagents: nm + CuX n = 1, X = halide, CN Cu CuM (CN)CuM (CN)CuM M = MgX, Li M = MgX, Li M = MgX, Li organocopper organocuprate (Gilman reagent) lower order cuprate higher order cuprate
3 eady; Catalysis Conjugate Addition: general CuM (M = MgX, Li) Most common for simple conjugate additions Soluble in Et, TF Thermally unstable (usually keep temp < o 0 for = aryl, vinyl; -78 o C for = alkyl) Cu I Cu(0) General reactivity trends X + CuLi Et or TF X = 1 o ~ o >3 o >>>alkynyl α or β sub. K α,β sub usually K β,β ususally difficult, but possible ketones>esters>amides Me CuLi 98% C 3 3 CN aldehydes: 1, addition competes CuLi 75% 3 CN C 3 C 3 C 3 Ac Me CuLi 55% C 3 C 3 Ac LiCu 66%
4 eady; Catalysis Conjugate Addition: mechanism mechanism ecall: εc - εcu = 0.6 (polarization: C-Sn > C-Si ~ C-Cu > C-B) Li CuX Li CuLi LiX Li a Cu-enone intermediate has been observed spectoscopically and implicated kinetically. For lead references, see Krause, ACIEE, 1999, 1644 set Li Li Li Cu II Cu Cu III Li predictions: some - formation (observed, usually use >1 equiv cuprate) Lewis Acids may help Can use enolate for further reaction
5 eady; Catalysis Conjugate Addition: TMSCl Added TMSCl+MPA very common method MgBr + 5 mol% CuBr-Me S additive Nakamura, Tet, 1989, 349 additive none TMSCl (.4 equiv) TMSCl + MPA (.4 equiv) <1 enals usually very difficult b/c 1, addition competes using MgBr + 5 mol% CuBr-Me S with added TMSCl/MPA: TMS TMS TMS 3 C %Y E/Z hex 83 94: :9 %Y E/Z hex 89 96: :4 C 3 %Y E/Z Bu 89 87: :8
6 eady; Catalysis Conjugate Addition-10 Problem with CuLi: Waste 1 equiv Solution: nontransferable ligand t-bu 1. MeLi. CuI 3. MeLi t-bu Cu C 3 Li 76% Whitesides, JACS, 1973, 3893 n-c 3 7 Cu Li + Corey, JACS, 197, 710 =, tbu, vinyl Y > 90% eagents of the form 'Cu have reactivity intermediate between Cu and ' Cu n-bu Cu P Li more reactive than Bu CuLi Stable at 0-5 o C Especially good for alkylations with alkyl halides similar: tbucu(s)li Posner, JACS, 1973, n-bu 88% Bertz, JACS, 198, 585 rigin of selectivity with mixed cuprates: Nakamra, JACS, 005, 4697
7 eady; Catalysis Conjugate Addition-11 alkyl-cyano-cuprates review: Tet, 1984, 5005 Cu(CN)Li - lower order cuprates Cu(CN)Li - igher order cuprates Lower order cuprates have reactivity intermediate between Cu and CuLi i.e. CN similar to alkyne (non-transferable ligand) usually moderate reactivity for 1,4 addns, but K for SN' + Li(NC)Cu C 'single product' 95% yield Trost, JC, 1980, 456
8 eady; Catalysis Conjugate Addition-1 alkyl-cyano-cuprates review: Tet, 1984, 5005 Cu(CN)Li - lower order cuprates Cu(CN)Li - igher order cuprates experimental observation that higher order cuprates display improved reactivity relative to Gilman reagents. I 'Cu' Bu 'Cu' Bu CuLi LiI only none Bu Cu(CN)Li 90% C Et 'Cu' C Et 'Cu' yield CuLi 38 Cu(CN)Li 75 Et n-c % 85% 88%
9 eady; Catalysis Conjugate Addition-13 The structure of higher-order cuprates has been debated: From Chem Comm. 1996
10 eady; Catalysis Conjugate Addition-14 Brief review: Krause, ACIEE, 1999, 79 So why are higher order cuprates more effective?? Not clear
11 eady; Catalysis Conjugate Addition-15 Copper-mediated addition of alkylzinc reagents ften characterized by complicated reaction mixtures: Ms C 3 NBoc 'MeCu' C Me 'MeCu' NBoc C Me NBoc C Me MeCu(CN)MgBr Me Cu(CN)(ZnCl) Mg(Br)Cl 3LiCl MeMgBr + ZnCl --> white suspension MeMgBr + ZnCl + LiCl --> solution TL 199, 3783 for conjugate addtions: review: Chem ev. 1993, 117 NC 65% Piv ZnX + CuCN LiCl soluble in TF TMS 59% Cu(CN)ZnX LiX good thermal stability (reflux TF) Less reactive than MgBr or Li-derived no rxn with epoxides only 1 o iodides Allows functional groups not compatible with MgBr or Li C Me 95% i-pr C 93% P P' n-c %
12 eady; Catalysis Conjugate Addition-16
13 eady; Catalysis Conjugate Addition-17 Problem: often hard to alkylate intermediate Li enolate Solution: transmetalate to Sn 1 (Bu 3 P)Cu C 5 11 TBS (C ) 3 C Me TBS 3 I 3 SnCl (C ) 3 C Me TBS TBS C %, d.r. Noyori, JACS, 1988, 4718 w/o Sn, get complex mixture resulting from enolate equilibration. Li Li Li Li TBS TBS TBS TBS Propose Sn(Cl) 3 Li less basic
14 eady; Catalysis Conjugate Addition-19 1,4 additions to alkynones CuLi Cu Li Cu Cu Cu ' ' ' ' ' ' cis-addn ' trans-addn Note: -Cu can add to unactivated alkynes: Br EtCu(Me S) MgBr + C6 13 C 5 Cu C 5 C 6 13 'carbocupration' get less-hindered organocopper C % rg. Syn. 1985, 64, 1 so 1,4 addn mechanistically between 1,4 addn and carbocupration
15 eady; Catalysis Conjugate Addition-0 alkynones and alkynoates are more reactive than enones, so Cu is enough C Me MgBr/CuBr Me C C Me Et TP somtimes isomerization is good: C Me + C Me Et CuLi Me C = alkyl, vinyl, phenyl, TMS Chem Lett, 1981, 905 and 1363 Cu TP Et Et 81%:1 E:Z TL 1976, 345 C Me Crimmins, JC, 1984, %
16 eady; Catalysis Conjugate Addition- more examples of enoate addn C Et 1. Me CuLi. allyl-br 3. + syn comm. 1980, 751 C Et (TMS) Cu(CN)Li Me 3 Si C Et Marshall, Syn. Comm. 1983, 531 Mg(0) 65 o C MgCl C Me C Me Cl CuI, TMEDA ppolzer, TL, 1986, 5471
17 eady; Catalysis Conjugate Addition-1 Asymmetric 1,4 addn Breakthrough came with observation that Zn are effective alkyl sources -functionalized Nu's -can do catalytically without competing rxns of nucleophile Zn Cu(II) L*, Zn details of mechanism not known stereoselectivity not understood Zn L* Cu(I) Proposals Concerted transfer (ie no Cu(III) intermediate: Noyori, Bull. Chem. Soc. 000, 999; Kitamura, Chem Lett. 003, 4. M = Cu, Zn M Zn CuL* ate limiting A: Gennari, JMC, 005, 689, 169 ate limiting E: Schrader, Chem-E.J. 004, a poorly-defiined complex
18 eady; Catalysis Conjugate Addition-3 two best systems: n n + Zn + Zn Cu(Tf) ( mol%) Feringa, Accts, 000, 346 P [Cu(Tf)] (1 mol%) N P i-pr N N 4 mol% NBn Bn.4 mol% overall best ligand, can optimize for individual substrates n n n ee Ac Et Et Et Et ipr n ee Et ipr Et ipr Et ipr oveyda, JACS, 001, 755
19 eady; Catalysis Conjugate Addition Asymmetric addition of Grignards Chemo- and stereoselectivity Feringa: PNAS, 004, 5834 JACS, 004, 1784 ACIEE, 005, 75
20 eady; Catalysis Conjugate Addition-4 SN' additions β LG Nu - β Nu Nu β γ α γ α SN γ SN' α issues: regioselectivity (α vs γ) (usually SN') SN' favored: Cu-BF 3 ; CuLi-ZnCl ; Cu; Cu(Cl or Br)(MgBr), TF SN favored: CuI(MgBr) ; Et stereoselectivity (syn vs anti) (usually anti) substrates X X X = halide, Ac, C, P()(), S()
21 eady; Catalysis Conjugate Addition-5
22 eady; Catalysis Conjugate Addition-6 SN' addns: examples ' ''MgBr/CuBr DMS '' ' C regio 98: 70-90% -'' = alkyl JC, 1986, 161 Me CuLi + Ac cis trans TBS MeCu(CN)Li BF3 LiBr Ms E TBS C Me Ms C Me C Me MgBr minimize A 1,3 JACS, 1986, 740 N 3 Me CuCN N 3 Me Trost, JACS, 1995, 10143
23 eady; Catalysis Conjugate Addition-7 SN' addns involving alkynes Me n-c 8 17 MgBr 10% CuBr n-c 8 17 TMS BuCu Bu S()C 3 sulfinate TMS 91% N EtCu Et N 60%
24 eady; Catalysis Conjugate Addition-8 best systems to date: t-bu C N N NBn note: similar but different than ligand for 1,4 addn P()(Et) + Zn or -AliBu ' or -B(Pin) NC-based ligands Mes N N Me Et = Me, 74% ee 91% ee = Et, 86% ee Cu(Tf) oveyda, JACS, 004, solid state: N N Cu Cu N N oveyda, JACS, 004, JACS, 014, 149 c-c 6 11 c-c 6 1 Me or Et Et 93% ee 94% ee N N S Cu or = Et = e poor, neutral ee's >90 3 C >80%ee >80% ee Me = Me ee's ~ 90 C 3 Boc N >80% ee
25 eady; Catalysis Conjugate Addition-31 h-catalyzed 1,4 addns of boronic acids - a remarkable rxn review: ayashi Chem ev. 003, 89 original report: Miyaura M, 1997, 49 B() + h(acac)(c) dppb /Me moderate yields high temp long times enantioselective version B() + [h()(binap)] /dioxane 35 o C n n 1 3 4Me- 4-F- exenyl ee % ee 9% ee n-c 5 11
26 eady; Catalysis Conjugate Addition-3 Mechanism of h-catalyzed 1,4 addn P = BINAP P h hp No xidation state change on h! P h B() B() 3 hp P h hydrolysis faster than β-hydride elimination P h
27 eady; Catalysis Conjugate Addition-33 h-catalyzed 1,4 addn: other substrates Esters and amides: C Me C Me NBn 89% ee 98% ee 98% ee 98% ee (requires K C 3 ) osphonates P 96% ee Et uses B B B nitro olefins N NaC 3 N 98% ee 9:1 dr initial product 98% ee
28 eady; Catalysis Conjugate Addition-34 ow to do 3 component coupling? + B cat. [h(me)(cod)] BINAP toluene B BuLi, allyl-br n n only for n = 1, n 98% ee D C n D Ti(iPr) 3 TMS n cat. [h()(binap)] 1. LiiPr. TMSCl Ti(i-Pr) 3 n TMS n ee(%) 1 3 4F- 4Me % ee TMS
29 Pd-Catalyzed addition of aryl boronic acids: formation of quaternary stereocenters Stoltz, JACS, 011, 690 Pd(CCF 3 ) ClC C Cl, o C B() PdL B() n + B() N N tbu L n Pd eck-type addition No possibility of!-hydride elimination yields mostly >80%, ee's mostly >90% Me Me Me Me Me (no -substituted) C Me Br Me CF 3 Cl Bn Me Me
30 eady; Catalysis Conjugate Addition-35 1,4-addn of nucleophiles Bergman and Toste, JACS, 003, 8696 EWG PMe 3 (5 mol%) ' EWG ' EWG Y (%) CMe Me 56 CEt Me 77 CMe Me Me 71 CN Me 79 CMe Me 0
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
transmetallate displace ox. add. M + (insert) (β-elim.)
 Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent
 Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]
![o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)] o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]](/thumbs/85/92754396.jpg) 3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
Zr-Catalyzed Carbometallation
 -Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
-Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Synthesis of Amphidinolide X and an Exploration of Key Reactions
 PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Nickel-Catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides
 ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Chem 253 Problem Set 7 Due: Friday, December 3, 2004
 Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
A. Organometallic Mechanisms
 Dr. P. Wipf - Chem 2320 1 4/10/2006 II. Special Topics IIC. Organometallics Boger Notes: p. 395-426 (Chapter XII) Carey/Sundberg: B p. 477-546 (Chapter B 8) A. Organometallic Mechanisms Oxidation State:
Dr. P. Wipf - Chem 2320 1 4/10/2006 II. Special Topics IIC. Organometallics Boger Notes: p. 395-426 (Chapter XII) Carey/Sundberg: B p. 477-546 (Chapter B 8) A. Organometallic Mechanisms Oxidation State:
Kinetic Resolutions. Some definitions and examples Resolution: A process leading to the separation of enantiomers, or derivatives thereof.
 Material outline: For the Scientist in you: Definitions Theoretical treatment Kinetic esolutions General eferences: Vedejs, ACIEE, 2005, 3974 Jacobsen, Adv. Syn. Cat. 2001, 5 Kagan, Topics in Stereochemistry,
Material outline: For the Scientist in you: Definitions Theoretical treatment Kinetic esolutions General eferences: Vedejs, ACIEE, 2005, 3974 Jacobsen, Adv. Syn. Cat. 2001, 5 Kagan, Topics in Stereochemistry,
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
CH 3 TMG, DMF N H 3 CO 2 S. (PPh 3 ) 2 Pd 0
 1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
Organomagnesium (Grignard) and organolithium reagents
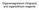 rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 852 Final Exam. May 5, 2011
 CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
Catalysis by Group IV Elements CHEM 966 (Tunge) Good reference: Titanium and Zirconium in Organic Synthesis Ilan Marek Ed., 2002.
 Catalysis by Group IV Elements CEM 966 (Tunge) Good reference: Titanium and Zirconium in rganic Synthesis Ilan Marek Ed., 2002. Electronegativity: Ti(1.54); (1.33); f(1.3) Much of the chemistry is dominated
Catalysis by Group IV Elements CEM 966 (Tunge) Good reference: Titanium and Zirconium in rganic Synthesis Ilan Marek Ed., 2002. Electronegativity: Ti(1.54); (1.33); f(1.3) Much of the chemistry is dominated
Literature Report IX. Cho, S. H. et al. Org. Lett. 2016, 18, Cho, S. H. et al. Angew. Chem. Int. Ed. 2017, 56,
 Literature Report IX ynthesis of β-aminoboronates by Copper(I)- Catalyzed Addition of Diborylalkanes to Imines Reporter Checker Date : hubo Hu : Xiaoyong Zhai : 2017-9-1818 Cho,. H. et al. rg. Lett. 2016,
Literature Report IX ynthesis of β-aminoboronates by Copper(I)- Catalyzed Addition of Diborylalkanes to Imines Reporter Checker Date : hubo Hu : Xiaoyong Zhai : 2017-9-1818 Cho,. H. et al. rg. Lett. 2016,
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Reactivity Umpolung-1 Ready
 eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Rhodium Catalyzed Alkyl C-H Insertion Reactions
 Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Mechanistic Studies in Copper Catalysis
 chanistic Studies in Copper Catalysis Jen Alleva May 1st 2013 Timeline of Achievements in Copper Chemistry General istorical verview first cross-couplings 1869 Ullmann Goldberg Glaser 1903 Glaser, C. Ann.
chanistic Studies in Copper Catalysis Jen Alleva May 1st 2013 Timeline of Achievements in Copper Chemistry General istorical verview first cross-couplings 1869 Ullmann Goldberg Glaser 1903 Glaser, C. Ann.
Chem 634. Metal Mediated Substitution Chemistry. Reading: Heg Ch 1 2 (handout), CS-B 7.1, , 11.3, Grossman Ch 6
 Chem 634 tal diated Substitution Chemistry eading: Heg Ch 1 2 (handout), CS-B 7.1, 8.2 8.3, 11.3, Grossman Ch 6 Announcements Problem Set 1 due NW. Mary Beth Kramer Lectureship 101 Brown Laboratory September
Chem 634 tal diated Substitution Chemistry eading: Heg Ch 1 2 (handout), CS-B 7.1, 8.2 8.3, 11.3, Grossman Ch 6 Announcements Problem Set 1 due NW. Mary Beth Kramer Lectureship 101 Brown Laboratory September
A Stille or Suzuki reaction is a good choice for this coupling O O because they are functional group tolerant, no radical chemistry F
 Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Rachel Whittaker Dong Group Literature Talk October 10, Ref: Perez-Temprano, M.H, Casares, J.A., Espinet, P., Chem. Eur. J. 2012, 18, 1864.
 Rachel Whittaker Dong Group Literature Talk October 10, 2013 Ref: Perez-Temprano, M.H, Casares, J.A., Espinet, P., Chem. Eur. J. 2012, 18, 1864. Overview Introduction Group Exchange C-C Bond Forming Reactions
Rachel Whittaker Dong Group Literature Talk October 10, 2013 Ref: Perez-Temprano, M.H, Casares, J.A., Espinet, P., Chem. Eur. J. 2012, 18, 1864. Overview Introduction Group Exchange C-C Bond Forming Reactions
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction Mechanisms. Group Meeting Aaron Bailey 12 May 2009
 Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
12.5 Organometallic Compounds
 12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
Organo-transition Metal Chemistry
 Prof. Dr. Burkhard König, nstitut für rganische Chemie, Universität egensburg 1 rgano-transition tal Chemistry 1. Some Basics Chemistry involves intermediates containing transition-metal carbon bonds tal-carbon
Prof. Dr. Burkhard König, nstitut für rganische Chemie, Universität egensburg 1 rgano-transition tal Chemistry 1. Some Basics Chemistry involves intermediates containing transition-metal carbon bonds tal-carbon
Investigations of Organocuprates
 Investigations of rganocuprates Department of Chemistry University of North Carolina at Charlotte Andy Thomas Rapid injection NMR Why is RINMR so useful? Time intervals between spectra can be modified
Investigations of rganocuprates Department of Chemistry University of North Carolina at Charlotte Andy Thomas Rapid injection NMR Why is RINMR so useful? Time intervals between spectra can be modified
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Sonogashira: in situ, metal assisted deprotonation
 M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Synthetic Methodology. Using Tertiary Phosphines. as Nucleophilic Catalysts
 Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
CEM 852 Final Exam. May 6, 2010
 CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
Mechanism Problem. 1. NaH allyl bromide, THF N H
 Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Development of Chiral Phosphine Olefin Ligands and Their Use in Asymmetric Catalysis
 Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Copper-mediated asymmetric transformations*
 Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Catalytic Reactions in Organic Synthesis
 17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13
 2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming eaction 1/13 Lewis acid B Al Zn Cu etc rganometallics Lewis base -Li -MgX -Na etc + Ate complex Central
2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming eaction 1/13 Lewis acid B Al Zn Cu etc rganometallics Lewis base -Li -MgX -Na etc + Ate complex Central
Enantioselective Borylations. David Kornfilt Denmark Group Meeting Sept. 14 th 2010
 Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
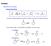 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Synthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives
 ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
Huang, C.; Gevorgyan, V. J. Am. Chem. Soc. 2009, 131, Daniel Tzvi Cohen Short Literature Feb. 23, MeO HO OH. COOH ( )-Plicatic Acid OH OH
 Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Functionalization of C O Bonds. Stefan McCarver. MacMillan Lab Group Meeting
 Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes
 Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Decarboxylation of allylic β-ketoesters
 M.C. White, Chem 253 π-allyl chemistry -224- Week of ovember 8, 2004 Decarboxylation of allylic β-ketoesters Indicate the mechanism of the following transformation: d 2 dba 3 2.5 mol% h 3 10-20 mol% TF,
M.C. White, Chem 253 π-allyl chemistry -224- Week of ovember 8, 2004 Decarboxylation of allylic β-ketoesters Indicate the mechanism of the following transformation: d 2 dba 3 2.5 mol% h 3 10-20 mol% TF,
A Stereoselective Synthesis of (+)-Gonyautoxin 3
 A Stereoselective Synthesis of (+)-Gonyautoxin 3 Mulcahy, J. V.; Du Bois, J. J. Am. Chem. Soc. 2008, 130, 12630-12631 Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via
A Stereoselective Synthesis of (+)-Gonyautoxin 3 Mulcahy, J. V.; Du Bois, J. J. Am. Chem. Soc. 2008, 130, 12630-12631 Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Conjugate (1,4-) addition
 1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
eatles Oasis - 199
 eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
Multicatalyst Promoted Asymmetric Tandem Reactions
 Literature presentation Kishor Mohanan Multicatalyst Promoted Asymmetric Tandem Reactions Features of Tandem Catalysis Reduces the yield losses associated with the purification of intermediates, Save time,
Literature presentation Kishor Mohanan Multicatalyst Promoted Asymmetric Tandem Reactions Features of Tandem Catalysis Reduces the yield losses associated with the purification of intermediates, Save time,
An Analysis of the Total Syntheses of Aphidicolin
 An Analysis of the Total Syntheses of Aphidicolin An Evans Group Afternoon Seminar Krista Beaver March 20, 1998 A B D C Aphidicolin Isolated from the fungus Cephalosporium aphidicola (esp, Chem. Comm.1972,
An Analysis of the Total Syntheses of Aphidicolin An Evans Group Afternoon Seminar Krista Beaver March 20, 1998 A B D C Aphidicolin Isolated from the fungus Cephalosporium aphidicola (esp, Chem. Comm.1972,
MECHANISMS. Croomine. Key reaction is the vinylogous Mannich reaction. (CH 2 ) 4 Br H N P. CO 2 Me. Iminium ion formation via decarboxylation
 MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid
 Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S
![[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S [3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S](/thumbs/94/119475672.jpg) Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Chapter 14. Principles of Catalysis
 Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Nickel-Catalyzed Multicomponent Coupling of Alkynes. -Recent development in methodologies and applications. Zhenjie Lu. Department of Chemistry, MSU
 28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
Problem session (3) Daiki Kuwana. Please fill in the blank and explain reaction mechanisms and stereoselectivities.
 Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
