Prof. F O Shode CHEM 241/ 11. Applied Organic Chemistry for Chemical Engineers. Shode F O 1
|
|
|
- Solomon Warren
- 6 years ago
- Views:
Transcription
1 Prof. F O Shode CHEM 241/ 11 Applied Organic Chemistry for Chemical Engineers Shode F O 1
2 General Introduction Shode F O 2
3 Course Information Course materials T/Bk, Notes, etc Appointment of Class Rep: Practicals: Two groups (A & B) on Thursdays Tests Dates: Friday 11 th March/29 th April 2011 Worksheets Home study (see Course Outline & Contents plus Supplementary Notes to be provided during the course). Lecture Periods: Tuesday (4 th & 5 th ); Friday (7th ) Shode F O 3
4 CHEM 241/ SCHEDULE OF PRACTICALS DATE GROUP PRAC 10/02/11 A Locker Checking 17/02/11 A Prac 1 24/02/11 B Prac 1 3/03/11 A Prac 2A (cancelled) 10/03/11 B Prac 2A 17/03/11 A Prac 2A 24/03/11 B Prac2B/Prac3 31/03/11 A Prac 2B/Prac 3 7/04/11 B Prac 4 14/04/11 A Prac 4 MID-TERM BREAK NO PRAC NO PRAC [21/04/11 2/05/11] 5/05/11 B Prac 5 12/05/11 A Prac 5 Shode F O 4
5 Course Outline Unit 1: Fundamental aspects of organic chemistry - Revision (Chapters 1 & 2). Unit 2: The chemistry of aromatic compounds: Benzene & substituted Benzene (Chapter 14). Unit 3: Chemistry of carbonyl compounds (I& II) (Chapters 16 & 17). Unit 4: Oxidation/Reduction reactions (Chapter 19). Unit 5: Structure elucidation using combined spectroscopic methods MS, IR, UV/Vis & NMR (Chapters 12 & 13) Unit 6: Synthetic Polymers (Chapter 28) Shode F O 5
6 ANY QUESTIONS OR COMMENTS? Shode F O 6
7 What is Chemistry? Chemistry is the study of matter (any substance that occupies space and has mass). The business of chemistry transforms the natural raw materials of the earth, sea and air into products that we use everyday. It creates products that brings major societal benefits to quality of life, health, productivity, convenience and safety. The value chain of the business of chemistry is shown below: Shode F O 7
8 The Value Chain of the Business of Chemistry Chemistry Discovery & Innovation Better Products Social & Economic Benefits from Better Products Improved Quality of Life, Safety & Well- Being Peace of Mind, Security & Enjoyment of Life Shode F O 8
9 Thus, the business of chemistry involves problem solving companies providing solutions to improve the world. It is a science and technology, knowledge-based industry that is key to a sustainable world economy and improved health and nutrition. Shode F O 9
10 The Outline of Chemical Industry Mining Inorganic Raw Materials Organic Raw Materials Oil & Gas Production Heat Air Water Chemical Processing Chemical Building Blocks Shode F O 10
11 Chemical Building Blocks Heat Air Water Chemical Processing Chemical Intermediates & Finished Products Other Manufacturers Consumers Exports Shode F O 11
12 Chemistry combines organic and inorganic materials from the earth, with heat, air, and water to make products useful in modern civilization. Organic chemicals begin with raw materials containing hydrocarbons such as oil, natural gas, and coal. Inorganic chemicals do not contain carbon but are made from ores taken from the earth, such as salt. Shode F O 12
13 Why do we study organic chemistry? What is organic chemistry? Simply, organic chemistry is the chemistry of CARBON and its compounds (except carbonates & its oxides) Several millions of carbon compounds are known. Many organic compounds (and materials made from them) play important roles in our lives. In fact, life without organic compounds is not possible. Shode F O 13
14 Assignment #1 3/Organic-Chemistry.html Shode F O 14
15 "I JUST WANT TO SAY ONE WORD TO YOU PLASTICS." SIGNIFYING THAT PLASTICS WERE THE WAVE OF THE FUTURE, THESE WORDS WERE UTTERED TO DUSTIN HOFFMAN'S CHARACTER IN THE 1967 FILM THE GRADUATE. (The Kobal Collection. Reproduced by permission.) Shode F O 15
16 CHEM 241 Unit 1 Revision of Some fundamental Concepts: [Bruice] Chapters 1 & 2 Shode F O 16
17 Chapter 1: Electronic Structure & Bonding Section Concept Page 1.1 The Structure of an atom Electronic Configuration Ionic & Covalent bonds How a structure of a compound is represented Atomic Orbitals Molecular Orbital Theory (MOT) Shode F O 17
18 Covalent Bonds: Theories, Types, & Theories: Properties Valence-Bond Theory (VBT): Sharing of valence electrons by two atoms to form single bond, double bond, and triple bond. Molecular Orbital Theory (MOT): Covalent bonds are formed by overlapping of atomic orbitals leading to sigma (σ) bond, and pi (π) bonds. VSEPR (valence-shell electron-pair repulsion)model: combines VBT, MOT, and the minimisation of electron repulsion Shode F O 18
19 Hybridization [Bruice: pg 29-35] This concept means that some atoms, prior to form bonds, mix their atomic orbitals to form new equal energy hybrid orbitals with stereochemical consequences. There are three common types of hybridization in Organic Molecules namely: sp 3 hybridization sp 2 hybridization sp hybridization. Shode F O 19
20 Covalent Bond Type (VBT) Single Bond ( ) Double Bond ( ) Triple Bond ( ) Molecular Orbitals(MOT) σ(bonding) and σ* (anti-bonding) σ(bonding) + π(bonding) and σ*(antibonding) + π* (anti-bonding) σ(bonding) + 2 x π(bonding) + 2 x π* (anti-bonding) + σ* (anti-bonding) Shode F O 20
21 Continuum of Bond Types Electronegativity differences Shode F O 21
22 Understanding bond polarity is critical to understanding how organic reactions occur: RULE - electron-rich atoms/molecules are attracted to electron-deficient atoms/molecules Shode F O 22
23 Bond Properties: Revise Bond length Bond angle Bond energies Bond rotation Shode F O 23
24 Simple Molecular Structures Space-filling MODELS Shode F O 24
25 Representation of Molecular Structures Electron-Dot formula (Lewis structures) Valence-Bond formula (Kekulé structures) Condensed formula Bond-Line formula Shode F O 25
26 Assignment 1 1. Problem #20 (Bruice, p28) 2. What is the hybridization of each of the carbon and oxygen atoms in the following compounds? HO OH O O H 3 C N O CH 3 N HO OH O N N vitamin C CH 3 caffeine Shode F O 26
27 Chemistry of Aromatic Compounds: Benzene & Substituted Benzene UNIT 2 Shode F O 27
28 Introduction BENZENE[CAS Number: ] It is a high volume chemical with production exceeding 1 million pounds annually in the U.S. Shode F O 28
29 The History of Benzene Benzene was discovered in 1825 by the English scientist Michael Faraday, who isolated it from oil gas and gave it the name bicarburet of hydrogen. In 1833, the German chemist Eilhard Mitscherlich produced it via the distillation of benzoic acid (from gum benzoin) and lime. Mitscherlich gave the compound the name benzin. Molecular structure deduced in 1834 by Mitcherlich In 1845, the English chemist Charles Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Shode F O 29
30 Introduction: Which industries use Benzene Laboratory Chemicals Pesticide Mfg (Herbicides) Pesticide Mfg (Insecticides) Pharmaceuticals Mfg Printing Pulp and Paper Manufacture How is it used? Solvents - Dilution Solvents - Herbicide Manufacture Solvents - Insecticide Manufacture Solvents - Pharmaceuticals Solvents for Equipment Cleaning Solvents for de-inking paper Wood Stains and Varnishes Vanish Solvents Shode F O 30
31 Chemistry of benzene Physical properties: It is a colourless liquid, bp 80 o C (mp 5 o C). It is highly toxic! It has a sweetsmelling aroma (a fragrant liquid). Shode F O 31
32 Structure of Benzene Molecular Formula: C 6 H 6 It is highly unsaturated. [Compare alkanes, alkenes, & alkynes]. It does not react like alkenes & alkynes. [No addition reaction products] In 1858, August Kekulé proposed a cyclic structure for benzene based on a supernatural revelation: Shode F O 32
33 Structure of Benzene Cont d H H H H H H This will imply simply alternating C-C and C=C bonds (1.47Å & 1.34Å) Shode F O 33
34 The Grandfathers of benzene chemistry Shode F O 34
35 The Kekulé Model August Kekule proposed a rapid oscillation (I & II) between the 3 C=C and the 3 alternating C-C of hexagonal benzene (C 6 H 6 ). Shode F O 35
36 Flaws in Kekulé s Model Why does benzene possess 150 kj/mol less energy than predicted? Shode F O 36
37 2. Addition versus Substitution: Compounds with C=C (alkenes) typically undergo addition reactions such as bromination. Shown below are reactions of ethene/bromine and benzene/bromine: Shode F O 37
38 3. X-Ray studies revealed the following bond lengths: 0.153nm for typical C-C, 0.134nm for typical C=C, and 0.139nm for the 6 carbon- carbon bonds in benzene. Why does benzene possess 6 bonds intermediate between C=C and C-C? Shode F O 38
39 Resonance Theory Resonance To help explain molecules like benzene, Linus Pauling proposed resonance theory in 1931 (Pauling also gave us hybrid orbitals, electronegativity, and valence bond theory). As a result of Pauling's resonance, benzene is viewed as a hybrid of III& IV and represented by V. Shode F O 39
40 Shode F O 40
41 The six carbons are arranged in a hexagon with one hydrogen atom attached to each carbon. The 12 atoms of benzene are planar with carbon in the sp2 hybrid state and 120 bond angles. Because the six bond lengths are equivalent, Kekule's rapid equilibrium must be ruled out. To describe benzene we need to use contributingstructures III & IV or one structure with a circle in the middle (V). Structures III &IV do not exist! Whatever benzene might be, it displays characteristics of its contributing structures. For an analogy, consider crossing a bloodhound with a greyhound: Although the offspring is unique, it displays characteristics (smell& speed) of its parents--it does not flip flop between a greyhound one instant and a bloodhound the next. Shode F O 41
42 Bloodhound Greyhound Shode F O 42
43 Why does benzene possess 150 kj/mol less energy than predicted? This missing energy, called resonance energy, is attributed to overlapof p- orbitals. Structures VI and VII show pi bonding for the contributing structures while VIII illustrates delocalizationof pi electrons over the 6 carbon atoms. Shode F O 43
44 Benzene can be represented as IX using molecular models with p-orbitals. The circle in the middle of V is an abbreviated way to represent the delocalization of the 6 pi electrons. Resonance energy is the difference in energies between III (or IV) and V(V has lower energy). Due to resonance, benzene is 150 kj/mol more stable than calculations would predict. Shode F O 44
45 Why does benzene resist addition and prefer substitution? If benzene were to undergo addition, resonance would be disrupted and this would render the structure less stable. Therefore benzene prefers to substitute (Br for H) and maintain resonance. Why does benzene possess 6 bonds intermediate between C=C and C-C? According to resonance, the bonds are not C-C or C=C but a hybrid of the two. X-ray studies confirm this with the intermediate bond lengths. Shode F O 45
46 Aromaticity: Reactions of Benzene For a compound to be aromatic, it must obey the following criteria: 1. The molecule must be cyclic. 2. The molecule must be planar. 3. It must contain (4n + 2) π electrons, called Huckel s rule.[n = 0,1,2,3, etc] Why? This results in an uninterrupted cyclic cloud of π electrons, a characteristic feature of aromatic compounds. π bonding results from the overlap of p orbitals. Shode F O 46
47 Why are the following molecules aromatic? N H O N Benzene Napthalene Pyridine Pyrrole Furan Shode F O 47
48 Benzene:planar, cyclic, 4n + 2 πe - s where n = 1 Naphthalene:cyclic, planar, 4n + 2πe - s where n = 2. Pyridine:cyclic, planar, 4n + 2πe - s (n = 1), lone pair on N is non-bonding. Pyrrole:cyclic, planar, 4n + 2πes - (n = 1), lone pair on N involved in p orbital overlap. Furan: cyclic, planar, 4n + 2πe - s (n = 1), one lone pair on O involved in p orbital overlap and the other is nonbonding. Shode F O 48
49 Why are the following molecules not aromatic? cyclobutadiene cycloheptatriene cyclopropenyl anion Cyclobutadiene: not 4n + 2 π e - s cycloheptatriene: has an sp 3 carbon atom, not planar cyclopropenyl anion: not 4n + 2 π e - s Shode F O 49
50 Exercises: Are the following compounds aromatic or non-aromatic? H C + H C:- Cyclopentadiene Cyclopentadienyl cation Cyclopentadienyl anion Shode F O 50
51 Reactivity considerations Why does the aromatic ring undergo electrophilic substitution reactions rather than electrophilic addition reactions? An electrophile (Y + ) is attracted to the π electrons. When it attaches itself, a carbocation intermediate results. H + Y + Y carbocation intermediate This carbocation intermediate can follow two routes. What are they? Shode F O 51
52 + Y + H Y Z - Z Y r carbocation intermediate a non-aromatic product of electrophilic addition H + Y + Y Z - Y a carbocation intermediate an aromatic product of electrophilic substitution The non-aromatic product is much less stable that the aromatic product. Benzene undergoes electrophilic substitution reactions that preserve aromatic stabilisation. Shode F O 52
53 The five most common electrophilic aromatic substitution reactions are: 1. Halogenation 2. Nitration 3. Sulphonation + Y + 4. Friedel-Crafts acylation 5. Friedel-Crafts alkylation H carbocation intermediate They take place by the same two step mechanism. Y Z - Y an aromatic product of electrophilic substitution They differ only in how the electrophile (Y + ) that is needed to start the reaction is generated. Shode F O 53
54 Halogenation of benzene bromination + Br 2 FeBr 3 Br chlorination + Cl 2 FeCl 3 bromobenzene Cl iodination I 2 HNO 3 2 I + chlorobenzene + I + I iodobenzene Shode F O 54
55 Mechanism for bromination Br Br + FeBr 3 Br Br FeBr 3 + Br Br FeBr 3 Lewis acid weakens Br-Br bond H Br + FeBr 4 - B Br + HB + Mechanism for chlorination (replace Br for Cl above) Mechanism for iodination I 2 oxidising agent 2 I e - + I + H I B I + HB + Shode F O 55
56 Nitration of benzene HNO 3 NO 2 H 2 SO 4 Mechanism H HO NO 2 + H OSO 3 H HO nitric acid nitrobenzene NO + 2 NO 2 + H 2 O nitronium ion + NO 2 + NO 2 H B NO 2 + HB + Shode F O 56
57 Sulphonation of Benzene SO 3 H + H 2 SO 4 + H 2 O O O O H O HO S OH + HO S OH HO S OH + - O S OH O O O O O SO 3 + H 2 O HO S + H 2 O O HO 3 S H B SO 3 H + SO 3 H + HB + Shode F O 57
58 Desulphonation SO 3 H H 3 O + / 100 o C + SO 3 + H + Mechanism SO 3 H + H + SO 3 H H + SO 3 H Shode F O 58
59 FRIEDEL-CRAFTS ACYLATION OF BENZENE R O C acyl group O O + R C Cl acyl chloride 1. AlCl 3 2. H 2 O R + HCl O + O R C O O C R 1. AlCl 3 2. H 2 O R + HCl acid anhydride Shode F O 59
60 Mechanism for Friedel Crafts Acylation O H 3 C C Cl AlCl 3 H 3 C C O H 3 C C O acylium ion + AlCl 4 - H B O C O CH 3 C + H 3 C C O CH 3 + HB + Shode F O 60
61 Friedel Crafts Alkylation + RCl AlCl 3 R + HCl Mechanism R = alkyl group CH 3 CH 2 Cl + AlCl 3 CH 3 CH AlCl CH 3 CH 2 + CH 2 CH 3 H B CH 2 CH 3 + HB + Shode F O 61
62 Stability of carbocations: Tertiary > Secondary > Primary + CH 3 CH 2 CH 2 CH 2 Cl CH 3 CH 2 CHCH 2 H 1-chlorobutane AlCl 3 0 o C 1,2 hydride shift CH 3 CH 2 CHCH 3 CH 3 + CH 3 CCH 2 Cl AlCl 3 2-phenylbutane rearranged alkyl subsituent 65% CH 3 CHCH 2 CH 3 CH 3 C + CH 3 CH 2 CH 3 1-phenylbutane unrearranged alkyl subsituent 35% + CH 2 CH 2 CH 2 CH 3 CH 3 CH 2 CCH 3 CH 3 CH 3 100% rearranged 0% unrearranged Reason: 1,2-methyl shift CH 3 CH 3 CH 3 C CH 2 CH 3 C CH 2 CH 3 CH 3 Shode F O 62
63 What can be done about this problem? Answer: Acylation followed by reduction. O + CH 3 CH 2 CH 2 CCl AlCl 3 CCH 2 CH 2 CH 3 O reduction CH 2 CH 2 CH 2 CH 3 Two methods of reduction: Clemmenson reduction: Zn(Hg), HCl, heat Wloff-Kishner reduction: H 2 NNH 2, OH -, heat Shode F O 63
BENZENE AND AROMATIC COMPOUNDS
 BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
Benzene and Aromatic Compounds
 1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES Dr Ali El-Agamey. Organic Chemistry, 7 th Edition L. G. Wade, Jr.
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES 11-12 Dr Ali El-Agamey Organic Chemistry, 7 th Edition L. G. Wade, Jr. Amines 2010, Prentice Hall Reactions N,N-Disubstituted amides 2 o amine
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES 11-12 Dr Ali El-Agamey Organic Chemistry, 7 th Edition L. G. Wade, Jr. Amines 2010, Prentice Hall Reactions N,N-Disubstituted amides 2 o amine
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Organic Mechanisms 1
 Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Fundamentals of Organic Chemistry
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
Frost Circles a Great Trick
 Aromatics Frost Circles a Great Trick Inscribe a polygon of the same number of sides as the ring to be examined such that one of the vertices is at the bottom of the ring The relative energies of the MOs
Aromatics Frost Circles a Great Trick Inscribe a polygon of the same number of sides as the ring to be examined such that one of the vertices is at the bottom of the ring The relative energies of the MOs
4. AROMATIC COMPOUNDS
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
11/26/ Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds
 9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
Electrophilic Aromatic Substitution. Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi
 Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Chapter 4: Aromatic Compounds. Bitter almonds are the source of the aromatic compound benzaldehyde
 Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or
 Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
Treatment of cyclooctatetrene with potassium gives you a dianion. Classify the starting material and product as aromatic, antiaromatic or nonaromatic? 1 2 Classify cyclononatetrene and it s various ions
Organic Chemistry, 7 L. G. Wade, Jr. 2010, Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 16 Aromatic Compounds 2010, Prentice Hall Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized
More Nomenclature: Common Names for Selected Aromatic Groups. Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings.
 More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine.
 The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Benzenes & Aromatic Compounds
 Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Benzene and Aromaticity
 Benzene and Aromaticity Why this Chapter? Reactivity of substituted aromatic compounds is tied to their structure Aromatic compounds provide a sensitive probe for studying relationship between structure
Benzene and Aromaticity Why this Chapter? Reactivity of substituted aromatic compounds is tied to their structure Aromatic compounds provide a sensitive probe for studying relationship between structure
Introduction to Aromaticity
 Introduction to Aromaticity Historical Timeline: 1 Spotlight on Benzene: 2 Early 19 th century chemists derive benzene formula (C 6 H 6 ) and molecular mass (78). Carbon to hydrogen ratio of 1:1 suggests
Introduction to Aromaticity Historical Timeline: 1 Spotlight on Benzene: 2 Early 19 th century chemists derive benzene formula (C 6 H 6 ) and molecular mass (78). Carbon to hydrogen ratio of 1:1 suggests
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Aromatic Compounds I
 2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Electrophilic Aromatic Substitution
 Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Organic Chemistry SL IB CHEMISTRY SL
 Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
Plymstock School. Arenes. P.J.McCormack
 Plymstock School 1 A2 Chemistry F324: Rings, Polymers & Analysis 4.1.1 - Arenes Arenes P.J.McCormack 2 4.1.1 Arenes Objective Checklist Draw the structure of benzene Explain the terms arene and aromatic
Plymstock School 1 A2 Chemistry F324: Rings, Polymers & Analysis 4.1.1 - Arenes Arenes P.J.McCormack 2 4.1.1 Arenes Objective Checklist Draw the structure of benzene Explain the terms arene and aromatic
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Chem!stry. The Chemistry of Benzene C 6H 6 Macroconcept Models
 Chem!stry Name: ( ) Class: Date: / / The Chemistry of Benzene C 6H 6 Macroconcept Models This booklet covers essential information concerning the chemistry of benzene, including its structure, bonding
Chem!stry Name: ( ) Class: Date: / / The Chemistry of Benzene C 6H 6 Macroconcept Models This booklet covers essential information concerning the chemistry of benzene, including its structure, bonding
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Chapter 17 Reactions of Aromatic Compounds
 rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
Upon successful completion of this course, the student will be able to:
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
12/27/2010. Chapter 14 Aromatic Compounds
 Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Q.1 Draw out suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
6.1.1 Aromatic Compounds
 6.1.1 Aromatic ompounds There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon ams with delocalised
6.1.1 Aromatic ompounds There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon ams with delocalised
Arenes occur naturally in many substances, and are present in coal and crude oil. Aspirin, for example, is an aromatic compound, an arene: HO
 Naming Aromatic compounds contain one or more benzene rings (while aliphatic compounds do not contain benzene rings). Another term for a compound containing a benzene ring is arene. The basic benzene ring,
Naming Aromatic compounds contain one or more benzene rings (while aliphatic compounds do not contain benzene rings). Another term for a compound containing a benzene ring is arene. The basic benzene ring,
Electrophilic Aromatic Substitution
 Chem 263 Sept 29, 2016 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable (36 kcal/mole more) and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes,
Chem 263 Sept 29, 2016 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable (36 kcal/mole more) and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes,
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
BIOB111 - Tutorial activities for session 8
 BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
08. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16
 08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
08. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 6 th edition, Chapter 16 Benzene is a nucleophile p electrons make benzene nucleophile, like alkenes.
Module III: Aromatic Hydrocarbons (6 hrs)
 Module III: Aromatic Hydrocarbons (6 hrs) Nomenclature and isomerism in substituted benzene. Structure and stability of benzene: Kekule, resonance and molecular orbital description. Mechanism of aromatic
Module III: Aromatic Hydrocarbons (6 hrs) Nomenclature and isomerism in substituted benzene. Structure and stability of benzene: Kekule, resonance and molecular orbital description. Mechanism of aromatic
IGNORE Just benzene has a delocalised ring Benzene does not have C=C double bonds Any references to shape/ bond angles. Acceptable Answers Reject Mark
 1(a) All carbon to carbon bonds same length/ longer C-C and shorter C=C not present 1 IGNORE Just benzene has a delocalised ring Benzene does not have C=C double bonds Any references to shape/ bond angles
1(a) All carbon to carbon bonds same length/ longer C-C and shorter C=C not present 1 IGNORE Just benzene has a delocalised ring Benzene does not have C=C double bonds Any references to shape/ bond angles
Chapter 16 Aromatic Compounds. Discovery of Benzene
 Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C: ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C: ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Unit 1 Module 1 Forces of Attraction page 1 of 10 Various forces of attraction between molecules
 Unit 1 Module 1 Forces of Attraction page 1 of 10 Various forces of attraction between molecules 1. Ionic bonds 2. Covalent bonds (also co-ordinate covalent bonds) 3. Metallic bonds 4. Van der Waals forces
Unit 1 Module 1 Forces of Attraction page 1 of 10 Various forces of attraction between molecules 1. Ionic bonds 2. Covalent bonds (also co-ordinate covalent bonds) 3. Metallic bonds 4. Van der Waals forces
Benzene and aromaticity
 aromaticity The word "benzene" derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 15th century
aromaticity The word "benzene" derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 15th century
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Benzene a remarkable compound. Chapter 14 Aromatic Compounds. Some proposed structures for C 6 H 6. Dimethyl substituted benzenes are called xylenes
 Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Give the major product(s) of the following reaction.
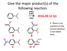 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
3.10 Benzene : Aromatic Hydrocarbons / Arenes
 3.10 Benzene : Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six
3.10 Benzene : Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB
 SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
AROMATIC CHEMISTRY BENZENE
 AROMATIC CHEMISTRY BENZENE Definition of AROMATIC/ALIPHATIC?? The structure of benzene Percentage composition by mass gave the empirical formula as C 6 H 6, which led Kekulé to suggest the Structure shown.
AROMATIC CHEMISTRY BENZENE Definition of AROMATIC/ALIPHATIC?? The structure of benzene Percentage composition by mass gave the empirical formula as C 6 H 6, which led Kekulé to suggest the Structure shown.
2. Examining the infrared spectrum of a compound allows us to:
 CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
Copyright McGraw-Hill Education. Permission required for reproduction or display : A force that holds atoms together in a molecule or compound
 : Chemical Bonding 8-1 8.1 Types of Bonds : A force that holds atoms together in a molecule or compound Two types of chemical bonds Ionic Bonds Covalent Bonds 8-2 1 8.1 Types of Bonds 8-3 8.1 Types of
: Chemical Bonding 8-1 8.1 Types of Bonds : A force that holds atoms together in a molecule or compound Two types of chemical bonds Ionic Bonds Covalent Bonds 8-2 1 8.1 Types of Bonds 8-3 8.1 Types of
Chapter 16. Aromatic Compounds
 Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Chapter 17 Aromati ti S u stit tit t u i tion Reactions
 Chapter 17 Aromatic Substitution Reactions 1 17.1 Mechanism for Electricphilic Aromatic Substitution Arenium ion resonance stabilization 2 Example 1. Example 2. 3 Example 2. Mechanism of the nitration
Chapter 17 Aromatic Substitution Reactions 1 17.1 Mechanism for Electricphilic Aromatic Substitution Arenium ion resonance stabilization 2 Example 1. Example 2. 3 Example 2. Mechanism of the nitration
Chapter 17: Reactions of Aromatic Compounds
 1 Chapter 17: Reactions of Aromatic Compounds I. Introduction to Electrophilic Aromatic Substitution (EAS) A. General Mechanism II. Reactions of Electrophilic Aromatic Substitution A. Halogenation (E =
1 Chapter 17: Reactions of Aromatic Compounds I. Introduction to Electrophilic Aromatic Substitution (EAS) A. General Mechanism II. Reactions of Electrophilic Aromatic Substitution A. Halogenation (E =
Hour Examination # 1
 CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Aromatic Hydrocarbons / Arenes
 Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon atoms
Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon atoms
Carbon Compounds. Electronegativity. Chemical Bonding Part 1c. Bond Polarity. Bond Polarity
 Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Chapter 8 Chemical Bonding
 Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
Theoretically because there are 3 double bonds one might expect the amount of energy to be 3 times as much.
 18. Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon ams with delocalised bonding.
18. Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six carbon ams with delocalised bonding.
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
How Does Molecular Orbital Theory Explain Ionic And Covalent Bonding
 How Does Molecular Orbital Theory Explain Ionic And Covalent Bonding Valence bond and molecular orbital theories are used to explain chemical bonding. According to VB theory, a covalent bond forms from
How Does Molecular Orbital Theory Explain Ionic And Covalent Bonding Valence bond and molecular orbital theories are used to explain chemical bonding. According to VB theory, a covalent bond forms from
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives
 C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
CHEMISTRY Matter and Change Section 8.1 The Covalent Bond
 CHEMISTRY Matter and Change Section Chapter 8: Covalent Bonding CHAPTER 8 Table Of Contents Section 8.2 Section 8.3 Section 8.4 Section 8.5 Naming Molecules Molecular Structures Molecular Shapes Electronegativity
CHEMISTRY Matter and Change Section Chapter 8: Covalent Bonding CHAPTER 8 Table Of Contents Section 8.2 Section 8.3 Section 8.4 Section 8.5 Naming Molecules Molecular Structures Molecular Shapes Electronegativity
ALKYNE, AROMATIC HYDROCARBONS OR ARENES ALKYNES. Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C)
 ALKYNES Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C) Their general formula is Cn H2n-2 They are also called as a acetylenes STRUCTURE OF ALKYNES Each
ALKYNES Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C) Their general formula is Cn H2n-2 They are also called as a acetylenes STRUCTURE OF ALKYNES Each
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
Chapter 5. Aromatic Compounds
 Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chemistry 52 Exam #1. Name: 22 January This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages.
 Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
18.1 Intro to Aromatic Compounds
 18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
Topic 10 Organic Chemistry. Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School
 Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
What Is Organic Chemistry?
 What Is Organic Chemistry? EQ: What is Organic Chemistry? Read: pages 1-3 Answer the questions in your packet Basics of Organic Chem 1 Chapter 1: Structure and Bonding Key terms Organic Chemistry Inorganic
What Is Organic Chemistry? EQ: What is Organic Chemistry? Read: pages 1-3 Answer the questions in your packet Basics of Organic Chem 1 Chapter 1: Structure and Bonding Key terms Organic Chemistry Inorganic
