Nomenclature of Tocopherols and Related Compounds
|
|
|
- Sheena Strickland
- 6 years ago
- Views:
Transcription
1 Page 1 of 6 INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY and INTERNATIONAL UNION OF BIOCHEMISTRY AND MOLECULAR BIOLOGY IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of Tocopherols and Related Compounds (Recommendations 1981) World Wide Web version prepared by G. P. Moss Department of Chemistry, Queen Mary University of London, Mile End Road, London, E1 4NS, UK g.p.moss@qmul.ac.uk These Rules are as close as possible to the published version [see Arch. Biochem. Biophys., 1982, 218, ; Eur. J. Biochem., 1982, 123, ; Mol. Cell. Biochem., 1982, 49, ; Pure Appl. Chem., 1982, 54, ; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages ; Copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. Any comments should be sent to any member of the Committee Important Note: This version is formatted using the font symbol for Greek letters. If you cannot see a Delta (a triangle) in quotation marks next " " click here for a version where Greek letters are created using graphic images. The structure of natural α-tocopherol, the most potent natural source of vitamin E activity, was elucidated in At the same time the first synthesis of a biologically active product, consisting of a mixture of the natural diastereoisomer and its 2-epimer, was reported. Work in the following years revealed the existence in nature of a whole family of structurally related compounds with qualitatively identical biological action. Recommendations for the nomenclature of the vitamins, including the tocopherols, were published in 1966 [1]; in that document the configuration of the natural α-tocopherol, 2R,4'R,8'R, is mentioned in a footnote, followed by the sentence 'The designation of other stereoisomers is under consideration'. The work of Isler and his co-workers [2-5] provided a description of the configuration of all compounds of this class, but it proved difficult to devise a system for their nomenclature, because some of the important tocopherols are mixtures of diastereoisomers. Nevertheless recommendations, based on drafts prepared by W. Klyne and O. Hoffmann-Ostenhof after wide consultations, were published in 1974 [6], to replace section M-3 of the 1966 recommendations [1], and these use the RS system for designating stereoisomers [8]. The present recommendations take into account points raised about the previous ones [6]; although they contain no new recommendations they explain more fully the relation of present
2 Page 2 of 6 designations (especially on stereochemistry in section 12) to older ones. They incorporate agreements reached in 1976 between representatives of the predecessor of JCBN and of the Commision 1/I of the International Union of Nutritional Sciences (IUNS); they are in agreement with the IUNS Recommendations of 1976 [10]. RECOMMENDATIONS 1.1. Vitamin E. The term vitamin E should be used as the generic descriptor for all tocol and tocotrienol derivatives exhibiting qualitatively the biological activity of α-tocopherol. This term should be used in derived terms such as vitamin E deficiency, vitamin E activity, vitamin E antagonist Tocol. The term tocol is the trivial designation for 2-methyl-2-(4,8,12-trimethyltridecyl)chroman-6-ol (I, R 1 = R 2 = R 3 = H) 1.3. Tocopherol(s). The term tocopherol(s) should be used as a generic descriptor for all mono, di, and trimethyltocols. Thus, this term is not synonymous with the term vitamin E. 2. Compound I (R 1 = R 2 = R 3 = Me), known as α-tocopherol, is designated α-tocopherol (note 1) or 5,7,8-trimethyltocol. For the designation of the configuration of α-tocopherol see Recommendations 11 and 12 (note 2). 3. Compound I (R 1 = R 3 = Me; R 2 = H), known as, β-tocopherol, is designated, β-tocopherol (note 1) or 5,8-dimethyltocol. 4. Compound I (R 1 = H; R 2 = R 3 = Me), known as γ-tocopherol, is designated γ-tocopherol (note 1) or 7,8-dimethyltocol. 5. Compound I (R 1 = R 2 = H; R 3 = Me), known as δ-tocopherol, is designated δ-tocopherol (note 1) or 8-methyltocol (note 3). 6. Compound II (R 1 = R 2 = R 3 = H), 2-methyl-2-(4,8,12-trimethyltrideca-3,7,11-trienyl)chroman-6-ol, is designated tocotrienol [only the all-trans (E,E)-tocotrienol has been found occurring in nature].
3 Page 3 of 6 7. Compound II (R 1 = R 2 = R 3 = Me), formerly known as ζ 1 or ζ 2 -tocopherol, is designated 5,7,8- trimethyltocotrienol (note 3) or α-tocotrienol (note 1). The name tocochromanol-3 (cf. paragraph 3.2 in [11]) has also been used. 8. Compound II (R 1 = R 2 = Me; R 2 = H), formerly known as ε-tocopherol, is designated 5,8- dimethyltocotrienol (note 3) or β-tocotrienol (note 1). 9. Compound II (R 1 = H; R 2 = R 3 = Me), formerly known as η-tocopherol, is designated 7,8- dimethyltocotrienol (note 3) or γ-tocotrienol (note 1). The name plastochromanol-3 (cf. paragraph 3.2 in [11]) has also been used. 10. Compound II (R 1 = R 2 = H; R 3 = Me) is designated 8-methyltocotrienol (note 3) or δ-tocotrienol (note 1). 11. The only naturally occurring stereoisomer of α-tocopherol hitherto discovered (III) has the configuration 2R,4'R,8'R according to the sequence-rule system [8]. Its semisystematic name is therefore (2R,4'R,8'R)-α-tocopherol. The same system can be applied to all other individual stereoisomers of tocopherols. 12. Trivial designations are sometimes desirable to indicate briefly the configuration of important stereoisomers of α-tocopherol and especially of mixtures of such stereoisomers. Some of these materials are of considerable commercial and therapeutic importance. The use of the following trivial designations for the most important materials of this class is recommended. a) The above-mentioned α-tocopherol with the configuration 2R,4'R,8'R, formerly known as d-αtocopherol, should be called RRR-α-tocopherol. b) The diastereoisomer of RRR-α-tocopherol, formerly known as l-α-tocopherol, being the epimer of RRR-α-tocopherol at C-2 with the configuration 2S,4'R,8'R, should be called 2-epi-α-tocopherol. c) A mixture of RRR-α-tocopherol and 2-epi-α-tocopherol (obtained by synthesis using phytol (note 4) and the appropriate achiral hydroquinone derivative), should be called 2-ambo-α-tocopherol (notes 5 and 6): this mixture was formerly known as dl-α-tocopherol until the optical activity of phytol was recognized when dl-α-tocopherol was restricted to all-rac-α-tocopherol (see 12e). It is probable that the
4 Page 4 of 6 asymmetric reaction involved in this partial synthesis would only by chance lead to the formation of equimolar proportions of the two possible epimers [12]. However, 2-ambo-α-tocopherol obtained by this method closely approaches equimolar proportions [13-16]. The acetate of 2-ambo-α-tocopherol acetate) was the former international standard vitamin E activity [17]. d) The reduction product of natural 5,7,8-tocotrienol, in which the double bonds at 3', 7' and 11' are hydrogenated and two new asymmetric centres are created at C-4' and C-8', is a mixture in unspecified proportions of four diastereoisomeric α-tocopherols, having the configurations 2R,4'R,8'R: 2R,4'S,8'R; 2R,4'S,8'S; and 2R,4'R,8'S. The material should be called 4'-ambo,8'-ambo-α-tocopherol e) The totally synthetic vitamin E, obtained without any control of stereochemistry, is a mixture in unspecified proportions (in preparations examined the proportions closely approached equimolar [13-16]) of four racemates or pairs of enantiomers (i.e. eight diastereoisomers). It should be called all-rac-αtocopherol (it was formerly known as dl-α-tocopherol, although this designation was previously used for 2-ambo-α-tocopherol, see 12c). 13. Esters of tocopherols and tocotrienols should be called tocopheryl esters and tocotrienyl esters, respectively (e.g. α-tocopheryl acetate, α-tocotrienyl acetate). Recommended names are listed in the Table. Table 1. List of the trivial names for some α-tocopherols Description of material Configuration Recommended trivial name 1. The compound having the configuration shown in the next column, exemplified by the only isomer of α-tocopherol yet found in nature 3. α-tocopherol with the natural configuration at C-4' and C-8' but both configurations at C-2, such as may be obtained semisynthetically from phytol (note 4) 4. Synthetic α-tocopherol where there has been no control of configuration at C-2, C- 4' or C-8' 5. Synthetic α-tocopherol with the natural configuration at C-2, but both configurations at C-4' and C-8' 2R,4'R,8'R 2S,4'R,8'R 2R,4'R,8'R and 2S,4'R,8'R a mixture, not necessarily equimolar a mixture, not necessarily equimolar, of all four possible racemates (i.e. of all the four pairs of enantiomers) a mixture, not necessarily equimolar, of the four isomers 2R,4'R,8'R; 2R,4'S,8'R; 2R,4'R,8'S; 2R,4'S,8'S RRR-α-tocopherol 2. The isomer epimeric only at C-2 with RRRα-tocopherol 2-epi-αtocopherol 2-ambo-αtocopherol (note 4) all-rac-αtocopherol 4'-ambo-8'-amboα-tocopherol
5 Page 5 of 6 Notes References 1. Recommended for nutritional usage by IUNS [10]. 2. The name α-tocopherol should never be used without a stereochemical designation when referring to a specific compound. 3. Recommended for chemical usage. 4. The name 'phytol' designates the suhstance with 7R,11R-configuration. 5. From 'ambo', Latin for 'both'. 6. A more complete name would be 2-ambo-(4'R,8'R)-α-tocopherol but in order to keep trivial names short it is to be assumed that the configuration at each centre in the tocopherols is R unless stated otherwise. The term α-tocopherol, by itself a generic descriptor without stereochemical implication, thus obtains a stereochemical meaning when preceded by a stereochemical prefix like ambo or epi. 1. IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Tentative rules, section on Trivial names of miscellaneous compounds of importance in biochemistry. 1964, Arch. Biochem. Biophys. 118, (1967), Biochem. J. 102, (1967), Biochem. Biophys. Acta, 107, l-4 (1965), Bull. Soc. Chim. Biol. (in French) 44, (1967), Eur. J. Biochem. 2, l-2 (1967), Hoppe-Seyler's Z. Physiol. Chem. (in German) 348, (1967). IUPAC Inf. Bull. 25, (1966), and J. Biol. Chem. 241, (1966). 2. Schudel. P., Mayer, H., Metzger. J., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta, 46, Schudel. P., Mayer, H., Metzger, J., Ruegg. R. & Isler, O. (1963) Helv. Chim. Acta, 46, Mayer, H., Schudel, P., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta, 46, Mayer, H., Schudel, P., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta. 46, IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Nomenclature of tocopherols and related compounds. Recommendations 1973, Arch. Biochem. Biophys. 165, 6-8 (1974), Biochem. J. 147, (1975), Eur. J. Biochem. 46, (1974), and IUPAC Inf. Bull., Append. Provisional Nomencl. Symb. Units Stand. No. 47 (1975); also on pages in [7]. 7. International Union of Biochemistry (1978) Biochemical Nomenclature and Related Documents, The Biochemical Society, London. [Now as the 1992 edition] 8. IUPAC Commission on Nomenclature of Organic Chemistry (1976) Rules for the nomenclature of organic chemistry, Section E: Stereochemistry (Recommendations 1974) Pure Appl. Chem. 45, 11-30; also on pages 1-18 in [7] and section E in [9]. 9. International Union of Pure and Applied Chemistry (1979) Nomenclature of Organic Chemistry,
6 Page 6 of 6 Sections A, B, C, D, E, F and H, Pergamon Press, Oxford. 10. International Union of Nutritional Sciences, Committee 1/I. Nomenclature (1978) Nutr. Abstr. Rev. 48A, IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Nomenclature of quinones with isoprenoid side-chains, Recommendations 1973, Arch. Biochem. Biophys. 165, 1-5 (1974), Biochem. J. 147, (1975), Biochim. Biophys. Acta, 387, (1975), Eur. J. Biochem. 53, (1975), and Pure Appl. Chem. 38, (1974); also on pages in [7]. 12. Morrison, J. D. & Mosher, H. S. (1971) Asymmetric Organic Reactions, Prentice Hall, Englewood Cliffs, New Jersey. 13. Ames, S. R. (1979) J. Nutr. 109, Slover, H. T. & Thompson. R. H., Jr (1981) Lipids, 16, Weiser, H. & Vecchi, M. (1981) Int. J. Vitam. Nutr. Res. 51, Cohen, N., Scott, C. G., Neukom, C., Lopresti, R. J., Weber, G. & Saucy, G. (1981) Helv. Chim. Acta. 64, Hume, E. M. (1941) Nature (Lond.) 148, ; see also World Health Organ. Tech. Rep. Ser. 259, 58 (1963). Return to main IUBMB Biochemical Nomenclature home page Return to main IUPAC Chemical Nomenclature home page Return to Biochemical Nomenclature Committees home page
FOR FIVE- AND SIX-MEMBERED RING FORMS OF MONOSACCHARIDES AND
 Pure & Appi. Chum., Vol.53, pp.1901 1905. 00334545/81/10190105$02.00/0 Printed in Great Britain. Pergamon Press Ltd INTERNATIONAL UNION OF PURE AND APPLIED CEMISTRY and PROVISIONAL INTERNATIONAL UNION
Pure & Appi. Chum., Vol.53, pp.1901 1905. 00334545/81/10190105$02.00/0 Printed in Great Britain. Pergamon Press Ltd INTERNATIONAL UNION OF PURE AND APPLIED CEMISTRY and PROVISIONAL INTERNATIONAL UNION
10/4/2010. Sequence Rules for Specifying Configuration. Sequence Rules for Specifying Configuration. 5.5 Sequence Rules for Specifying.
 5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
Nomenclature of Retinoids
 Eur. J. Biochem. 129, 1-5 (1982) f-3 FEBS 1982 IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of Retinoids Recommendations 1981 CONTENTS Preamble Ret-1 1.1 Ret-2 2.1 2.2 2.3
Eur. J. Biochem. 129, 1-5 (1982) f-3 FEBS 1982 IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of Retinoids Recommendations 1981 CONTENTS Preamble Ret-1 1.1 Ret-2 2.1 2.2 2.3
Organic Chemistry. Chemical Bonding and Structure (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
18 Isomerism and stereochemistry
 s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
s manual for Burrows et.al. hemistry Third edition 8 Isomerism and stereochemistry s to worked examples WE 8. Structural isomers (on p. 88 in hemistry ) For the following four compounds, A D, identify
(S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer
 C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
C h a p t e r F i v e: Stereoisomerism N 2 2 N (S)-(-)-Dopa, used to treat Parkinson's disease, and its medically ineffective (R)-(+) enantiomer CM 321: Summary of Important Concepts YConcepts for Chapter
-SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION
 -SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION -Unit Number- 3481996 -Superclass- -Title- RD CONCEPTS IN ORGANIC CHEMISTRY -------------------------------
-SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION -Unit Number- 3481996 -Superclass- -Title- RD CONCEPTS IN ORGANIC CHEMISTRY -------------------------------
240 Chem. Stereochemistry. Chapter 5
 240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
The Total Synthesis of Vitamin B12
 The Total Synthesis of Vitamin B12 The most advanced synthetic intermediate as of 1968 Nathan S. Werner Denmark Group Meeting September 28 th, 2010 Biology of Vitamin B 12 Vitamin B 12, common name cobalamin,
The Total Synthesis of Vitamin B12 The most advanced synthetic intermediate as of 1968 Nathan S. Werner Denmark Group Meeting September 28 th, 2010 Biology of Vitamin B 12 Vitamin B 12, common name cobalamin,
Basic Stereochemical Considerations
 Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
1. Complete the template below to show the stereochemistry of (3R,4R) 3-chloro-4,5,5- trimethyl-hexane-1,4-diol.
 Chemistry 51 Exam 1, Fall 2004 This is a closed book exam. The exam lasts 50 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer sheet
Chemistry 51 Exam 1, Fall 2004 This is a closed book exam. The exam lasts 50 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer sheet
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Three-Dimensional Structures of Drugs
 Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Three-Dimensional Structures of Drugs Moore, T. (2016). Acids and Bases. Lecture presented at PHAR 422 Lecture in UIC College of Pharmacy, Chicago. Chiral drugs are sometimes sold as one enantiomer (pure
Due Date: 2) What is the relationship between the following compounds?
 Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Chapter 6. Isomers and Stereochemistry
 hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
hapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Lesson 4. Molecular Geometry and Isomers II. Lesson 4 CH 3 HO H OH
 Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
HO C. Explain briefly (in one or two short sentences) the meaning of the following basic stereochemical terms.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
Chapter 4: Stereochemistry
 Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C 7 16 : C 3 C 3 2-methylhexane 3-methylhexane C 2-methylhexane Bu
Chapter 5 Stereochemistry
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
ORGANIC CHEMISTRY 1 CHEM A FALL 2004 SYLLABUS
 ORGANIC CHEMISTRY 1 CHEM A300-001 FALL 2004 SYLLABUS DR. D. ANDREW KNIGHT Office: Monroe 231 Phone: 865-2269 E-mail: daknight@loyno.edu Web: http://www.loyno.edu/~knightgr INTRODUCTION AND OBJECTIVES Organic
ORGANIC CHEMISTRY 1 CHEM A300-001 FALL 2004 SYLLABUS DR. D. ANDREW KNIGHT Office: Monroe 231 Phone: 865-2269 E-mail: daknight@loyno.edu Web: http://www.loyno.edu/~knightgr INTRODUCTION AND OBJECTIVES Organic
Assign (R) or (S) configurations to the chiral carbons in the following molecules: enantiomers
 CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
CAPTER 5: STERECEMISTRY (cont.) Assign (R) or (S) configurations to the chiral carbons in the following molecules: 3 C 3 C N 2 Molecules With Multiple Chiral Atoms. 1-chloro-2-methylcyclohexane has four
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chapter 6. Isomers and Stereochemistry
 Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
InChI keys as standard global identifiers in chemistry web services. Russ Hillard ACS, Salt Lake City March 2009
 InChI keys as standard global identifiers in chemistry web services Russ Hillard ACS, Salt Lake City March 2009 Context of this talk We have created a web service That aggregates sources built independently
InChI keys as standard global identifiers in chemistry web services Russ Hillard ACS, Salt Lake City March 2009 Context of this talk We have created a web service That aggregates sources built independently
independent, long-lived existence (in "separate bottles") on the basis of the above
 Text Related to Segment 4.01 2002 Claude E. Wintner It is first among the C 7 16 cases we run into a new and wonderful! complication, which perhaps may be best introduced by the statement that instead
Text Related to Segment 4.01 2002 Claude E. Wintner It is first among the C 7 16 cases we run into a new and wonderful! complication, which perhaps may be best introduced by the statement that instead
Measuring enzyme (enantio)selectivity
 Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
1. Name the following compound. Use the IUPAC system and include the stereochemical designations.
 Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Option II: Chiral + Achiral = Optically Active Diastereomers
 Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
4Types of Isomers. 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B.
 4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
(W'' Nomenclature of Organic Chemistry. Section F : Natural Products and Related Compounds
 Eur. J. Biochem. 86, 1-8 (1978) IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) Nomenclature of Organic Chemistry. Section F : Natural Products and Related Compounds Recommendations 1976
Eur. J. Biochem. 86, 1-8 (1978) IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) Nomenclature of Organic Chemistry. Section F : Natural Products and Related Compounds Recommendations 1976
Style guide for chemical structures
 Style guide for chemical structures Although there are a number of ways that chemical structures can be drawn based on individual preferences, our journals aim to use consistent styling wherever possible.
Style guide for chemical structures Although there are a number of ways that chemical structures can be drawn based on individual preferences, our journals aim to use consistent styling wherever possible.
Lecture Topics: I. Stereochemistry Stereochemistry is the study of the three dimensional structure of molecules
 Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
geometric isomers (diastereomers)
 Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Eliel, E.L.: Wilen, S.H. Stereochemistry of Organic Compounds, Wiley, New York, 1994.
 Chem 233 Course Glossary George O Doherty For an authoritative treatment of Organic stereochemistry see: Eliel, E.L.: Wilen, S.H. Stereochemistry of Organic Compounds, Wiley, New York, 1994. Relationships
Chem 233 Course Glossary George O Doherty For an authoritative treatment of Organic stereochemistry see: Eliel, E.L.: Wilen, S.H. Stereochemistry of Organic Compounds, Wiley, New York, 1994. Relationships
STEREOCHEMISTRY. 2. Define the following, and tell whether or not a given compound or structure fits the description or possesses the feature.
 A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
A STUDENT SOULD BE ABLE TO: STEREOEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define each of the following terms, and give examples of
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Topic 5 Stereochemistry and optical isomers Isomerism
 Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6
 GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
* * * * a3 a5 A. (5 pts.) Identify each functional group by name:
 Chemistry 11 Fall 2010 Examination #3 ANSWER KEY 1. (10 pts. total) Consider the derivative structure of Lovastatin below, a potent inhibitor of an important enzyme in the biosynthesis of cholesterol.
Chemistry 11 Fall 2010 Examination #3 ANSWER KEY 1. (10 pts. total) Consider the derivative structure of Lovastatin below, a potent inhibitor of an important enzyme in the biosynthesis of cholesterol.
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Copyright 2009 James K Whitesell
 Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
Copyright 2009 James K Whitesell 5-1 These two molecules, cyclopropylcyclopentane and cyclobutycyclobutane have the same number of carbon and hydrogen atoms and thus they are constitutional isomers. 5-2
Stereochemistry Terminology for two pure isomeric compounds, both of which are chiral? A pair of stereoisomers
 Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
First Name MIKE. Chem 30A Winter 2005 MIDTERM #2 (50 Min) Weds March 2nd
 Last Name First Name MI Student I Number: Total Score Circle the name of your TA: MIKE ROB iscussion Section ay: Time: / 100 Chem 30A Winter 2005 MITERM #2 (50 Min) Weds March 2nd INTERPRETATION OF THE
Last Name First Name MI Student I Number: Total Score Circle the name of your TA: MIKE ROB iscussion Section ay: Time: / 100 Chem 30A Winter 2005 MITERM #2 (50 Min) Weds March 2nd INTERPRETATION OF THE
Chapter 5 Stereochemistry
 Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
CHEM 261 Feb. 2, Pheromone: from Greek pherein horman meaning to carry excitement. Only about 50 % of the population can smell this compound
 70 EM 61 eb., 017 Pheromone: from Greek pherein horman meaning to carry excitement O Only about 50 % of the population can smell this compound omenclature of Alkynes Rules: - ind longest chain with max
70 EM 61 eb., 017 Pheromone: from Greek pherein horman meaning to carry excitement O Only about 50 % of the population can smell this compound omenclature of Alkynes Rules: - ind longest chain with max
CHM 251 Organic Chemistry 1
 CM 251 rganic Chemistry 1 NAME Fall 2008 omework #2 Due: Tuesday, ctober 7, 2008 at 7 am Print out this assignment and complete all of your work on these pages. Question #1 Rank the following alkenes in
CM 251 rganic Chemistry 1 NAME Fall 2008 omework #2 Due: Tuesday, ctober 7, 2008 at 7 am Print out this assignment and complete all of your work on these pages. Question #1 Rank the following alkenes in
(2/94)(6,7,9/95)(8,9/97)(12/99)(1/00) Neuman Chapter 4
 4: Stereochemistry Tetrahedral Carbon Configurations Stereoisomers and R,S Assignments The Number and Types of Stereoisomers Drawing Structures of Stereoisomers Cyclic Molecules Optical Activity Preview
4: Stereochemistry Tetrahedral Carbon Configurations Stereoisomers and R,S Assignments The Number and Types of Stereoisomers Drawing Structures of Stereoisomers Cyclic Molecules Optical Activity Preview
Exam 2 Chem 109a Fall 2004
 Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Isomerism. Introduction
 Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
CHEM 263 Oct 18, Do they have the same molecular formula?
 EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
Dr Daniel Allwood. Independent Guided Study
 MODULE DESCRIPTOR TITLE Fundamentals of Organic Chemistry SI MODULE CODE 66-4837-00L CREDITS 20 LEVEL 4 JACS CODE F160 SUBJECT GROUP Biosciences DEPARTMET Biosciences MODULE LEADER Dr Daniel Allwood MODULE
MODULE DESCRIPTOR TITLE Fundamentals of Organic Chemistry SI MODULE CODE 66-4837-00L CREDITS 20 LEVEL 4 JACS CODE F160 SUBJECT GROUP Biosciences DEPARTMET Biosciences MODULE LEADER Dr Daniel Allwood MODULE
Organic Chemistry Chapter 5 Stereoisomers H. D. Roth
 Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout
 Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout So far we have built upon our understanding of the questions I and II, which deal with the number of
Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout So far we have built upon our understanding of the questions I and II, which deal with the number of
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark]
![Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark] Answer the following questions 1. Define the following : [ ( 6x2) + ( 2x4)= 20 mark]](/thumbs/89/97487476.jpg) Benha University Time : 2 hrs. Faculty of Science 1 st Term (2014/2015) Chemistry Department Date : 1 /1/2015 (Jun.2014) Organic photo and Stereochemistry Final Exam. ( 415 Ch.) ; for 4 th level Answer
Benha University Time : 2 hrs. Faculty of Science 1 st Term (2014/2015) Chemistry Department Date : 1 /1/2015 (Jun.2014) Organic photo and Stereochemistry Final Exam. ( 415 Ch.) ; for 4 th level Answer
The structures and common names of two amino acids are shown. Draw the structure of the zwitterion of proline.
 Q1.(a) The structures and common names of two amino acids are shown. (i) Draw the structure of the zwitterion of proline. Draw the structure of the tripeptide formed when a proline molecule bonds to two
Q1.(a) The structures and common names of two amino acids are shown. (i) Draw the structure of the zwitterion of proline. Draw the structure of the tripeptide formed when a proline molecule bonds to two
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Stereochemistry. 3-dimensional Aspects of Tetrahedral Atoms
 Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Suggested solutions for Chapter 14
 s for Chapter 14 14 PRBLEM 1 Are these molecules chiral? Draw diagrams to justify your answer. 2 C 2 C Reinforcement of the very important criterion for chirality. Make sure you understand the answer.
s for Chapter 14 14 PRBLEM 1 Are these molecules chiral? Draw diagrams to justify your answer. 2 C 2 C Reinforcement of the very important criterion for chirality. Make sure you understand the answer.
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
MEDCHEM 562 Kent Kunze Lecture 2. Physicochemical Properties of Drugs and Drug Disposition. Stereochemistry (Double the trouble. or double the fun?
 15 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
15 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
CHE 321 Summer 2010 Exam 2 Form Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III
 CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
Problem Set 7: Stereochemistry-ANSWER KEY
 Problem Set 7: Stereochemistry-ANSWER KEY Chemistry 260 Organic Chemistry 1. The answer is (2). Circled isomers have a stereogenic carbon (*) and hence a stereogenic centre. C 2 C 2 C 2 C 2 C 2 C 2 C 2
Problem Set 7: Stereochemistry-ANSWER KEY Chemistry 260 Organic Chemistry 1. The answer is (2). Circled isomers have a stereogenic carbon (*) and hence a stereogenic centre. C 2 C 2 C 2 C 2 C 2 C 2 C 2
The Final Learning Experience
 Chemistry 212 Fall Semester 1996 The Final Learning Experience University of Missouri Columbia, Prof. Rainer Glaser Monday, December 9, 1996, 103 Schlundt all, 12:40-2:30 Your Name: Max. Question 1 (Sugars)
Chemistry 212 Fall Semester 1996 The Final Learning Experience University of Missouri Columbia, Prof. Rainer Glaser Monday, December 9, 1996, 103 Schlundt all, 12:40-2:30 Your Name: Max. Question 1 (Sugars)
Massachusetts Institute of Technology. Chemistry 5.43 February 28 th, Exam #1. Question 1a /4 points Question 2b /10 points
 Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Nomenclature of Vitamin D
 Eur I Biocheni 124, 223-227 (1982) 'i FEBS 1982 IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of Vitamin D ecommendations 1981 CONTENTS Introduction 1. Class name 2. Semisystematic
Eur I Biocheni 124, 223-227 (1982) 'i FEBS 1982 IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of Vitamin D ecommendations 1981 CONTENTS Introduction 1. Class name 2. Semisystematic
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3
 Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3 COURSE DESCRIPTION: This course emphasizes introductory Organic Chemistry and Biochemistry. The examination
Montgomery County Community College CHE 122 General Chemistry-Organic (For the Non-Science Major) 4-3-3 COURSE DESCRIPTION: This course emphasizes introductory Organic Chemistry and Biochemistry. The examination
CHEM 261 Oct 11, Diastereomers. Enantiomers. Pheromones: from Greek pherein horman meaning to carry excitement. Discovered by Adolf Butenanot.
 EM 26 ct, 208 REALL: is Trans Trans Diastereomers Enantiomers Enantiomers have opposite stereochemistry at every stereocenter (chiral center) Diastereomers are all stereoisomers that are not enantiomers
EM 26 ct, 208 REALL: is Trans Trans Diastereomers Enantiomers Enantiomers have opposite stereochemistry at every stereocenter (chiral center) Diastereomers are all stereoisomers that are not enantiomers
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop
 Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Assigning Stereochemistry I What is stereochemistry?
 S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3
 Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3 COURSE DESCRIPTION: This course will present a brief overview of Nuclear Chemistry. The major portion of the semester will
Montgomery County Community College CHE 132 Chemistry for Technology II 4-3-3 COURSE DESCRIPTION: This course will present a brief overview of Nuclear Chemistry. The major portion of the semester will
9. Stereochemistry: Introduction to Using Molecular Models
 9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
9. Stereochemistry: Introduction to Using Molecular Models The first part of this document reviews some of the most important stereochemistry topics covered in lecture. Following the introduction, a number
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
9. Stereochemistry. Stereochemistry
 9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
Final Exam. Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions:
 CHEM 232 Final Exam May 10, 2014 RedID number: Signature: Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions: 1. In fairness to all, do not open this test until
CHEM 232 Final Exam May 10, 2014 RedID number: Signature: Your lab section and TA name: (if you are not in a lab section write no lab ) Instructions: 1. In fairness to all, do not open this test until
CHEM 2423 Unit 8 Homework Answers
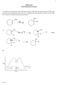 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
ORGANIC CHEMISTRY CHEM 2210 SECOND REVIEW EXAM FALL *A*
 ORGANI EMISTRY EM 2210 SEOND REVIEW EXAM ALL 1999 - *A* 1. What is the IUPA name of the compound shown? O A. cis-1-chloro-4-cyclohexanol B. cis-4-chlorocyclohexanol. trans-1-chloro-4-cyclohexanol trans-4-chlorocyclohexanol
ORGANI EMISTRY EM 2210 SEOND REVIEW EXAM ALL 1999 - *A* 1. What is the IUPA name of the compound shown? O A. cis-1-chloro-4-cyclohexanol B. cis-4-chlorocyclohexanol. trans-1-chloro-4-cyclohexanol trans-4-chlorocyclohexanol
Two enantiomers of a racemic carboxylic acid (to be separated)
 7.8 FISCER PRJECTINS 237 (R)-RC 2 (S)-RC 2 Two enantiomers of a racemic carboxylic acid (to be separated) Figure 7.5 RESLUTIN F A RACEMIC CARBYLIC ACID. (S)-RN 2 ne enantiomer of a chiral amine + (R)-RC
7.8 FISCER PRJECTINS 237 (R)-RC 2 (S)-RC 2 Two enantiomers of a racemic carboxylic acid (to be separated) Figure 7.5 RESLUTIN F A RACEMIC CARBYLIC ACID. (S)-RN 2 ne enantiomer of a chiral amine + (R)-RC
