A new method for multicomponent activity coefficients of electrolytes in aqueous atmospheric aerosols
|
|
|
- Liliana Johnston
- 6 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 11,, doi:1.129/2jd681, 25 A new method for multiomponent ativity oeffiients of eletrolytes in aqueous atmospheri aerosols Rahul A. Zaveri and Rihard C. Easter Atmospheri Sienes and Gloal Change Division, Paifi Northwest National Laoratory, Rihland, Washington, USA Anthony S. Wexler Departments of Mehanial and Aeronautial Engineering, Civil and Environmental Engineering, and Land, Air, and Water Resoures, University of California, Davis, California, USA Reeived 23 Feruary 2; revised 1 Otoer 2; aepted 26 Otoer 2; pulished 21 January 25. [1] Three-dimensional models of atmospheri inorgani aerosols need aurate and omputationally effiient parameterizations of ativity oeffiients of various eletrolytes in multiomponent aqueous solutions. In this paper, we extend the Taylor s series expansion mixing rule used y C. Wagner in 1952 for estimating ativity oeffiients in dilute alloy solutions to aqueous eletrolyte solutions at any onentration. The resulting method, alled the multiomponent Taylor expansion method (MTEM), estimates the mean ativity oeffiient of an eletrolyte in a multiomponent solution on the asis of its values in inary solutions of all the eletrolytes present in the mixture at the solution water ativity a w, assuming a w is equal to the amient relative humidity. MTEM is applied here for atmospheri aerosol systems ontaining H +,NH +,Na +,Ca 2+,SO 2, HSO,NO 3, and Cl ions. The aerosol water ontent is alulated using the Zdanovskii-Stokes-Roinson (ZSR) method. For self-onsisteny, most of the MTEM and ZSR parameters are derived using the omprehensive Pitzer-Simonson-Clegg model at K and are valid for an a w range of Beause CaSO is sparingly solule, it is treated as a solid in the model over the entire a w range. MTEM is evaluated for several multiomponent systems representing various ontinental and marine aerosols and is ontrasted against the mixing rule of C. L. Kusik and H. P. Meissner and of L. A. Bromley and the newer approah of S. Metzger and olleagues. Preditions of MTEM are found to e generally within a fator of of the omprehensive Pitzer-Simonson-Clegg model and are shown to e signifiantly more aurate than preditions of the other three methods. MTEM also yields a noniterative solution of the isulfate ion dissoiation in sulfate-rih systems: a major omputational advantage over other ioni-strength-ased methods that require an iterative solution. CPU time requirements of MTEM relative to other methods for sulfate-poor and sulfate-rih systems are also disussed. Citation: Zaveri, R. A., R. C. Easter, and A. S. Wexler (25), A new method for multiomponent ativity oeffiients of eletrolytes in aqueous atmospheri aerosols, J. Geophys. Res., 11,, doi:1.129/2jd Introdution [2] Solule inorgani onstituents in tropospheri aerosols typially inlude various eletrolytes of ammonium, sodium, alium, sulfate, nitrate, and hloride. Threedimensional aerosol models need aurate and omputationally effiient parameterizations of ativity oeffiients of these eletrolytes in multiomponent aqueous solutions to relialy predit the solid-liquid equiliria within the aerosol phase and the partitioning of semivolatile speies Copyright 25 y the Amerian Geophysial Union /5/2JD681$9. etween the gas and the aerosol phases. The parameterizations must e appliale over a wide range of ompositions and onentrations, whih are ommonly found in tropospheri aerosols of different types exposed to amient relative humidity (RH) values ranging from less than 2% to near 1%. [3] Multiomponent ativity oeffiient models suh as that of Bromley [1973] and Kusik and Meissner [1978] (KM) are ased on mixing rules that make use of singleeletrolyte (also alled a inary system) ativity oeffiients at the same solution ioni strength (molal-sale). These models have een widely used in aerosol models eause of their simpliity and reasonaly good auray [Bassett 1of23
2 and Seinfeld, 1983; Saxena et al., 1986; Kim et al., 1993a; Pilinis et al., 1987; Jaoson et al., 1996; Nenes et al., 1998]. The Pitzer model, ased on ion interations, has also een widely used to predit ativity oeffiients in rine hemistry [Pitzer and Mayorga, 1973]. Although more aurate than the KM and Bromley mixing rules, appliaility of Pitzer s method, whih is also ased on the molal sale, is typially limited to onentrations less than 1 15 mol kg 1. More reently, Pitzer, Simonson, and Clegg (PSC) developed a mole-fration-ased ion interation model that overomes the limitation of Pitzer s approah, and is appliale over the entire onentration range [Pitzer and Simonson, 1986; Clegg et al., 1992]. The PSC model has een inorporated into the Aerosol Inorganis Model (AIM), and has een parameterized for a numer of multiomponent systems ommonly found in atmospheri aerosols using a large ody of experimental data at susaturated, saturated, and supersaturated onditions [Clegg et al., 1998a, 1998; Wexler and Clegg, 22, and referenes therein]. The AIM thermodynami models are availale for use on the Worldwide We, and are the most omprehensive and aurate over the entire range of ompositions and relative humidities; however, they are also the most omputationally expensive, and remain too slow to e used in three-dimensional atmospheri hemistry models [Wexler and Clegg, 22]. [] While the KM and Bromley type mixing rules are muh faster than the PSC model and reasonaly aurate for susaturated solutions, their appliaility for saturated and supersaturated multiomponent solutions is limited y maximum ioni strengths up to whih the mean inary ativity oeffiient parameterizations are valid. Also, eause the inary ativity oeffiients are expressed as a funtion of ioni strength, these mixing rules usually entail expensive, repeated evaluations of the inary ativity oeffiients as the ioni strength hanges in an iterative numerial solution for gas/liquid/solid partitioning. The approah of Metzger et al. [22] partially overomes these limitations y expressing mean inary ativity oeffiients as a funtion of water ativity (assuming a w = RH); however, it simply assumes that the multiomponent ativity oeffiient of eah eletrolyte is equal to its mean inary ativity oeffiient. This assumption may e reasonale for dilute solutions (RH > 8%), ut generally tends to reak down for onentrated solutions. [5] In this paper we present a new method that omines the auray of the omprehensive PSC model and the effiieny of the Metzger et al. [22] approah. The new method, alled multiomponent Taylor expansion method (MTEM) extends the mixing rule ased on the Taylor s series expansion approah of Wagner [1952] for dilute alloy systems to aqueous eletrolytes at any onentration. MTEM is applied here for onentrated aqueous systems ontaining H + -NH + -Na + -Ca 2+ -SO 2 -HSO -NO 3 -Cl ions at K, and is evaluated for several test ases representing ommonly found multiomponent systems in tropospheri aerosols. It is also ontrasted against the other approahes mentioned aove with regard to auray and omputational effiieny. The ativity oeffiient parameterizations presented here are intended for omputationally intensive three-dimensional tropospheri aerosol modeling appliations, and are used in a new model, Model for Simulating Aerosol Interations and Chemistry (MOSAIC), whih will e desried elsewhere. 2. Model Development 2.1. Derivation [6] The molal-sale ativity of an eletrolyte A ontaining the ation and anion a in an aqueous solution an e expressed as a A ¼ a v ð ava a ¼ g m Þ v ðg a m a ¼ g va A mv mva a where susripts and a represent ations and anions, respetively; m and m a are the ation and anion molalities, respetively; g and g a are the molal-sale ativity oeffiients of the ation and anion, respetively; v and v a are the numer of moles of ation and anion, respetively, per mole of eletrolyte A; and v A = v + v a. Thus the mean ioni ativity oeffiient of the eletrolyte is related to the individual ion ativity oeffiients y g A ¼ g v gva a Þ va ð1þ 1=vA ð2þ The mean ativity oeffiient of a pure eletrolyte A in aqueous solution is denoted y g A. Now onsider an aqueous solution ontaining N strong eletrolytes in equilirium with amient relative humidity so that the water ativity a w = RH. Let the ioni mole fration of an eletrolyte A e defined as z A ¼ P N E¼1 v An A v E n E where n E is the numer of moles of any eletrolyte E in the solution. Note that this definition of ioni mole fration exludes the solvent itself (i.e., water), so that P ze = 1 (the rationale for this partiular definition of z is illustrated in susetion 2.3 with the use of a simple example). [7] The following derivation is ased on the Taylor s series expansion approah used y Wagner [1952] who developed a mixing rule for ativity oeffiients for alloys in dilute multiomponent systems [Lewis and Randall, 1961]. In this work, we extend this approah to aqueous eletrolyte solutions in equilirium with the amient water vapor so that RH is equal to the solution water ativity a w. Let log g A e denoted as f A. At a given a w a Taylor s series expansion of f A in terms of the (N 1) independent ioni mole frations, an then e written as f A ðz B ; z C ;...; a w Þ ¼ f Að a wþþ X z E A ðz B ; z C ;...; a w E where the terms involving seond and higher derivatives are negleted, and f A (a w )=f A (,,..., a w ). Expressing the partial derivatives as finite differenes over the entire range ð3þ ðþ 2of23
3 of ioni mole frations ( to 1), the aove equation is rewritten as f A ðz B ; z C ;...; a w Þ ¼ f Að a wþþ X z E E6¼A ¼ f A ðz E ¼ 1; ;...; a w Þ f Að a wþ 1 1 X E6¼A z E!f Að a wþ þ X z E f A ðz E ¼ 1; ;...; a w Þ E6¼A ¼ z A f Að a wþþ X z E f A ðz E ¼ 1; ;...; a w Þ E6¼A The term f A (a w ) implies that z A = 1, and is therefore equal to log g A (a w ), i.e., the mean inary ativity oeffiient of A at a w. Dropping ioni mole frations from the argument list of f A in equation (5) for simpliity, and sustituting log g A ak for f A, the aove equation redues to a general form: ð5þ log g A ða w Þ ¼ XN z E log g A Eð a wþ ð6þ E¼1 where g A E (a w ) is the mean ativity oeffiient of eletrolyte A in a inary aqueous solution of eletrolyte E at a w. Note that when E = A, g A A (a w )=g A (a w ). [8] Thus, at a given water ativity, the mean ativity oeffiient of A in a multiomponent solution ontaining N eletrolytes may e estimated from a linear omination of its values in ternary solutions of A-A-H 2 O, A-B-H 2 O, A-C-H 2 O, et., as the amount of eletrolyte A approahes zero. Although equation (6) is derived with the Taylor s series expansion approah used y Wagner [1952], the final forms of the two mixing rules are quite different. Wagner s mixing rule is ased on mole fration of solutes (inluding the solvent) and the partial derivatives are taken for the limiting ase of zero onentration of all solutes where as MTEM is ased on ioni mole frations (exluding the solvent) and the partial derivatives are taken over their entire range at a given water ativity. It should also e noted that MTEM is appliale at any a w and that it is speifially designed for atmospheri aerosols for whih a w is known from the assumption a w = RH Eletrolyte Composition [9] Beause eletrolytes ionize either ompletely or partially in aqueous solution, many different ominations of eletrolytes are possile for a given ioni omposition. A unique eletrolyte mixture is needed for the purpose of alulating the ioni mole frations (z) for equation (6) as well as the total aerosol water ontent, W, whih is omputed here y the widely used ZSR mixing rule [Zdanovskii, 198; Stokes and Roinson, 1966]: W ¼ XN E¼1 n E m Eð a wþ ð7þ where m E (a w ) is the inary eletrolyte molality of E at the solution water ativity, assuming a w = RH. Two eletrolyte formation domains are possile in the H + -NH + -Na + -Ca 2+ - SO 2 -HSO -NO 3 -Cl -H 2 O system as defined y the sulfate ratio, X T : X T ¼ n NH þ þ n Na þ þ 2n Ca 2þ n SULF where n SULF = n SO 2 + n HSO, i.e., total S(VI). In the sulfatepoor domain, where X T 2, S(VI) is ompletely neutralized y NH +,Na +, and Ca 2+, and therefore exists as SO 2. All the eletrolytes that form in this domain are strong, and ionize ompletely in aqueous solution. On the other hand, in the sulfate-rih domain (X T < 2) S(VI) is not ompletely neutralized y NH +,Na +, and Ca 2+, resulting into the formation of isulfate ion (HSO ), whih is a weak aid that exists in equilirium with H + and SO 2. Algorithms for determining a unique eletrolyte omposition in the two domains are desried elow Sulfate-Poor Domain: X T 2 [1] In the ase of strong eletrolytes, whih ionize ompletely in aqueous solution, the eletrolyte omposition is determined using the equivalent frations e and e a for ations and anions, respetively, whih are first omputed as: e ¼ P z n and e a ¼ P z an a z n z a n a where z i is the magnitude of the harge on ion i, and n i is the numer of moles of ion i present in the solution. The numer of moles of an eletrolyte A onsisting of the ation and anion a is then omputed as n A ¼ e n a M a þ e a n M M A a ð8þ ð9þ ð1þ where M a and M are the moleular weights of the anion and ation, respetively, and M A is the moleular weight of the eletrolyte A, suh that M A = v a M a + v M. This rule is ommonly used in ion solution thermodynamis to partition the ions into eletrolytes. [11] Sine CaSO is sparingly solule [Sheikholeslami and Ong, 23], it is treated as solid over the entire RH range in the model. Thus, for a given mixture of various ations and anions, the numer of moles of solid CaSO is first alulated as n CaSO = min(n CA 2+, n SO 2 ), and the moles of other eletrolytes are then omputed from the remaining ions in the aqueous phase using the algorithm desried aove. Tale 1 lists all the ations and anions onsidered in the sulfate-poor domain, the possile eletrolytes resulting from the various ominations thereof, and their v E values to e used in omputing ioni mole frations. [12] The total water ontent (W) an e omputed from equation (7) one the moles (n E ) of individual eletrolytes are determined as desried aove. The resulting multiomponent eletrolyte molalities are omputed then simply as m E = n E /W (individual ioni molalities an also e omputed in a similar manner). The H + ion molality (m H +), whih is needed in the sulfate-poor domain to 3of23
4 Tale 1. List of Ions and Possile Aqueous Eletrolytes in the Sulfate-Poor Domain (X T 2) Ion i z i M i 1 H NH Na Ca SO NO Cl Eletrolyte E v E M E 1 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl ompute gas-liquid partitioning of HNO 3 and HCl, an e approximated from the eletroneutrality ondition as dissoiation of the isulfate ion, and potentially requires an expensive iterative solution to ompute a unique eletrolyte omposition y the algorithm desried aove. Instead, a simpler noniterative approah is used here that works quite well for alulating the ioni mole frations needed for MTEM as well as for estimating the total water ontent using the ZSR method. [1] Beause the vapor pressure of sulfuri aid over partially and ompletely neutralized aqueous solutions is extremely low (less than 1 12 atm) at amient tropospheri onditions [Marti et al., 1997], it essentially resides in the aerosol phase, and displaes the relatively more volatile HNO 3 and HCl aids. As a result, none of the nitrate and hloride eletrolytes an exist in the sulfate-rih domain, exept for negligily small amounts of HNO 3 and HCl that may form in the highly aidi aerosol phase to maintain equilirium with their gas phase ounterparts. As efore, CaSO is assumed to exist ompletely in the solid phase, and eause n Ca 2+ is always less than n SULF in the sulfaterih domain, alium is not allowed to exist in the aqueous phase (note that alium isulfate formation is not onsidered in the model). The aqueous eletrolyte formation then depends on the modified sulfate ratio, m H þ ¼ m OH þ 2mSO 2 þ m NO 3 þ m Cl m NH þ þ m Na þ þ 2m Ca 2þ : ð11þ Sine the strong eletrolytes are ompletely ionized, molalities of all the ions exept OH are readily known. Molality of OH is related to m H + through the water dissoiation reation, H 2 O Ð K w H + +OH,as ðm H þm OH Þðg H þg OH Þ ¼ a w K w ð12þ where the dissoiation onstant is K w = mol 2 kg 2 at K. By assuming the produt g H +g OH = 1, and omining equations (11) and (12), we an estimate m H + y solving a quadrati equation of the form m 2 H + + m H + + = for the positive root, where ¼ 2m SO 2 þ m NO 3 þ m Cl ¼ a w K w : m NH þ þ m Na þ þ 2m Ca 2þ ; ð13þ ð1þ One m H + is known, the HNO 3 and HCl partial pressures are alulated as p HNO3 ¼ m H þm NO 3 g2 HNO 3 K HNO3 ; and ð15þ XT ¼ n NH þ þ n Na þ n SULF ð17þ where n SULF = n SULF n CaSO, and on the relative frations of Na + and NH +, whih are respetively defined as f Na þ ¼ n Na þ n Na þ þ n NH þ and f NH þ ¼ n NH þ n Na þ þ n NH þ ð18þ Tale 2 displays the eletrolyte formation formulae as a funtion of XT. In this approah unique eletrolytes are assumed to form at XT =, 1, 1.5, and 2. For example, note that H 2 SO forms at XT = ; a mixture of NH HSO and NaHSO forms at XT = 1; a mixture of (NH ) 3 H(SO ) 2 and Na 3 H(SO ) 2 forms at XT = 1.5; and finally a mixture of (NH ) 2 SO and Na 2 SO forms at XT = 2. A linear omination of these eletrolytes is assumed to form at intermediate values of XT depending on its sudomain. Also shown in Tale 2 are the values of v E that are to e used in omputing ioni mole frations of the listed eletrolytes. [15] In the sulfate-rih domain the H + ion molality is needed to ompute solid-liquid partitioning of the salts shown in Tale 2, and to ompute gas-liquid partitioning of HNO 3, HCl, and NH 3. However, unlike efore, m H + in the sulfate-rih domain must e omputed y expliitly solving the partial isulfate ion dissoiation p HCl ¼ m H þm Cl g 2 HCl K HCl ; ð16þ where the Henry s law onstants are K HNO3 = mol 2 kg 2 atm 1, and K HCl = mol 2 kg 2 atm 1 at K [Kim et al., 1993a] Sulfate-Rih Domain: X T < 2 [13] The sulfate-rih domain is relatively more omplex than the sulfate-poor domain, eause it involves partial HSO ÐKd H þ þ SO 2 ð19þ where the equilirium onstant K d = mol kg 1 at K [Clegg et al., 1998a]. This equilirium is mathematially expressed as K d ¼ m H þm SO m HSO!! gh þg SO : ð2þ g HSO of23
5 Tale 2. Aqueous Eletrolyte Formation in the Sulfate-Rih Domain (X T <2) a Eletrolyte E v E X T <1 1 X T < X T <2 Eletrolyte Moles n E H 2 SO 3 NH HSO 2 (1 X T)n SULF X Tn SULFf + NH (3 2X T)n SULFf + NH NaHSO 2 X Tn SULFf NA + (3 2X T)n SULFf Na + (NH ) 3 H(SO ) 2 5 (X T 1)nSULFf + NH (2 X T)n + SULFf NH Na 3 H(SO ) 2 5 (X T 1)nSULFf Na + (2 X T)n SULFf Na + (NH ) 2 SO 3 (2X T 3)n + SULFf NH Na 2 SO 3 (2X T 3)n SULFf Na + a Major ions for this domain are as follows: H +,NH +,Na +,SO 2, and HSO. Relatively small amounts of NO 3 and Cl may also exist in equilirium with gas-phase HNO 3 and HCl, respetively. Ignoring the OH ion in the highly aidi sulfate-rih domain, the eletroneutrality ondition at equilirium an e written as m H þ ¼ 2m SO 2 þ m HSO þ m NO 3 þ m Cl mnh þ þ m Na þ : ð21þ Equations (2) and (21) must e solved simultaneously with m SULF = m SO 2 + m HSO to determine m H +, where m SULF = n SULF/W T. Sine MTEM does not predit individual ion ativity oeffiients, we use the following expression to estimate the ioni ativity oeffiient produt in equation (2) [Kim et al., 1993]: g H þg SO ¼ g2 Hþg SO ¼ g3 H 2SO g HSO g H þg HSO g 2 : ð22þ HHSO In the aove equation oth H 2 SO and HHSO are to e viewed as ion pairs 2H + -SO 2 and H + -HSO, respetively, that exist in sulfate-rih systems and not as unique eletrolytes that an exist independently. It should e noted that in the aove development the aqueous sulfuri aid isulfate reversile reation is negleted, whih ould eome signifiant at very low X T ratios and high sulfate onentrations. [16] Now, sine g H2 SO and g HHSO depend on the ioni omposition of the solution, an iterative numerial tehnique is typially required to solve for m H + when multiomponent ioni-strength-ased ativity oeffiient models suh as KM, Bromley, PSC, and Pitzer are used. However, eause MTEM predits the multiomponent g H2 SO and g HHSO diretly as a funtion of eletrolyte omposition and a w (assuming a w = RH), we an ompute m H + noniteratively 2 y simply solving a quadrati equation of the form m H + + m H + + = for the positive root, where g 2 HHSO ¼ K d g 3 þ m Na þ þ m NH þ m NO m 3 Cl m SULF ; and H 2SO ð23þ g 2 HHSO ¼ K d g 3 m Na þ þ m NH þ m NO m 3 Cl 2m SULF : ð2þ H 2SO The equilirium partial pressure of NH 3 over aqueous solutions is governed y the following equilirium reations: NH 3g ð Þ Ð KH NH 3aq ð Þ ; and ð25þ K NH NH 3aq ð Þ þ H 2 O ðaqþ Ð NH þ þ OH ð26þ where the Henry s law onstant of NH 3, K H = 57.6 mol kg 1 atm 1, and the dissoiation onstant of aqueous NH 3, K NH = mol kg 1 at K [Kim et al., 1993a]. Reations (25) and (26) an e omined with H 2 O Ð H + +OH to eliminate OH and NH 3(aq), and express the partial pressure of NH 3 as P NH3 ¼ m NH þ gnh þ K w ð27þ m H þ g H þ K NH K H Again, sine MTEM does not predit individual ion ativity oeffiients, we use the following expression to estimate the ioni ativity oeffiient ratio in the aove equation: g NH þ g H þ ¼ g NH þ g HSO g H þg HSO ¼ g2 NH HSO g 2 HHSO ð28þ In summary, with the eletrolyte formation proedure desried aove, we an diretly alulate the multiomponent ativity oeffiients of all the eletrolytes present in the sulfate-rih domain, and ompute the equilirium m H + and P NH3 in a noniterative manner Example: (NH ) 2 SO -NH NO 3 -H 2 O System [17] To etter understand the underlying rationale of the new mixing rule, we illustrate here with an example the ehavior of mean ioni ativity oeffiients of (NH ) 2 SO and NH NO 3 in the ternary (NH ) 2 SO -NH NO 3 -H 2 O system. The omprehensive PSC model was used to alulate the multiomponent ativity oeffiients for varying (NH ) 2 SO to NH NO 3 molar ratio at four different water ativities:.3,.5,.7, and.9. The results are plotted in Figure 1 as a funtion of ioni mole fration. Interestingly, oth log g (NH ) 2SO and log g NH NO 3 vary almost linearly with z (NH ) 2SO and z NH NO 3, respetively. Thus, at a given water ativity, one an approximate g (NH ) 2SO from a linear equation in terms of its values at z = (i.e., log g (NH ) 2 SO -NH NO 3 ) and z = 1 (i.e., log )as g (NH ) 2 SO -(NH ) 2 SO log gð NH Þ 2 SO ¼ 1 zð NH Þ 2 SO log g ðnh Þ 2 SO NH NO 3 þ zð NH Þ 2 SO log g ðnh Þ 2 SO ðnh Þ 2 SO ; ð29þ ut sine z (NH ) 2 SO + z NH NO 3 = 1, the aove equation redues to a form that is idential to the new mixing rule: log gð NH Þ 2 SO ¼ z NHNO 3 log g ðnh Þ 2 SO NH NO 3 þ zð NH Þ 2 SO log g ðnh Þ 2 SO ðnh Þ 2 SO : ð3þ 5of23
6 Figure 1. Plots of log g as a funtion of ioni mole fration z at different water ativities for the ternary (NH ) 2 SO -NH NO 3 -H 2 O system: (a) (NH ) 2 SO and () NH NO 3. Open and solid irles are used for larity. A similar expression an e written for log g NH NO 3. It should now e evident that we hose to define z as ioni mole fration (equation (3)), eause the variation of log g with this definition of z appears to e almost linear over the entire RH range. On the other hand, variation of log g was not as linear when z was defined simply as eletrolyte mole fration in the aove example. Thus the hoie of z is ased on the empirial ehavior of the given multiomponent system rather than on ion solution thermodynami theory. 2.. Model Implementation [18] Implementation of MTEM for multiomponent solutions requires knowledge of the two-eletrolyte system ativity oeffiients, log g A E as a funtion of a w. For a given A-E-H 2 O system, the PSC model is used to alulate the ativity oeffiient of A in the inary E-H 2 O system over the desired range of RH (assuming a w = RH), and the resulting log g A E (a w ) are fit with approximating polynomials. Clegg et al. [1998] determined the PSC model parameters for the H + -NH + -Na + -SO 2 -NO 3 -Cl -H 2 O system at K while the parameters for the H + -Ca 2+ -NO 3 -H 2 O and H + -Ca 2+ -Cl -H 2 O systems are availale from Clegg et al. [1992]. Ternary ion PSC model parameters of type W ii j, U ii j, and Q 1,ii j for solutions ontaining Ca 2+ and other ions are urrently not availale, and are therefore set to zero in the model. Negleting these parameters would distort the model preditions in highly onentrated solutions to some degree. Tale 3. Coeffiients for Polynomial Fits of Mole Fration (x E ) of Pure Eletrolyte E in Aqueous Solution as a Funtion of Water Ativity (a w ) at K, Assuming a w =RH a Eletrolyte A A 1 A 2 A 3 A A 5 Soure 1 (NH ) 2 SO CW 2 (NH ) 3 H(SO) CW 3 NH HSO CW NH NO CW 5 NH Cl CH 6 Na 2 SO CW 7 Na 3 H(SO ) CW 8 NaHSO CW 9 NaNO CW 1 NaCl CW 11 Ca(NO 3 ) KS 12 CaCl KS 13 H 2 SO CW 1 HNO CW 15 HCl CW a The fits are valid a w =.2.98 unless noted otherwise. x E (a w )=A + A 1 a w + A 2 a 2 w + A 3 a 3 w + A a w + A 5 a 5 w. Soure: CW, Clegg et al. [1998] and Wexler and Clegg [22]; KS, Kim and Seinfeld [1995]; CH, Chan and Ha [1999]. Minimum valid a w =.1. 6of23
7 Tale. Coeffiients for Polynomial Fits of log g A E (a w ) at K for Sulfate-Poor Domain a Eletrolyte E B B 1 B 2 B 3 B B 5 Eletrolyte A = (NH ) 2 SO (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = NH NO 3 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = NH Cl (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = Na 2 SO (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = NaNO 3 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = NaCl (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl of23
8 Tale. (ontinued) Eletrolyte E B B 1 B 2 B 3 B B 5 Eletrolyte A = Ca(NO 3 ) 2 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = CaCl 2 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = HNO 3 (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl Eletrolyte A = HCl (NH ) 2 SO NH NO NH Cl Na 2 SO NaNO NaCl Ca(NO 3 ) CaCl HNO HCl a The fits are valid from a w =.2 to.97 unless noted otherwise. Here, log g A E (a w )=B + B 1 a w + B 2 a 2 w + B 3 a 3 w + B a w + B 5 a 5 w. These eletrolyte pairs are not allowed in the model sine solid-phase CaSO is assumed to form in the presene of Ca 2+ and SO 2. Polynomials for A-HNO 3 and A-HCl pairs are valid to a w =.1. [19] Although MTEM does not expliitly require the aerosol water ontent in omputing ativity oeffiients y equation (6), it is needed to ompute ion molalities, whih are used along with the ativity oeffiients in equilirium or dynami gas/solid/liquid partitioning alulations. For self-onsisteny, the inary eletrolyte mole frations, x E,as a funtion of a w were also omputed using the PSC model for all the eletrolytes exept for NH Cl, Ca(NO 3 ) 2, and CaCl 2. The inary molality parameterization of Chan and Ha [1999] was used for NH Cl as it is superior to the PSC preditions at high supersaturation (<5% RH), and the data given y Kim and Seinfeld [1995] were used for Ca(NO 3 ) 2 and CaCl 2. The oeffiients for x E (a w ) polynomial fits are listed in Tale 3, and the inary molalities required for the ZSR equation are then omputed as m E = (55.59 x E )/(1 x E ). The oeffiients for the resulting polynomial fits of log g A E (a w ) for all the possile two-eletrolyte systems (A-E) in the sulfate-poor and sulfate-rih domains are listed in Tale and Tale 5, respetively. The log g A E (a w ) fits are valid for water ativities ranging from.2 to.97 unless noted otherwise; all eletrolytes exept H 2 SO are assumed to e solid elow 2% RH, and the ativity oeffiients are assumed to e unity eyond 97% RH. [2] Figure 2 illustrates the strong dependene of the mean inary ativity oeffiients (g A ) on water ativity (same as RH) for all the eletrolytes onsidered in the model, omputed using the polynomials, g A E (a w ), in Tales and 5 when E = A. Here g H2 SO and g HHSO are evaluated in inary H 2 SO solution (i.e., E =H 2 SO ) onsidering HSO ion dissoiation. Note that values signifiantly depart from unity for many of the eletrolytes as a w dereases. Thus signifiant nonideal ehavior is expeted 8of23
9 Tale 5. Coeffiients for Polynomial Fits of log g A E (a w ) at K for Sulfate-Rih Domain a Eletrolyte E B B 1 B 2 B 3 B B 5 Eletrolyte A = H 2 SO H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl Eletrolyte A = HHSO H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl Eletrolyte A = NH HSO H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl Eletrolyte A = (NH ) 3 H(SO ) 2 H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl Eletrolyte A = (NH ) 2 SO H 2 SO NH HSO (NH ) 3 H(SO ) NaHSO Na 3 H(SO ) Eletrolyte A = NaHSO H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl Eletrolyte A = Na 3 H(SO ) 2 H 2 SO NH HSO (NH ) 3 H(SO ) (NH ) 2 SO NaHSO Na 3 H(SO ) Na 2 SO HNO HCl of23
10 Tale 5. (ontinued) Eletrolyte E B B 1 B 2 B 3 B B 5 Eletrolyte A = Na 2 SO H 2 SO NH HSO (NH ) 3 H(SO ) NaHSO Na 3 H(SO ) Eletrolyte A = HNO 3 H 2 SO NH HSO (NH ) 3 H(SO ) NaHSO Na 3 H(SO ) Eletrolyte A = HCl H 2 SO NH HSO (NH ) 3 H(SO ) NaHSO Na 3 H(SO ) a The fits are valid for a w =.2 to.97 unless noted otherwise. Minimum valid a w for all the A-H 2 SO, A-HNO 3, and A-HCl pairs is.1. in multiomponent systems, espeially at low amient relative humidities. Performane of MTEM for multiomponent systems is evaluated next. 3. Results and Disussion [21] The present model is evaluated for 16 test ases listed in Tale 6, representing different relative ioni ompositions ommonly found in uran and marine tropospheri aerosols. Tale 7 displays the relative eletrolyte ompositions for these test ases as omputed using the algorithms desried in susetion 2.2 for sulfate-poor and sulfaterih multiomponent systems. The PSC model is used as the enhmark, and the results are ontrasted against the KM and Bromley mixing rules and the Metzger et al. [22] approah, whih is areviated as MBAC for mean inary ativity oeffiients at the solution water ativity, g A (a w ), assuming a w = RH. The inary ativity oeffiients g A (shown in Figure 2) needed in KM and Bromley methods were parameterized as a funtion of ioni strength (I) using the PSC model output. This ensures perfet agreement etween all the methods for inary eletrolyte solutions. A similar approah was also adopted y Lin and Taazadeh [21], who updated the inary ativity oeffiient polynomials in EQUISOLV-II using AIM2 data. The maximum valid ioni strengths are signifiantly different for different eletrolytes, ranging from 15 mol kg 1 for HCl to 35 mol kg 1 for NH NO 3 as shown in Figure 3. As mentioned earlier, this potentially limits the appliaility of KM and Bromley to a maximum multiomponent solution ioni strength that is equal to the lowest valid ioni strength amongst all the individual omponents present in the solution. In the following omparison, however, g A (I max ) is used when the atual multiomponent solution ioni strength is higher than I max for a given eletrolyte A. [22] Test ases 1 are simple multiomponent systems onsisting of omparale amounts of two salts. The first two ases represent ontinental uran aerosols with different relative amounts of (NH ) 2 SO and NH NO 3. Test ases 3 and represent freshly emitted and aged sea-salt aerosol, respetively, with different relative amounts of NaCl and Na 2 SO. The next four test ases 5 8 are relatively more omplex systems onsisting of omparale amounts of (NH ) 2 SO,NH NO 3,NH Cl, NaCl, NaNO 3, and Na 2 SO. Suh multiomponent systems are usually enountered in oastal uran loations and marine environments downwind of large uran soures where the sea-salt aerosols an reat signifiantly with NH 3, HNO 3 and H 2 SO. The test ases 9 and 1 represent reative rustal aerosol (dust) ontaining alium salts of nitrate and hloride with other eletrolytes. Finally, the test ases 11 through 16 are sulfate-rih multiomponent systems with different NH + to Na + molar ratios, and X T ranging from.3 to 1.9. [23] Relatively small amounts of HCl and/or HNO 3 are usually present in oth sulfate-poor and sulfate-rih aerosols so that HNO 3 and HCl in the gas phase an remain in equilirium with the aerosol NO 3 and Cl, respetively. Gas-liquid partitioning of these aids is partiularly important for sulfate-poor aerosols in determining the aerosol ph. It is therefore important to aurately alulate the mean ioni ativity oeffiients of HNO 3 and HCl even though their amounts may e negligile ompared to other eletrolytes in the aerosol Auray [2] In the following analysis, the auray of different methods is assessed in terms of performane ratios of ativity oeffiients omputed y eah method to the PSC model. Thus, for a given method X, the performane ratio (PR) for an eletrolyte A at water ativity a w is alulated as PR ¼ gx A ð a wþ g PSC A ða w Þ ð31þ 1 of 23
11 Figure 2. Mean inary ativity oeffiients (g A ) for individual eletrolytes as a funtion of a w omputed using the polynomials g A E (a w ) in (a) Tale and () Tale 5 when E = A; with E =H 2 SO for g H2 SO and g HHSO. Solid and dotted lines are used for larity. Performane ratios for MTEM, KM, and MBAC are plotted as a funtion of a w (same as RH) in Figures a for the 1 sulfate-poor test ases. Bromley results were found to e very similar to KM, and are therefore not inluded in the plots to maintain larity, ut will e summarized later. For eah test ase, plots are shown only for the eletrolytes present in the solution as indiated y Tale 7 along with HNO 3 and HCl. Also shown in these figures are aritrary.8 and 1.25 performane ratio ounds. [25] In the sulfate-poor test ases, the performane ratios of all the major salts for MTEM are generally loser to unity than for the KM method, and rarely exeed the ounds over the entire a w range while the PR in the MBAC approah generally exeed the ounds for a w <.5. The largest deviations of PR from unity are seen for HNO 3 and HCl for all three methods. PR for KM range from less than.2 to 2. for these aids at a w =.5 while MBAC performs even worse, with PR values exeeding 11 of 23
12 Tale 6. Relative Aqueous Phase Ioni Composition in Sulfate- Poor and Sulfate-Rih Test Cases Test Case NH + Ion Moles Na + Ca 2+ SULF NO 3 Cl Sulfate-Poor Domain Sulfate-Rih Domain at a w =.5. MTEM performs muh etter than the other two methods for oth the aids, with PR generally within the ounds for a w >.5, and never worse than.2 and 5. at a w =.2. [26] Figures 5a 5 displays the performane ratios of ativity oeffiients of all the eletrolytes that an form in the sulfate-rih domain (test ases 11 16) along with the performane ratios of m H + and P NH3. As disussed efore, in the sulfate-rih domain it is not only important to aurately predit the mean ioni ativity oeffiients, ut also to aurately solve the isulfate ion dissoiation and ompute the resulting equilirium H + ion onentration and NH 3 partial pressure. While KM and MBAC show aeptale auray for prediting ativity oeffiients of all the pertinent salts, oth the methods show poor performane in prediting the multiomponent g H2 SO and g HHSO, resulting in large errors in the equilirium m H + and P NH3 values (PR values for these variales range from less than.2 to more than 5.). On the other hand, MTEM onsistently performs quite well in prediting ativity oeffiients of all the eletrolytes as well as in estimating m H + and P NH3 over the entire XT range and also near the oundary of the sulfate-rih and sulfate-poor domains. Reent laoratory experiments show that the yield of seondary organi aerosol (SOA) formation via aid-atalyzed heterogeneous reations is sensitive to partile aidity [Jang and Kamens, 21; Czoshke et al., 23; Noziere and Riemer, 23; Iinuma et al., 2]. While the hemial mehanisms of SOA are still under investigation, it will e important for a given inorgani aerosol thermodynami module to relialy predit the partile ph in the next generation of SOA models. [27] Figure 6 shows the multiomponent ioni strengths as a funtion of RH for the 16 test ases. Ioni strengths in ammonium-rih aerosols (test ases 1, 2, 5, and 6) exeed 3 mol kg 1 at water ativity values around or less than.6. Appliaility of the KM mixing rule in suh highly onentrated solutions eomes questionale for eletrolytes suh as NaCl, HNO 3 and HCl whose maximum valid ioni strengths are lower than 3 mol kg 1. Although the ioni strengths in sodium-rih (test ases 3,, 7, and 8) and alium-rih (test ases 9 and 1) aerosols are relatively lower, they an still easily exeed the maximum ioni strengths of HNO 3 and HCl at low RH values. Similar oservations an e made for the sulfate-rih solutions (test ases 11 16) where the ioni strength at a given water ativity is seen to inrease as the modified sulfate ratio XT inreases. The maximum valid ioni strength for the g H2 SO (I ) and g HHSO (I ) polynomials, whih are derived from pure H 2 SO solution, is only 23 mol kg 1, whih limits the appliaility of the KM and Bromley mixing rules to high RH or very low XT values. Nevertheless, large errors in multiomponent g H2 SO and g HHSO are seen even under these onditions (see test ases 11 and 12 in Figures 5a 5). Tale 7. Eletrolyte Compositions for the Ioni Compositions Given in Tale 6 Sulfate-Poor Domain a Test Case (NH ) 2 SO, mol NH NO 3, mol NH Cl, mol NaCl, mol Na 2 SO, mol NaNO 3, mol Ca(NO 3 ) 2, mol CaCl 2, mol Sulfate-Rih Domain Test Case (NH ) 2 SO, mol (NH ) 3 H(SO ) 2, mol NH HSO, mol H 2 SO, mol Na 2 SO, mol Na 3 H(SO ) 2, mol NaHSO, mol X T a Eletrolyte omposition omputed using equation (1). Eletrolyte omposition omputed as shown in Tale of 23
13 Figure 3. Maximum valid ioni strengths (I max ) for individual eletrolytes omputed at a w =.2 using the polynomials in Tale 2. The maximum valid ioni strength for H 2 SO is omputed at a w =.1. [28] The auray results for all the methods (inluding Bromley) are summarized in Tale 8 in terms of the logarithmi root mean square (LRMS) of the performane ratio for eah speies, whih is defined as LRMS PR ¼ 1 rffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi 1 M P M ½logðPR i ÞŠ 2 i¼1 ð32þ where M is the sample size. We use this performane statisti to give equal weighting to performane ratios less than and greater than unity. For example, performane ratios of 2 and 1 = 2 oth orrespond to an LRMS PR of 2. Benkovitz and Shwartz [1997] used a similar statisti, whih they alled ratio harateristi spread, for omparing simulated and oserved mixing ratios of SO 2 and sulfate. To etter reflet the performane of the three methods over the entire RH range, the LRMS PR values are omputed for three water ativity ranges: low,.2 to.5; middle,.5 to.7; and high,.7 to.97. It an e readily seen that MTEM is generally more aurate than KM and Bromley for all three a w ranges, exept for Ca(NO 3 ) 2 and CaCl 2, for whih MTEM is either slightly less aurate or omparale. Furthermore, LRMS PR values for MBAC are onsistently and signifiantly higher than those for KM and Bromley for all three a w ranges. Thus MTEM s overall auray and appliaility are generally etter than KM, Bromley, and MBAC for all the different types of sulfate-poor and sulfaterih aerosol systems over the entire a w range. [29] In summary, MTEM should not only prove to e more aurate than KM, Bromley, and MBAC for prediting the solid-liquid equiliria for all the salts, ut also for prediting equilirium partial pressures of HCl, HNO 3, and NH 3 over aqueous aerosols, and alulating the equilirium ph within the aerosols Computational Effiieny [3] Apart from eing more aurate, we will now show that MTEM is as or more effiient than the KM and Bromley mixing rules when used in three-dimensional dynami setional aerosol models. Setional models typially employ eight or more size ins, with eah in requiring internal (solid-liquid) equilirium alulations and dynami gas-liquid partitioning performed several times during eah 3-D model time step (typially around 5 to 1 min). Eah gas/solid/liquid partitioning alulation typially involves an iterative numerial solution, whih requires repeated evaluation of the ativity oeffiients. [31] For example, any given solid-liquid and gas-liquid equilirium numerial solver typially requires 3 or more iterations per in to onverge, whih we denote as level 1. A disussion on the algorithms of our numerial solvers is eyond the sope of the present paper, and is deferred to future puliations. Our goal here is to estimate the relative CPU time requirements for different ativity oeffiient methods themselves, irrespetive of the solver osts. Thus, instead of atually solving any partiular type of equilirium system (solid/liquid or gas/liquid), we will simply re-evaluate the ativity oeffiients for a hosen multiomponent system for the speified numer of level-1 iteration to mimi an iterative solution. [32] Furthermore, sine the ioni strength in a sulfaterih solution is dependent on H + ion molality, the multiomponent ativity oeffiients are impliit nonlinear funtions of H +. Therefore a sulfate-rih system needs an additional level of iterations for every level-1 iteration to solve the isulfate dissoiation equilirium when using ioni-strength-ased methods suh as KM, Bromley, and PSC. These iterations are referred to as level-2 iterations (note that our usage of level-1/2 iteration loops differs somewhat from Jaoson [1999]). We use the isetion and seant methods in series to numerially solve the dissoiation equilirium, whih require a total of aout 1 iterations for m H + to onverge to a solution with relative error less than.1%. Sine there are aout ten g A (I) oeffiients for oth the sulfate-poor and sulfate-rih domains, eah level-1 iteration potentially entails 1 to 1 evaluations depending on the sulfate domain of eah in. On the other hand, eause RH is usually assumed to remain onstant during a model time step, the 166 g A E (a w ) oeffiients in the MTEM method have to e omputed only one at every grid point at the eginning of the time step. Sine MTEM yields a noniterative solution of the isulfate ion dissoiation in sulfate-rih systems, it ompletely eliminates the level-2 iterations in sulfate-rih systems. [33] Finally, instead of evaluating expensive polynomials during eah iteration, the omputational effiieny of MTEM, KM, and Bromley is inreased y reating an internal array of prealulated g A (I) and g A E (a w ) at very fine inrements of ioni strength (.1 mol kg 1 ) and water ativity (.1), respetively, whih an e queried during runtime as is done in ISORROPIA [Nenes et al., 1998]. One g A (I) and g A E (a w ) are updated, the KM and Bromley mixing rules require 2N summations while MTEM requires N 2 summations to ompute the multiomponent g A where N is the numer of eletrolytes present in the solution. [3] Figure 7 shows plots of the relative CPU time for KM and PSC with respet to MTEM as a funtion of numer of level-1 iterations. For a sulfate-poor system (test 13 of 23
Mean Activity Coefficients of Peroxodisulfates in Saturated Solutions of the Conversion System 2NH 4. H 2 O at 20 C and 30 C
 Mean Ativity Coeffiients of Peroxodisulfates in Saturated Solutions of the Conversion System NH 4 Na S O 8 H O at 0 C and 0 C Jan Balej Heřmanova 5, 170 00 Prague 7, Czeh Republi balejan@seznam.z Abstrat:
Mean Ativity Coeffiients of Peroxodisulfates in Saturated Solutions of the Conversion System NH 4 Na S O 8 H O at 0 C and 0 C Jan Balej Heřmanova 5, 170 00 Prague 7, Czeh Republi balejan@seznam.z Abstrat:
General Equilibrium. What happens to cause a reaction to come to equilibrium?
 General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
General Equilibrium Chemial Equilibrium Most hemial reations that are enountered are reversible. In other words, they go fairly easily in either the forward or reverse diretions. The thing to remember
A simple expression for radial distribution functions of pure fluids and mixtures
 A simple expression for radial distribution funtions of pure fluids and mixtures Enrio Matteoli a) Istituto di Chimia Quantistia ed Energetia Moleolare, CNR, Via Risorgimento, 35, 56126 Pisa, Italy G.
A simple expression for radial distribution funtions of pure fluids and mixtures Enrio Matteoli a) Istituto di Chimia Quantistia ed Energetia Moleolare, CNR, Via Risorgimento, 35, 56126 Pisa, Italy G.
Homework Assignment Number Two. where, for the oxygen molecule κ = ,
 D. eer, MSE 506, Dept. o Materials Siene & Engineering, University o Tennessee, noville Homework Assignment Numer Two Prolem. Consider the Peng-Roinson Equation o state as given elow. The ritial temperature
D. eer, MSE 506, Dept. o Materials Siene & Engineering, University o Tennessee, noville Homework Assignment Numer Two Prolem. Consider the Peng-Roinson Equation o state as given elow. The ritial temperature
Math 151 Introduction to Eigenvectors
 Math 151 Introdution to Eigenvetors The motivating example we used to desrie matrixes was landsape hange and vegetation suession. We hose the simple example of Bare Soil (B), eing replaed y Grasses (G)
Math 151 Introdution to Eigenvetors The motivating example we used to desrie matrixes was landsape hange and vegetation suession. We hose the simple example of Bare Soil (B), eing replaed y Grasses (G)
ELECTROCHEMISTRY Lecture/Lession Plan -1
 Chapter 4 ELECTROCHEMISTRY Leture/Lession Plan -1 ELECTROCHEMISTRY 4.1 Conept of eletrohemistry Eletrohemistry is a branh of hemistry where we will study how hemial energy an be transformed into eletrial
Chapter 4 ELECTROCHEMISTRY Leture/Lession Plan -1 ELECTROCHEMISTRY 4.1 Conept of eletrohemistry Eletrohemistry is a branh of hemistry where we will study how hemial energy an be transformed into eletrial
Analysis of discretization in the direct simulation Monte Carlo
 PHYSICS OF FLUIDS VOLUME 1, UMBER 1 OCTOBER Analysis of disretization in the diret simulation Monte Carlo iolas G. Hadjionstantinou a) Department of Mehanial Engineering, Massahusetts Institute of Tehnology,
PHYSICS OF FLUIDS VOLUME 1, UMBER 1 OCTOBER Analysis of disretization in the diret simulation Monte Carlo iolas G. Hadjionstantinou a) Department of Mehanial Engineering, Massahusetts Institute of Tehnology,
A population of 50 flies is expected to double every week, leading to a function of the x
 4 Setion 4.3 Logarithmi Funtions population of 50 flies is epeted to doule every week, leading to a funtion of the form f ( ) 50(), where represents the numer of weeks that have passed. When will this
4 Setion 4.3 Logarithmi Funtions population of 50 flies is epeted to doule every week, leading to a funtion of the form f ( ) 50(), where represents the numer of weeks that have passed. When will this
Buckling loads of columns of regular polygon cross-section with constant volume and clamped ends
 76 Bukling loads of olumns of regular polygon ross-setion with onstant volume and lamped ends Byoung Koo Lee Dept. of Civil Engineering, Wonkwang University, Iksan, Junuk, 7-79, Korea Email: kleest@wonkwang.a.kr
76 Bukling loads of olumns of regular polygon ross-setion with onstant volume and lamped ends Byoung Koo Lee Dept. of Civil Engineering, Wonkwang University, Iksan, Junuk, 7-79, Korea Email: kleest@wonkwang.a.kr
UPPER-TRUNCATED POWER LAW DISTRIBUTIONS
 Fratals, Vol. 9, No. (00) 09 World Sientifi Publishing Company UPPER-TRUNCATED POWER LAW DISTRIBUTIONS STEPHEN M. BURROUGHS and SARAH F. TEBBENS College of Marine Siene, University of South Florida, St.
Fratals, Vol. 9, No. (00) 09 World Sientifi Publishing Company UPPER-TRUNCATED POWER LAW DISTRIBUTIONS STEPHEN M. BURROUGHS and SARAH F. TEBBENS College of Marine Siene, University of South Florida, St.
An Improved Model for Calculating Heats of Dilution and Equilibrium Constants for High Temperature Aqueous Electrolyte Solutions
 Brigham Young University BYU SholarsArhive All Theses and Dissertations 2007-01-08 An Improved Model for Calulating Heats of Dilution and Equilibrium Constants for High Temperature Aqueous Eletrolyte Solutions
Brigham Young University BYU SholarsArhive All Theses and Dissertations 2007-01-08 An Improved Model for Calulating Heats of Dilution and Equilibrium Constants for High Temperature Aqueous Eletrolyte Solutions
Chapter 14. The Concept of Equilibrium and the Equilibrium Constant. We have for the most part depicted reactions as going one way.
 Chapter 14 The Conept of Equilibrium and the Equilibrium Constant In hapter 1 we dealt with Physial Equilibrium Physial Changes HO 2 (l) HO 2 (g) In hapter 14 we will learn about Chemial Equilibrium. We
Chapter 14 The Conept of Equilibrium and the Equilibrium Constant In hapter 1 we dealt with Physial Equilibrium Physial Changes HO 2 (l) HO 2 (g) In hapter 14 we will learn about Chemial Equilibrium. We
Millennium Relativity Acceleration Composition. The Relativistic Relationship between Acceleration and Uniform Motion
 Millennium Relativity Aeleration Composition he Relativisti Relationship between Aeleration and niform Motion Copyright 003 Joseph A. Rybzyk Abstrat he relativisti priniples developed throughout the six
Millennium Relativity Aeleration Composition he Relativisti Relationship between Aeleration and niform Motion Copyright 003 Joseph A. Rybzyk Abstrat he relativisti priniples developed throughout the six
The Complete Energy Translations in the Detailed. Decay Process of Baryonic Sub-Atomic Particles. P.G.Bass.
 The Complete Energy Translations in the Detailed Deay Proess of Baryoni Su-Atomi Partiles. [4] P.G.Bass. PGBass P12 Version 1..3 www.relativitydomains.om August 218 Astrat. This is the final paper on the
The Complete Energy Translations in the Detailed Deay Proess of Baryoni Su-Atomi Partiles. [4] P.G.Bass. PGBass P12 Version 1..3 www.relativitydomains.om August 218 Astrat. This is the final paper on the
STATIC SIMULATION PROGRAM OF COPPER SOLVENT EXTRACTION CONFIGURATIONS USING MICROSOFT EXCEL SOLVER
 STATIC SIMULATION PROGRAM OF COPPER SOLVENT EXTRACTION CONFIGURATIONS USING MICROSOFT EXCEL SOLVER Joseph Kafumbila This textbook provides the proedure for design stati simulation programs using Exel Solver
STATIC SIMULATION PROGRAM OF COPPER SOLVENT EXTRACTION CONFIGURATIONS USING MICROSOFT EXCEL SOLVER Joseph Kafumbila This textbook provides the proedure for design stati simulation programs using Exel Solver
Appendix A Market-Power Model of Business Groups. Robert C. Feenstra Deng-Shing Huang Gary G. Hamilton Revised, November 2001
 Appendix A Market-Power Model of Business Groups Roert C. Feenstra Deng-Shing Huang Gary G. Hamilton Revised, Novemer 200 Journal of Eonomi Behavior and Organization, 5, 2003, 459-485. To solve for the
Appendix A Market-Power Model of Business Groups Roert C. Feenstra Deng-Shing Huang Gary G. Hamilton Revised, Novemer 200 Journal of Eonomi Behavior and Organization, 5, 2003, 459-485. To solve for the
5.2 THE EFFECT OF TURBULENCE ON CLOUD MICROSTRUCTURE, PRECIPITATION FORMATION AND THE ORGANISATION OF STRATOCUMULUS AND SHALLOW CUMULUS CONVECTION
 5.2 THE EFFECT OF TURBULENCE ON CLOUD MICROSTRUCTURE, PRECIPITATION FORMATION AND THE ORGANISATION OF STRATOCUMULUS AND SHALLOW CUMULUS CONVECTION Charmaine N. Franklin * Centre for Australian Weather
5.2 THE EFFECT OF TURBULENCE ON CLOUD MICROSTRUCTURE, PRECIPITATION FORMATION AND THE ORGANISATION OF STRATOCUMULUS AND SHALLOW CUMULUS CONVECTION Charmaine N. Franklin * Centre for Australian Weather
DIGITAL DISTANCE RELAYING SCHEME FOR PARALLEL TRANSMISSION LINES DURING INTER-CIRCUIT FAULTS
 CHAPTER 4 DIGITAL DISTANCE RELAYING SCHEME FOR PARALLEL TRANSMISSION LINES DURING INTER-CIRCUIT FAULTS 4.1 INTRODUCTION Around the world, environmental and ost onsiousness are foring utilities to install
CHAPTER 4 DIGITAL DISTANCE RELAYING SCHEME FOR PARALLEL TRANSMISSION LINES DURING INTER-CIRCUIT FAULTS 4.1 INTRODUCTION Around the world, environmental and ost onsiousness are foring utilities to install
Copyright 2018 Society of Photo-Optical Instrumentation Engineers (SPIE). One print or electronic copy may be made for personal use only.
 Copyright 018 Soiety of Photo-Optial Instrumentation Engineers (SPIE) One print or eletroni opy may be made for personal use only Systemati reprodution and distribution, dupliation of any material in this
Copyright 018 Soiety of Photo-Optial Instrumentation Engineers (SPIE) One print or eletroni opy may be made for personal use only Systemati reprodution and distribution, dupliation of any material in this
Examining Applied Rational Functions
 HiMAP Pull-Out Setion: Summer 1990 Eamining Applied Rational Funtions Flod Vest Referenes Environmental Protetion Agen. Gas Mileage Guide. (Copies an usuall e otained from a loal new ar dealer.) Information
HiMAP Pull-Out Setion: Summer 1990 Eamining Applied Rational Funtions Flod Vest Referenes Environmental Protetion Agen. Gas Mileage Guide. (Copies an usuall e otained from a loal new ar dealer.) Information
QCLAS Sensor for Purity Monitoring in Medical Gas Supply Lines
 DOI.56/sensoren6/P3. QLAS Sensor for Purity Monitoring in Medial Gas Supply Lines Henrik Zimmermann, Mathias Wiese, Alessandro Ragnoni neoplas ontrol GmbH, Walther-Rathenau-Str. 49a, 7489 Greifswald, Germany
DOI.56/sensoren6/P3. QLAS Sensor for Purity Monitoring in Medial Gas Supply Lines Henrik Zimmermann, Mathias Wiese, Alessandro Ragnoni neoplas ontrol GmbH, Walther-Rathenau-Str. 49a, 7489 Greifswald, Germany
On the Buckling of Axially Restrained Steel Columns in Fire. University of Bath, Bath BA2 7AY, UK. University of Sheffield, Sheffield S1 3JD, UK
 Title On the Bukling of Axially Restrained Steel Columns in Fire Authors Affiliations P.G. Shepherd a * and I.W. Burgess a Department of Arhiteture & Civil Engineering University of Bath, Bath BA2 7AY,
Title On the Bukling of Axially Restrained Steel Columns in Fire Authors Affiliations P.G. Shepherd a * and I.W. Burgess a Department of Arhiteture & Civil Engineering University of Bath, Bath BA2 7AY,
A Cubic Equation of State for Reservoir Fluids
 6th IASME/WSEAS International Conferene on HEAT TRANSFER, THERMAL ENGINEERING and ENVIRONMENT (HTE'08) Rhodes, Greee, August 0-, 008 A Cubi Equation of State for Reservoir Fluids AMIR AHMAD SHIRAZIMANESH,
6th IASME/WSEAS International Conferene on HEAT TRANSFER, THERMAL ENGINEERING and ENVIRONMENT (HTE'08) Rhodes, Greee, August 0-, 008 A Cubi Equation of State for Reservoir Fluids AMIR AHMAD SHIRAZIMANESH,
Determination of the reaction order
 5/7/07 A quote of the wee (or amel of the wee): Apply yourself. Get all the eduation you an, but then... do something. Don't just stand there, mae it happen. Lee Iaoa Physial Chemistry GTM/5 reation order
5/7/07 A quote of the wee (or amel of the wee): Apply yourself. Get all the eduation you an, but then... do something. Don't just stand there, mae it happen. Lee Iaoa Physial Chemistry GTM/5 reation order
Pressure drop of gas liquid Taylor flow in round micro-capillaries for low to intermediate Reynolds numbers
 Mirofluid Nanofluid (200) 8:33 45 DOI 0.007/s0404-009-0448-z RESERCH PPER Pressure drop of gas liquid Taylor flow in round miro-apillaries for low to intermediate Reynolds numers M. J. F. Warnier Æ M.
Mirofluid Nanofluid (200) 8:33 45 DOI 0.007/s0404-009-0448-z RESERCH PPER Pressure drop of gas liquid Taylor flow in round miro-apillaries for low to intermediate Reynolds numers M. J. F. Warnier Æ M.
Supporting Information
 Supporting Information Olsman and Goentoro 10.1073/pnas.1601791113 SI Materials Analysis of the Sensitivity and Error Funtions. We now define the sensitivity funtion Sð, «0 Þ, whih summarizes the steepness
Supporting Information Olsman and Goentoro 10.1073/pnas.1601791113 SI Materials Analysis of the Sensitivity and Error Funtions. We now define the sensitivity funtion Sð, «0 Þ, whih summarizes the steepness
2 How far? Equilibrium Answers
 How far? Equilibrium Answers ratie: pages 37 39 1 Answer is D. Only a hange in temperature harges the value of the equilibrium onstant. Answer is D. [B] /[A] so [B] [A] or [B] [A] 1/ 3 Answer is B. Amounts
How far? Equilibrium Answers ratie: pages 37 39 1 Answer is D. Only a hange in temperature harges the value of the equilibrium onstant. Answer is D. [B] /[A] so [B] [A] or [B] [A] 1/ 3 Answer is B. Amounts
Coupled-cluster with active space selected higher amplitudes: Performance of seminatural orbitals for ground and excited state calculations
 THE JOURNAL OF CHEMICAL PHYSICS 125, 174110 2006 Coupled-luster with ative spae seleted higher amplitudes: Performane of seminatural oritals for ground and exited state alulations Andreas Köhn a and Jeppe
THE JOURNAL OF CHEMICAL PHYSICS 125, 174110 2006 Coupled-luster with ative spae seleted higher amplitudes: Performane of seminatural oritals for ground and exited state alulations Andreas Köhn a and Jeppe
Process engineers are often faced with the task of
 Fluids and Solids Handling Eliminate Iteration from Flow Problems John D. Barry Middough, In. This artile introdues a novel approah to solving flow and pipe-sizing problems based on two new dimensionless
Fluids and Solids Handling Eliminate Iteration from Flow Problems John D. Barry Middough, In. This artile introdues a novel approah to solving flow and pipe-sizing problems based on two new dimensionless
Gluing Potential Energy Surfaces with Rare Event Simulations
 This is an open aess artile published under an ACS AuthorChoie Liense, whih permits opying and redistribution of the artile or any adaptations for non-ommerial purposes. pubs.as.org/jctc Gluing Potential
This is an open aess artile published under an ACS AuthorChoie Liense, whih permits opying and redistribution of the artile or any adaptations for non-ommerial purposes. pubs.as.org/jctc Gluing Potential
Optimization of Statistical Decisions for Age Replacement Problems via a New Pivotal Quantity Averaging Approach
 Amerian Journal of heoretial and Applied tatistis 6; 5(-): -8 Published online January 7, 6 (http://www.sienepublishinggroup.om/j/ajtas) doi:.648/j.ajtas.s.65.4 IN: 36-8999 (Print); IN: 36-96 (Online)
Amerian Journal of heoretial and Applied tatistis 6; 5(-): -8 Published online January 7, 6 (http://www.sienepublishinggroup.om/j/ajtas) doi:.648/j.ajtas.s.65.4 IN: 36-8999 (Print); IN: 36-96 (Online)
Eigenvalues of tridiagonal matrix using Strum Sequence and Gerschgorin theorem
 Eigenvalues o tridiagonal matrix using Strum Sequene and Gershgorin theorem T.D.Roopamala Department o Computer Siene and Engg., Sri Jayahamarajendra College o Engineering Mysore INDIA roopa_td@yahoo.o.in
Eigenvalues o tridiagonal matrix using Strum Sequene and Gershgorin theorem T.D.Roopamala Department o Computer Siene and Engg., Sri Jayahamarajendra College o Engineering Mysore INDIA roopa_td@yahoo.o.in
23.1 Tuning controllers, in the large view Quoting from Section 16.7:
 Lesson 23. Tuning a real ontroller - modeling, proess identifiation, fine tuning 23.0 Context We have learned to view proesses as dynami systems, taking are to identify their input, intermediate, and output
Lesson 23. Tuning a real ontroller - modeling, proess identifiation, fine tuning 23.0 Context We have learned to view proesses as dynami systems, taking are to identify their input, intermediate, and output
An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2.
![An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2. An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2.](/thumbs/89/98214175.jpg) 1 An exess of a given reagent is added to eah of the following pairs of aqueous metal ions. For eah metal ion, state the initial olour of the solution and the final oservation that you would make. In eah
1 An exess of a given reagent is added to eah of the following pairs of aqueous metal ions. For eah metal ion, state the initial olour of the solution and the final oservation that you would make. In eah
LEARNING OBJECTIVES: UDOL.STES Discuss how mercury poisoning has affected the natural environment and human society.
 Multiple Choie 1. What is the primary reason for the ourrene of merury in the human ody? a. It is iologially inative and dormant.. It provides vital iologial funtions in trae amounts.. It is needed to
Multiple Choie 1. What is the primary reason for the ourrene of merury in the human ody? a. It is iologially inative and dormant.. It provides vital iologial funtions in trae amounts.. It is needed to
Chapter 15: Chemical Equilibrium
 Chapter 5: Chemial Equilibrium ahoot!. At eq, the rate of the forward reation is the rate of the reverse reation. equal to, slower than, faster than, the reverse of. Selet the statement that BEST desribes
Chapter 5: Chemial Equilibrium ahoot!. At eq, the rate of the forward reation is the rate of the reverse reation. equal to, slower than, faster than, the reverse of. Selet the statement that BEST desribes
FINITE WORD LENGTH EFFECTS IN DSP
 FINITE WORD LENGTH EFFECTS IN DSP PREPARED BY GUIDED BY Snehal Gor Dr. Srianth T. ABSTRACT We now that omputers store numbers not with infinite preision but rather in some approximation that an be paed
FINITE WORD LENGTH EFFECTS IN DSP PREPARED BY GUIDED BY Snehal Gor Dr. Srianth T. ABSTRACT We now that omputers store numbers not with infinite preision but rather in some approximation that an be paed
Notation 2, 8, 1 2, 8, 2 2, 8
 Page 90 Atomi struture 2 1 a Contains 3 protons (1); and 4 neutrons (1) Page 90 Eletroni struture 2 a 2, 8 Type of reation Ionisation Nulear fission Nulear fusion Change in mass of nuleus Stays the same
Page 90 Atomi struture 2 1 a Contains 3 protons (1); and 4 neutrons (1) Page 90 Eletroni struture 2 a 2, 8 Type of reation Ionisation Nulear fission Nulear fusion Change in mass of nuleus Stays the same
Surfactants in cloud droplet activation: mixed organic-inorganic particles
 Atmos. Chem. Phys., 0, 5663 5683, 200 www.atmos-hem-phys.net/0/5663/200/ doi:94/ap-0-5663-200 Author(s) 200. CC Attribution 3.0 Liense. Atmospheri Chemistry and Physis Surfatants in loud droplet ativation:
Atmos. Chem. Phys., 0, 5663 5683, 200 www.atmos-hem-phys.net/0/5663/200/ doi:94/ap-0-5663-200 Author(s) 200. CC Attribution 3.0 Liense. Atmospheri Chemistry and Physis Surfatants in loud droplet ativation:
Chemistry (Physical chemistry) Lecture 10.
 Chemistry (Physial hemistry) Leture 0. EPM, semester II by Wojieh Chrzanowsi, PhD, DS Wyłady współfinansowane ze środów Unii Europejsiej w ramah EFS, UDA-POKL 04.0.02.-00-37/-00 Absolwent Wydziału Chemiznego
Chemistry (Physial hemistry) Leture 0. EPM, semester II by Wojieh Chrzanowsi, PhD, DS Wyłady współfinansowane ze środów Unii Europejsiej w ramah EFS, UDA-POKL 04.0.02.-00-37/-00 Absolwent Wydziału Chemiznego
Conveyor trajectory discharge angles
 University of Wollongong Researh Online Faulty of Engineering - Papers (Arhive) Faulty of Engineering and Information Sienes 007 Conveyor trajetory disharge angles David B. Hastie University of Wollongong,
University of Wollongong Researh Online Faulty of Engineering - Papers (Arhive) Faulty of Engineering and Information Sienes 007 Conveyor trajetory disharge angles David B. Hastie University of Wollongong,
JF Physical Chemistry JF CH 1101: Introduction to Physical Chemistry.
 JF Physial Chemistry 010-011. JF CH 1101: Introdution to Physial Chemistry. Dr Mike Lyons. Shool of Chemistry Trinity College Dublin. melyons@td.ie A ompendium of past examination questions set on Physial
JF Physial Chemistry 010-011. JF CH 1101: Introdution to Physial Chemistry. Dr Mike Lyons. Shool of Chemistry Trinity College Dublin. melyons@td.ie A ompendium of past examination questions set on Physial
Methods of evaluating tests
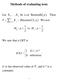 Methods of evaluating tests Let X,, 1 Xn be i.i.d. Bernoulli( p ). Then 5 j= 1 j ( 5, ) T = X Binomial p. We test 1 H : p vs. 1 1 H : p>. We saw that a LRT is 1 if t k* φ ( x ) =. otherwise (t is the observed
Methods of evaluating tests Let X,, 1 Xn be i.i.d. Bernoulli( p ). Then 5 j= 1 j ( 5, ) T = X Binomial p. We test 1 H : p vs. 1 1 H : p>. We saw that a LRT is 1 if t k* φ ( x ) =. otherwise (t is the observed
RESEARCH ON RANDOM FOURIER WAVE-NUMBER SPECTRUM OF FLUCTUATING WIND SPEED
 The Seventh Asia-Paifi Conferene on Wind Engineering, November 8-1, 9, Taipei, Taiwan RESEARCH ON RANDOM FORIER WAVE-NMBER SPECTRM OF FLCTATING WIND SPEED Qi Yan 1, Jie Li 1 Ph D. andidate, Department
The Seventh Asia-Paifi Conferene on Wind Engineering, November 8-1, 9, Taipei, Taiwan RESEARCH ON RANDOM FORIER WAVE-NMBER SPECTRM OF FLCTATING WIND SPEED Qi Yan 1, Jie Li 1 Ph D. andidate, Department
Sample Teaching Sequence (Hong Kong Secondary 4 6 Chemistry)
 Revised (1 Sept 009 Sample Teahing Suene (Hong Kong Seondary 4 6 Chemistry Topi: Chemial Equilibrium Teahing Suene Content 1.1 Reversible reations Examples of reversible reation; forward reation; reverse
Revised (1 Sept 009 Sample Teahing Suene (Hong Kong Seondary 4 6 Chemistry Topi: Chemial Equilibrium Teahing Suene Content 1.1 Reversible reations Examples of reversible reation; forward reation; reverse
Theory of Thermodynamic Variables of Rubber Band Heat Engine
 Journal of Physis: onferene Series PAPER OPEN AESS Theory of Thermodynami Variales of Ruer Band Heat Engine To ite this artile: Nurhidayah Muharayu et al 016 J. Phys.: onf. Ser. 739 01091 Related ontent
Journal of Physis: onferene Series PAPER OPEN AESS Theory of Thermodynami Variales of Ruer Band Heat Engine To ite this artile: Nurhidayah Muharayu et al 016 J. Phys.: onf. Ser. 739 01091 Related ontent
Morphological Stability of Diffusion Couples Under Electric Current
 Journal of ELECTRONIC MATERIALS, Vol. 39, No. 2, 2 DOI:.7/s664--368- Ó 2 TMS Morphologial Staility of Diffusion Couples Under Eletri Current PERRY LEO,2 ANA RASETTI,3. Department of Aerospae Engineering
Journal of ELECTRONIC MATERIALS, Vol. 39, No. 2, 2 DOI:.7/s664--368- Ó 2 TMS Morphologial Staility of Diffusion Couples Under Eletri Current PERRY LEO,2 ANA RASETTI,3. Department of Aerospae Engineering
Answer: Easiest way to determine equilibrium concentrations is to set up a table as follows: 2 SO 2 + O 2 2 SO 3 initial conc change
 Problem #1 6 mol of SO and 4 mol of O are plaed into a 1 L flask at temperature, T. The equilibrium onentration of SO is found to be 4 mol/l. Determine K. SO (g) + O (g) SO (g) K = [SO ] / [SO ] [O ] Answer:
Problem #1 6 mol of SO and 4 mol of O are plaed into a 1 L flask at temperature, T. The equilibrium onentration of SO is found to be 4 mol/l. Determine K. SO (g) + O (g) SO (g) K = [SO ] / [SO ] [O ] Answer:
Click here to order this book in one of two formats: softcover ISBN: $50.00 ISBN: $50.00
 ere is a sample hapter from Letures on Radiation Dosimetry Physis: A Deeper Look into the Foundations of Clinial Protools his sample hapter is opyrighted and made availale for personal use only No part
ere is a sample hapter from Letures on Radiation Dosimetry Physis: A Deeper Look into the Foundations of Clinial Protools his sample hapter is opyrighted and made availale for personal use only No part
The Effectiveness of the Linear Hull Effect
 The Effetiveness of the Linear Hull Effet S. Murphy Tehnial Report RHUL MA 009 9 6 Otober 009 Department of Mathematis Royal Holloway, University of London Egham, Surrey TW0 0EX, England http://www.rhul.a.uk/mathematis/tehreports
The Effetiveness of the Linear Hull Effet S. Murphy Tehnial Report RHUL MA 009 9 6 Otober 009 Department of Mathematis Royal Holloway, University of London Egham, Surrey TW0 0EX, England http://www.rhul.a.uk/mathematis/tehreports
Ordering Generalized Trapezoidal Fuzzy Numbers. Using Orthocentre of Centroids
 International Journal of lgera Vol. 6 no. 69-85 Ordering Generalized Trapezoidal Fuzzy Numers Using Orthoentre of Centroids Y. L. P. Thorani P. Phani Bushan ao and N. avi Shanar Dept. of pplied Mathematis
International Journal of lgera Vol. 6 no. 69-85 Ordering Generalized Trapezoidal Fuzzy Numers Using Orthoentre of Centroids Y. L. P. Thorani P. Phani Bushan ao and N. avi Shanar Dept. of pplied Mathematis
A Stochastic Analysis of Liquid Mixing in Bubble Column
 Amerian Journal of Fluid Dynamis 013, 3(3): 75-79 DOI: 193/j.ajfd.0130303.04 A Stohasti Analysis of Liquid Mixing in Bubble Column Rajeev Parmar, Subrata Kumar Majumder * Department of Chemial Engineering,
Amerian Journal of Fluid Dynamis 013, 3(3): 75-79 DOI: 193/j.ajfd.0130303.04 A Stohasti Analysis of Liquid Mixing in Bubble Column Rajeev Parmar, Subrata Kumar Majumder * Department of Chemial Engineering,
A NETWORK SIMPLEX ALGORITHM FOR THE MINIMUM COST-BENEFIT NETWORK FLOW PROBLEM
 NETWORK SIMPLEX LGORITHM FOR THE MINIMUM COST-BENEFIT NETWORK FLOW PROBLEM Cen Çalışan, Utah Valley University, 800 W. University Parway, Orem, UT 84058, 801-863-6487, en.alisan@uvu.edu BSTRCT The minimum
NETWORK SIMPLEX LGORITHM FOR THE MINIMUM COST-BENEFIT NETWORK FLOW PROBLEM Cen Çalışan, Utah Valley University, 800 W. University Parway, Orem, UT 84058, 801-863-6487, en.alisan@uvu.edu BSTRCT The minimum
Supplementary Materials
 Supplementary Materials Neural population partitioning and a onurrent brain-mahine interfae for sequential motor funtion Maryam M. Shanehi, Rollin C. Hu, Marissa Powers, Gregory W. Wornell, Emery N. Brown
Supplementary Materials Neural population partitioning and a onurrent brain-mahine interfae for sequential motor funtion Maryam M. Shanehi, Rollin C. Hu, Marissa Powers, Gregory W. Wornell, Emery N. Brown
Complexity of Regularization RBF Networks
 Complexity of Regularization RBF Networks Mark A Kon Department of Mathematis and Statistis Boston University Boston, MA 02215 mkon@buedu Leszek Plaskota Institute of Applied Mathematis University of Warsaw
Complexity of Regularization RBF Networks Mark A Kon Department of Mathematis and Statistis Boston University Boston, MA 02215 mkon@buedu Leszek Plaskota Institute of Applied Mathematis University of Warsaw
The Laws of Acceleration
 The Laws of Aeleration The Relationships between Time, Veloity, and Rate of Aeleration Copyright 2001 Joseph A. Rybzyk Abstrat Presented is a theory in fundamental theoretial physis that establishes the
The Laws of Aeleration The Relationships between Time, Veloity, and Rate of Aeleration Copyright 2001 Joseph A. Rybzyk Abstrat Presented is a theory in fundamental theoretial physis that establishes the
Singular Event Detection
 Singular Event Detetion Rafael S. Garía Eletrial Engineering University of Puerto Rio at Mayagüez Rafael.Garia@ee.uprm.edu Faulty Mentor: S. Shankar Sastry Researh Supervisor: Jonathan Sprinkle Graduate
Singular Event Detetion Rafael S. Garía Eletrial Engineering University of Puerto Rio at Mayagüez Rafael.Garia@ee.uprm.edu Faulty Mentor: S. Shankar Sastry Researh Supervisor: Jonathan Sprinkle Graduate
the cold 5-eV electrons. The energy E* of the
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 88, NO. A10, PAGES 8097-8102, OCTOBER 1, 1983 THE NON-MAXWELLIAN ENERGY DISTRIBUTION OF IONS IN THE WARM IO TORUS John D. Rihardson and George L. Sisoe Department
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 88, NO. A10, PAGES 8097-8102, OCTOBER 1, 1983 THE NON-MAXWELLIAN ENERGY DISTRIBUTION OF IONS IN THE WARM IO TORUS John D. Rihardson and George L. Sisoe Department
MultiPhysics Analysis of Trapped Field in Multi-Layer YBCO Plates
 Exerpt from the Proeedings of the COMSOL Conferene 9 Boston MultiPhysis Analysis of Trapped Field in Multi-Layer YBCO Plates Philippe. Masson Advaned Magnet Lab *7 Main Street, Bldg. #4, Palm Bay, Fl-95,
Exerpt from the Proeedings of the COMSOL Conferene 9 Boston MultiPhysis Analysis of Trapped Field in Multi-Layer YBCO Plates Philippe. Masson Advaned Magnet Lab *7 Main Street, Bldg. #4, Palm Bay, Fl-95,
Ab initio structures and energetics of selected hydrogenated silicon clusters containing six to ten silicon atoms
 9 July 1999 Ž. Chemial Physis Letters 307 1999 527 532 www.elsevier.nlrloaterplett A initio strutures and energetis of seleted hydrogenated silion lusters ontaining six to ten silion atoms Mark T. Swihart
9 July 1999 Ž. Chemial Physis Letters 307 1999 527 532 www.elsevier.nlrloaterplett A initio strutures and energetis of seleted hydrogenated silion lusters ontaining six to ten silion atoms Mark T. Swihart
The prediction of conveyor trajectories
 University of Wollongong Researh Online Faulty of Engineering - Papers (Arhive) Faulty of Engineering and Information Sienes 007 The predition of onveyor trajetories David B. Hastie University of Wollongong,
University of Wollongong Researh Online Faulty of Engineering - Papers (Arhive) Faulty of Engineering and Information Sienes 007 The predition of onveyor trajetories David B. Hastie University of Wollongong,
EFFECTIVE STRESS LAW FOR THE PERMEABILITY OF CLAY-RICH SANDSTONES
 SCA22-5 1/6 EFFECTIVE STRESS LAW FOR THE PERMEABILITY OF CLAY-RICH SANDSTONES Widad Al-Wardy and Robert W. Zimmerman Department of Earth Siene and Engineering Imperial College of Siene, Tehnology and Mediine
SCA22-5 1/6 EFFECTIVE STRESS LAW FOR THE PERMEABILITY OF CLAY-RICH SANDSTONES Widad Al-Wardy and Robert W. Zimmerman Department of Earth Siene and Engineering Imperial College of Siene, Tehnology and Mediine
A Heuristic Approach for Design and Calculation of Pressure Distribution over Naca 4 Digit Airfoil
 IOSR Journal of Engineering (IOSRJEN) ISSN (e): 2250-3021, ISSN (p): 2278-8719 PP 11-15 www.iosrjen.org A Heuristi Approah for Design and Calulation of Pressure Distribution over Naa 4 Digit Airfoil G.
IOSR Journal of Engineering (IOSRJEN) ISSN (e): 2250-3021, ISSN (p): 2278-8719 PP 11-15 www.iosrjen.org A Heuristi Approah for Design and Calulation of Pressure Distribution over Naa 4 Digit Airfoil G.
KINETICS OF IRON OXIDE DIRECT REDUCTION BY COAL E.R. ABRIL 1
 KINETICS OF IRON OXIDE DIRECT REDUCTION BY COAL E.R. ABRIL 1 CIMM- Av.Velez Sarsfield 1561 C.P.5000 Córdoba, Argentina. aabril@intiemor.gov.ar Abstrat - A new interpretation to the kinetis of iron oxide
KINETICS OF IRON OXIDE DIRECT REDUCTION BY COAL E.R. ABRIL 1 CIMM- Av.Velez Sarsfield 1561 C.P.5000 Córdoba, Argentina. aabril@intiemor.gov.ar Abstrat - A new interpretation to the kinetis of iron oxide
The Impact of Information on the Performance of an M/M/1 Queueing System
 The Impat of Information on the Performane of an M/M/1 Queueing System by Mojgan Nasiri A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master
The Impat of Information on the Performane of an M/M/1 Queueing System by Mojgan Nasiri A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master
CEE 670 TRANSPORT PROCESSES IN ENVIRONMENTAL AND WATER RESOURCES ENGINEERING. Kinetics Lecture #1
 Updated: 8 Deember 0 Print version CEE 670 TRNSPORT PROCESSES IN ENVIRONMENTL ND WTER RESOURCES ENGINEERING Kinetis Leture # Introdution: Simple Rate Laws Clark, 9.-9.6 Brezonik, pp.-39 Introdution Kinetis
Updated: 8 Deember 0 Print version CEE 670 TRNSPORT PROCESSES IN ENVIRONMENTL ND WTER RESOURCES ENGINEERING Kinetis Leture # Introdution: Simple Rate Laws Clark, 9.-9.6 Brezonik, pp.-39 Introdution Kinetis
SRC Research. Report. An Efficient Matching Algorithm for a High-Throughput, Low-Latency Data Switch. Thomas L. Rodeheffer and James B.
 Novemer 5, 998 SRC Researh Report 6 An Effiient Mathing Algorithm for a High-Throughput, Low-Lateny Data Swith Thomas L Rodeheffer and James B Saxe Systems Researh Center 30 Lytton Avenue Palo Alto, CA
Novemer 5, 998 SRC Researh Report 6 An Effiient Mathing Algorithm for a High-Throughput, Low-Lateny Data Swith Thomas L Rodeheffer and James B Saxe Systems Researh Center 30 Lytton Avenue Palo Alto, CA
Characterisation of Balcony Spill Plume Entrainment using Physical Scale Modelling
 Charaterisation of Balony Spill Plume Entrainment using Physial Sale Modelling ROGER HARRISON and MICHAEL SPEARPOINT Department of Civil and Natural Resoures Engineering University of Canterury Christhurh,
Charaterisation of Balony Spill Plume Entrainment using Physial Sale Modelling ROGER HARRISON and MICHAEL SPEARPOINT Department of Civil and Natural Resoures Engineering University of Canterury Christhurh,
Where as discussed previously we interpret solutions to this partial differential equation in the weak sense: b
 Consider the pure initial value problem for a homogeneous system of onservation laws with no soure terms in one spae dimension: Where as disussed previously we interpret solutions to this partial differential
Consider the pure initial value problem for a homogeneous system of onservation laws with no soure terms in one spae dimension: Where as disussed previously we interpret solutions to this partial differential
Variation Based Online Travel Time Prediction Using Clustered Neural Networks
 Variation Based Online Travel Time Predition Using lustered Neural Networks Jie Yu, Gang-Len hang, H.W. Ho and Yue Liu Abstrat-This paper proposes a variation-based online travel time predition approah
Variation Based Online Travel Time Predition Using lustered Neural Networks Jie Yu, Gang-Len hang, H.W. Ho and Yue Liu Abstrat-This paper proposes a variation-based online travel time predition approah
Multicomponent analysis on polluted waters by means of an electronic tongue
 Sensors and Atuators B 44 (1997) 423 428 Multiomponent analysis on polluted waters by means of an eletroni tongue C. Di Natale a, *, A. Maagnano a, F. Davide a, A. D Amio a, A. Legin b, Y. Vlasov b, A.
Sensors and Atuators B 44 (1997) 423 428 Multiomponent analysis on polluted waters by means of an eletroni tongue C. Di Natale a, *, A. Maagnano a, F. Davide a, A. D Amio a, A. Legin b, Y. Vlasov b, A.
Gas/aerosol partitioning: 1. A computationally efficient model
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D16, 4312, 10.1029/2001JD001102, 2002 Gas/aerosol partitioning: 1. A computationally efficient model Swen Metzger, 1 Frank Dentener, 2 Spyros Pandis, 3 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D16, 4312, 10.1029/2001JD001102, 2002 Gas/aerosol partitioning: 1. A computationally efficient model Swen Metzger, 1 Frank Dentener, 2 Spyros Pandis, 3 and
Supplementary Materials for A universal data based method for reconstructing complex networks with binary-state dynamics
 Supplementary Materials for A universal ata ase metho for reonstruting omplex networks with inary-state ynamis Jingwen Li, Zhesi Shen, Wen-Xu Wang, Celso Greogi, an Ying-Cheng Lai 1 Computation etails
Supplementary Materials for A universal ata ase metho for reonstruting omplex networks with inary-state ynamis Jingwen Li, Zhesi Shen, Wen-Xu Wang, Celso Greogi, an Ying-Cheng Lai 1 Computation etails
Modeling of Threading Dislocation Density Reduction in Heteroepitaxial Layers
 A. E. Romanov et al.: Threading Disloation Density Redution in Layers (II) 33 phys. stat. sol. (b) 99, 33 (997) Subjet lassifiation: 6.72.C; 68.55.Ln; S5.; S5.2; S7.; S7.2 Modeling of Threading Disloation
A. E. Romanov et al.: Threading Disloation Density Redution in Layers (II) 33 phys. stat. sol. (b) 99, 33 (997) Subjet lassifiation: 6.72.C; 68.55.Ln; S5.; S5.2; S7.; S7.2 Modeling of Threading Disloation
Advances in Radio Science
 Advanes in adio Siene 2003) 1: 99 104 Copernius GmbH 2003 Advanes in adio Siene A hybrid method ombining the FDTD and a time domain boundary-integral equation marhing-on-in-time algorithm A Beker and V
Advanes in adio Siene 2003) 1: 99 104 Copernius GmbH 2003 Advanes in adio Siene A hybrid method ombining the FDTD and a time domain boundary-integral equation marhing-on-in-time algorithm A Beker and V
Coastal Engineering 64 (2012) Contents lists available at SciVerse ScienceDirect. Coastal Engineering
 Coastal Engineering 64 (2012) 87 101 Contents lists available at SiVerse SieneDiret Coastal Engineering journal homepage: www.elsevier.om/loate/oastaleng Probability distribution of individual wave overtopping
Coastal Engineering 64 (2012) 87 101 Contents lists available at SiVerse SieneDiret Coastal Engineering journal homepage: www.elsevier.om/loate/oastaleng Probability distribution of individual wave overtopping
Sufficient Conditions for a Flexible Manufacturing System to be Deadlocked
 Paper 0, INT 0 Suffiient Conditions for a Flexile Manufaturing System to e Deadloked Paul E Deering, PhD Department of Engineering Tehnology and Management Ohio University deering@ohioedu Astrat In reent
Paper 0, INT 0 Suffiient Conditions for a Flexile Manufaturing System to e Deadloked Paul E Deering, PhD Department of Engineering Tehnology and Management Ohio University deering@ohioedu Astrat In reent
Stochastic boundary conditions to the convection-diffusion equation including chemical reactions at solid surfaces.
 Stohasti boundary onditions to the onvetion-diffusion equation inluding hemial reations at solid surfaes. P. Szymzak and A. J. C. Ladd Department of Chemial Engineering, University of Florida, Gainesville,
Stohasti boundary onditions to the onvetion-diffusion equation inluding hemial reations at solid surfaes. P. Szymzak and A. J. C. Ladd Department of Chemial Engineering, University of Florida, Gainesville,
An Adaptive Optimization Approach to Active Cancellation of Repeated Transient Vibration Disturbances
 An aptive Optimization Approah to Ative Canellation of Repeated Transient Vibration Disturbanes David L. Bowen RH Lyon Corp / Aenteh, 33 Moulton St., Cambridge, MA 138, U.S.A., owen@lyonorp.om J. Gregory
An aptive Optimization Approah to Ative Canellation of Repeated Transient Vibration Disturbanes David L. Bowen RH Lyon Corp / Aenteh, 33 Moulton St., Cambridge, MA 138, U.S.A., owen@lyonorp.om J. Gregory
Determining. quantitation levels for regulatory purposes. A quantitation level is proposed that should be quantifiable by a majority of laboratories.
 REGULATORY GRIDLOCK Determining quantitation levels for regulatory purposes A quantitation level is proposed that should e quantifiale y a majority of laoratories. Paul F. Sanders, R. Lee Lippinott, and
REGULATORY GRIDLOCK Determining quantitation levels for regulatory purposes A quantitation level is proposed that should e quantifiale y a majority of laoratories. Paul F. Sanders, R. Lee Lippinott, and
Edexcel GCSE Maths Foundation Skills Book Ratio, proportion and rates of change 1
 Guidane on the use of odes for this mark sheme ethod mark A C P ao oe ft Auray mark ark awarded independent of method Communiation mark Proof, proess or justifiation mark Corret answer only Or equivalent
Guidane on the use of odes for this mark sheme ethod mark A C P ao oe ft Auray mark ark awarded independent of method Communiation mark Proof, proess or justifiation mark Corret answer only Or equivalent
V. Interacting Particles
 V. Interating Partiles V.A The Cumulant Expansion The examples studied in the previous setion involve non-interating partiles. It is preisely the lak of interations that renders these problems exatly solvable.
V. Interating Partiles V.A The Cumulant Expansion The examples studied in the previous setion involve non-interating partiles. It is preisely the lak of interations that renders these problems exatly solvable.
Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene
 Exerpt from the Proeedings of the OMSOL onferene 010 Paris Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene M. Bekmann-Kluge 1 *,. errero 1, V. Shröder 1, A. Aikalin and J. Steinbah
Exerpt from the Proeedings of the OMSOL onferene 010 Paris Improvements in the Modeling of the Self-ignition of Tetrafluoroethylene M. Bekmann-Kluge 1 *,. errero 1, V. Shröder 1, A. Aikalin and J. Steinbah
Complete Shrimp Game Solution
 Complete Shrimp Game Solution Florian Ederer Feruary 7, 207 The inverse demand urve is given y P (Q a ; Q ; Q ) = 00 0:5 (Q a + Q + Q ) The pro t funtion for rm i = fa; ; g is i (Q a ; Q ; Q ) = Q i [P
Complete Shrimp Game Solution Florian Ederer Feruary 7, 207 The inverse demand urve is given y P (Q a ; Q ; Q ) = 00 0:5 (Q a + Q + Q ) The pro t funtion for rm i = fa; ; g is i (Q a ; Q ; Q ) = Q i [P
Answers to Coursebook questions Chapter J2
 Answers to Courseook questions Chapter J 1 a Partiles are produed in ollisions one example out of many is: a ollision of an eletron with a positron in a synhrotron. If we produe a pair of a partile and
Answers to Courseook questions Chapter J 1 a Partiles are produed in ollisions one example out of many is: a ollision of an eletron with a positron in a synhrotron. If we produe a pair of a partile and
7.1 Roots of a Polynomial
 7.1 Roots of a Polynomial A. Purpose Given the oeffiients a i of a polynomial of degree n = NDEG > 0, a 1 z n + a 2 z n 1 +... + a n z + a n+1 with a 1 0, this subroutine omputes the NDEG roots of the
7.1 Roots of a Polynomial A. Purpose Given the oeffiients a i of a polynomial of degree n = NDEG > 0, a 1 z n + a 2 z n 1 +... + a n z + a n+1 with a 1 0, this subroutine omputes the NDEG roots of the
Lightning electromagnetic environment in the presence of a tall grounded strike object
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL.,, doi:.9/4jd555, 5 Lightning eletromagneti environment in the presene of a tall grounded strike objet Yoshihiro Baba Department of Eletrial Engineering, Doshisha University,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL.,, doi:.9/4jd555, 5 Lightning eletromagneti environment in the presene of a tall grounded strike objet Yoshihiro Baba Department of Eletrial Engineering, Doshisha University,
Review of classical thermodynamics
 Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
Review of lassial thermodynamis Fundamental Laws, roperties and roesses () First Law - Energy Balane hermodynami funtions of state Internal energy, heat and work ypes of paths (isobari, isohori, isothermal,
Failure Assessment Diagram Analysis of Creep Crack Initiation in 316H Stainless Steel
 Failure Assessment Diagram Analysis of Creep Crak Initiation in 316H Stainless Steel C. M. Davies *, N. P. O Dowd, D. W. Dean, K. M. Nikbin, R. A. Ainsworth Department of Mehanial Engineering, Imperial
Failure Assessment Diagram Analysis of Creep Crak Initiation in 316H Stainless Steel C. M. Davies *, N. P. O Dowd, D. W. Dean, K. M. Nikbin, R. A. Ainsworth Department of Mehanial Engineering, Imperial
arxiv:gr-qc/ v2 6 Feb 2004
 Hubble Red Shift and the Anomalous Aeleration of Pioneer 0 and arxiv:gr-q/0402024v2 6 Feb 2004 Kostadin Trenčevski Faulty of Natural Sienes and Mathematis, P.O.Box 62, 000 Skopje, Maedonia Abstrat It this
Hubble Red Shift and the Anomalous Aeleration of Pioneer 0 and arxiv:gr-q/0402024v2 6 Feb 2004 Kostadin Trenčevski Faulty of Natural Sienes and Mathematis, P.O.Box 62, 000 Skopje, Maedonia Abstrat It this
UTC. Engineering 329. Proportional Controller Design. Speed System. John Beverly. Green Team. John Beverly Keith Skiles John Barker.
 UTC Engineering 329 Proportional Controller Design for Speed System By John Beverly Green Team John Beverly Keith Skiles John Barker 24 Mar 2006 Introdution This experiment is intended test the variable
UTC Engineering 329 Proportional Controller Design for Speed System By John Beverly Green Team John Beverly Keith Skiles John Barker 24 Mar 2006 Introdution This experiment is intended test the variable
A Longitudinal Aerodynamic Data Repeatability Study for a Commercial Transport Model Test in the National Transonic Facility
 NASA Tehnial Paper 3522 A Longitudinal Aerodynami Data Repeatability Study for a Commerial Transport Model Test in the National Transoni Faility R. A. Wahls and J. B. Adok Langley Researh Center Hampton,
NASA Tehnial Paper 3522 A Longitudinal Aerodynami Data Repeatability Study for a Commerial Transport Model Test in the National Transoni Faility R. A. Wahls and J. B. Adok Langley Researh Center Hampton,
DEVELOPMENT OF A MULTI-FEED P-T WELLBORE MODEL FOR GEOTHERMAL WELLS
 PROCEEDINGS, Thirty-First Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 3-February 1, 6 SGP-TR-179 DEVELOPMENT OF MULTI-FEED P-T WELLBORE MODEL FOR GEOTHERML
PROCEEDINGS, Thirty-First Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 3-February 1, 6 SGP-TR-179 DEVELOPMENT OF MULTI-FEED P-T WELLBORE MODEL FOR GEOTHERML
Calculation of acid dissociation constants
 Retrospetive Theses and Dissertations Iowa State University apstones, Theses and Dissertations 1964 alulation of aid dissoiation onstants Wayne Woodson Dunning Iowa State University Follow this and additional
Retrospetive Theses and Dissertations Iowa State University apstones, Theses and Dissertations 1964 alulation of aid dissoiation onstants Wayne Woodson Dunning Iowa State University Follow this and additional
Phase Diffuser at the Transmitter for Lasercom Link: Effect of Partially Coherent Beam on the Bit-Error Rate.
 Phase Diffuser at the Transmitter for Laserom Link: Effet of Partially Coherent Beam on the Bit-Error Rate. O. Korotkova* a, L. C. Andrews** a, R. L. Phillips*** b a Dept. of Mathematis, Univ. of Central
Phase Diffuser at the Transmitter for Laserom Link: Effet of Partially Coherent Beam on the Bit-Error Rate. O. Korotkova* a, L. C. Andrews** a, R. L. Phillips*** b a Dept. of Mathematis, Univ. of Central
( ) ( ) Volumetric Properties of Pure Fluids, part 4. The generic cubic equation of state:
 CE304, Spring 2004 Leture 6 Volumetri roperties of ure Fluids, part 4 The generi ubi equation of state: There are many possible equations of state (and many have been proposed) that have the same general
CE304, Spring 2004 Leture 6 Volumetri roperties of ure Fluids, part 4 The generi ubi equation of state: There are many possible equations of state (and many have been proposed) that have the same general
Developing Excel Macros for Solving Heat Diffusion Problems
 Session 50 Developing Exel Maros for Solving Heat Diffusion Problems N. N. Sarker and M. A. Ketkar Department of Engineering Tehnology Prairie View A&M University Prairie View, TX 77446 Abstrat This paper
Session 50 Developing Exel Maros for Solving Heat Diffusion Problems N. N. Sarker and M. A. Ketkar Department of Engineering Tehnology Prairie View A&M University Prairie View, TX 77446 Abstrat This paper
Likelihood-confidence intervals for quantiles in Extreme Value Distributions
 Likelihood-onfidene intervals for quantiles in Extreme Value Distributions A. Bolívar, E. Díaz-Franés, J. Ortega, and E. Vilhis. Centro de Investigaión en Matemátias; A.P. 42, Guanajuato, Gto. 36; Méxio
Likelihood-onfidene intervals for quantiles in Extreme Value Distributions A. Bolívar, E. Díaz-Franés, J. Ortega, and E. Vilhis. Centro de Investigaión en Matemátias; A.P. 42, Guanajuato, Gto. 36; Méxio
Calibration of Piping Assessment Models in the Netherlands
 ISGSR 2011 - Vogt, Shuppener, Straub & Bräu (eds) - 2011 Bundesanstalt für Wasserbau ISBN 978-3-939230-01-4 Calibration of Piping Assessment Models in the Netherlands J. Lopez de la Cruz & E.O.F. Calle
ISGSR 2011 - Vogt, Shuppener, Straub & Bräu (eds) - 2011 Bundesanstalt für Wasserbau ISBN 978-3-939230-01-4 Calibration of Piping Assessment Models in the Netherlands J. Lopez de la Cruz & E.O.F. Calle
An Integrated Architecture of Adaptive Neural Network Control for Dynamic Systems
 An Integrated Arhiteture of Adaptive Neural Network Control for Dynami Systems Robert L. Tokar 2 Brian D.MVey2 'Center for Nonlinear Studies, 2Applied Theoretial Physis Division Los Alamos National Laboratory,
An Integrated Arhiteture of Adaptive Neural Network Control for Dynami Systems Robert L. Tokar 2 Brian D.MVey2 'Center for Nonlinear Studies, 2Applied Theoretial Physis Division Los Alamos National Laboratory,
