Chapter 2. Atoms, Molecules, and Ions
|
|
|
- Adam Wiggins
- 5 years ago
- Views:
Transcription
1 Chapter 2 Atoms, Molecules, and Ions
2 Section 2.1 The Early History of Chemistry Early History of Chemistry Greeks were the first to attempt to explain why chemical changes occur. Alchemy dominated for 2000 years. Several elements discovered. Mineral acids prepared. Robert Boyle was the first chemist. Performed quantitative experiments. Developed first experimental definition of an element. Copyright Cengage Learning. All rights reserved 2
3 Section 2.2 Fundamental Chemical Laws Three Important Laws Law of conservation of mass (Lavoisier): Mass is neither created nor destroyed in a chemical reaction. Law of definite proportion (Proust): A given compound always contains exactly the same proportion of elements by mass. Copyright Cengage Learning. All rights reserved 3
4 Section 2.2 Fundamental Chemical Laws Three Important Laws (continued) Law of multiple proportions (Dalton): When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers. Copyright Cengage Learning. All rights reserved 4
5 Section 2.3 Dalton s Atomic Theory Dalton s Atomic Theory (1808) Each element is made up of tiny particles called atoms. Copyright Cengage Learning. All rights reserved 5
6 Section 2.3 Dalton s Atomic Theory Dalton s Atomic Theory (continued) The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ways. Copyright Cengage Learning. All rights reserved 6
7 Section 2.3 Dalton s Atomic Theory Dalton s Atomic Theory (continued) Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms. Copyright Cengage Learning. All rights reserved 7
8 Section 2.3 Dalton s Atomic Theory Dalton s Atomic Theory (continued) Chemical reactions involve reorganization of the atoms changes in the way they are bound together. The atoms themselves are not changed in a chemical reaction. Copyright Cengage Learning. All rights reserved 8
9 Section 2.3 Dalton s Atomic Theory Gay-Lussac and Avogadro ( ) Gay Lussac Measured (under same conditions of T and P) the volumes of gases that reacted with each other. Avogadro s Hypothesis At the same T and P, equal volumes of different gases contain the same number of particles. Volume of a gas is determined by the number, not the size, of molecules. Copyright Cengage Learning. All rights reserved 9
10 Section 2.3 Dalton s Atomic Theory Representing Gay Lussac s Results Copyright Cengage Learning. All rights reserved 10
11 Section 2.3 Dalton s Atomic Theory Representing Gay Lussac s Results Copyright Cengage Learning. All rights reserved 11
12 Section 2.3 Dalton s Atomic Theory Representing Gay Lussac s Results Copyright Cengage Learning. All rights reserved 12
13 Section 2.3 Dalton s Atomic Theory Representing Gay Lussac s Results Copyright Cengage Learning. All rights reserved 13
14 Section 2.4 Early Experiments to Characterize the Atom J. J. Thomson ( ) Postulated the existence of negatively charged particles, that we now call electrons, using cathode-ray tubes. Determined the charge-to-mass ratio of an electron. The atom must also contain positive particles that balance exactly the negative charge carried by electrons. Copyright Cengage Learning. All rights reserved 14
15 Section 2.4 Early Experiments to Characterize the Atom Cathode-Ray Tube Copyright Cengage Learning. All rights reserved 15
16 Section 2.4 Early Experiments to Characterize the Atom Robert Millikan (1909) Performed experiments involving charged oil drops. Determined the magnitude of the charge on a single electron. Calculated the mass of the electron ( kg). Copyright Cengage Learning. All rights reserved 16
17 Section 2.4 Early Experiments to Characterize the Atom Millikan Oil Drop Experiment To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright Cengage Learning. All rights reserved 17
18 Section 2.4 Early Experiments to Characterize the Atom Henri Becquerel (1896) Discovered radioactivity by observing the spontaneous emission of radiation by uranium. Three types of radioactive emission exist: Gamma rays (ϒ) high energy light Beta particles (β) a high speed electron Alpha particles (α) a particle with a 2+ charge Copyright Cengage Learning. All rights reserved 18
19 Section 2.4 Early Experiments to Characterize the Atom Ernest Rutherford (1911) Explained the nuclear atom. The atom has a dense center of positive charge called the nucleus. Electrons travel around the nucleus at a large distance relative to the nucleus. Copyright Cengage Learning. All rights reserved 19
20 Section 2.5 The Modern View of Atomic Structure: An Introduction The atom contains: Electrons found outside the nucleus; negatively charged. Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge. Neutrons found in the nucleus; no charge; virtually same mass as a proton. Copyright Cengage Learning. All rights reserved 20
21 Section 2.5 The Modern View of Atomic Structure: An Introduction The nucleus is: Small compared with the overall size of the atom. Extremely dense; accounts for almost all of the atom s mass. Copyright Cengage Learning. All rights reserved 21
22 Section 2.5 The Modern View of Atomic Structure: An Introduction Nuclear Atom Viewed in Cross Section Copyright Cengage Learning. All rights reserved
23 Section 2.5 The Modern View of Atomic Structure: An Introduction Isotopes Atoms with the same number of protons but different numbers of neutrons. Show almost identical chemical properties; chemistry of atom is due to its electrons. In nature most elements contain mixtures of isotopes. Copyright Cengage Learning. All rights reserved 23
24 Section 2.5 The Modern View of Atomic Structure: An Introduction Two Isotopes of Sodium Copyright Cengage Learning. All rights reserved
25 Section 2.5 The Modern View of Atomic Structure: An Introduction Isotopes are identified by: Atomic Number (Z) number of protons Mass Number (A) number of protons plus number of neutrons Copyright Cengage Learning. All rights reserved 25
26 Section 2.5 The Modern View of Atomic Structure: An Introduction EXERCISE! A certain isotope X contains 23 protons and 28 neutrons. What is the mass number of this isotope? Identify the element. Mass Number = 51 Vanadium Copyright Cengage Learning. All rights reserved 26
27 Section 2.6 Molecules and Ions Chemical Bonds Covalent Bonds Bonds form between atoms by sharing electrons. Resulting collection of atoms is called a molecule. Copyright Cengage Learning. All rights reserved 27
28 Section 2.6 Molecules and Ions Covalent Bonding To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright Cengage Learning. All rights reserved 28
29 Section 2.6 Molecules and Ions Chemical Bonds Ionic Bonds Bonds form due to force of attraction between oppositely charged ions. Ion atom or group of atoms that has a net positive or negative charge. Cation positive ion; lost electron(s). Anion negative ion; gained electron(s). Copyright Cengage Learning. All rights reserved 29
30 Section 2.6 Molecules and Ions Molecular vs Ionic Compounds To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright Cengage Learning. All rights reserved 30
31 Section 2.6 Molecules and Ions EXERCISE! A certain isotope X + contains 54 electrons and 78 neutrons. What is the mass number of this isotope? 133 Copyright Cengage Learning. All rights reserved 31
32 Section 2.6 Molecules and Ions CONCEPT CHECK! Which of the following statements regarding Dalton s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestructible. Copyright Cengage Learning. All rights reserved 32
33 Section 2.7 An Introduction to the Periodic Table The Periodic Table Metals vs. Nonmetals Groups or Families elements in the same vertical columns; have similar chemical properties Periods horizontal rows of elements Copyright Cengage Learning. All rights reserved 33
34 Section 2.7 An Introduction to the Periodic Table The Periodic Table
35 Section 2.7 An Introduction to the Periodic Table Groups or Families Table of common charges formed when creating ionic compounds. Group or Family Charge Alkali Metals (1A) 1+ Alkaline Earth Metals (2A) 2+ Halogens (7A) 1 Noble Gases (8A) 0 Copyright Cengage Learning. All rights reserved 35
36 Section 2.8 Naming Simple Compounds Naming Compounds Binary Compounds Composed of two elements Ionic and covalent compounds included Binary Ionic Compounds Metal nonmetal Binary Covalent Compounds Nonmetal nonmetal Copyright Cengage Learning. All rights reserved 36
37 Section 2.8 Naming Simple Compounds Binary Ionic Compounds (Type I) 1. The cation is always named first and the anion second. 2. A monatomic cation takes its name from the name of the parent element. 3. A monatomic anion is named by taking the root of the element name and adding ide. Copyright Cengage Learning. All rights reserved 37
38 Section 2.8 Naming Simple Compounds Binary Ionic Compounds (Type I) Examples: KCl Potassium chloride MgBr 2 Magnesium bromide CaO Calcium oxide Copyright Cengage Learning. All rights reserved 38
39 Section 2.8 Naming Simple Compounds Binary Ionic Compounds (Type II) Metals in these compounds form more than one type of positive ion. Charge on the metal ion must be specified. Roman numeral indicates the charge of the metal cation. Transition metal cations usually require a Roman numeral. Elements that form only one cation do not need to be identified by a roman numeral. Copyright Cengage Learning. All rights reserved 39
40 Section 2.8 Naming Simple Compounds Binary Ionic Compounds (Type II) Examples: CuBr Copper(I) bromide FeS Iron(II) sulfide PbO 2 Lead(IV) oxide Copyright Cengage Learning. All rights reserved 40
41 Section 2.8 Naming Simple Compounds Polyatomic Ions Must be memorized (see Table 2.5 on pg. 65 in text). Examples of compounds containing polyatomic ions: NaOH Sodium hydroxide Mg(NO 3 ) 2 Magnesium nitrate (NH 4 ) 2 SO 4 Ammonium sulfate Copyright Cengage Learning. All rights reserved 41
42 Section 2.8 Naming Simple Compounds Formation of Ionic Compounds To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright Cengage Learning. All rights reserved 42
43 Section 2.8 Naming Simple Compounds Binary Covalent Compounds (Type III) Formed between two nonmetals. 1. The first element in the formula is named first, using the full element name. 2. The second element is named as if it were an anion. 3. Prefixes are used to denote the numbers of atoms present. 4. The prefix mono- is never used for naming the first element. Copyright Cengage Learning. All rights reserved 43
44 Section 2.8 Naming Simple Compounds Prefixes Used to Indicate Number in Chemical Names Copyright Cengage Learning. All rights reserved 44
45 Section 2.8 Naming Simple Compounds Binary Covalent Compounds (Type III) Examples: CO 2 Carbon dioxide SF 6 Sulfur hexafluoride N 2 O 4 Dinitrogen tetroxide Copyright Cengage Learning. All rights reserved 45
46 Section 2.8 Naming Simple Compounds Flowchart for Naming Binary Compounds Copyright Cengage Learning. All rights reserved 46
47 Section 2.8 Naming Simple Compounds Overall Strategy for Naming Chemical Compounds Copyright Cengage Learning. All rights reserved 47
48 Section 2.8 Naming Simple Compounds Acids Acids can be recognized by the hydrogen that appears first in the formula HCl. Molecule with one or more H + ions attached to an anion. Copyright Cengage Learning. All rights reserved 48
49 Section 2.8 Naming Simple Compounds Acids If the anion does not contain oxygen, the acid is named with the prefix hydro and the suffix ic. Examples: HCl HCN H 2 S Hydrochloric acid Hydrocyanic acid Hydrosulfuric acid Copyright Cengage Learning. All rights reserved 49
50 Section 2.8 Naming Simple Compounds Acids If the anion does contain oxygen: The suffix ic is added to the root name if the anion name ends in ate. Examples: HNO 3 H 2 SO 4 HC 2 H 3 O 2 Nitric acid Sulfuric acid Acetic acid Copyright Cengage Learning. All rights reserved 50
51 Section 2.8 Naming Simple Compounds Acids If the anion does contain oxygen: The suffix ous is added to the root name if the anion name ends in ite. Examples: HNO 2 Nitrous acid H 2 SO 3 Sulfurous acid HClO 2 Chlorous acid Copyright Cengage Learning. All rights reserved 51
52 Section 2.8 Naming Simple Compounds Flowchart for Naming Acids Copyright Cengage Learning. All rights reserved
53 Section 2.8 Naming Simple Compounds EXERCISE! Which of the following compounds is named incorrectly? a) KNO 3 potassium nitrate b) TiO 2 titanium(ii) oxide c) Sn(OH) 4 tin(iv) hydroxide d) PBr 5 phosphorus pentabromide e) CaCrO 4 calcium chromate Copyright Cengage Learning. All rights reserved 53
AP* Chapter 2. Atoms, Molecules, and Ions. Monday, September 16, 13
 AP* Chapter 2 Atoms, Molecules, and Ions AP Learning Objectives LO 1.1 The student can justify the observation that the ratio of the masses of the constituent elements in any pure sample of that compound
AP* Chapter 2 Atoms, Molecules, and Ions AP Learning Objectives LO 1.1 The student can justify the observation that the ratio of the masses of the constituent elements in any pure sample of that compound
Atoms, Molecules, and Ions
 Chapter 2 Atoms, Molecules, l and Ions Chapter 2 Table of Contents 2.1 The Early History of Chemistry 2.2 Fundamental Chemical Laws 23 2.3 Dalton s Atomic Theory 2.4 Early Experiments to Characterize the
Chapter 2 Atoms, Molecules, l and Ions Chapter 2 Table of Contents 2.1 The Early History of Chemistry 2.2 Fundamental Chemical Laws 23 2.3 Dalton s Atomic Theory 2.4 Early Experiments to Characterize the
Chapter 2 Atoms, Molecules and Ions
 Sec$on 2.1 The Early History of Chemistry Chapter 2 Atoms, Molecules and Ions Sec$on 2.1 The Early History of Chemistry Early History of Chemistry Greeks were the first to a?empt to explain why chemical
Sec$on 2.1 The Early History of Chemistry Chapter 2 Atoms, Molecules and Ions Sec$on 2.1 The Early History of Chemistry Early History of Chemistry Greeks were the first to a?empt to explain why chemical
Chemistry Chapter 2. Atoms, Molecules, and Ions Section Periodic Table Ions Chemical Bonds Nomenclature
 Chemistry Chapter 2 Atoms, Molecules, and Ions Section 2 2.6-2.8 Periodic Table Ions Chemical Bonds Nomenclature Organization of the Periodic Table Columns, Groups or Families elements in the same vertical
Chemistry Chapter 2 Atoms, Molecules, and Ions Section 2 2.6-2.8 Periodic Table Ions Chemical Bonds Nomenclature Organization of the Periodic Table Columns, Groups or Families elements in the same vertical
3/1/2017. Chapter 5. Nomenclature. Naming Compounds. Metal nonmetal. Section 5.1. Binary Compounds: Composed of two elements. Binary Ionic Compounds
 Chapter 5 Nomenclature Section 5.1 Naming Compounds Binary Compounds: Composed of two elements Binary Ionic Compounds Metal nonmetal Copyright Cengage Learning. All rights reserved 2 1 Binary Ionic Compounds
Chapter 5 Nomenclature Section 5.1 Naming Compounds Binary Compounds: Composed of two elements Binary Ionic Compounds Metal nonmetal Copyright Cengage Learning. All rights reserved 2 1 Binary Ionic Compounds
Chapter 2. Atoms, Molecules, and Ions. Copyright 2017 Cengage Learning. All Rights Reserved.
 Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 2. Atoms, Molecules, and Ions. Copyright 2018 Cengage Learning. All Rights Reserved.
 Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 1. Chemical Foundations
 Chapter 1 Chemical Foundations Chapter 1 Table of Contents (1.1) (1.2) (1.3) (1.4) (1.5) (1.6) (1.7) Chemistry: An atoms-first approach The scientific method The early history of chemistry Fundamental
Chapter 1 Chemical Foundations Chapter 1 Table of Contents (1.1) (1.2) (1.3) (1.4) (1.5) (1.6) (1.7) Chemistry: An atoms-first approach The scientific method The early history of chemistry Fundamental
Chapter 2: Atoms, Molecules and Ions
 Chapter 2: Atoms, Molecules and Ions Sep 24 10:24 AM 2.1 The Early History of Chemistry The Greek did not use experiments to establish the veracity of their ideas. Alchemy "gold making juice" Khem the
Chapter 2: Atoms, Molecules and Ions Sep 24 10:24 AM 2.1 The Early History of Chemistry The Greek did not use experiments to establish the veracity of their ideas. Alchemy "gold making juice" Khem the
Atoms, Molecules and Ions
 Atoms, Molecules and Ions Atomic Theory of Matter Modern Atomic Theory Relation to Periodic Table Isotopes & Mass Number Periodic Table Ionic Compounds Molecular Compounds Acid Compounds The Atomic Theory
Atoms, Molecules and Ions Atomic Theory of Matter Modern Atomic Theory Relation to Periodic Table Isotopes & Mass Number Periodic Table Ionic Compounds Molecular Compounds Acid Compounds The Atomic Theory
Chapter 2. Atoms, Molecules and Ions
 Chapter 2 Atoms, Molecules and Ions Atoms Ancient Greek philosophers had to invent the idea of atoms The only way to explain change: The rearrangement of unchanging pieces, with space between them Usually
Chapter 2 Atoms, Molecules and Ions Atoms Ancient Greek philosophers had to invent the idea of atoms The only way to explain change: The rearrangement of unchanging pieces, with space between them Usually
p38 Chapter Two: ATOMS, MOLECULES, AND IONS
 p38 Chapter Two: ATOMS, MOLECULES, AND IONS 2-1 The Early History of Chemistry p39 2-2 Fundamental Chemical Laws p41 Three Important Laws Law of conservation of mass Mass is neither created nor destroyed
p38 Chapter Two: ATOMS, MOLECULES, AND IONS 2-1 The Early History of Chemistry p39 2-2 Fundamental Chemical Laws p41 Three Important Laws Law of conservation of mass Mass is neither created nor destroyed
Chapter Two: Early History of Chemistry. Three Important Laws. Dalton s Atomic Theory (1808) Three Important Laws (continued) Greek Explanation
 Greek Explanation Chapter Two: ATOMS, MOLECULES, AND IONS Notes 2.1 In the Greek model they theorized there were four elements earth, water, air, and fire. These elements were characterized by the following
Greek Explanation Chapter Two: ATOMS, MOLECULES, AND IONS Notes 2.1 In the Greek model they theorized there were four elements earth, water, air, and fire. These elements were characterized by the following
Chapter 2. Atoms, Molecules, and Ions. Copyright 2018 Cengage Learning. All Rights Reserved.
 Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 2 Atoms, Molecules, and Ions Chapter 2 Table of Contents (2.1) (2.2) (2.3) (2.4) (2.5) (2.6) (2.7) (2.8) The early history of chemistry Fundamental chemical laws Dalton s atomic theory Early experiments
Chapter 2 : Atoms, Molecules, and Ions
 Chapter 2 : Atoms, Molecules, and Ions Parmenides (BC 515? BC 445?) : < 있는것 ( 토에온 )> 은있고 < 없는것 ( 토메에온 )> 은없다고하는전제 ( 前提 ) 에서불생불멸 불가분 불변부동이며, 완결된둥근공과비슷하다고하는 < 있는것 > 의속성을끌어내고, < 있는것 > 을우리에게보여주는이성만이진리를포착하며생성
Chapter 2 : Atoms, Molecules, and Ions Parmenides (BC 515? BC 445?) : < 있는것 ( 토에온 )> 은있고 < 없는것 ( 토메에온 )> 은없다고하는전제 ( 前提 ) 에서불생불멸 불가분 불변부동이며, 완결된둥근공과비슷하다고하는 < 있는것 > 의속성을끌어내고, < 있는것 > 을우리에게보여주는이성만이진리를포착하며생성
4. What is the law of constant composition (also known as the law of definite proportion)?
 Name: Exercises #1: 1. What is the law of conservation of mass? 2. Show that the results of the following experiments illustrate the law of conservation of mass. Experiment #1: a 5.00-g sample of pure
Name: Exercises #1: 1. What is the law of conservation of mass? 2. Show that the results of the following experiments illustrate the law of conservation of mass. Experiment #1: a 5.00-g sample of pure
Atoms, Molecules and Ions
 Atoms, Molecules and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
Atoms, Molecules and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
CHAPTER #2 - Atoms, Molecules, and Ions
 CHAPTER #2 - Atoms, Molecules, and Ions 2.1 The Early History of Chemistry - Ancient Greeks - thought matter was composed to 4 substances earth, air, fire, water ( vs. ) Alchemy - (discovered many elements;
CHAPTER #2 - Atoms, Molecules, and Ions 2.1 The Early History of Chemistry - Ancient Greeks - thought matter was composed to 4 substances earth, air, fire, water ( vs. ) Alchemy - (discovered many elements;
Voltage source. Voltage source. Voltage source
 Chapter 2 Atoms, Molecules, and Ions History Greeks Democritus and Leucippus atomos Aristotle elements Alchemy 1660 Robert Boyle experimental definition of element. Lavoisier Father of modern chemistry
Chapter 2 Atoms, Molecules, and Ions History Greeks Democritus and Leucippus atomos Aristotle elements Alchemy 1660 Robert Boyle experimental definition of element. Lavoisier Father of modern chemistry
4. What is the law of constant composition (also known as the law of definite proportion)?
 Name: Exercises #1: 1. What is the law of conservation of mass? 2. Show that the results of the following experiments illustrate the law of conservation of mass. Experiment #1: a 5.00-g sample of pure
Name: Exercises #1: 1. What is the law of conservation of mass? 2. Show that the results of the following experiments illustrate the law of conservation of mass. Experiment #1: a 5.00-g sample of pure
Chemical Bonding and Naming Compounds. Ionic. Acid. Base. Oct 4 7:40 PM
 Chemical Bonding and Naming Compounds (Chapter 9) Types of Bonds Ionic Bonds Molecular Bonds Types of Compounds Ionic Molecular Acid Base Chemical Bonding Atoms will bond together using their valence electrons.
Chemical Bonding and Naming Compounds (Chapter 9) Types of Bonds Ionic Bonds Molecular Bonds Types of Compounds Ionic Molecular Acid Base Chemical Bonding Atoms will bond together using their valence electrons.
Chemical Nomenclature
 Chemical Nomenclature! The first names for chemicals were common names: Sugar, quicklime, Epsom salts, milk of magnesia, gypsom, laughing gas Simple, but not practical, the tell us little about the chemicals
Chemical Nomenclature! The first names for chemicals were common names: Sugar, quicklime, Epsom salts, milk of magnesia, gypsom, laughing gas Simple, but not practical, the tell us little about the chemicals
Chapter 2. Atoms, Molecules, and Ions
 Chapter 2 Atoms, Molecules, and Ions The atomic theory Topics The structure of the atom Atomic number, mass number and isotopes The Periodic Table The atomic mass scale and the average atomic mass Molecules
Chapter 2 Atoms, Molecules, and Ions The atomic theory Topics The structure of the atom Atomic number, mass number and isotopes The Periodic Table The atomic mass scale and the average atomic mass Molecules
Atoms, Molecules and Ions
 Atoms, Molecules and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
Atoms, Molecules and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
Atoms, Molecules and Ions. Chapter 2
 Atoms, Molecules and Ions Chapter 2 2.1 The Atomic Theory of Matter Democritus [460-370 BCE] Described tiny, indivisible particles Called them atomos Differed from Aristotle 17th century - idea of atoms
Atoms, Molecules and Ions Chapter 2 2.1 The Atomic Theory of Matter Democritus [460-370 BCE] Described tiny, indivisible particles Called them atomos Differed from Aristotle 17th century - idea of atoms
Experiment #3: When 2.0 g of sodium hydroxide reacts with 2.2 g carbon dioxide, 4.2 g of baking soda (sodium bicarbonate) is produced.
 Name: Dalton s Atomic Theory: (1) Matter is composed of very small units called atoms. Atom is the smallest unit that possesses the chemical property of an element. (2) An element contains only one type
Name: Dalton s Atomic Theory: (1) Matter is composed of very small units called atoms. Atom is the smallest unit that possesses the chemical property of an element. (2) An element contains only one type
Chapter 6 and 15 Ionic Compounds
 Chapter 6 and 15 Ionic Compounds Chapter 6 Ionic compounds 6.3, 6.4 6.1: Intro to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
Chapter 6 and 15 Ionic Compounds Chapter 6 Ionic compounds 6.3, 6.4 6.1: Intro to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
H 2 O. Chapter 9 Chemical Names and Formulas
 H 2 O Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions OBJECTIVES: Identify the charges on monatomic ions by using the periodic table, and name the ions. Section 9.1 Naming Ions OBJECTIVES:
H 2 O Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions OBJECTIVES: Identify the charges on monatomic ions by using the periodic table, and name the ions. Section 9.1 Naming Ions OBJECTIVES:
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch. 2 Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which formula/name pair is incorrect? 1) A) Fe2(SO4)3 iron(iii) sulfide
Ch. 2 Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which formula/name pair is incorrect? 1) A) Fe2(SO4)3 iron(iii) sulfide
CHAPTER 2. ATOMS, MOLECULES, AND IONS REMEMBER correct in a different color. Questions
 CHAPTER 2 ATOMS, MOLECULES, AND IONS REMEMBER correct in a different color Questions 17. A compound will always contain the same numbers (and types) of atoms. A given amount of hydrogen will react only
CHAPTER 2 ATOMS, MOLECULES, AND IONS REMEMBER correct in a different color Questions 17. A compound will always contain the same numbers (and types) of atoms. A given amount of hydrogen will react only
Bonding and Nomenclature notes.notebook
 Chemical Bonding & Nomenclature Objectives: Distinguish between covalent and ionic bonding Explain the process of bonding Name ionic and covalent compounds and acids Write chemical formulas for ionic and
Chemical Bonding & Nomenclature Objectives: Distinguish between covalent and ionic bonding Explain the process of bonding Name ionic and covalent compounds and acids Write chemical formulas for ionic and
Chapter 5 Molecules and Compounds
 Chapter 5 Molecules and Compounds 1 Chemical Formulas Atomic/Molecular Elements Ionic/Molecular Compounds Naming Ionic Compounds Naming Molecular Compounds Naming Acids Formula Mass Atomic / Molecular
Chapter 5 Molecules and Compounds 1 Chemical Formulas Atomic/Molecular Elements Ionic/Molecular Compounds Naming Ionic Compounds Naming Molecular Compounds Naming Acids Formula Mass Atomic / Molecular
2.1 Atomic Theory of Matter
 Chapter 2 2.1 Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter re-emerged in the early nineteenth century, championed by John Dalton. Law of Conservation of Mass
Chapter 2 2.1 Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter re-emerged in the early nineteenth century, championed by John Dalton. Law of Conservation of Mass
Atoms, Molecules, and Ions
 Atoms, Molecules, and Ions Atomic Theory of Matter Postulates of Dalton s Atomic Theory All matter is composed of indivisible atoms. An atom is an extremely small particle of matter that retains its identity
Atoms, Molecules, and Ions Atomic Theory of Matter Postulates of Dalton s Atomic Theory All matter is composed of indivisible atoms. An atom is an extremely small particle of matter that retains its identity
Chapter 2. Law of Definite Proportions. Law of Definite Proportions. Law of Conservation of Mass 10/7/2011 WATER H 2 O
 Chapter 2 Fundamental Chemical Laws (2.2) Dalton s Atomic Theory (2.3) Defining the Atom (2.5) Atomic Structure (2.6) Molecules and Ions (2.7) The Periodic Table (2.8) Nomenclature (2.9) Law of Conservation
Chapter 2 Fundamental Chemical Laws (2.2) Dalton s Atomic Theory (2.3) Defining the Atom (2.5) Atomic Structure (2.6) Molecules and Ions (2.7) The Periodic Table (2.8) Nomenclature (2.9) Law of Conservation
Chemical Names & Formulas. Water Ammonia Methane 1
 Chemical Names & Formulas Water Ammonia Methane 1 Why Systematic Names? # atomic particles 3 (p, n, e) # elements 110+ # elements in 8 earth s crust (99%) # elements in all 25 living things # compounds
Chemical Names & Formulas Water Ammonia Methane 1 Why Systematic Names? # atomic particles 3 (p, n, e) # elements 110+ # elements in 8 earth s crust (99%) # elements in all 25 living things # compounds
10/1/2017. General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy. Chapter 2. Chemistry: Atoms, Molecules and Ions
 General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 2 Chemistry: Atoms, Molecules and Ions 1 The Structure of the Atom Dalton s Atomic Theory (1808) o Compounds
General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 2 Chemistry: Atoms, Molecules and Ions 1 The Structure of the Atom Dalton s Atomic Theory (1808) o Compounds
Chapter 9. Chemical Names and Formulas
 Chapter 9 Chemical Names and Formulas 9.1 - Naming Ions Monatomic ions: Single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons. - Cations: Groups
Chapter 9 Chemical Names and Formulas 9.1 - Naming Ions Monatomic ions: Single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons. - Cations: Groups
Law of Definite Proportion** (Proust): A given compound always contains exactly the same proportion of elements by mass.
 # 10 Notes Unit 2: Laws, Properties & Mols Ch. Atoms, Molecules, Ions 5 Chemical Laws: I. Law of Conservation of Mass** (Lavoisier): Mass cannot be created or destroyed. II. III. IV. Law of Definite Proportion**
# 10 Notes Unit 2: Laws, Properties & Mols Ch. Atoms, Molecules, Ions 5 Chemical Laws: I. Law of Conservation of Mass** (Lavoisier): Mass cannot be created or destroyed. II. III. IV. Law of Definite Proportion**
Lecture Presentation. Chapter 2. Atoms, Molecules, and Ions. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 2 James F. Kirby Quinnipiac University Hamden, CT Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early nineteenth
Lecture Presentation Chapter 2 James F. Kirby Quinnipiac University Hamden, CT Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early nineteenth
Using the Periodic Table
 Quick Review - Dimensional Analysis - Density - Classification of Matter (end of Chapter 1 material) - Fundamental Laws - Dalton s Atomic Theory - Cathode-Ray Tube Experiment (J.J. Thomson) - Plum Pudding
Quick Review - Dimensional Analysis - Density - Classification of Matter (end of Chapter 1 material) - Fundamental Laws - Dalton s Atomic Theory - Cathode-Ray Tube Experiment (J.J. Thomson) - Plum Pudding
Memorize For SOL. Ionization Energy = lose or remove electrons
 Memorize For SOL Understanding The Periodic Table: Ionization Energy = lose or remove electrons Electronegativity = attract or steal electrons Atomic radius = Size Electron affinity = gain electrons Charge
Memorize For SOL Understanding The Periodic Table: Ionization Energy = lose or remove electrons Electronegativity = attract or steal electrons Atomic radius = Size Electron affinity = gain electrons Charge
Chapter 2. and Ions. Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 2 John D. Bookstaver St. Charles Community College Cottleville, MO Atomic Theory of Matter
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 2 John D. Bookstaver St. Charles Community College Cottleville, MO Atomic Theory of Matter
1/28/13. Naming and Writing Formulas > for Ionic Compounds
 chemistry 1 of 29 Naming and Writing Formulas A recipe is a formula for the sauce a complete list of ingredients and their proportions. Chemistry also uses formulas. Once you know the rules, you can write
chemistry 1 of 29 Naming and Writing Formulas A recipe is a formula for the sauce a complete list of ingredients and their proportions. Chemistry also uses formulas. Once you know the rules, you can write
CHAPTER 2: ATOMS, MOLECULES AND IONS. -matter (element) is composed of indivisible and indestructible particles termed atoms
 CHAPTER 2: ATOMS, MOLECULES AND IONS DALTON's ATOMIC THEORY -matter (element) is composed of indivisible and indestructible particles termed atoms -all atoms of the same element are identical. atoms of
CHAPTER 2: ATOMS, MOLECULES AND IONS DALTON's ATOMIC THEORY -matter (element) is composed of indivisible and indestructible particles termed atoms -all atoms of the same element are identical. atoms of
The Components of Matter
 The Components of Matter Elements the basic building blocks of matter Ancient Greeks four elements: earth, air, fire and water The atomic idea Democritus there are atoms and void. Boyle (17 th century)
The Components of Matter Elements the basic building blocks of matter Ancient Greeks four elements: earth, air, fire and water The atomic idea Democritus there are atoms and void. Boyle (17 th century)
Chapter 2. Atoms, Molecules, and Ions. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 2 John D. Bookstaver St. Charles Community College Cottleville, MO Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in
Lecture Presentation Chapter 2 John D. Bookstaver St. Charles Community College Cottleville, MO Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in
CHEM 103 Naming Compounds
 CHEM 103 Naming Compounds Lecture Notes February 7, 2006 Prof. Sevian 1 Agenda How we name compounds depends on what kind of compounds they are Ionic compounds Molecular compounds Acids are molecular compounds
CHEM 103 Naming Compounds Lecture Notes February 7, 2006 Prof. Sevian 1 Agenda How we name compounds depends on what kind of compounds they are Ionic compounds Molecular compounds Acids are molecular compounds
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Module 2 Practice Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Elements exhibit similar physical and chemical properties. 1) A) on
Module 2 Practice Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Elements exhibit similar physical and chemical properties. 1) A) on
Chapter 02 - Atoms, Molecules, and Ions
 1. According to the law of definite proportions, a. the ratio of the masses of the elements in a compound is always the same. b. it is not possible for the same two elements to form more than one compound.
1. According to the law of definite proportions, a. the ratio of the masses of the elements in a compound is always the same. b. it is not possible for the same two elements to form more than one compound.
9.4 Naming and Writing. Formulas for Acids and Bases. Chapter 9 Chemical Names and Formulas. 9.4 Naming and Writing Formulas for Acids and Bases
 Chapter 9 Chemical Names and Formulas 9.1 Naming Ions 9.2 Naming and Writing Formulas for Ionic Compounds 9.3 Naming and Writing Formulas for Molecular Compounds 9.4 Naming and Writing Formulas for Acids
Chapter 9 Chemical Names and Formulas 9.1 Naming Ions 9.2 Naming and Writing Formulas for Ionic Compounds 9.3 Naming and Writing Formulas for Molecular Compounds 9.4 Naming and Writing Formulas for Acids
- Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS
 63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
This chapter deals with matter, in its various forms, on the nanoscale.
 Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with matter, in its various forms, on the nanoscale. While we believe that all matter is composed of protons, electrons and, usually, neutrons,
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with matter, in its various forms, on the nanoscale. While we believe that all matter is composed of protons, electrons and, usually, neutrons,
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A molecule of water contains hydrogen and oxygen in a 1:8 ratio by mass. This is a statement
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A molecule of water contains hydrogen and oxygen in a 1:8 ratio by mass. This is a statement
Chapter 2 Atoms, Molecules, and Ions
 hapter 2 Atoms, Molecules, and Ions 2.1 The Atomic Theory of Matter Is there such thing like a indivisible particle of matter (atom)? Based on the following observations: 1. Law of conservation of mass/matter
hapter 2 Atoms, Molecules, and Ions 2.1 The Atomic Theory of Matter Is there such thing like a indivisible particle of matter (atom)? Based on the following observations: 1. Law of conservation of mass/matter
NAMING IONIC COMPOUNDS
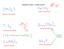 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes.
 Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes. What is matter? Matter is anything that has mass and occupies space. Chemists use a scientific method to study matter.
Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes. What is matter? Matter is anything that has mass and occupies space. Chemists use a scientific method to study matter.
Chapter 1. Study of chemistry Classification of Matter Properties of Mater Unit of Measurements Uncertainty of Measurements Dimensional Analysis
 Chapter 1 Completed Chapter Study of chemistry Classification of Matter Properties of Mater Unit of Measurements Uncertainty of Measurements Dimensional Analysis Quiz Next Thurs- Problems taken from Book
Chapter 1 Completed Chapter Study of chemistry Classification of Matter Properties of Mater Unit of Measurements Uncertainty of Measurements Dimensional Analysis Quiz Next Thurs- Problems taken from Book
Ionic Compounds. And Acids
 CHAPTER 7 LANGUAGE OF CHEMISTRY CLASSIFICATION OF COMPOUNDS Inorganic compounds does not contain the element carbon, but there are exception to this rule, CO 2 (carbon dioxide), CO 3 2 (carbonate), and
CHAPTER 7 LANGUAGE OF CHEMISTRY CLASSIFICATION OF COMPOUNDS Inorganic compounds does not contain the element carbon, but there are exception to this rule, CO 2 (carbon dioxide), CO 3 2 (carbonate), and
Compounds Element = 1 type of atom Compound = more than 1 type of atom (over 8 million) Chemical Bond = glue that links atoms together in a compound
 Compounds Element = 1 type of atom Compound = more than 1 type of atom (over 8 million) Chemical Bond = glue that links atoms together in a compound Ionic Compounds Ionic Bonds = atoms switch e- forming
Compounds Element = 1 type of atom Compound = more than 1 type of atom (over 8 million) Chemical Bond = glue that links atoms together in a compound Ionic Compounds Ionic Bonds = atoms switch e- forming
Chemical Nomenclature
 Chemical Nomenclature Learn names you will Review: Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that
Chemical Nomenclature Learn names you will Review: Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that
Big Idea: Matter & Atoms
 Big Idea: Matter & Atoms Naming Ionic Compounds Naming Covalent Compounds Naming Acids Naming Hydrates The cation (positive ion) is written first Takes the same name as the element if only forms one charge
Big Idea: Matter & Atoms Naming Ionic Compounds Naming Covalent Compounds Naming Acids Naming Hydrates The cation (positive ion) is written first Takes the same name as the element if only forms one charge
Nomenclature. Ex. For sodium the oxidation number is +1. For oxygen the oxidation number is -2.
 Nomenclature 1. BONDING CAPACITY (VALENCE) The number of bonds an atom can make. For a Cation, the bonding capacity is the number of electrons lost to become stable. For an Anion, the bonding capacity
Nomenclature 1. BONDING CAPACITY (VALENCE) The number of bonds an atom can make. For a Cation, the bonding capacity is the number of electrons lost to become stable. For an Anion, the bonding capacity
AP CHEMISTRY THINGS TO KNOW
 AP CHEMISTRY THINGS TO KNOW Diatomic Molecules H2-hydrogen gas (do not write H) N2-nitrogen gas (do no write N) O2-oxygen gas (do not write O) F2-fluorine gas (do not write F) Cl2-chlorine gas (do not
AP CHEMISTRY THINGS TO KNOW Diatomic Molecules H2-hydrogen gas (do not write H) N2-nitrogen gas (do no write N) O2-oxygen gas (do not write O) F2-fluorine gas (do not write F) Cl2-chlorine gas (do not
Chapter 2. Chapter 2 Atoms, Molecules and Ions
 Chapter 2 Atoms, Molecules and Ions How can we classify these substances? What are their distinguishing characteristics? Can we classify them simply on the basis of appearance? 2.1 Atomic Theory How do
Chapter 2 Atoms, Molecules and Ions How can we classify these substances? What are their distinguishing characteristics? Can we classify them simply on the basis of appearance? 2.1 Atomic Theory How do
Chapter 2 Atoms, Molecules, and Ions
 hapter 2: Phenomena Phenomena: Some elements, such as A and B, are known to form several compounds. Data on the masses of A and B were collected on different sample sizes of the three compounds. What patterns
hapter 2: Phenomena Phenomena: Some elements, such as A and B, are known to form several compounds. Data on the masses of A and B were collected on different sample sizes of the three compounds. What patterns
Chapter 2: Atoms, Molecules, and Ions
 Chapter 2: Atoms, Molecules, and Ions 1. Which of the following pairs of compounds can be used to illustrate the law of multiple proportions? A) NH 4 and NH 4 Cl B) ZnO 2 and ZnCl 2 C) H 2 O and HCl D)
Chapter 2: Atoms, Molecules, and Ions 1. Which of the following pairs of compounds can be used to illustrate the law of multiple proportions? A) NH 4 and NH 4 Cl B) ZnO 2 and ZnCl 2 C) H 2 O and HCl D)
Atomic Theory of Matter
 Chapter 2: Atoms, Molecules, and Ions Learning outcomes: Learn the basic postulates of Dalton s atomic theory. Describe the key experiments that led to the discovery of electrons and the nuclear model
Chapter 2: Atoms, Molecules, and Ions Learning outcomes: Learn the basic postulates of Dalton s atomic theory. Describe the key experiments that led to the discovery of electrons and the nuclear model
Chemistry 51 Chapter 5 OCTET RULE & IONS
 OCTET RULE & IONS Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. In the formation of a
OCTET RULE & IONS Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. In the formation of a
Chemical Names and Formulas
 Chemical Names and Formulas ELECTRONS AND THE STRUCTURE OF ATOMS BONDING AND INTERACTIONS 91 Naming Ions For students using the Foundation edition, assign problems 4, 8 15 Essential Understanding Ions
Chemical Names and Formulas ELECTRONS AND THE STRUCTURE OF ATOMS BONDING AND INTERACTIONS 91 Naming Ions For students using the Foundation edition, assign problems 4, 8 15 Essential Understanding Ions
Ionic Compounds - Examples
 Quick Review - Classification of Matter (end of Chapter 1 material) - Fundamental Laws - Dalton s Atomic Theory - Cathode-Ray Tube Experiment (J.J. Thomson) - Plum Pudding Model - Alpha-Particle Bombardment
Quick Review - Classification of Matter (end of Chapter 1 material) - Fundamental Laws - Dalton s Atomic Theory - Cathode-Ray Tube Experiment (J.J. Thomson) - Plum Pudding Model - Alpha-Particle Bombardment
Experiment #4. Chemical Nomenclature
 Experiment #4. Chemical Nomenclature Many everyday and historically important chemical compounds have common names. For example, water is the common name for H 2 O, baking soda is the common name for NaHCO
Experiment #4. Chemical Nomenclature Many everyday and historically important chemical compounds have common names. For example, water is the common name for H 2 O, baking soda is the common name for NaHCO
Naming and Formula Writing
 + Naming and Formula Writing + Chemical Formulas Shows the kind and number of atoms in the smallest piece of a substance Use subscripts to show the number of atoms per element Molecular formula- number
+ Naming and Formula Writing + Chemical Formulas Shows the kind and number of atoms in the smallest piece of a substance Use subscripts to show the number of atoms per element Molecular formula- number
Chapter 2: Atomic Theory
 Chapter 2: Atomic Theory all things are made of atoms - little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed
Chapter 2: Atomic Theory all things are made of atoms - little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed
Naming Simple Compounds
 Naming Simple Compounds Ionic Compounds Ionic compounds consist of positive and negative ions. have attractions called ionic bonds between positively and negatively charged ions. have high melting and
Naming Simple Compounds Ionic Compounds Ionic compounds consist of positive and negative ions. have attractions called ionic bonds between positively and negatively charged ions. have high melting and
AP Chemistry Chapter 2
 AP Chemistry Chapter 2 Atoms, Molecules & Ions The Atomic Theory of Maer > Democritus (460 370 BC) > Greek philosopher > Thought that material was made up of ny indivisible parcles called atomos > Aristotle
AP Chemistry Chapter 2 Atoms, Molecules & Ions The Atomic Theory of Maer > Democritus (460 370 BC) > Greek philosopher > Thought that material was made up of ny indivisible parcles called atomos > Aristotle
NOMENCLATURE AND WRITING FORMULAS
 NOMENCLATURE AND WRITING FORMULAS PART I--FORMULAS AND NOMENCLATURE OF IONIC COMPOUND Composed of Cations and Anions. Types of Cations (positive ions): A. Metals lose electrons to form positive ions. These
NOMENCLATURE AND WRITING FORMULAS PART I--FORMULAS AND NOMENCLATURE OF IONIC COMPOUND Composed of Cations and Anions. Types of Cations (positive ions): A. Metals lose electrons to form positive ions. These
NAMING IONIC COMPOUNDS. ammonium sulfide. iron(ii) carbonate. barium phosphate. titanium(iv) sulfide. Spelling matters!
 67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
Chapter 4 (part 1) Chemical Foundations: Elements, Atoms, and Ions. Copyright Cengage Learning. All rights reserved 1
 Chapter 4 (part 1) Chemical Foundations: Elements, Atoms, and Ions Copyright Cengage Learning. All rights reserved 1 Section 4.1 The Elements 118 known: 88 found in nature, others are made in laboratories.
Chapter 4 (part 1) Chemical Foundations: Elements, Atoms, and Ions Copyright Cengage Learning. All rights reserved 1 Section 4.1 The Elements 118 known: 88 found in nature, others are made in laboratories.
Atoms, Molecules, and Ions
 Atoms, Molecules, and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
Atoms, Molecules, and Ions Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Dalton s Atomic Theory (1808) 1. Elements are composed of extremely small
Chapter 2: Atoms, Molecules, and Ions
 Chapter 2: Atoms, Molecules, and Ions 1. According to the law of definite proportions, A) the ratio of the masses of the elements in a compound is always the same. B) it is not possible for the same two
Chapter 2: Atoms, Molecules, and Ions 1. According to the law of definite proportions, A) the ratio of the masses of the elements in a compound is always the same. B) it is not possible for the same two
Ch.2: Atoms, Molecules, and Ions
 Ch.2: Atoms, Molecules, and Ions Naming Recall Ionic Bond = electrostatic attraction due to the transfer of vse - s between a metal and nonmetal Covalent Bond = sharing of valence electrons between nonmetals
Ch.2: Atoms, Molecules, and Ions Naming Recall Ionic Bond = electrostatic attraction due to the transfer of vse - s between a metal and nonmetal Covalent Bond = sharing of valence electrons between nonmetals
CHEM 103 Naming Compounds
 CHEM 103 Naming Compounds Lecture Notes February 9, 2006 Prof. Sevian Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda How we
CHEM 103 Naming Compounds Lecture Notes February 9, 2006 Prof. Sevian Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda How we
Chemistry CRT Study Guide First Quarter
 Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
Name Date Class. 3 charge, whereas Group 2A metals form ions with a 4 3. charge, and Group 3A metals form ions with a 5 charge. 4.
 9.1 NAMING IONS Section Review Objectives Determine the charges of monatomic ions by using the periodic table and write the names of the ions Define a polyatomic ion and write the names and formulas of
9.1 NAMING IONS Section Review Objectives Determine the charges of monatomic ions by using the periodic table and write the names of the ions Define a polyatomic ion and write the names and formulas of
Chapter 5. Naming Compounds Writing Formulas
 Chapter 5 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Chapter 5 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Chemistry--Unit 2: Chemical Names and Formulas Test Review
 vocab anion binary compound cation chemical formula formula unit ion ionic compound law of definite proportions law of multiple proportions molecular formula polyatomic ion representative particle ternary
vocab anion binary compound cation chemical formula formula unit ion ionic compound law of definite proportions law of multiple proportions molecular formula polyatomic ion representative particle ternary
Name: 1. Show all work on Math Problems!!! Significant Figures and Calculations (*all math problems will require the use of sig figs)
 Name: 1 AP Chemistry Summer Assignment The goal of this assignment is to make sure that everyone has the fundamentals that they will need to be successful in Chemistry II & AP Chemistry. You should have
Name: 1 AP Chemistry Summer Assignment The goal of this assignment is to make sure that everyone has the fundamentals that they will need to be successful in Chemistry II & AP Chemistry. You should have
Science Naming and Writing Formulas for Chemical Compounds NAME:
 Science 1206 - Naming and Writing Formulas for Chemical Compounds NAME: IUPAC! International Union of Pure and Applied Chemists! This is a global organization that sets the standards in chemistry.! One
Science 1206 - Naming and Writing Formulas for Chemical Compounds NAME: IUPAC! International Union of Pure and Applied Chemists! This is a global organization that sets the standards in chemistry.! One
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
9/19/07. Chemistry 6A Fall 2007 Dr. J. A. Mack. Molar Masses. Avagagro s s Number. Avogadro s Number and the Mole
 Chemistry 6A Fall 007 Dr. J. A. Mack Avogadro s Number and the Mole The concept of a mole is defined so that we may equate the amount of matter (mass) to the number of particles (mole). The Standard is
Chemistry 6A Fall 007 Dr. J. A. Mack Avogadro s Number and the Mole The concept of a mole is defined so that we may equate the amount of matter (mass) to the number of particles (mole). The Standard is
Ch 2: Atoms, Molecules, and Ions
 AP Chemistry: Atoms, Molecules, and Ions Lecture Outline 2.1 The Atomic Theory of Matter Greek philosophers: Can matter be subdivided into fundamental particles? Democritus (460 370 BC): All matter can
AP Chemistry: Atoms, Molecules, and Ions Lecture Outline 2.1 The Atomic Theory of Matter Greek philosophers: Can matter be subdivided into fundamental particles? Democritus (460 370 BC): All matter can
Chemical Nomenclature Chapter 2.5-8
 Chemical Nomenclature Chapter 2.5-8 Octet Rule An octet is 8 valence electrons is associated with the stability of the noble gases does not occur with He, which is stable with two valence electrons (duet)
Chemical Nomenclature Chapter 2.5-8 Octet Rule An octet is 8 valence electrons is associated with the stability of the noble gases does not occur with He, which is stable with two valence electrons (duet)
1). Ionic bond electron from Na is transferred to Cl. Na is a metal and Cl is a nonmetal
 Chemical Bonds 1). Ionic bond electron from Na is transferred to Cl. Na is a metal and Cl is a nonmetal Salt versus Molecules A metal cation and nonmetal anion are joined together by an ionic bond called
Chemical Bonds 1). Ionic bond electron from Na is transferred to Cl. Na is a metal and Cl is a nonmetal Salt versus Molecules A metal cation and nonmetal anion are joined together by an ionic bond called
Books at Amazon.com on Nanotechnology
 Books at Amazon.com on Nanotechnology http://www.amazon.com/s/103-9040246- 0256655?ie=UTF8&keywords=NANOTECHNOLOGY%20&tag =thenanotecinscie&index=books&search=search&link%5fcode=qs Ions Ions are charged
Books at Amazon.com on Nanotechnology http://www.amazon.com/s/103-9040246- 0256655?ie=UTF8&keywords=NANOTECHNOLOGY%20&tag =thenanotecinscie&index=books&search=search&link%5fcode=qs Ions Ions are charged
Atomic Theory of Matter
 Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter John Dalton (1766-1844) Postulates of Dalton s Theory 1) Each element is composed of extremely small particles
Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter John Dalton (1766-1844) Postulates of Dalton s Theory 1) Each element is composed of extremely small particles
Chapter 2 Atoms, Molecules, and Ions. 許富銀 ( Hsu Fu-Yin)
 Chapter 2 Atoms, Molecules, and Ions 許富銀 ( Hsu Fu-Yin) 1 The Atomic Theory of Matter Democritus (460 370 bce) and other early Greek philosophers described the material world as made up of tiny, indivisible
Chapter 2 Atoms, Molecules, and Ions 許富銀 ( Hsu Fu-Yin) 1 The Atomic Theory of Matter Democritus (460 370 bce) and other early Greek philosophers described the material world as made up of tiny, indivisible
Unit 2. Atoms, Molecules, and Ions
 Unit 2. Atoms, Molecules, and Ions Upon successful completion of this unit, the students should be able to: 2.1 State and be able to apply the Law of Conservation of Mass, Law of Definite Proportions,
Unit 2. Atoms, Molecules, and Ions Upon successful completion of this unit, the students should be able to: 2.1 State and be able to apply the Law of Conservation of Mass, Law of Definite Proportions,
Topic 5: The Language of Chemistry
 Topic 5: The Language of Chemistry Chemical Formulas & Chemical Compounds (Chapter 7 in Modern Chemistry) A Chemical Formula Recall that a chemical formula indicates the relative number of atoms of each
Topic 5: The Language of Chemistry Chemical Formulas & Chemical Compounds (Chapter 7 in Modern Chemistry) A Chemical Formula Recall that a chemical formula indicates the relative number of atoms of each
