Total Synthesis of Celastrol, Celastroid Natural Products
|
|
|
- Agnes Campbell
- 5 years ago
- Views:
Transcription
1 Total Synthesis of Celastrol, Development of a Platform to Access Celastroid Natural Products Reporter: Jie Wang Checker: Bo Song Date: 2015/12/01 Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
2 Contents 1 Introduction 2 Total Synthesis of Celastrol, Wilforol A, Wilforic acid 3 Total Synthesis of dl-alnusenone, ctahydropicene 4 Summary 2
3 Introduction De Santana, C. F. Rev. Inst Antibiot. Recife 1971, 11, 37. 3
4 Introduction C Celastrol Tripterygium wilfordii Initially isolated from Tripterygium wilfordii Activities relevant to neuronal degeneration, inflammation Kiaei, M. et al. Neurodegener. Dis. 2005, 2,
5 Retrosynthesis of Celastrol C 2 Me Me Celastrol Polyene cascade Br Me Me Br FeCl 3,C 2 Cl 2 Me Me Me Me 5
6 Total Synthesis of Celastrol Br Me 2 N a) LDA, Me 2 NCMe TF, -78 o C, 91% b) MeLi, TF 89% 6 Br 7 NMe 2 8 c) Triethyl phosphonoacetate NaMDS, PhMe, 59% E/Z =6:1 Et 2 C oner-wadsworth-emmons olefination d) n BuLi, Ph 3 PMeBr 85% Et 2 C 9 10 Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
7 Total Synthesis of Celastrol Robinson annulation Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
8 Total Synthesis of Celastroid k) LiAl 4,Et 2, dr = 1.4:1 Me 99% Me Me Me Polyene cascade l) FeCl 3,C 2 Cl 2 38% Me m) B 3, 2 2,Na n) Jones reagent, Me 2 C Me Me 60% 2 step Me Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
9 Total Synthesis of Celastrol Two rounds of alkylation Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
10 Total Synthesis of Celastrol C 2 Me C 2 Me a) K, 1,4-dioxane/ 2, 99% b) BBr 3, C 2 Cl 2, 69% Me 21 wilforic acid 22 DDQ C 2 C 2 Tautomerization celastrol 1 23 Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
11 Total Synthesis of Celastrol Selenoxide elimination Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
12 Total Synthesis of Celastrol Siegel, D. et al. J. Am. Chem. Soc. 2015, 137,
13 Total Synthesis of dl-alnusenone C 2 5 Robinson annulation C 2 5 C a) K aq. C 3 72% C 3 29 Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
14 Total Synthesis of ctahydropicenes 33, 37 C 2 5 C 2 5 a) (C 2 5 ) 2 AlCN, C 6 6 ; K t Bu, t Bu, 90% CN C 3 C b) (C 6 5 ) 3 P=C 2,C % C 2 5 C 2 5 C 3 f) ( i Bu) 2 Al, C 6 6 ; N 2 4 2Cl, N 2 4 2, TEG, K 66% C 3 CN Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
15 Total Synthesis of ctahydropicenes 33, 37 Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
16 Total Synthesis of ctahydropicenes 33, 37 C 2 5 C 2 5 C 3 a) (C 2 5 ) 3 Al, CN, TF 86% 29 C 3 34 CN b) C 3 MgI, SCl 2, pyridine 95% C 2 5 C 2 5 C 3 c) ( i Bu) 2 Al, C 6 6 ; N 2 4 2Cl, N 2 4 2, TEG, K 67% C CN Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
17 Total Synthesis of ctahydropicenes 33, 37 Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
18 Total Synthesis of dl-alnusenone C 2 5 C 2 5 e) LiPPh 2,TF 90% C 3 ctahydropicene f) Li, N 3,C 3 I, 42% g) LiAl( t Bu) 3, 85% h) C 2 I 2,Zn(Cu) i) Cr 3 2Py C % C 3 39 Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
19 Total Synthesis of dl-alnusenone i) K t Bu, C 3 I j) Li, N 3 ;N 4 Cl 66% C 3 C k) LiAl 4, TF-Et 2 l) n BuLi, MPA-NEt 3, ClP(NMe 2 ) 2 m) Li, N 3, 47% 3 steps n) K t Bu, t Bu, C 3 I 67% dl-alnusenone 42 Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
20 Summary Pentacyclic framework of the celastroid class FeCl 3 -mediated polyene cascade 31 steps linear sequence, 0.5% yield Related : wilforic acid, wilforol A Celastrol Siegel, D. et al. J. Am. Chem. Soc. 2015, 137, Pentacyclic system of dl-alnusenone type Robinson annulation, Friedel-Crafts reaction 17 steps linear sequence, 0.9% yield dl-alnusenone Ireland, R. E. et al. J. Am. Chem. Soc. 1973, 95,
21 Celastrol was initially isolated from Tripterygium wilfordii (thunder of god vine) and later identified in a variety of plant species in the Celastracae family. The natural product has a wide array of promising activities relevant to neuronal degeneration, inflammation, diseases caused byprotein misfolding, cancer, and obesity. The related celastroid natural product, tingenone, has been examined in clinical trials for skin, stomach, and lymphoepithelioma and shown to possess moderate activity with minimal side effects. Medicinal chemistry optimization of the triterpene oleanolic acid arrived at CDD methyl ester. While not a member of the celastroid family, CDD methyl ester is similarly related, as it possesses a strong Michael acceptor within a triterpene scaffold and continues to be used in multiple clinical trials. 21
22 In conclusion, a platform utilizing a polyene cascade was developed to provide access to the pentacyclic framework of the celastroid class of triterpenoids, lending to the total synthesis of celastrol and related natural products wilforic acid, and wilforol A. The allylic alcohol cyclization precursor is accessed in >5 g quantities in 12 steps (longest linear) with an overall yield of 21%. The developed cascade employs ferric chloride as an activator in a dilute solution of C 2 Cl 2 to generate the pentacycle in 38% yield on gram scale and showcases the utility of this reagent for polyene cyclizations. Through this intermediate, the first syntheses of celastrol were completed in 31 and 32 (longest linear steps, respectively) as well as wilforic acid and wilforol A. 22
Reporter: Yue Ji. Date: 2016/12/26
 Literature Report (11) Total Synthesis of Rubriflordilactone B Reporter: Yue Ji Checker: Mu-Wang Chen Date: 2016/12/26 Yang, P.; Yao, M.; Li, J.; Li, Y.; Li, A.* Angew. Chem. Int. Ed. 2016, 55, 6964. Laboratory
Literature Report (11) Total Synthesis of Rubriflordilactone B Reporter: Yue Ji Checker: Mu-Wang Chen Date: 2016/12/26 Yang, P.; Yao, M.; Li, J.; Li, Y.; Li, A.* Angew. Chem. Int. Ed. 2016, 55, 6964. Laboratory
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
EXAM #3 EXTRA PROBLEMS
 Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
OChem2 Course Pack. Practice Problems by Chapter. Practice Exams
 Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
CHAPTER 25 HW: AMINES
 CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans or E/Z
CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans or E/Z
CH310N Spring Anslyn. March 23, Exam 2
 C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
Large-Scale Synthesis of the Anti-Cancer Marine Natural Product (+)-Discodermolide
 61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives
 C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Total Synthesis of (±)-Cephanolides B and C via a Palladium-Catalyzed Cascade Cyclization and Late-Stage sp 3 C H Bond Oxidation
 Total Synthesis of (±)-Cephanolides B and C via a Palladium-Catalyzed Cascade Cyclization and ate-stage sp 3 C Bond xidation un Xu, Chao Wang, Ziwei Gao, and Yu-Ming Zhao* Cameron McConnell Professor S.-Y.
Total Synthesis of (±)-Cephanolides B and C via a Palladium-Catalyzed Cascade Cyclization and ate-stage sp 3 C Bond xidation un Xu, Chao Wang, Ziwei Gao, and Yu-Ming Zhao* Cameron McConnell Professor S.-Y.
Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization
![Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization](/thumbs/82/85235286.jpg) Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Problem session (3) Daiki Kuwana. Please fill in the blank and explain reaction mechanisms and stereoselectivities.
 Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Baran Group Meeting 04/07/04 The Total Synthesis of (+)-Ryanodol
 Baran Group eting 04/07/04 The Total Synthesis of ()-Ryanodol A. Bélanger; D. Berney;. Borschber; R. Brousseau; A. Doutheau; R. Durand;. Katayama; R. Lapalme; D. Leturc; C. Liao; F. MacLachlan; J. Maffrand;
Baran Group eting 04/07/04 The Total Synthesis of ()-Ryanodol A. Bélanger; D. Berney;. Borschber; R. Brousseau; A. Doutheau; R. Durand;. Katayama; R. Lapalme; D. Leturc; C. Liao; F. MacLachlan; J. Maffrand;
Literature Report 2. Total Synthesis of Longeracinphyllin A and (-)-Calyciphylline N. Date :
 Literature Report 2 Total Synthesis of Longeracinphyllin A and (-)-Calyciphylline N Reporter : Kui Liu Checker : Shao-Jie Cheng Date : 2018-09-03 Ang Li. et al. J. Am. Chem. Soc. 2017, 139, 14893 14896
Literature Report 2 Total Synthesis of Longeracinphyllin A and (-)-Calyciphylline N Reporter : Kui Liu Checker : Shao-Jie Cheng Date : 2018-09-03 Ang Li. et al. J. Am. Chem. Soc. 2017, 139, 14893 14896
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
Graphical Abstract. Tandem Epoxysilane Rearrangement/Wittig-Type Reactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane
 Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
An Analysis of the Total Syntheses of Aphidicolin
 An Analysis of the Total Syntheses of Aphidicolin An Evans Group Afternoon Seminar Krista Beaver March 20, 1998 A B D C Aphidicolin Isolated from the fungus Cephalosporium aphidicola (esp, Chem. Comm.1972,
An Analysis of the Total Syntheses of Aphidicolin An Evans Group Afternoon Seminar Krista Beaver March 20, 1998 A B D C Aphidicolin Isolated from the fungus Cephalosporium aphidicola (esp, Chem. Comm.1972,
Renaud Group Exercise Set
 Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Strategies for Stereocontrolled Synthesis
 Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
CHAPTER 24 HW: CARBONYL CONDENSATIONS
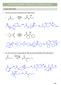 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Chapter 18 Ketones and Aldehydes
 Chapter 18 Ketones and Aldehydes omenclature o Ketones have priority over alcohols Find the longest chain with the carbonyl in it. ame the parent and replace the e with one Say where the carbonyl is. octan-3-one
Chapter 18 Ketones and Aldehydes omenclature o Ketones have priority over alcohols Find the longest chain with the carbonyl in it. ame the parent and replace the e with one Say where the carbonyl is. octan-3-one
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
C h a p t e r T w e n t y - o n e : Enols, Enolates, and Aldol-like Condensations
 h a p t e r T w e n t y o n e : Enols, Enolates, and Aldollike ondensations Li LDA 1. R TF R 2. 3 R A directed aldol reaction, used in the synthesis of periplanone B, a cockroach attractant M 323: Summary
h a p t e r T w e n t y o n e : Enols, Enolates, and Aldollike ondensations Li LDA 1. R TF R 2. 3 R A directed aldol reaction, used in the synthesis of periplanone B, a cockroach attractant M 323: Summary
Chapter 20: Aldehydes and Ketones
 Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Synthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives
 ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
Literature Report. A 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed Dehydro Diels-Alder Reaction
 Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Hirsutene and Δ 9(12) Capnellene. D. P. Curran (1986) Presenter: Mehdi Moemeni
 irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems
 Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
Chemistry 39. Exam 3
 Chemistry 39 Exam 3 Wednesday, April 0, 005 Name (printed) 6:30-7:45 p.m. S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 5 questions on this exam.
Chemistry 39 Exam 3 Wednesday, April 0, 005 Name (printed) 6:30-7:45 p.m. S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 5 questions on this exam.
Total Syntheses of Nominine
 Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
Comparative Synthesis of Ingenol. Tyler W. Wilson SED Group Meeting
 Comparative Synthesis of Ingenol Tyler W. Wilson SED Group eting 2.27.07 Ingenol: Biology, Isolation, and istory During WWII a latex was manufactured by precipitating the milky juice of the Euphorbia Ingen
Comparative Synthesis of Ingenol Tyler W. Wilson SED Group eting 2.27.07 Ingenol: Biology, Isolation, and istory During WWII a latex was manufactured by precipitating the milky juice of the Euphorbia Ingen
Olefin-Forming Reactions I
 C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
Chemistry 3720, Spring 2004 Exam 3 Name:
 Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
Name: Student No: Page 1 of 10
 Name: Student No: Page 1 of 10 CEM 2220 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter Session 08R Paper 102 University centre, Room 210 224 Tuesday April 15, 2008 9:00 am 12:00 pm Students
Name: Student No: Page 1 of 10 CEM 2220 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter Session 08R Paper 102 University centre, Room 210 224 Tuesday April 15, 2008 9:00 am 12:00 pm Students
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
STRATEGIES IN SYNTHESIS
 STRATEGIES I SYTESIS Professor T. J. Donohoe MT 2006 6 Lectures: Tuesday at 10 am; Thursday at 9 am (weeks 6-8) DP: Lecture Theatre Monensin Me 7 Et Me Me Me Me Me Me C 2 1 Me Kishi J. Am. Chem. Soc, 1979,
STRATEGIES I SYTESIS Professor T. J. Donohoe MT 2006 6 Lectures: Tuesday at 10 am; Thursday at 9 am (weeks 6-8) DP: Lecture Theatre Monensin Me 7 Et Me Me Me Me Me Me C 2 1 Me Kishi J. Am. Chem. Soc, 1979,
Synthesis of Azadirachtin: A Long but Successful Journey
 ynthesis of Azadirachtin: A Long but uccessful Journey Gemma E. Veitch, Edith Beckmann, Brenda J. Burke, Alistair Boyer, arah L. Maslen, and teven V. Ley Angew. Chem. Int. Ed. 2007, Early View And Gemma
ynthesis of Azadirachtin: A Long but uccessful Journey Gemma E. Veitch, Edith Beckmann, Brenda J. Burke, Alistair Boyer, arah L. Maslen, and teven V. Ley Angew. Chem. Int. Ed. 2007, Early View And Gemma
Synthesis of Double Bonds
 Synthesis of Double Bonds Wittig eaction!!! Georg Wittig: Nobel Prize 1979 For their development of the use of boronand phosphorous-containing compounds respectively, into important reagents in organic
Synthesis of Double Bonds Wittig eaction!!! Georg Wittig: Nobel Prize 1979 For their development of the use of boronand phosphorous-containing compounds respectively, into important reagents in organic
Total Synthesis towards Maoecrystal V
 Total Synthesis towards Maoecrystal V isolated from the leaves of a Chinese medicinal herb, Isodon eriocalyx in 2004. structure was determined by comprehensive NMR and MS spectroscopic analysis and confirmed
Total Synthesis towards Maoecrystal V isolated from the leaves of a Chinese medicinal herb, Isodon eriocalyx in 2004. structure was determined by comprehensive NMR and MS spectroscopic analysis and confirmed
Synthesis of Atisine-type Alkaloids
 Literature Report III Synthesis of Atisine-type Alkaloids Reporter : Yang Zhao Checker : Zhou-ao Zhu Date : 2018-7-16 Ma, D. et al. Angew. Chem. Int. Ed. 2018, 57, 6676 Baran, P. S. et al. J. Am. Chem.
Literature Report III Synthesis of Atisine-type Alkaloids Reporter : Yang Zhao Checker : Zhou-ao Zhu Date : 2018-7-16 Ma, D. et al. Angew. Chem. Int. Ed. 2018, 57, 6676 Baran, P. S. et al. J. Am. Chem.
Enantioselective Syntheses of Insect Pheromones with a Methyl-branched Skeleton Verified by Chiral HPLC Analyses
 29 th ISCE Annual eting lbourne, Australia (August 20, 2013) Enantioselective Syntheses of Insect Pheromones with a thyl-branched Skeleton Verified by Chiral PLC Analyses Ts T. Ando, T. Taguri, Y. Muraki,
29 th ISCE Annual eting lbourne, Australia (August 20, 2013) Enantioselective Syntheses of Insect Pheromones with a thyl-branched Skeleton Verified by Chiral PLC Analyses Ts T. Ando, T. Taguri, Y. Muraki,
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EAMINATIN CEMISTRY 353 April 25 th, 2000 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EAMINATIN CEMISTRY 353 April 25 th, 2000 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Short Literature 8/9/10
 Short Literature 8/9/10 Reiter, M.; Torssell, S.; Lee, S.; MacMillan, D. W. C. The organocatalytic three-step total synthesis of ()-frondosin B Chem. Sci. 2010, 37-42. Spangler, J. E.; Carson, C. A.; Sorensen,
Short Literature 8/9/10 Reiter, M.; Torssell, S.; Lee, S.; MacMillan, D. W. C. The organocatalytic three-step total synthesis of ()-frondosin B Chem. Sci. 2010, 37-42. Spangler, J. E.; Carson, C. A.; Sorensen,
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 29 th, 2002 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 29 th, 2002 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
Nine-Step Enantioselective Total Synthesis of (+)-Minfiensine
 ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Literature Report 2. Stereocontrolled Synthesis of Kalihinol C. Reiher, C. A.; Shenvi, R. A. J. Am. Chem. Soc. 2017, 139,
 Literature Report 2 Stereocontrolled Synthesis of Kalihinol C Reporter: Huanping Xie Checker: Xiang Gao Date: 2017-04-17 Reiher, C. A.; Shenvi, R. A. J. Am. Chem. Soc. 2017, 139, 3647 3650. Contents 1
Literature Report 2 Stereocontrolled Synthesis of Kalihinol C Reporter: Huanping Xie Checker: Xiang Gao Date: 2017-04-17 Reiher, C. A.; Shenvi, R. A. J. Am. Chem. Soc. 2017, 139, 3647 3650. Contents 1
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Modern Organic Synthesis an Introduction
 Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Progress toward the Total Synthesis of Pleurotin
 Progress toward the Total Synthesis of Pleurotin SINYA IIMURA Wipf Research Group Meeting July 9th, 2005 Shinya Iimura @ Wipf Group 1 7/10/2005 Presentation utline! Isolation and Structure! Biological
Progress toward the Total Synthesis of Pleurotin SINYA IIMURA Wipf Research Group Meeting July 9th, 2005 Shinya Iimura @ Wipf Group 1 7/10/2005 Presentation utline! Isolation and Structure! Biological
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Free Radical Reactions in Synthetic Organic Chemistry
 Free adical eactions in ynthetic rganic Chemistry C549.M. Williams Free radical intermediates have been utilized with varying degrees of success in the stereocontrolled formation of C-C bonds. Among the
Free adical eactions in ynthetic rganic Chemistry C549.M. Williams Free radical intermediates have been utilized with varying degrees of success in the stereocontrolled formation of C-C bonds. Among the
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis
 Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis C 3 C3 Liffert, R., Linden, A., Gademann, K. J. Am. Chem. Soc. 2017, 139, 16096 Presented by: Brendyn Smith CEM 852
Total Synthesis of the Sesquiterpenoid Periconianone A Based on a Postulated Biogenesis C 3 C3 Liffert, R., Linden, A., Gademann, K. J. Am. Chem. Soc. 2017, 139, 16096 Presented by: Brendyn Smith CEM 852
Final Exam /415 ( CHEM 6352 Fall %) Name
 Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
REACTIONS OF AROMATIC COMPOUNDS
 A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
A STUDENT SHOULD BE ABLE TO: REACTIONS OF AROMATIC COMPOUNDS 1. Predict the product(s) of Electrophilic aromatic substitution (EAS): halogenation, sulfonation, nitration, Friedel- Crafts alkylation and
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)-
 Literature Report Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)- aseseazines A and B Reporter: Mu-Wang Chen Checker: Zhang-Pei Chen Date: 2013-05-28 Reisman,
Literature Report Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)- aseseazines A and B Reporter: Mu-Wang Chen Checker: Zhang-Pei Chen Date: 2013-05-28 Reisman,
I. Liu Lab. Ka<e Boknevitz 1
 A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 350
 Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Prof. Ang Li. Literature Seminar Kosuke Minagawa (D2)
 Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Total Synthesis of all ( )-Agelastatin Alkaloids
 Total Synthesis of all ( )-Agelastatin Alkaloids Mohammad Movassaghi,* Dustin S. Siegel and Sunkyu an Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United
Total Synthesis of all ( )-Agelastatin Alkaloids Mohammad Movassaghi,* Dustin S. Siegel and Sunkyu an Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
CEM 852 Final Exam. May 6, 2010
 CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
(please print and circle your last name) CHEMISTRY 332 FINAL EXAM. December 11, 2006
 Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
Literature Report 3. Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A. Date :
 Literature Report 3 Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A Reporter : Xiao-Yong Zhai Checker : Shubo u Date : 2017-10-30 Pritchett, B. P.; Donckele, E. J.; Stoltz, B. M. Angew.
Literature Report 3 Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A Reporter : Xiao-Yong Zhai Checker : Shubo u Date : 2017-10-30 Pritchett, B. P.; Donckele, E. J.; Stoltz, B. M. Angew.
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage
 henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
Syntheses of Leucascandrolide A. Supergroup Meeting August 4 th, 2004 Yu Yuan
 Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Chem 253 Problem Set 7 Due: Friday, December 3, 2004
 Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
