Free Radical Reactions in Synthetic Organic Chemistry
|
|
|
- Oswald Malone
- 5 years ago
- Views:
Transcription
1 Free adical eactions in ynthetic rganic Chemistry C549.M. Williams Free radical intermediates have been utilized with varying degrees of success in the stereocontrolled formation of C-C bonds. Among the potential problems with free radical intermediates is the possibility for bond rotation (resulting in loss of stereochemical integrity), quenching of the radical prior to C-C bond formation, side reactions (such as reactions with adventitious oxygen or other radical traps), and the possibility of homo-coupling (dimerization). In addition, carbon-centered free radicals are generally highly reactive species and methods to tame their selectivity have been a challenging endeavor. owever, judicious choices of substrate structure and reaction conditions can often lead to synthetically useful transformations. Primarily through the work of Walling, Ingold, Beckwith, Barton, Julia, Giese, tork and Curran, many methods now exist for the generation of radical species have been realized. A few examples are illustrated below. The Barton eaction The Barton reaction is a radical-based method to remotely functionalize an unactivated C- bond as shown below: The general reaction involves the formation and photochemical rearrangement of a nitroxyl intermediate that tautomerizes to an oxime; hydrolysis affords the corresponding aldehyde of ketone hn 3. 3 hn - 3 oxime Initially designed for the remote functionalization of carbon atoms in steroids as shown by the example below, the Barton reaction has also been put to advantage in natural products total syntheses. Ac hn 3. 3 An example of the latter application is a synthesis of grandisol (the boll weevil pheromone) by obbs and Magnus as shown below. C Ac
2 = 1. hn, n-hexane 2% aq. 2. i-pr, D Et 2, acetone 51% overall 3 P=C 2 Ac 67% 2, 20% Pd-C 90% ( 2 CC) 2 B, TF; a, 2 2 Ac 95% C hn, ac 3 obbs, P.D.; Magnus, P.D., J. Am. chem. oc. 1976, 98, 4594 The Barton-McCombie Deoxygenation eaction 1. Ac 2, py. 2. P 3, py. 59% Ac Ac 1. h( 3 P) 3 2. LA 60% 75% Cr 3 -py 41% grandisol Barton & McCombie have devised a useful synthetic method for the radical-based deoxygenation of alcohols. The method involves the formation of thiohydroxamates or xanthates which upon reaction with a trialkyltin hydride, leads to the net replacement of the alcohol group with a hydrogen atom. The general reaction is shown below: 1 Z X X n-bu 3 n tol., D 1 2 The mechanism of the Barton-McCombie deoxygenation reaction is illustrated below on the 2,4,6- trichlorothionocarbonate species py., 1 2 nn-bu 3 n-bu 3 n tol., D 1 2 nn-bu 3 n-bu 3 n 2 1 nn-bu3 Xanthates can also be used in this reaction and are the easiest to prepare and are the least expensive. 1. C 2, a 2 2. I n-bu 3 n tol., D 1 2 xanthate Three specific examples are shown below:
3 n- 3 n, AIB toluene /reflux (81%) 3 steps 40% BBu 2 ~1:25 C 2 2 /-78 C 53% 2, Pd 2 Et-TF n-bu 2 BTf, Et 3 C 2 2 /0 C 2 C 2 C a(i 3 ) 2 TF -78 C Æ T 38% Williams,.M.; Im, M-.; Cao, J., J.Am.Chem.oc., 1991, 113, 6976~6981 (6-hydroxymethyl-2,6-diaminopimelic acid, a natural antibiotic from Micromonospora chalcea) C % Br, D 2. Et, D 91% 2 C 2 6-MDAP C 2 2 4% a, C 2 2, C 2, I 3 n, AIB toluene /reflux 1. 2, Pd 2 Et-TF 2. Et, reflux 2 C 2 C 2 2 Williams,.M.; Yuan, C., J. rg. Chem., 1992, 57, TB Ac 1. C n 4, Et 2 2. Ac 2, py. 54% Ac 1. Jones ox a 91% Ac 1. s 4, M n-bu 3 n, AIB 74% C 2 Ac 1. BP, Et 3 C 2 allyl 2. ( 3 P) 4 Pd 53% Uno,.; Baldwin, J.E.; ussell, A.T., J.Am.Chem.oc., 1994, 116, 2139~2140. The Barton Thiohydroxamic Ester Chemistry Ac 1. 2, Pd-C,, 2. Et 3 i, py., Ac % F, C 70% lactacystin Barton has also exploited the facile homolysis of thiohydroxamic esters to generate caron-centered free radicals that can be trapped by a variety of agents forming new functionality. The general mechanism is shown below: C 2 Ac
4 pypy a hn or D C 2 radical trap The driving forces for the Barton thiohydroxamate system are: (1) enthalpic gain in converting the weaker thiocarbonyl group into a carbonyl group (C 2 ); (2) aromatization of the pyridine nucleus; (3) the gain in entropy in forming three components from a single molecular entity. -X -Br - BrC 3 C n-bu 3 n or t-bu 3 C-I ee e Intramolecular traps can be used for this reaction as in the cyclization reaction shown below: -I hn 82% Another utility of the Barton thiohydroxamate chemistry is in the setting of a modification of a unsdiecker reaction as illustrated below. The net result is an oxidative decarboxylation reaction. C 2 DCC, DMAP BrC 3, D (Barton-modified unsdiecker) 34~43% BrC 3 Br 1 equiv. a, 0 o C ~2:1 spirotryprostatin B 12-epi-spirotryprostatin B ebahar, P..; Williams,.M., J. Am. Chem. oc. 2000, 122, 5666~5667
5 Perhaps the greatest utility of free radical reactions has been realized in cyclization reactions and cyclization reaction cascades. ere, the conformational rigidity of the substrate can be exploited to tame the intrinsically high reactivity of the free radical instermediates. In general, the formation of five- and sixmembered rings has proven the most successful based on entropic considerations. P 5 C 4 dihydroagarofuran isodihydroagarofuran 3 : 7 (67%) n-bu 3 n, hn, D AIB, cyclohexane (20%) 6-exo-trig Büchi, G..; Wüest,., J. rg. Chem. 1979, 44, 546 Like virtually all radical reactions, we can understand the presence of the undesired (reduced) uncyclized product by a careful accounting of the mechanism. adical reactions typically occur in three stages: (1) initiation (2) propagation (3) termination In accord with this mechanism, the amount of uncyclized product increases with increasing tin hydride concentration which serves as the ultimate -atom donor. nbu 3 termination AIB = C C free radical initiator D - 2 initiation 2 C nbu 3 C nbu 3 cyclization nbu 3 propagation A solution to the poor facial selectivity of the above system was found by altering the intramolecular trap functionality to give a vinylsilane intermediate. n-bu 3 n, hn, D 6-exo-dig AIB, cyclohexane 72% TM nbu 3 pts = TM 92% dihydroagarofuran Büchi, G..; Wüest,., J. rg. Chem. 1979, 44, 546
6 tork has reported an elegant example of free radical-based cyclization reaction in the total synthesis of prostaglandin F2a as shown below. Et Et I Et Et 0.1 eq. n-bu 3 n Bu 3 n TM 2 eq. acb 3, hn C 5 11 TB TB TB TB TM C TM C 5 11 Et TM 140 o C Et Pd(Ac) 2, C Et C 5 11 TB C 5 11 (Brook rearrangement) TB C 5 11 TM (aegusa oxidation) TB 1. ()-BIAL-, TF, -100 o C (oyori asymmetric reduction) 2., TF, 2 C P C 2 PGF2a C 5 11 tork, G.; her, P.M.; Chen,.-L., J. Am. Chem. oc. 1986, 108, 6384 Thiols, sulfoxides and sulfones all readily eliminate when situated b- to a radical forming olefins. This was nicely illustrated by Boger and Coleman in their approach to CC-1065 as shown below. Br 2 n-bu 3 n, AIB Bn, D Bn 2 Bn - Bn CC-1065 Boger, D.L.; Coleman,.., J. Am. Chem. oc. 1988, 110, 4796 Arylselenides can also be utilized as substrates for the generation of free radical species. A particularly impressive example is the total synthesis of tunicamycin reported by Myers, et al. as shown below:
7 TB BM Ac BC e i n-bu 3 n, Et 3 B tol. 0 o C TB BM Ac i BC 7-endo-trig TB BM Ac i BC (C 3 ) 2 C(C 2 ) 9 5 steps Ac tunicamycin V Myers, A.G.; Gin, D.Y.; Widdowson, K.L., J. Am. Chem. oc. 1991, 113, 9661 ne of the first examples of a tandem radical cyclization cascade was reported by tork in the synthesis of a butenolide as shown below: Br n-bu 3 n 5-exo-dig 5-exo-trig AIB,, D n-bu 3 n 1. a o, TF, 3 (l) 2. PCC, C DBU tork, G.; Mook,., J. Am. Chem. oc. 1983, 105, 6765 This concept has since been extensively exploited by many workers in the synthesis of fused and spirocyclic carbocycles. Another example is the synthesis of hirsutene by Curran as shown below: 13 steps I n-bu 3 n. AIB, D 5-exo-trig 5-exo-dig n-bu 3 n hirsutene Curran, D.P.; akiewicz, D.M., J.Am.Chem. oc., 1985, 107, 1448
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
R.M. Williams. S Me O R H
 C549.M. Williams xidations in ynthetic rganic Chemistry The Moffat and wern xidation eactions The classical Moffat oxidation (see: Pfitzner, K.E.; Moffatt, J.G., J. Am. Chem. oc. 1963, 85, 3027~3028),
C549.M. Williams xidations in ynthetic rganic Chemistry The Moffat and wern xidation eactions The classical Moffat oxidation (see: Pfitzner, K.E.; Moffatt, J.G., J. Am. Chem. oc. 1963, 85, 3027~3028),
Olefin-Forming Reactions I
 C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
C549 R.M. Williams lefin-forming Reactions I The McMurry lefination Reaction. An important alternative to the acyloin condensation is the McMurry olefination which is a free radical coupling reaction initiated
Hirsutene and Δ 9(12) Capnellene. D. P. Curran (1986) Presenter: Mehdi Moemeni
 irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
irsutene and Δ 9(12) Capnellene D. P. Curran (1986) Presenter: Mehdi Moemeni Back Ground of Radical Structure In a modern context the first organic free radical identified was triphenylmethyl radical.
CEM 852 Final Exam. May 6, 2010
 CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
Recent Development in. Tandem Radical Reactions (TRR)
 ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Literature Report. A 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed Dehydro Diels-Alder Reaction
 Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
Literature Report 11-Steps Total Synthesis of Magellanine through a Gold(І)-Catalyzed ehydro iels-lder Reaction Reporter: ong-qiang Shen Checker: Cong Liu ate: 2017/06/19 McGee, P.; Bétournay, G.; Barabé,
Although we won t go into this, the reactions can be regioselective if non-symmetrical alkenes are used.
 2.1 rganic ynthesis A. Armstrong - 2004-2005 Functional Group Interconversions - Lecture 5 ection 5: xidation of C- bonds bearing no heteroatom 5.1 xidation of allylic positions Many reagents will do this
2.1 rganic ynthesis A. Armstrong - 2004-2005 Functional Group Interconversions - Lecture 5 ection 5: xidation of C- bonds bearing no heteroatom 5.1 xidation of allylic positions Many reagents will do this
CEM 852 Exam LDA, THF, 0 C, 15 min; then
 CEM 85 Exam- April, 005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. Provide examples of the following
CEM 85 Exam- April, 005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. Provide examples of the following
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Problem session (3) Daiki Kuwana. Please fill in the blank and explain reaction mechanisms and stereoselectivities.
 Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Reporter: Yue Ji. Date: 2016/12/26
 Literature Report (11) Total Synthesis of Rubriflordilactone B Reporter: Yue Ji Checker: Mu-Wang Chen Date: 2016/12/26 Yang, P.; Yao, M.; Li, J.; Li, Y.; Li, A.* Angew. Chem. Int. Ed. 2016, 55, 6964. Laboratory
Literature Report (11) Total Synthesis of Rubriflordilactone B Reporter: Yue Ji Checker: Mu-Wang Chen Date: 2016/12/26 Yang, P.; Yao, M.; Li, J.; Li, Y.; Li, A.* Angew. Chem. Int. Ed. 2016, 55, 6964. Laboratory
Massachusetts Institute of Technology Organic Chemistry 5.512
 Massachusetts Institute of Technology rganic Chemistry 5.512 Problem Set 6 Solutions Stereocontrolled Carbonyl Reduction and Aldol Reactions May 5, 2006 Lucy Kohnen ( The target compound was used as an
Massachusetts Institute of Technology rganic Chemistry 5.512 Problem Set 6 Solutions Stereocontrolled Carbonyl Reduction and Aldol Reactions May 5, 2006 Lucy Kohnen ( The target compound was used as an
Total synthesis of marine natural products without using protecting groups
 Total synthesis of marine natural products without using protecting groups ature (London, United Kingdom), Vol 446, March 2007, p. 404-408 Phil S. Baran, Thomas J. Maimone & Jeremy M. Richter Presented
Total synthesis of marine natural products without using protecting groups ature (London, United Kingdom), Vol 446, March 2007, p. 404-408 Phil S. Baran, Thomas J. Maimone & Jeremy M. Richter Presented
EWG EWG EWG EDG EDG EDG
 Functional Group Interconversions Lecture 4 2.1 rganic Synthesis A. Armstrong 20032004 3.4 eduction of aromatic systems We can reduce aromatic systems to cyclohexanes under very forcing hydrogenolytic
Functional Group Interconversions Lecture 4 2.1 rganic Synthesis A. Armstrong 20032004 3.4 eduction of aromatic systems We can reduce aromatic systems to cyclohexanes under very forcing hydrogenolytic
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
CEM 852 Final Exam. May 5, 2011
 CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Timeless Methods for Radical Cyclizations in Total Synthesis. Patricia Zhang MacMillan Group Meeting Wednesday September 25th, 2013
 Timeless thods for adical Cyclizations in Total Synthesis Patricia Zhang MacMillan Group eting Wednesday September 25th, 2013 ! A little history! 1900: Moses Gomberg "Ph 3 C" rather than "Ph 3 C " trivalent
Timeless thods for adical Cyclizations in Total Synthesis Patricia Zhang MacMillan Group eting Wednesday September 25th, 2013 ! A little history! 1900: Moses Gomberg "Ph 3 C" rather than "Ph 3 C " trivalent
CEM 852 Exam-2 April 11, 2015
 CEM 852 Exam-2 April 11, 2015 This exam consists of 6 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 100. In answering your questions,
CEM 852 Exam-2 April 11, 2015 This exam consists of 6 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 100. In answering your questions,
JOC: 1985 Year in Review
 Baran Group eting JC: 1985 Year in eview Syntheses discussed: thodoligies discussed: Quadrone 2 C (+)-irsutic Acid C (±) Coriolin (!)-Longifolene (!)-astanecine Manganese (III)-mediated "-lactone annulation
Baran Group eting JC: 1985 Year in eview Syntheses discussed: thodoligies discussed: Quadrone 2 C (+)-irsutic Acid C (±) Coriolin (!)-Longifolene (!)-astanecine Manganese (III)-mediated "-lactone annulation
Suggested solutions for Chapter 27
 uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
uggested solutions for Chapter 27 27 PRBLEM 1 uggest a mechanism for this reaction, commenting on the selectivity and the stereochemistry. Me 2 1. t-buk 2. Raney Ni Et The opportunity to explore the consequences
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
R 2 R 4 Ln catalyst. This manuscript describes the methods for the synthesis and application of group 4 metallocene bis(trimethylsilyl)acetylene
 VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
I. Liu Lab. Ka<e Boknevitz 1
 A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Topic 18: Nucleophilic Sigma Bonds
 Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Synthesis of Atisine-type Alkaloids
 Literature Report III Synthesis of Atisine-type Alkaloids Reporter : Yang Zhao Checker : Zhou-ao Zhu Date : 2018-7-16 Ma, D. et al. Angew. Chem. Int. Ed. 2018, 57, 6676 Baran, P. S. et al. J. Am. Chem.
Literature Report III Synthesis of Atisine-type Alkaloids Reporter : Yang Zhao Checker : Zhou-ao Zhu Date : 2018-7-16 Ma, D. et al. Angew. Chem. Int. Ed. 2018, 57, 6676 Baran, P. S. et al. J. Am. Chem.
Synthesis of the Phomoidrides (CP 225,917 & CP 263,114) Chem. Rev. 2003, 103, 2691.
 ynthesis of the Phomoidrides (P 225,917 & P 263,114) hem. Rev. 2003, 103, 2691. 15 7 26 2 7 16 14 1 2 5 7 2 7 1 2 1 4 2 6 1 5 9 1 7 1 9 2 9 1 5 (+)-P hom oidr ide A ; 7 = (P- 225,917) (+)-P hom oidr ide
ynthesis of the Phomoidrides (P 225,917 & P 263,114) hem. Rev. 2003, 103, 2691. 15 7 26 2 7 16 14 1 2 5 7 2 7 1 2 1 4 2 6 1 5 9 1 7 1 9 2 9 1 5 (+)-P hom oidr ide A ; 7 = (P- 225,917) (+)-P hom oidr ide
CHAPTER 23 HW: ENOLS + ENOLATES
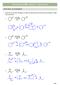 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
VINBLASTINE. H MeO 2 C MeO. OAc. CO 2 Me. Me H
 VIBLATIE 2 C 1 C 2 Ac a 3: catharanthine C 2 Ac C 2 2: ( )-vindoline xidation 5' 2 C 3' 16' 20' Ac C 2 1: (+)-vinblastine b 4 C 2 TFAA, -50 C Polonovski fragmentation 6' 5' 16' C 2 5 TFA 4' 3' 15' 16'
VIBLATIE 2 C 1 C 2 Ac a 3: catharanthine C 2 Ac C 2 2: ( )-vindoline xidation 5' 2 C 3' 16' 20' Ac C 2 1: (+)-vinblastine b 4 C 2 TFAA, -50 C Polonovski fragmentation 6' 5' 16' C 2 5 TFA 4' 3' 15' 16'
Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and Notoamide B
 Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and otoamide B (+)-avrainvillamine (+)-notoamide B 20 51 21 55 (-)-stephacidin B In 2002, Bristol-Myers Squibb reported the biologically
Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and otoamide B (+)-avrainvillamine (+)-notoamide B 20 51 21 55 (-)-stephacidin B In 2002, Bristol-Myers Squibb reported the biologically
Organic Tutorials 3 rd Year Xmas Vac
 rganic Tutorials 3 rd Year Xmas Vac Third Year Reactive Intermediates: Radicals, Arynes, Carbenes etc. Radicals References: Moody and Whitham Reactive Intermediates, xford Chemistry Primer 8; Carey and
rganic Tutorials 3 rd Year Xmas Vac Third Year Reactive Intermediates: Radicals, Arynes, Carbenes etc. Radicals References: Moody and Whitham Reactive Intermediates, xford Chemistry Primer 8; Carey and
2,3-Sigmatropic Rearrangements in Organic Synthesis
 2,3-igmatropic Rearrangements in rganic ynthesis ctober 25, 2006 Matt aley Crimmins roup igmatropic Rearrangements -concerted pericyclic reactions traditionally thought to be governed by orbital symmetry
2,3-igmatropic Rearrangements in rganic ynthesis ctober 25, 2006 Matt aley Crimmins roup igmatropic Rearrangements -concerted pericyclic reactions traditionally thought to be governed by orbital symmetry
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
JOC Year-in-Review, 1984
 Baran Lab Group eting JC Year-in-eview, 198 Y oshihiro Ishihara Statistics for J. rg. Chem. 198, Volume 9, Issues 1-26: 1313 Papers 1 erbert C. Brown 8 Albert Padwa 8 Leo A. Paquette 7 Dale L. Boger 7
Baran Lab Group eting JC Year-in-eview, 198 Y oshihiro Ishihara Statistics for J. rg. Chem. 198, Volume 9, Issues 1-26: 1313 Papers 1 erbert C. Brown 8 Albert Padwa 8 Leo A. Paquette 7 Dale L. Boger 7
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Total Syntheses of Nominine
 Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
Total Syntheses of ominine i Literature t Group eting Lizzie Bryan ctober 17, 2007 Aconite Alkaloids Atidane V eatchane Cycloveatchane Aconitane etisan Muratake,. M.; atsume, M.; akai,. Tetrahedron, 2006,
CHEM 153 PRACTICE TEST #1 ANSWER KEY
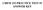 CEM 153 PACTICE TEST #1 ASWE KEY Provide a mechanism for the following transformation, indicating the electron count and oxidation state of each organometallic intermediate: u 3 (C) 12 (5 mol%) TF, 135
CEM 153 PACTICE TEST #1 ASWE KEY Provide a mechanism for the following transformation, indicating the electron count and oxidation state of each organometallic intermediate: u 3 (C) 12 (5 mol%) TF, 135
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
OC IV: Organic Photochemistry Exercise 1 Exercise class Page 1 of 11. Exercise 1: Fundamentals, H-Abstraction reactions
 C IV: rganic otochemistry Exercise 1 Exercise class Page 1 of 11 Exercise 1: Fundamentals, -Abstraction reactions 1. Draw the energy potential curve of the ground state and the first two excited states
C IV: rganic otochemistry Exercise 1 Exercise class Page 1 of 11 Exercise 1: Fundamentals, -Abstraction reactions 1. Draw the energy potential curve of the ground state and the first two excited states
Chem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below.
 hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
Chemistry 335 Supplemental Slides: Interlude 1. Reduction: addition of hydrogen to the substrate. Oxidation: addition of oxygen to the substrate
 Interlude 1: Oxidations, Reductions & Other Functional Group Interconversions (FGI) 1. Definition of Oxidation and Reduction For practical purposes in organic chemistry, oxidation and reduction are defined
Interlude 1: Oxidations, Reductions & Other Functional Group Interconversions (FGI) 1. Definition of Oxidation and Reduction For practical purposes in organic chemistry, oxidation and reduction are defined
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
OC II (FS 2013) Lecture 1 Prof. Bode. Oxidation
 C (F 2013) Lecture 1 Prof. Bode 1 xidation state of carbon xidation xidation is a process in which a chemical species loses electron. eduction is a process in which a chemical species gains electron. 3
C (F 2013) Lecture 1 Prof. Bode 1 xidation state of carbon xidation xidation is a process in which a chemical species loses electron. eduction is a process in which a chemical species gains electron. 3
Dr. P. Wipf Chem /26/2007
 I. Basic Principles I-L. Radicals & Carbenes Features of Radical Reactions Review: Curran, D. P. In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Ed.; Pergamon Press: Oxford, 1991; Vol.
I. Basic Principles I-L. Radicals & Carbenes Features of Radical Reactions Review: Curran, D. P. In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Ed.; Pergamon Press: Oxford, 1991; Vol.
Nine-Step Enantioselective Total Synthesis of (+)-Minfiensine
 ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
ine-step nantioselective Total Synthesis of (+)-Minfiensine Jones, S. B.; Simmons, B.; MacMillan, D. W. C.* J. Am. Chem. Soc. 2009, ASAP. DI: 10.1021/ja906472m Kara George Wipf Group - Current Literature
3.7. Pyridinium Chloro Chromate (PCC):
 xidation 3.7. Pyridinium hloro hromate (P): 97 l l r r 3 r l P is a very important oxidising reagent which will give controlled oxidation in the case of primary alcohol. It does not give over oxidation
xidation 3.7. Pyridinium hloro hromate (P): 97 l l r r 3 r l P is a very important oxidising reagent which will give controlled oxidation in the case of primary alcohol. It does not give over oxidation
Aldehydes and Ketones
 Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Strategies for Stereocontrolled Synthesis
 Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
Intramolecular Huisgen-Type Cyclization of Platinum-Bound Pyrylium Ions with Alkenes and Subsequent Insertion into a Benzylic C-H Bond
 Intramolecular uisgen-type Cyclization of Platinum-Bound Pyrylium Ions with Alkenes and Subsequent Insertion into a Benzylic C- Bond Chang o h,* Ji o Lee, Su Jin Lee, Jae Il Kim, and Chang Seop ong Department
Intramolecular uisgen-type Cyclization of Platinum-Bound Pyrylium Ions with Alkenes and Subsequent Insertion into a Benzylic C- Bond Chang o h,* Ji o Lee, Su Jin Lee, Jae Il Kim, and Chang Seop ong Department
Reduction. Ø Hydrogenation. Ø Boron Reagents. Ø Aluminium Reagents. Ø Tin Hydrides. Ø Silanes. Ø Dissolving Metal Reductions. Ø Diimide Reductions
 eduction Ø ydrogenation Ø Boron eagents Ø Aluminium eagents Ø Tin ydrides Ø Silanes Ø Dissolving Metal eductions Ø Diimide eductions C- 423 Course on rganic Synthesis; Course Instructor: Krishna P. Kaliappan
eduction Ø ydrogenation Ø Boron eagents Ø Aluminium eagents Ø Tin ydrides Ø Silanes Ø Dissolving Metal eductions Ø Diimide eductions C- 423 Course on rganic Synthesis; Course Instructor: Krishna P. Kaliappan
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
JACS 1982: A Survey of Papers with a Focus on Synthetic Organic Chemistry
 JAC 1982: A urvey of Papers with a Focus on ynthetic rganic Chemistry Baran Lab Group eting 15 ctober 2003 Carlos A. Guerrero eagents and thods 1 2 [] 2 2 1 1 PhC, Et 3 2 [] 2 1 2 1 By 1982, the [3 + 2]
JAC 1982: A urvey of Papers with a Focus on ynthetic rganic Chemistry Baran Lab Group eting 15 ctober 2003 Carlos A. Guerrero eagents and thods 1 2 [] 2 2 1 1 PhC, Et 3 2 [] 2 1 2 1 By 1982, the [3 + 2]
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Carbenes and Carbene Complexes I Introduction
 Carbenes and Carbene Complexes I Introduction A very interesting (honest) class of radical-like molecules Steadily becoming more important as they find far more synthetic applications We will primarily
Carbenes and Carbene Complexes I Introduction A very interesting (honest) class of radical-like molecules Steadily becoming more important as they find far more synthetic applications We will primarily
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Roxanne Atienza Short Literature Presentation January 24, 2011
 Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
CHEMISTRY Topic #8: Oxidation and Reduction Reactions Fall 2018 Dr. Susan Findlay
 CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
Renaud Group Exercise Set
 Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Palladium-Catalyzed Oxygenation of Unactivated sp 3 C-H Bonds
 Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
Palladium-Catalyzed xygenation of Unactivated sp 3 C- Bonds Pd(Ac) 2 5 mol% PhI(Ac) 2 1.1 eq. Pd 2 Ac Desai, L. P.; ull, K. L.; Sanford *, M. S. University of Michigan J. Am. Chem. Soc. 2004, 126, ASAP
CEM 852 Exam-2 April 2, 2016
 CM 852 xam-2 April 2, 2016 This exam consists of 6 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 100. In answering your questions, please
CM 852 xam-2 April 2, 2016 This exam consists of 6 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 100. In answering your questions, please
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Chapter 15. Reactions of Aromatic Compounds. 1. Electrophilic Aromatic Substitution Reactions
 hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
ROC Exam Problem 1
 RC Exam 2012 Problem 1 Silyl ether 1 is transformed into 2 in a two step process. Predict, through a detailed mechanistic rationale, the stereochemistry of the newly formed chiral center. TBS Si 1) Ms
RC Exam 2012 Problem 1 Silyl ether 1 is transformed into 2 in a two step process. Predict, through a detailed mechanistic rationale, the stereochemistry of the newly formed chiral center. TBS Si 1) Ms
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
There are 15 total pages to this exam. Please be sure your copy has 15 pages before you begin.
 Initials: 1 ame: Chem 633: Advanced rganic Chemistry Midterm 2 Please answer the following questions clearly and concisely. Write your answers in the space provided. Write your initials on each page you
Initials: 1 ame: Chem 633: Advanced rganic Chemistry Midterm 2 Please answer the following questions clearly and concisely. Write your answers in the space provided. Write your initials on each page you
Loudon Chapter 10 Review: Alcohols & Thiols Jacquie Richardson, CU Boulder Last updated 4/26/2016
 Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Practice Synthetic Problems: CHEM 235 Page 2
 Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
UNIVERSITY OF MANITOBA DEPARTMENT OF CHEMISTRY
 PAGE 1 of 11 UNVESTY F MANTBA DEPATMENT F CEMSTY 2.339 STUCTUAL TANSFMATNS N GANC CEMSTY FNAL EXAMNATN - PAPE # 432 Dr. Phil ultin Friday December 17, 2004. NAME: STUDENT NUMBE: 1. (20 Marks) For each
PAGE 1 of 11 UNVESTY F MANTBA DEPATMENT F CEMSTY 2.339 STUCTUAL TANSFMATNS N GANC CEMSTY FNAL EXAMNATN - PAPE # 432 Dr. Phil ultin Friday December 17, 2004. NAME: STUDENT NUMBE: 1. (20 Marks) For each
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Total Synthesis of the Chartellines
 Total Synthesis of the Chartellines X Y chartelline A, X = Y = chartelline B, X =, Y = chartelline C, X = Y = Mariam Shamszad ovember 1, 2006 Background Chartellines A, B, and C were isolated in the 1980s
Total Synthesis of the Chartellines X Y chartelline A, X = Y = chartelline B, X =, Y = chartelline C, X = Y = Mariam Shamszad ovember 1, 2006 Background Chartellines A, B, and C were isolated in the 1980s
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
James D. White. A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab. Education Experience
 A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab Education Experience Fraser Fleming University of Drexel Pavel agory University of Michigan Cambridge University,
A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab Education Experience Fraser Fleming University of Drexel Pavel agory University of Michigan Cambridge University,
