STUDIES OF NEW PARTICLE FORMATION IN THE EUROPEAN BOUNDARY LAYER
|
|
|
- Maria Stevenson
- 5 years ago
- Views:
Transcription
1 REPORT SERIES IN AEROSOL SCIENCE No 112 (2010) STUDIES OF NEW PARTICLE FORMATION IN THE EUROPEAN BOUNDARY LAYER AMAR HAMED Department of Physics and Mathematics Faculty of Science and Forestry University of Eastern Finland Kuopio, Finland Academic dissertation To be presented, with the permission of the Faculty of Science and Forestry of the University of Eastern Finland, for public criticism in auditorium L 22, Snellmania building, on August 26 th, 2010, at 12 o clock noon. Kuopio 2010
2 Author s Address: Department of Physics and Mathematics University of Eastern Finland P.O. Box 1627, Kuopio, Finland Amar.Hamed@uef.fi Supervisors: Professor Ari Laaksonen, Ph.D. Department of Physics and Mathematics University of Eastern Finland and Finnish Meteorological Institute Helsinki Finland Docent Jorma Joutsensaari, D.Sc. (Tech.) Department of Physics and Mathematics University of Eastern Finland Kuopio Finland Reviewers: Professor Jeffrey R. Pierce Department of Physics and Atmospheric Science Dalhousie University Halifax Canada Docent Jyrki Mäkelä, Ph.D. Tampere University of Technology, Institute of Physics, Tampere Finland Opponent: Professor Joachim Curtius Experimental Atmospheric Research Institute for Atmospheric and Environmental Sciences Goethe-University Frankfurt Frankfurt am Main Germany ISBN (printed version) ISSN Yliopistopaino Helsinki 2010 ISBN (PDF version) Helsinki 2010
3 Acknowledgments The research for this thesis was carried out at the Department of Physics and Mathematics in the University of Eastern Finland, Kuopio campus. I want to thank Prof. Pasi Karjalainen, the head of the department as well as Prof. Jari Hämäläinen, Mr. Tero Karjalainen and Prof. Jari Kaipio, former heads of the department, for providing me with the working facilities. First and foremost, I would like to express my gratitude to my supervisor Prof. Ari Laaksonen, the establisher of the Aerosol Physics research group of the University of Eastern Finland, for introducing me to aerosol science and for giving me a great opportunity to work with interesting and challenging topics in national and international top-ranked collaboration projects. I would also like to thank Ari for numerous inspiring scientific discussions and for his continued guidance, and encouragement for my work. During my very hard times, when life struck me with its cruelty, Ari was always there, providing great mental and moral support and understanding. I cannot put into words how grateful I am for all your support during that difficult time. I offer my special thanks to my second supervisor, Doc. Jorma Joutsensaari, whose advice and encouragement have been invaluable for me throughout this work. I am grateful to Jorma, who used hours of his time in helping me complete this work. I also thank Jorma, for his fruitful discussions on topics that were not necessarily related to our work. Prof. Jeffrey Pierce and Doc. Jyrki Mäkelä are gratefully acknowledged for carefully reviewing this thesis. I warmly thank Cathy Zuck for carefully proofreading this thesis. I am very thankful to the Magnus Ehrnrooth Foundation for financial support during the last two years of this work. The studies presented in this thesis would have not been possible without the cooperation and efforts of all my coauthors. Their irreplaceable expertise, help and support are warmly acknowledged. I am particularly thankful for the excellent collaboration with Prof. Cristina Facchini and her group, especially Stefano Decesari, Mihaela Mircea, and Claudio Carbon at the Institute of Atmospheric Sciences and Climate in Bologna, Italy. I continue to appreciate our ongoing collaboration and look forward to our future work together. I also thank Prof. Alfred Wiedensohler, the head of the Department of Physics at the Leibniz-Institute for Tropospheric Research, and his group. I especially thank Wolfram Birmili, the leader of the aerosol physics group, Birgit Wehner and Gerald Spindler for sharing their data, their brilliant ideas and giving valuable comments for current projects as well as goals to aim for in the future. I am very thankful to Prof. Kari Lehtinen and Prof. James Smith for always having time for my questions and sharing their brilliance and valuable scientific insight with me. Prof. Markku Kulmala is acknowledged for always having time for a careful reading of my papers and giving valuable comments. Thanks Markku for your precious time. Dr. Hannele Korhonen, Ms. Sanna Liisa Sihto, and Dr. Larisa Sogacheva are not only acknowledged for being among my wonderful coauthors but also for their friendship. I am grateful to all of my former and current colleagues in the Aerosol Physics Group for creating an unusually pleasant, positive, and thriving work atmosphere and for helping me out with various scientific and non-scientific problems. I especially thank Sami Romakkaniemi for his support and for worrying about the financial arrangements for me. My thanks are extended to all members of the Physics and Mathematics Department for maintaining a pleasant and friendly atmosphere. Although I was not able at least until now to learn the Finnish language I have always had the feeling of being very welcome.
4 There have been several professors who made an impact on my life as a student and to whom I will be always grateful. Prof. S.A Elwakil, the head of the Theoretical Physics Group at Mansoura University, is one of the best professors one can ever have. He was not only my dear professor who showed me how to love mathematics, but also my Godfather who taught me a lot about life. Prof. Magdy Tadros, the head of the Atmospheric Research Group, introduced me to programming, taught me the FORTRAN language, and encouraged me to develop my own ideas. I am grateful to Dr. Mossad El Metwally for inspiring me to pursue studies in atmospheric science. There is no way I could have come this far without the love, help, encouragement and inspiration of my family and my friends. I warmly thank my parents and my brothers for their love, their care, their support and for giving me the most basic building blocks of my values and world view. My heartfelt thanks go also to my friends for making the difficult days easier, and continuously reminding me in a most pleasant way that there is more to life than work. Kuopio, August 2010 Amar Hamed
5 Amar Hamed University of Eastern Finland 2010 Abstract Atmospheric aerosol particles have a significant impact on air quality, human health and global climate. A better understanding of these aerosol-related effects, especially with respect to long-term climate projections, requires more comprehensive knowledge on aerosol sources and their atmospheric transformation processes. An important process controlling the number concentration of atmospheric particles is the formation of new ultrafine particles typically 1 2 nm in size, through gas-to-particle conversion. Once thermodynamically stable, the new particles can grow through condensation and coagulation to sizes of nm where they can become cloud condensation nuclei (CCN). In this thesis we studied the formation of nanometer sized atmospheric aerosol particles and their subsequent growth to the CCN size range (i.e. 50 nm and larger) in different atmospheric environments. Our study was based on analyzing long time series of aerosol particle size distributions from different field measurements sites (San Pietro Capofiume (SPC), Italy; Melpitz, Germany; Hyytiälä, Finland). Additionally, we used theoretical approaches and modeling studies to support our detailed characterization of the formation and growth processes using field observations. Based on our study, we came to 7 main conclusions: 1) new particle formation (NPF) events are very intense and frequent in PoValley and, surprisingly, they indeed act as an important source of CCN in spite of the polluted nature and the strong anthropogenic activities of the region; 2) secondary particle formation from vapors is occurring in a rural polluted area (SPC, Italy) and in a rural area (Melpitz, Germany) as frequently as in a clean area (Hyytiälä, Finland); 3) between 1996/1997 and 2003/2006 a significant decrease of the anthropogenic SO 2 (-65%) in the Melpitz station shows a strong connection to the decrease in the frequency of new particle formation events (-45%) and in formation rates (-68%), and was in contrast to the increased growth rates of nucleated particles (+22%); 4) the production of CCN following NPF events in Melpitz between the two previously mentioned periods appears to have increased by tens of percent and this is most likely due to the increased particle growth rate, possibly due to organics; 5) field data from the Hyytiälä measurement station show that the maximum observed gas-phase sulphuric acid concentrations are limited to relative humidity (RH) values below 60% and that this is likely due to low hydroxyl radical (OH) concentrations at high RH; 6) theoretical calculations indicate that the increase of coagulational scavenging at elevated RH probably has a relatively small effect in masking the appearance of new particle formation events; 7) the model simulation results show that the decreased source term at high RH limits H 2 SO 4 levels in the air, and therefore high new particle formation rates (above ~1 cm -3 s -1 ) rarely occur above 80% RH. In this thesis a method for identifying NPF is presented, as are basic characteristics such as growth and formation rates, condensational and coagulation sinks. Also the possible effects of meteorological parameters and gas phase concentrations on new particle formation at different atmospheric environments are summarized. Methodology to estimate the production of CCN following NPF events is introduced for field measurements and is supported by modeling studies. The results are particularly useful when estimating, for instance, the role of SO 2, the main precursor of gaseous sulphuric acid (H 2 SO 4 ), which in turn is a main precursor for atmospheric particle nucleation, on particle formation and the growth process. This work gives a better understanding of the role of RH in inhibiting new particle formation. Keywords: Atmospheric aerosols, nucleation, particle formation and growth, CCN, sulfuric acid.
6 Contents 1 Introduction Background and motivation 6 2 Aerosol particle size distribution and concentration 11 3 Aerosol dynamical processes Nucleation and new particle formation (NPF) Condensation and condensational sink (CS) Coagulation and coagulation sink (CoagS) New particle formation and nucleation rates (J 3, J 1 ) 20 4 Gas phase species responsible for nucleation & NPF Sulphuric acid Organic vapours 28 5 On the role of condensation and coagulation sinks on NPF Influence of condensation sink on NPF Influence of coagulation sink on NPF 31 6 Analysis of atmospheric new particle formation events Identifying new particle formation events Event characterization Conditions favoring new particle formation in different atmospheric environments 39 7 Review of papers and the author s contribution 43 8 Discussion and conclusions 46 References 50
7 List of publications This thesis consists of an introductory review, followed by five research articles. In the introductory part, these papers are cited according to their Roman numerals. The papers are reproduced with the kind permission of the journals concerned. Paper I and Paper V are reproduced by permission of American Geophysical Union. Paper II and Paper III are reprinted under Creative Commons License. Paper IV is reproduced by permission from Boreal Environment Research Publishing Board. I Laaksonen A., Hamed A., Joutsensaari J., Hiltunen L., Cavalli F., Junkermann W., Asmi A., Fuzzi S., and Facchini M.C. (2005). Cloud condensation nucleus production from nucleation events at a highly polluted region. Geophys. Res. Lett., 32, 1-4. II Hamed A., Joutsensaari J., Mikkonen S., Sogacheva L., Dal Maso M., Kulmala M., Cavalli F., Fuzzi S., Facchini M. C., Decesari S., Mircea M., Lehtinen K.E.J., and Laaksonen A. (2007). Nucleation and growth of new particles in Po Valley, Italy. Atmos. Chem. Phys., 7, III Hamed A., Birmili W., Joutsensaari J., Mikkonen S., Asmi A., Wehner B., Spindler G., Jaatinen A., Wiedensohler A., Korhonen H., Lehtinen K. E. J., and Laaksonen A.(2010). Changes in the production rate of secondary aerosol particles in central Europe in view of decreasing SO2 emissions between 1996 and Atmos. Chem. Phys., 10, IV Jaatinen A., Hamed A., Joutsensaari J., Birmili W., Wehner B., Spindler G., Wiedensohler A., Decesari S., Mircea M., Facchini M.C., Junninen H., Kulmala M., Lehtinen K.E.J., and Laaksonen A. (2009). A comparison of new particle formation events at three different sites in the European boundary layer. Boreal Env. Res., 14, V Hamed A., Korhonen H., Sihto S.L., Joutsensaari J., Järvinen H., Petäjä T., Arnold F., Nieminen T., Kulmala M., Smith J.N., Lehtinen K.E.J., and Laaksonen A. (2010). The role of relative humidity in continental new particle formation. J. Geophys. Res., submitted.
8 1 Introduction 1.1 Background and motivation The term aerosol, by definition, refers to a mixture of solid and/or liquid particles suspended in a gaseous medium (Hinds, 1999). Some aerosols occur naturally, originating from volcanoes, dust storms, forest and grassland fires, living vegetation, and sea spray. Human activities, such as the burning of fossil fuels and the alteration of natural surface cover, also generate aerosols. Depending on their source, two groups of aerosols are defined: primary aerosol, which means aerosols that are emitted directly into the atmosphere by natural or anthropogenic processes (volcano activity, fossil fuel burning, biomass burning, pollen etc.), and secondary aerosol, which are produced in the atmosphere by gas-to-particle conversion. The precursor gases (e.g. sulphur dioxide, and volatile organics) converted to particles by chemical transformation (mostly oxidation reactions) into low volatility vapors followed by phase transformations: nucleation and condensation. Nucleation is the initiating process of the first-order phase transitions in which a substance changes its phase from one to another. One of the simplest examples is the situation where the vapor phase changes to the liquid phase. The first-order phase transitions do not necessarily start right away at equilibrium: they may need to first overcome an energy barrier, which is the work of formation of a small embryo or nucleus of the new phase, which emerge from fluctuations within the old phase. Condensation refers to mass increase from the uptake of low volatility atmospheric vapors, either directly or through surface- or volume-controlled reactions (Seinfeld and Pandis, 2006). Secondary aerosol formation via nucleation of atmospheric vapors accompanied by subsequent condensational growth of the particles (the focus of this work) are of interest for a number of reasons. It is a global phenomenon proven to take place in a wide variety of high and low altitude environments (Kulmala et al., 2004 and references therein; Paper II; Paper III; Paper IV). The process produces a large amount of particles that have significant effects on the climate and human health. Epidemiological research has made it clear that aerosols have a large impact on human health. Aerosols increase the mortality rate due to cardiopulmonary diseases (heart and lung diseases including asthma and bronchitis). Particles smaller than 100 nm are especially of interest for their ability to penetrate cell membranes and are believed to have serious health impacts (Raizenne et al., 1989; Raizenne et al., 1996; Stieb et al., 2002; Nel, 2005). Particles that form and grow to be a size larger than 100 nm in diameter are also important for their ability to directly scatter solar radiation back to the space, and moreover, to activate to cloud droplets, resulting in even greater scattering of radiation which leads to a cooling effect on the climate (Menon, 2004; 6
9 IPCC, 2007; Spracklen et al., 2008). Several recent studies (Kerminen et al., 2005; Paper I; Kuang et al., 2009; Wiedensohler et al., 2009) and modeling efforts (Pierce and Adams, 2007; Spracklen et al., 2008; Spracklen et al., 2010) have directly implicated newly formed particles from atmospheric new particle formation (NPF) events as an important source of cloud condensation nuclei (CCN). It is essential that NPF is sufficiently well understood since global climate models require accurate prediction of this phenomenon in order to realistically capture aerosol radiative effects. This naturally has made estimates of the particle source from secondary aerosol formation an important challenge, as information on mechanisms and the vapors responsible for NPF and growth would be needed for accurate predictions. Until recently, it had been expected that NPF is less favoured in the urban atmosphere than in the rural atmosphere. It was thought that a higher condensation sink (CS) formed by pre-existing particles caused the condensation of non-volatile species onto existing particles to be more favourable than particle formation by homogeneous nucleation (Mäkelä, et al., 1997; Mönkkönen et al. 2005). However, a number of recent studies show that NPF events occur frequently in urban and polluted rural areas (Harrison et al., 2000; Woo et al., 2001; Alam et al., 2003; Dunn et al., 2004; Stanier et al., 2004; McMurry et al., 2005; Watson et al., 2006; Paper I; Paper II; Paper III; Paper IV). However, even if it is proved that NPF occurs almost everywhere in the atmosphere, it is not yet possible to predict, a priori, rates at which particles form and grow, or even to know with certainty which chemical species are involved (Birmili and Wiedensohler, 2000; Harrison et al., 2000; Kulmala, et al., 2004; McMurry et al., 2005; Wehner et al., 2005; Laaksonen et al., 2008b; Paper IV). A major limitation in our understanding of the first steps of atmospheric particle formation has been the size range at which nucleation and the initial steps of particle growth occur. The key to this is understanding the difference between nucleation and new particle formation. Nucleation involves the production of stable nuclei at ~1 nm, whereas NPF refers to the detection of particles larger than about 3 nm in diameter, which is the minimum detectable size for the most commonly used aerosol instruments, (Kulmala et al., 2004). Stable nuclei need time to grow to 3 nm size and this time varies under different atmospheric conditions. Not all particles that nucleate reach the 3 nm size, since they can be scavenged by larger particles. Thus, recently a significant effort has been placed in developing instruments that also measure neutral atmospheric particles of 1 3 nm in diameter (Kulmala et al., 2005; Sipilä et al., 2008). Only very recently, progress in instrumental capabilities has enabled measurement down to approximately 2 nm and since there is an indication that particle 7
10 formation is initiated at about 1 nm we are getting near to measuring nucleating particles directly (Kulmala et al., 2007). In addition, mass spectrometry-based instruments are capable of detecting some of the neutral molecular clusters responsible for nucleation (Zhao et al., 2010). While much progress has been made in directly observing nucleation, in most cases atmospheric nucleation rates can only be inferred indirectly by measuring the particle formation rate at some larger size (e.g. 3 nm) and extrapolating this down to the nuclei size taking into account the coagulational loss of particles before they reach the detection limit. Another limit in the understanding is the identification of the condensing species, particularly organics - their thermophysical properties and their reaction mechanisms - that are responsible for the large observed growth rates. Over a decade ago, Weber et al. (1997) concluded that while nucleation might depend on sulphuric acid and water, particle growth rates required another, probably organic, component. Later on, many other studies came up with the same conclusion (Kulmala et al., 2004, McMurry et al., 2005; Sihto et al., 2006; Riipinen et al., 2007; Paper II ; Smith et al., 2007; Laaksonen et al., 2008b; Kuang et al., 2008; Paper III ; Sipilä et al., 2010). As a direct result of these limitations and despite comprehensive research efforts, substantial inconsistencies remain and conflicting results of laboratory studies, atmospheric observations and model studies still persist (Laaksonen et al., 2008a; Berndt et al., 2008). Several key questions about the predictability of atmospheric nucleation in general, including the substances that take part in nucleation and subsequent growth and the size and composition of the critical cluster, have not been resolved so far. In the past years, the commonly proposed candidates for the atmospheric nucleation mechanism have included kinetic (McMurry and Friedlander, 1979), binary (Doyle, 1961; Mirabel and Katz, 1974), ternary (Korhonen et al., 1999; Merikanto et al., 2007), ion-induced or ion-mediated nucleation (Yue and Hamill, 1979; Yu and Turco, 2000; Lee et al., 2003), and the activation and growth of persistent neutral or charged molecular clusters (Hoppel et al., 1994; Kulmala et al., 2000; Kulmala, 2003; Kulmala et al., 2006). These proposed particle formation schemes usually include water, sulphuric acid and sometimes ammonia or organic vapors. In addition, iodine oxides are thought to be responsible for nucleation in coastal areas (O Dowd et al., 1999). In ion-induced nucleation, atmospheric ions created by cosmic rays and radon radioactivity act as nucleation agents (see e.g., Laaksonen et al., 1995 and references therein). The composition of atmospheric particles is a major factor determining their effects on the climate (see e.g., Seinfeld and Pandis, 2006), visibility and human health. In the case of the smallest nanoparticles, their composition naturally reflects the mixture of vapors participating in secondary 8
11 aerosol formation and growth. Several laboratory and field studies suggest that sulfuric acid and sulphate clusters are likely participants in atmospheric nucleation and growth (Weber et al., 1996; Hanson and Eisele, 2002; Berndt et al., 2005; Sihto et al., 2006). However, there are also observations that imply that the ambient sulfuric acid concentrations cannot explain the observed nucleation and growth completely (Fiedler et al., 2005; Boy et al., 2005; Laaksonen et al., 2008b), particularly in rural environments. On the other hand, a clear link between atmospheric aerosol formation and the emissions of biogenic organics has been reported in several studies (O Dowd et al., 2002; Tunved et al., 2006; Laaksonen et al., 2008b). The relative roles of sulfur-containing and organic compounds in particle formation and growth can, among other things, be treated as a measure of the relative magnitudes of anthropogenic and biogenic effects on the secondary aerosol source. This is particularly true in continental regions, where most of the gaseous sulfur is of anthropogenic origin. In the work presented in this thesis we have studied atmospheric new particle formation in different sites (Papers I-V). The anthropogenic impact at these sites varies from polluted rural (SPC) to moderate rural (Melpitz) to clean conditions (Hyytiälä). Comparative study of variations of formation and growth rates of newly formed particle at these sites together with studying the effect of meteorological parameters and gas phase concentrations on NPF occurrence and predictability of atmospheric nucleation have been presented in Paper IV. Cloud condensation nucleus production following NPF events has been studied and presented in Paper I and Paper III. In particular, Paper III focuses on how the decreasing SO 2 emissions (the main precursor of gaseous sulfuric acid H 2 SO 4 which in turn is a main precursor for atmospheric particle nucleation) between 1996 and 2006 in Melpitz have affected the frequency of NPF events, formation rates, growth rates and CCN production. Based on many earlier studies, it has been observed that NPF takes place preferentially at low relative humidities in almost all the different environments. Paper V explores in detail the possible reasons for this interesting observation. The main objectives of the work presented in this thesis thus can be summarized as: 1) Quantitatively describing the occurrence and characteristics of atmospheric particle formation in different atmospheric environments in the European boundary layer (Paper I, II, III, IV). 2) Investigating the contribution of NPF events on producing cloud condensation nuclei (CCN) (Papers I, III). 9
12 3) Evaluating the influence of changing SO 2 concentrations (precursor of sulfuric acid, H 2 SO 4 ) on NPF and particle growth (Paper III). 4) Discussing the role of relative humidity (RH) in continental NPF (Paper V). 10
13 2 Aerosol particle size distribution and concentration Two important properties of aerosol particles are their concentration and their size. Aerosol particles are present practically everywhere in the Earth s atmosphere, and their concentrations vary by four or five orders of magnitude depending on the site. Lowest values, 1-10 cm 3, have been observed in Antarctica during wintertime (Shaw, 1988; Ito, 1993) and slightly higher values, 100 cm 3, during the Antarctic summer (Koponen et al., 2003). Slightly higher numbers, cm 3 and cm 3 have been observed at two clean locations in northern Finland (Komppula et al., 2003; Lihavainen et al., 2003). For a more polluted environment such as Pittsburgh, Pennsylvania, the average number concentration was about cm 3 (Stanier et al., 2004). This value was higher than the average total particle concentration at SPC, Italy, cm 3 (Paper II). Different environments are also characterized by different types of size distributions. The sizes of atmospheric aerosol particles range from nanometers up to hundreds of micrometers. Aerosols are usually classified into one of the following four size categories: nucleation mode particles, which are the smallest with a diameter less than about 30 nm; Aitken mode ( nm diameter); large particles, or accumulation mode (100 nm 1µm diameter); and giant particles or coarse particle mode (> 1 µm diameter). The main sources of coarse particles are windblown mineral dust from deserts and salt particles formed as a result of oceanic bubble bursting. These particles also dominate the mass spectrum of particles. The terms nucleation mode and accumulation mode refer to the mechanical and chemical processes by which aerosol particles in those size ranges are usually produced. The accumulation mode, is so named because particles in its size range have low deposition velocities and thus long lifetimes, causing particles to accumulate in this size range. Aerosols in the accumulation mode are generally produced by the coagulation of smaller particles, by heterogeneous condensation of vapors onto existing aerosol particles, and by chemical conversion of volatile species into nonvolatiles in cloud droplets (cloud processing of aerosols). In addition, there are primary sources that produce particles directly into the accumulation mode. The smallest aerosols, in the nucleation mode, are principally produced by atmospheric gas-to-particle conversion. Some aerosol particles in the nucleation mode are comprised of sulfur species, the result of the oxidation of sulfur containing precursor gases (like SO 2 to sulfate, SO 2-4 ). The concentration of nucleation mode particles varies strongly with the strengths of sources and sinks. As observed in several stations located in Northern Europe (Tunved et al., 2005; Dal Maso et al., 2007), the continental background site Melpitz in Eastern Germany (Engler et al., 2006) and in the 11
14 rural polluted site SPC (Sogacheva et al., 2007), freshly nucleated particles may rapidly form in clean air masses and grow to larger sizes, whereas coagulation and condensation to larger preexisting aerosols remove them. There are a number of different types of instruments that can be used for atmospheric aerosol size distribution measurements. The Differential Mobility Particle Sizer (DMPS) is currently the most widely used, and best (e.g., Wiedensohler et al., 1994) instrument for measuring ultrafine size distributions. In principle, the DMPS consists of a Differential Mobility Analyzer (DMA) and a Condensation Particle Counter (CPC) (see e.g., Stolzenburg and McMurry, 1991; McMurry, 2000). When the aerosol sample enters the device, it is electrically charged with a bipolar charger to a known charge distribution. The particles are then classified according to their electrical mobility in the DMA. The mobilities can be converted to particle sizes (all particle sizes in this thesis are expressed in mobility equivalent diameters, see, e.g., Mäkelä et al., 1996), and the concentrations in each size class are counted optically in a CPC after growing the particles to detectable sizes by condensing butanol vapor onto them. Data collected with the DMPS system are used in the analyses presented in Papers I V. Details about the specific DMPS systems employed for each station are presented in Papers I V. 3 Aerosol dynamical processes 3.1 Nucleation and new particle formation (NPF) Nucleation is the first and critical step of NPF from precursor vapors. The transformation of matter in the vapor phase to the liquid phase does not happen instantly when the vapor is saturated. First, small clusters of the new phase are formed in the supersaturated vapor. These clusters are considered to be ca. 1 nm in diameter (Kulmala et al., 2000), too small to be detected by conventional aerosol instrumentation (DMPS) systems. If the nascent particles do not grow fast enough, they may not be able to reach a detectable size (e.g., 3 nm, the lower size limit of typical DMPS systems) before they are scavenged by larger particles. Finally, Aitken mode particles comprise of particles formed in NPF events that have grown larger than 30 nm, and of primary particles produced by combustion. According to the current understanding, the species participating in atmospheric nucleation and early growth of the clusters are still to a large degree unknown, but sulfuric acid is assumed to be 12
15 the key precursor gas. After nucleation, other supersaturated substances, e.g., low vapor pressure organics, take part in the subsequent aerosol growth. Iodine oxides seem to be responsible for nucleation and growth observed in some coastal areas (O Dowd et al., 1999). The binary homogeneous nucleation mechanism is one of the most studied in atmospheric nucleation investigations (Jaecker-Voirol and Mirabel, 1987; Kulmala and Laaksonen, 1990; Viisanen et al., 1997; Vehkamäki et al., 2002; Curtius, 2006). The theoretical nucleation rates predicted for the binary sulfuric acid and water system in tropospheric conditions are too low to explain observations. Therefore, it is likely that some other compounds, for example ammonia or some organic acids, are also participating in atmospheric nucleation processes (see e.g. Ball et al., 1999; Napari et al., 2002; Paper II; Laaksonen et al., 2008b; Paper III). One specific possibility is the ternary nucleation mechanism (water-h 2 SO 4 -ammonia) that has been studied e.g. by Korhonen et al., (1999), Napari et al., (2000) and Merikanto et al., (2007). Another factor that is studied is the influence of electric charge on the nucleation physics (ion-induced nucleation, see, e.g., Yue and Hamill, 1979; Yu and Turco, 2000; Laakso et al., 2002; Lee et al., 2003; Lovejoy et al., 2004; Eisele et al., 2006 ). Nucleation mechanisms involving ions have been proposed to be important for aerosol formation in the atmosphere (e.g., Arnold, 1980; Eisele et al., 2006). In ion-induced nucleation the vapors condense around the ions. In this case, the nucleation barrier is reduced due to the stabilizing electrostatic interaction between the ionic charge and the other participating molecules. The reduction in barrier height makes the ion-induced process thermodynamically more favorable (Winkler et al., 2008). Ion production is driven by galactic cosmic rays over most of the atmosphere; however our understanding on the connection between galactic cosmic rays, ions and atmospheric aerosol formation and their impacts on climate has relied solely on a few model investigations (Kazil et al., 2006; Pierce and Adams, 2009). Recently, based on long-term experimental data, Kulmala et al. (2010) showed that ion-induced formation contributes less than 10% to the number of new particle formation days and the galactic cosmic rays appear to play a minor role for atmospheric aerosol formation events. The nucleation theorem (Oxtoby and Kashchiev, 1994) relates the number of molecules, n A, of the species, A, in the critical nucleus to the slope of the logarithm of the nucleation rate J nuc, as a function of the logarithm of the gaseous concentration of the nucleating species, [A], i.e., n A din J nuc /din[a]. If A denotes sulfuric acid, an exponent value of 2 indicates that the critical cluster contains two sulfuric acid molecules. This result is supported by the work of Hanson and Eisele (2002) in which their measurements of pre-nucleation molecular clusters indicate a critical cluster containing two H 2 SO 4 molecules. Recent studies on the dependence relationship between nucleation 13
16 rate and H 2 SO 4 concentrations for atmospheric measurements have shown that the number of sulfuric acid molecules in the critical cluster is one (Kulmala et al., 2006; Sihto et al., 2006; Riipinen et al., 2007) or two (McMurry et al., 2005; Kuang et al., 2008). In contrast, laboratory studies suggest that nucleation rates depend more strongly on sulfuric acid concentrations, corresponding to a critical nucleus of four to nine sulfuric acid molecules (Berndt et al., 2005). Interestingly, that larger number of sulfuric acid in the critical cluster agrees with predictions of classical nucleation theory although the concentrations used in such laboratory studies are typically far above those in the atmosphere (Laaksonen et al., 2008a). In laboratory conditions, the concentrations of the nucleating vapors are so high that these vapors are also responsible for condensational growth. In contrast, the nucleation of aerosols in the atmosphere can be kinetically limited by thermodynamically stable clusters (Kulmala et al., 2000) that are formed during intermediate steps of aerosol nucleation. Recently, Sipilä et al. (2010) managed to produce sulfuric acid at concentrations comparable to those found in the atmosphere in their laboratory tests on particle formation rates. Sipilä and colleagues found that the critical nucleus consists of one or two sulfuric acid molecules, which is similar to what has been observed under atmospheric conditions. The difference between the recent results and previous laboratory measurements can be explained by their improved instrumentation that can detect particles that are just about bigger than a single nm. Past attempts failed because the previous measurements were by low counting efficiently for particles smaller than 3 nm. Therefore, old laboratory experiments were only able to detect particles of 3 nm, resulting in appreciable underestimates of nucleation rates. Atmospheric particle formation and the initial growth processes take place below 3 nm. Whether or not we are able to observe NPF is mainly governed by the rates at which stable nuclei are lost by coagulation to preexisting particles and grow by condensation. Therefore, fast growth is needed for particle formation; otherwise, nucleated particles would be scavenged before growing into measurable size range above 3 nm (McMurry et al., 2005; Kuang et al., 2010). In other words, what we really observe are the clusters that survive loss by coagulation and grow by condensation to a size that can be detected. Figure 1 shows an example of new particle formation day that we can observe in the atmosphere. 14
17 Figure 1: New particle formation event as measured with a DMPS system on at the San Pietro Capofiume (SPC) station, Italy (Paper I). The figure illustrates a typical NPF and growth event as observed from nm particle size distributions measured by a DMPS system. The event has been recorded on at the SPC station in Italy (Paper I). SPC station is 40 km northeast from the city of Bologna, in the Po Valley. Po Valley is a densely populated, highly industrialized and known to have relatively high levels of anthropogenic pollution. Most of the field data analyses presented in this work (Papers I, Paper II) are based on measurements from this station. In addition to the SPC station, we present NPF data analysis from two other sites: Melpitz (rural site in Germany) and Hyytiälä (boreal forest in Finland) in Paper III and Paper V, respectively. Paper IV examines specifically the possible differences and similarities of NPF for the same period (July 1 st, 2003 June 30 th, 2005) at the aforementioned three sites. 3.2 Condensation and condensational sink (CS) Although nucleation as a process is still not well understood, condensation is generally very well understood. In laboratory conditions, when the growth or evaporation rate of droplets of a known vapor can be determined accurately, condensation theory can be used to acquire knowledge of the participating vapor properties. In the atmosphere, however, the condensing vapor is often unknown and the determination of the growth rate can be problematic. A simplified picture as to what could 15
18 happen in the atmosphere is that, following nucleation, the freshly nucleated stable clusters grow using the nucleated vapors (e.g., H 2 SO 4 ) as the driving substances. The growth of freshly nucleated particles due to condensation can be thought of in terms of the Kelvin equation (see e.g., Friedlander, 1977). There, the driving force is the difference between vapor pressure at the critical conditions of nucleation (corresponding to the vapor pressure at critical cluster size) and at the actual size of the growing cluster (which is lower due to the weakening of the Kelvin effect with size). In this case the growth will increase very rapidly and then stay relatively constant, since the Kelvin effect is sensitive to cluster (or particle) size. This is the dominating particle formation mechanism in many chamber studies and in nucleation experiments. There are several ways to calculate condensation of vapor onto particles. As an example, consider a new liquid phase particle once it has been formed. Its surface is continuously bombarded by gas molecules. If the droplet is not in equilibrium with the surrounding vapor, there is a net flux of molecules either towards (condensation) or away from (evaporation) the droplet surface. Condensation is driven by the difference of vapor pressure (or concentration) at the droplet surface P s, C s and the vapor pressure (concentration) far away from the droplet, P, C. The relation between the rate of the diameter change (dd p /dt) and the concentration difference (C - C s ) in the case of assuming spherical particles can be given as (Seinfeld and Pandis, 2006). (1) Where d p is the particle diameter, D i is the binary diffusion coefficient in air for the condensing vapor, and M is the molecular mass of the condensing vapor, is the density of the condensed molecules. M is the transitional correction factor, introduced to account for the difference in the condensation flux at small and large droplet sizes. There is no fundamental equation of the transitional correction factor M but several approximations that are in broad agreements. We have chosen to use the form presented by Fuchs and Sutugin (1970) in our calculations: (2) 16
19 is the mass accommodation coefficient (also called the sticking coefficient) which describes the probability of an impinging molecule sticking to the surface. Unless otherwise stated, is assumed to be unity, based on the results obtained by Winkler et al. (2004). The size scale is characterized by a dimensionless group, the Knudsen number (Kn) given by: (3) v is the effective mean free path of the condensing vapor molecules in the gas (air). At small sizes the particles see the vapor molecules as discrete entities (free molecular regime Kn> 10, M = ~3d p /4 v), while at large particle sizes the condensing vapor is seen as a continuous medium (continuum regime Kn<=1, M =1). We see from equation (1) that the particle growth rate depends on the concentration of the condensing vapor (C vapor ). By assuming constant C vapor during growth, the concentrations of the condensing vapor, C vapor, which is determined by its source Q, and condensation sink CS can be expressed by: (4) Q is the source rate and CS is the condensation sink. If no sudden injections or depletions of vapor are taking place, we can assume a steady state situation and set in equation (4), which gives for the vapor source rate Q: (5) The condensation sink (CS) is the sink formed by pre-existing particles to condensable vapors. Therefore CS (units s -1 ) describes the loss rate of molecules with diameter d p, diffusion coefficient D, and mean free path v onto a distribution n(d p ) of existing particle and thus, it is obtained by the integrating (or summing) over the size spectrum and can be calculated from Kulmala et al. (2001): (6) 17
20 In practice, the vapor is assumed to have very low vapor pressure at the surface of the particle, and molecular properties are assumed similar to those of sulfuric acid. Therefore, D is taken to be the diffusion coefficient of sulfuric acid (diffusion coefficient of sulfuric acid is cm -2 s -1 ) and the transitional correction factor M is typically calculated using the expression given by Eqn. (2). Here, d pi is the diameter of the particles in the i th size and N i is the number concentration in the respective size class measured with a DMPS system at dry relative humidity. By determining CS one can get an indication of how rapidly condensable vapor molecules will condense on the existing aerosol (the whole particle size distribution). It is known that particles grow as a function of RH because of uptake of water vapor by hygroscopic substances in the particles. Thus the surface area, to which low vapor-pressure gases can condense, also increases. In other words, the increased condensation sink at high RH removes gases such as H 2 SO 4 that would otherwise participate in nucleation. Therefore, we simply can write the CS at some specific RH as CS(RH) = CS GF(d p, RH), and the function GF(d p, RH) gives the hygroscopic growth factor (RH-dependence). The hygroscopicity of the aerosol population varies spatially and temporally, and thus it would be difficult to derive a universal formula for GF(d p, RH). In Paper V, we examined QUEST (Quantification of Aerosol Nucleation in the European Boundary Layer) field campaign data from spring 2003 at the SMEAR II station, Hyytiälä, Finland. We applied the formula of GF(d p, RH) that was given by Laakso et al. (2004) and adapted for Hyytiälä conditions: (7) Z(d p ) is a parameter derived by a least-square fit to hygroscopicity data measured during the BIOFOR campaign in Hyytiälä in 1999 (Hämeri et al., 2001), given by: (8) Figure 2a shows an applicable example of the hygroscopic growth factor (GF) of dry 100 nm particles as a function of RH by using Laakso et al. (2004) (formula described above). Figure 2b shows the obtained result of the estimated condensation sink values as a function of RH measured during the study period (note that the CS values have been calculated from dry size distributions using the method described by see Eqn.6) and by applying an RH correction using Laakso et al. (2004) parameterization given by Eqn.7& Eqn. 8). 18
21 (a) (b) Figure 2: (a) The hygroscopic growth factor (GF) of dry 100 nm particles as a function of RH using Laakso et al. (2004) formula (b) RH (%) versus condensation sink (s -1 ) estimated during spring 2003 QUEST field campaign in Hyytiälä, Finland (Paper V). 3.3 Coagulation and coagulation sink (CoagS) Coagulation is the sticking together of two colliding particles. It is the result of particles coming into contact due to Brownian diffusion or some other forces (electrostatic, gravitational settling, turbulence, etc.). Note that contact does not necessarily lead to coagulation, but must happen as a pre-requisite. Coagulation is also enhanced in shearing or turbulent flows, as these induce fast relative particle motion. In this thesis, coagulation refers solely to Brownian coagulation which is the dominating coagulation mechanism for sub-micron particles. The coagulation rate of a particle population depends on the particle concentration and the coagulation efficiency of the particles. The coagulation efficiency in turn depends on the relative velocity of the colliding particles and their interception cross area. The coagulation coefficient K ij (also called the coagulation kernel) describes the coagulation efficiency of two particles, designated by i and j. In this work, we used the calculation of the coagulation coefficient K ij that has been performed following the Fuchs (1964) treatment in which the coagulation coefficient is given by: 19
22 (9) r i and r j are the radii of coagulating particles, D i and D j are their diffusion coefficients, c i and c j are the particle mean thermal velocities and is a distance parameter resulting from the flux matching approach. The coagulation coefficient is smallest for particles of the same size and increases rapidly as the ratio between the particle diameters increases. Therefore, coagulation is most efficient between particles with a large difference in size when the big but slowly moving particle scavenges the small particle which moves with a high velocity. This means that just-formed, nanometer-sized particles are most susceptible to coagulation scavenging, and Brownian coagulation is one of the key processes determining whether particles produced by nucleation ever reach detectable sizes, let alone CCN sizes. Coagulation sink (CoagS) can be determined from Kulmala et al. (2001): (10) Here N j is the particle number concentration of the j th size bin, and K ij is the coagulation coefficient given by equation (9) between particles of the j th bin (diameter d pj ) and i th bin. (see e.g., Seinfeld and Pandis, 2006). The new particles formed during NPF events have short lifetimes owing to their coagulation with larger particles. As a result, the growth of these particles to larger sizes is a crucial process for the formation of observable particles. 3.4 New particle formation and nucleation rates (J 3, J 1 ) The relative importance of the contribution of the condensation and coagulation to the observed NPF rate is presented and discussed in this section. As mentioned previously, the freshly nucleated clusters are too small to be detected by conventional aerosol instrumentation systems with a detection limit of 3 nm (e.g., DMPS). Atmospheric nucleation cannot thus directly be observed with the DMPS system, and therefore the actual atmospheric nucleation rates at 1 nm, J 1, must be 20
23 calculated from the formation rates at 3 nm, J 3, accounting for particle loss by coagulation with larger particles during growth between 1 nm to 3 nm (Weber et al., 1997; Kerminen and Kulmala, 2002; Lehtinen et al., 2007). This calculation requires knowledge of the growth rates of particles below 3 nm as well as the actual size of the nucleating particles. The formation rate of 3 nm particles is usually in the range of cm -3 s - 1 in the boundary layer and up to 100 cm -3 s -1 in urban areas. However, in coastal areas and industrial plumes, formation rates as a high as 10 5 cm - 3 s -1 have been recorded (see Kulmala et al., 2004 and references therein). The time evolution of N 3-6 is described with a balance equation: (11) Equation 11 includes terms for the growth into the 3-6 nm range from 3 nm (the first term), growth out of the range beyond 6 nm (the second term) and the loss by coagulation scavenging (the third term). The growth by intermodal coagulation is assumed to be negligible compared to condensation. Here, GR 6 denotes the particle growth rate at 6 nm, and n d is a particle size distribution function, defined as n d = dn d /dd p with d p = particle diameter. CoagS 3-6 denotes the average coagulation sink for the 3-6 nm range (Kulmala et al., 2001). By rearranging the terms, and defining the first term on the right hand side of Eqn. (11) as J 3, the rate at which particles grow past the minimum detectable size (3 nm) by vapor condensation can be described by the following equation: (12) Coagulation loss Loss due to condensation Here the coagulation loss for the interval 3-6 nm has been approximated by a term representing the loss of 4 nm sized particles, with hygroscopicity effects estimated as in Laakso et al. (2004). The third term representing the condensation loss out of the size range 3-6 nm is obtained by approximating n 6 by N 3-6 /(6 nm - 3 nm). The GR 6 value used in the calculations was obtained from the DMPS data in the size range 3 7 nm. The nucleation rate of critical clusters, J 1, is then extrapolated from the formation rate of 3 nm particles, J 3, which is obtained from measured particle size distributions as given in Eqn.13. Particularly, we apply the method presented by Kerminen and Kulmala (2002) to get J 1 : 21
24 (13) Here is the reduced condensation sink (i.e in units m -2 ), GR 1-3 is the 1 3 nm growth rate (in nmh -1 ) and is a coefficient with a value of approximately 0.23 m 2 nm 2 h -1. Time delay t is estimated for each new particle formation day as the time delay between the rise in sulfuric acid concentrations and particle number concentration N 3-6. The NPF event is assumed to begin when [H 2 SO 4 ] begins to rise sharply, and N 3-6 particle concentrations begin to increase as well. In this study, time delay values between rise of sulfuric acid concentration [H 2 SO 4 ] and number of particles between 3 and 6 nm (N 3-6 ) varied between 20 minutes to one and half hours for different NPF days. An example (red arrow in Fig. 2) shows an estimation of t for a NPF event (11 th July 2009) at the SPC station, Italy. This is the time required for the clusters to grow from the nucleated size of 1 nm to the detectable size of 3 nm in diameter. 4 Gas Phase Species Responsible for nucleation & NPF 4.1 Sulfuric acid Sulfuric acid is formed in the atmosphere via the oxidation of SO 2 by OH. The currently accepted mechanism of atmospheric SO 2 oxidation is as follows (Finlayson-Pitts and Pitts Jr., 2000): OH + SO 2 HSO 3 HSO 3 + O 2 SO 3 + HO 2 SO 3 +2H 2 O H 2 SO4 + H 2 O The reaction of HSO 3 with O 2 is very fast (Gleason et al., 1987). The production of H 2 SO 4 is mainly initiated and governed by SO 2 oxidation by OH radical. Sulfuric acid, H 2 SO 4, has been identified as a potentially important component in aerosol formation and growth, both experimentally and theoretically (Korhonen et al., 1999; Kulmala, 2003; Berndt et al., 2005 ; McMurry et al, 2005; Kuang et al., 2008 ; Sipilä et al., 2010). The measurement of gas-phase H 2 SO 4 and nucleation-mode particles in the 3 6 nm size range is shown in Fig. 3. The strong correlation between the two quantities when taking into account the estimated time lag (81 minutes in this example) for the growth of the particles to 3 nm size, presents evidence that sulfuric acid is taking part in the nucleation process. 22
25 Figure 3: Gaseous sulfuric acid concentration (H 2 SO 4 ) and number of particles between 3 and 6 nm (N 3-6 ) during a NPF event at SPC station, Italy. Red arrow between the two curves shows the time lag of about 81 minutes for the freshly nucleated particles to grow to observable sizes (>3 nm). (Hamed et al., 2010) The dependence between H 2 SO 4 concentration and nucleation rate is assumed to follow a simple power law equation: (14) J nuc is the atmospheric nucleation rate and [H 2 SO 4 ] is the sulfuric acid concentration. The prefactor P and nucleation exponent n provide insight into the nucleation mechanism, where n values of 1 and 2 correspond to the activation mechanism (Sihto et al., 2006; Riipinen et al., 2007; Kulmala et al., 2006) and kinetic mechanism (McMurry and Friedlander, 1979; Kuang et al., 2008; Sipilä et al., 2010) respectively. The activation model assumes that nucleation occurs through the activation of small clusters containing one H 2 SO 4 molecule via one of several mechanisms, including heterogeneous nucleation and heterogeneous chemical reaction. The kinetic model assumes that critical clusters are formed through bimolecular collisions of sulfuric acid containing clusters. The prefactor for both models contain chemical and physical details of the nucleation process. Figure 4 shows the logarithm of the nucleation rate at 1 nm, J 1 (cm -3 s -1 ), estimated from particle measurements, versus the logarithm of the sulfuric acid concentration at the SPC site. The linear 23
26 fitting of the data shows that the nucleation exponent n=2.2 ± 0.02 which is in agreement with the results obtained by Weber et al. (1996) and Kuang et al. (2009). The exponent n is close to 2, which means the kinetic mechanism is more favorable during this SPC campaign period than the activation mechanism (when n=1). As mentioned previously, the nucleation theorem for multicomponent systems (Oxtoby and Kashchiev, 1994) states that an exponent of 2 implies that the critical cluster comprises of two sulfuric acid molecules through a kinetically controlled nucleation process. Figure 4: The nucleation rate, J 1 (cm -3 s -1 ), estimated from particle measurements, versus the sulfuric acid concentration measured at SPC station during campaign from June 26 th to July 12 th Black line shows the best linear fit, n=2.2± (Hamed et al., 2010) The magnitudes of the nucleation coefficient, P, seem to vary depending on the site, being generally of the order of s 1 for n=1 (Sihto et al., 2006; Riipinen et al., 2007) and s 1 cm 3 for n=2 (Kuang et al., 2008; Sipilä et al., 2010). The results imply that homogeneous nucleation of sulfuric acid cannot explain the observed phenomena, since the binary and ternary nucleation theories involving sulfuric acid, water and ammonia would predict exponents larger than 3 (Kulmala et al., 2006; Merikanto et al., 2007). Spracklen et al. (2006) implemented Eqn. 14 by the two conditions (n=1 and n=2) as the particle formation mechanisms in a global aerosol microphysics model. The model reproduced the observed secondary aerosol concentrations and the occurrence of NPF with good accuracy, proving that this kind of a simple parameterization has the potential to predict the occurrence of particle formation, at least in the boreal forest boundary layer. 24
27 Although a clear correlation between the atmospheric sulfuric acid concentrations and particle formation rates is observed, this cannot be directly interpreted as a sign of the dominance of sulfuric acid participating in particle formation and growth. First, the diurnal profile of gaseous sulfuric acid is strongly correlated with the ambient OH concentration and further with the UV radiation (Rohrer and Berresheim, 2006). This points toward the possibility that some other compounds, also correlated with OH, might be participating or even dominating the particle formation. Second, as pointed out by Laaksonen et al. (2008a), several laboratory studies report remarkably smaller sulfuric acid concentrations needed for the same particle formation rates if the sulfuric acid is produced via the photo-oxidation of SO 2 (Friend et al., 1980; Berndt et al., 2005; 2006) compared with studies using sulfuric acid directly vaporized from a liquid sample (Viisanen et al., 1997; Ball et al., 1999; Zhang et al., 2004b). The previous conditions naturally resemble those of the real atmosphere. (These differences are illustrated in Fig. 2 in Laaksonen et al., 2008a). The data analysis presented for Melpitz, Germany, in Paper III revealed that the drop in the anthropogenic SO 2 concentrations between years to years had an influence on the frequency of NPF events, number concentrations of nucleation mode particles and the observed formation rates of the 3 nm-particles (J 3 ). Figure 5 shows that between years to years , the number of NPF events observed in Melpitz, Germany, had dropped by 45%. This drop was associated with the drop of the SO 2 concentrations by 65% between the two periods. The relative difference of nucleation mode particle number concentrations, N 3-10, between the two periods showed similar magnitude as the reductions of the 3 nm-particles (J 3 ). In Paper III, we studied the combined parameter called sulfuric acid proxy (product of SO 2 and global solar radiation divided by condensational sink). Our results also showed that the change of SO 2 dominates over the changes of solar radiation and condensational sink in the estimated sulfuric acid proxy. It is notable also that the proxy correlates fairly well with the measured 3 nm particle formation rates (J 3 ) and the slope between the logarithms of these two quantities was close to unity (suggesting an activation mechanism). This result shows that our proxy variable behaves at least qualitatively similarly to the actual [H 2 SO 4 ]. 25
28 (a) (b) (c) (d) Figure 5: Monthly average (a) SO 2 concentrations ( gm 3 ), (b) frequency of NPF events, (c) the nucleation mode particles concentrations 3 10 nm (cm 3 ) for the particle formation events and (d) the particle formation rate of 3 nm-particles (J 3 ) in (cm 3 s 1 ) in Melpitz in years (blue) and years (red) (Paper III). In Paper V, we produced evidence that the concentration of H 2 SO 4 at high humidities is controlled by the reduced OH formation rate. Our data analysis from QUEST field campaign in spring 2003 at the SMEAR II station, Hyytiälä, Finland, shows that sulfuric acid concentrations (Fig. 6a) decreased significantly at RH above 60%. At high RH (> 80%), formation rates mostly were below 2 cm -3 s -1 where the H 2 SO 4 concentrations were below cm -3 (see Fig. 6c). The factors contributing to H 2 SO 4 formation (SO 2 *OH) and loss rates (CS) as a function of RH were tested separately. Overall, there was no significant RH dependence for the measured SO 2 concentration although the maximum SO 2 values decreased slightly with RH. On the other hand, the UV radiation intensity (Fig. 6b), which is a proxy for OH concentration, decreased clearly with increasing RH. The interesting finding is that the UV radiation trend was very similar to that of the measured H 2 SO 4 concentrations (Fig. 6a & Fig. 6b), which suggests that concentration of H 2 SO 4 at high humidities is controlled by the reduced OH formation rate. It should be noted that the sink term for H 2 SO 4 increased as RH increased however, it played a relatively small role in the inhibition of nucleation at high RH. 26
29 (a) (b) (c) Figure 6: (a) RH (%) versus H 2 SO 4 concentrations (molecule/cm3), (b) UV irradiation in Wm -2 observed during spring 2003 QUEST field campaign in Hyytiälä, Finland (c) Estimated nucleation rate at 1.5 nm (J 1.5 ) versus [H 2 SO 4 ] measured during the 2003 QUEST campaign. Colour code refers to relative humidity (RH) expressed as percent (Paper V). 27
30 4.2 Organic vapors As discussed in the previous section, the observed close connection between atmospheric nucleation rates and sulfuric acid vapor concentration indicates that it is likely that sulfur compounds are present at the early stages of particle formation and growth. On the other hand, as mentioned also previously, observations show that sulfuric acid condensation is not enough to explain the particle growth rates, pointing to the increasing role of organics in the later stages of the condensational growth processes. A clear link between atmospheric aerosol formation and the emissions of biogenic organics has been reported, particularly at continental sites (e.g., Tunved et al., 2006; Laaksonen et al., 2008b; Metzger et al., 2010). There are also significant amounts of laboratory data showing that the VOCs typically emitted by vegetation, for instance isoprene, monoterpenes (Pandis et al., 1991; Kanakidou et al., 2005; Hallquis et al., 2009), and sesquiterpenes (Bonn and Moortgat, 2003), can act as precursors for condensable vapors forming and growing secondary aerosol particles. Unfortunately, the concentrations used in the environmental chambers are typically orders of magnitude higher than observed ambient concentrations. However, interaction between sulfuric acid and organic acids seem to promote NPF (Zhang et al., 2004a). Thus, the organic compounds cannot be neglected in the formation process under atmospheric conditions. Boy et al. (2005) compared measured and modeled sulfuric acid concentrations and their contribution to the nucleation mode growth in Hyytiälä, Finland. According to their findings, on average, only 10% of the observed growth could be attributed to sulfuric acid in the Boreal Forest. On the other hand, Stolzenburg et al. (2005) was able to explain almost 100% of the observed growth by sulfuric acid in sulfur-rich Atlanta, USA, during four out of six case studies. In the remaining two days, in which growth took place in the afternoon, observed growth rates were by a factor of three larger than the calculated ones. This leaves a door open for other, possibly organic vapors, to contribute to the observed growth even in the environment where sulfuric acid concentrations are much higher than in rural Finland. Recently, Smith et al. (2010) reported direct measurements of the molecular composition of 8 30 nm diameter particles formed from nucleation during the field study in Tecamac, Mexico. They observed that organic compounds contributed to 90% of the observed growth of freshly nucleated particles and some of the species belonging to this organic fraction could be amines. Smith and colleagues explained that amines can form organic salts with organic and inorganic acids in newly formed particles, and thus by transforming these 28
31 species into ion pairs they would become essentially nonvolatile, thus contributing to particle growth. The estimated growth rate values determined in Paper II give one point of view to this discussion of the species and mechanisms responsible for growth. In Paper II, our analysis showed that the estimated growth rate values from the SPC station were relatively high throughout the whole period, with the maximum growth rate observed in May (22.9 nm h 1 ) and minimum in February (2.9 nmh 1 ). Overall, in winter and autumn, the monthly mean growth rate values were lower than in spring and summer. Interestingly, the concentrations of ozone, which are responsible for the formation of condensable species directly through reactions with volatile organic compound (VOCs) and indirectly by forming other oxidants (OH) upon photolysis, were also lower in winter and autumn than in spring and summer. This is possibly an indication of the role of VOC oxidation products in increasing the growth of newly formed particles more predominantly in summer and spring than in winter and autumn. On the other hand, the SO 2 (precursor for H 2 SO 4 which participates in the nucleation and most likely also in the growth of nanoparticles) concentrations were the other way around (i.e. higher in winter and autumn than in spring and summer). This indicates that most likely H 2 SO 4 is not enough to explain the high observed particle growth rates in SPC. In Paper III, we showed that although the drop in the anthropogenic SO 2 in Melpitz, Germany, was reflected in the frequency of NPF events and formation rates, the growth rates of newly formed particles have actually increased. Moreover, although the number of days with new particle formation decreased from years to years , the formation events were quite intense in the later period so that the production of CCN following NPF events has increased by tens of percent. This is most likely due to increased particle growth rates. This finding was supported by our modeling studies as well. The possible explanation to the increased growth rates was speculated to be the increased production of the oxidation products of biogenic VOC s in This assumption was based on our results that showed that the average temperatures of NPF days were higher in than in In addition to high temperature in the later period, O 3 concentrations were also higher. Biogenic VOC emissions are strongly temperature dependent and, as described above, increased O 3 concentrations may speed up the oxidation of VOC s. As a result there may have been a higher concentration of condensable organics available to contribute to particle growth in compared with
32 5 On the role of condensation and coagulation sinks on NPF 5.1 Influence of condensation sink on NPF In general, low condensation sink (CS) values have been found to favor nucleation in clean areas (see e.g., Mäkelä, et al., 1997; Kulmala et al., 2004). This can be explained by the fact that high CS formed by pre-existing particles causes condensation of non-volatile species onto existing particles to be more favourable than particle formation by nucleation. The estimated condensation sink values in Paper II and Paper III for Melpitz (rural, Germany) and for SPC (urban, Italy), were on average lower on NPF days than on non-event days, but much higher than in Hyytiälä (Paper IV). However, nucleation can take place despite the high condensation sink if sulfuric acid concentrations are high enough to support the formation and growth. This might be one explanation for the fact that in Melpitz the CS was observed to be rather high during some NPF events (Paper III and Paper IV). The fact that the production term of H 2 SO 4 can overcome the sink term and therefore nucleation can still be observed despite the high CS was discussed in more detail in Paper V. In particular, remarkable differences are observed between the CS values at event start time (lower CS) and during the events (higher CS) as observed at SPC and Melpitz stations. On the other hand, the high value of the growth rate in SPC (Paper II) and Melpitz (Paper III) stations might possibly be due to the large degree of pollution coming from nearby big cities (Bologna is about 40 km from SPC station, and Leipzig is about 46 km from Melpitz). Since the evolution of the nucleation mode size distribution results from competition between growth and scavenging onto background aerosols, fast growth is needed for particle formation; otherwise, nucleated particles would be scavenged before growing into measurable size range above 3 nm. In Paper V, we explored the reason why the increased CS at high relative humidity (RH) can possibly suppress NPF. We applied the RH-correction to the CS (where CS represents the condensation sink at dry conditions that have been calculated from dry size distributions using Eqn. 6). During the QUEST campaign, the average CS increased roughly by a factor of 3 between 30% and 90% RH (Paper V). Thus as RH gets higher, the CS gets higher and therefore this increase of CS at high RH can possibly suppress NPF. In reality CS and Coagulation Sink (CoagS) values are not independent and studying their relative importance on the inhibition of nucleation (indicated by how low J 1 values are) is in practice quite difficult if done directly from the measurements. Luckily this problematic issue can be addressed by using an aerosol dynamics model, where CS and CoagS can be fixed separately. In Paper V, using the box model (UHMA), we investigated how much the 30
33 increase in the CS due to higher RH affects the H 2 SO 4 concentrations and thus nucleation rate (J 1 ) and new particle formation rate (J 3 ). The results from the model show that the formation rate and therefore the estimated nucleation rate have been affected somewhat by increasing CS as a function of RH; however the CS increase was not the dominant reason for NPF suppression. Our model study indicates that the effect of OH dominates the effect of the CS and the CoagS. 5.2 Influence of coagulation sink on NPF The effect of increased coagulation sink on the NPF rates at high humidities is difficult to evaluate directly from the field measurements and therefore in Paper V we discussed theoretically how increases in the coagulation sink at high relative humidity increases the depletion of freshly nucleated particles before they have grown to the observation limit of 3 nm. However, we make our calculations based on observed variables affecting coagulational scavenging of freshly formed nuclei. We considered two conditions; an average condition (average growth values of the newly formed particles and average CoagS) and an extreme condition (slow growth which is accompanied with high sinks for the newly formed particles). We used the revised formulation of the nucleation rate presented earlier by Kerminen and Kulmala (2002) (see Eqn. 13). The new formula was presented by Lehtinen et al. (2007), in which the authors tried to avoid having vapor properties (i.e., CS in Eqn.13) and instead used coagulation sink. Therefore, the Lehtinen et al. (2007) equation was useful for our purpose of examining the increase of CoagS due to increasing RH (note that we applied the Laakso et al., 2004 formula) on the estimated nucleation rate (J 1 ) and therefore the formation rate of 3 nm particles (J 3 ). Figure 7 reveals that dry coagulation sink is large enough in the extreme case, which is used here to represent very unfavourable conditions for nucleation, so that it can cause masking of the event, as J 3 is smaller than J 1 by a factor of more than Thus, it appears that increased coagulation sink, under certain conditions, may cause the observed suppression of NPF events at high RH values. On the other hand, in the average conditions the ratio J 3 /J 1 varies between about 0.5 and 0.15 and, therefore, it is quite improbable that high RH values could mask NPF events very efficiently under average conditions. This theoretical result again supports the idea that even though the increased uptake of water by particles does affect the concentration of nucleating vapors and survival of nucleating clusters to some extent, these effects (CS and CoagS) play a minor role in inhibiting new particle formation. This finding is in agreement with the analysis from the field measurements and 31
34 with the model study as well. Thus, it appears that in the extreme conditions no NPF can take place even at low RH, and the increase of CoagS as a function of RH thus has no consequences. Figure7: (a) Normalized J 3 (J 3 divided by J 3 at 10% RH) and (b) J 3 /J 1.5 as a function of RH for the average conditions on left axis (solid line) and for extreme conditions on right axis (dashed line). Average conditions correspond to GR = 3 nm h -1 and CS = 8.6 h -1, extreme conditions GR = 0.5 nm h -1 and CS = 30 h -1. (Paper V). 32
35 6 Analysis of atmospheric new particle formation events Before going into more detail about the analysis of atmospheric new particle formation events, a few words shall be devoted to the sites considered in this thesis. Data sets of particle size distribution and number concentrations were used from three different sites in the European boundary layer. The sites span latitudinal range from Southern Europe (44 o 3'N, 11 o 37'E, 10 m a.s.l.) for SPC; Central Europe (51 o 32'N, 12 o 54'E, 87 m a.s.l.) for Melpitz and Northern Europe (61 o 51'N, 24 o 17'E, 170 m a.s.l.) for Hyytiälä (see Fig. 8). The stations differ also in terms of their surrounding ecosystems, varying from Boreal forest (Hyytiälä) to rural agricultural areas (Melpitz) and highly polluted rural areas (San Pietro Capofiume). Figure 8: Map of the measurement sites. Sites from north to south: Hyytiälä (Finland), Melpitz (Germany), and San Pietro Capofiume (Italy). San Pietro Capofiume (SPC), Italy, (44 39 N, E, 10 m a.s.l.): SPC station is located about 40 km northeast from the city of Bologna, in the Po Valley. The Po Valley is the largest industrial, trading and agricultural area in Italy with a high population density. Because of this, the station can be described as a highly polluted rural site. For more details about this station see Papers I and II. The particle size distribution measurements in SPC were carried out using a twin Differential Mobility Particle Sizer (DMPS) system. The first system detects particles from 3 nm in diameter and the second one from 15 nm, with the maximum detectable diameter being 600 nm. In addition to particle size measurements, several gas and meteorological parameters were measured at the SPC 33
36 station: SO 2, NO, NO 2, NO x, O 3, temperature, relative humidity, wind direction, wind speed, global radiation, precipitation, and atmospheric pressure (Paper II). Melpitz, Germany (51 32 N, E, 87 m a.s.l.): The Melpitz station is operated by Leibniz- Institute for Tropospheric Research (IfT), and is situated on a flat meadow surrounded by agricultural land. The station is located in Northern Saxony, 41 km northeast of Leipzig near Torgau; however, the station itself is surrounded by agricultural land and the site can be described as situated in a rural polluted continental area. Melpitz is equipped with in-situ meteorological instrumentation as well as continuous gas (O 3, NO, NO 2, and SO 2 ) and aerosol measurements. The particle size distribution was determined by a twin DMPS system, the first system detects particles from 3 nm in diameter and the second one from 11 nm, maximum detectable diameter being 750 nm. For more details see Birmili and Wiedensohler (2000) and Paper III. Hyytiälä (SMEAR II), Finland, (61 51 N, E, 170 m a.s.l.): The station is operated by the University of Helsinki, and is located in Southern Finland, about 50 km northeast of Tampere, the nearest urban location. The station is in the middle of the boreal forest and can therefore be described to be situated in a relatively clean area. The particle size distribution measurements were performed using a twin DMPS system that is similar to the systems in the two other stations since January Particles are measured in the size range of nm. The station has also facilities to measure gases such as CO 2, H 2 O, SO 2, O 3 and NO x concentrations as well as temperature, wind speed and direction, radiation, rain, relative humidity and air pressure. For more details see e.g., Mäkelä et al. (1997); Kulmala et al. (1998) and Paper IV. 6.1 Identifying new particle formation events The first step in the analysis of particle formation events is the identification and categorization of particle size distribution data into days with NPF events and days without particle formation (nonevent days). The day is considered a NPF event day if the formation of new aerosol particles starts in the nucleation mode size range and subsequently grows, and the formation and growth is observed over a period of several hours. In practice, a NPF event can be seen as an increase in the particle concentrations in the smallest channels of the DMPS system. These newly formed particles then experience subsequent growth that can be seen to occur typically at a rate of few nanometers per hour during the rest of the day. If the aerosol size distribution for a given day exhibits these signs, the day can be classified as a typical NPF event day. Figure 1 (see section 3.1) gives a good example of a typical NPF event (class 1). This shape is sometimes referred to as a `banana plot' due 34
37 to its appearance when plotted as a colored surface plot. In addition to NPF days, days with an absence of particles in the nucleation mode are also interesting because they facilitate the study of the cause of NPF through the comparison of the conditions during event and non-event periods. In Paper II, we have described in more detail the classification method used for all our analysis. Here, however, we give a short summary to the NPF event classes: classes 1, 2 and 3 refer to strong, intermediate, and weak events, respectively. Class 3 differs from classes 1 and 2, in that NPF has taken place, but either NPF or growth rates cannot be determined due to unsteady conditions. The days with no particle formation are classified as non-event days (NE). The days which do not fulfill the criteria to be classified as the event and non-event days were combined into one group called undefined days. To show how frequently NPF has been observed throughout different months, we presented NPF event frequency. The frequency has been calculated by dividing the total number of days with new particle formation (classes 1, 2, 3) by the total number when the DMPS was working efficiently (we excluded the days when the DMPS system malfunctioned). Figure 9 gives an example of new particle event frequency calculated for Hyytiälä (Finland), Melpitz (Germany) and SPC (Italy) for the period of two years (July 2003 June 2005). Figure 9: Monthly frequency of NPF events at Hyytiälä, Melpitz and San Pietro Capofiume (SPC) for the period of two years (July 2003 June 2005) (Paper IV). 35
38 6.2 Event characterization The features associated with NPF events are very important for characterizing the NPF. These features include: the start and cut-off (end time) of the particle bursts above the detection limit of 3 nm, giving the new particle event duration, the NPF rate or (J 3, cm -3 s -1 ), nucleation rate or (J 1, cm -3 s -1 ), growth rate or (GR, nmh -1 ), coagulation sink (CoagS, cm -3 s -1 ) and condensational sink (CS,s -1 ). NPF event characterization can be estimated directly from the measured particle size distributions by the DMPS system. In Papers I-V, we have presented the event characterization parameters for all sites we used in our study. Theoretical background for these parameters was described in more detail in Section 3. For GR analysis, we determined the growth rates visually from the DMPS data plots by tracking the evolution of the nucleation mode as a function of time. The minimum growth time that we used for estimation of the GR was three hours, and the GR was estimated up to a period of about eight hours. Figure 10 gives an example of a typical NPF event day (class 1), where the fitted growth rate (white line) together with start and end time of the event (vertical blue and green lines respectively) are shown. In order to check the reliability of our methods, we used the procedure described by Dal Maso et al. (2005) to calculate the growth rates for clear NPF events for one complete year. The estimated values for GR using both methods are very similar. Accurate estimation for starting and ending times of the NPF events is sometimes difficult because of the fluctuation in the smallest size classes due to measurement uncertainties. Only particles larger than the current DMPS detection limit (i.e., 3 nm) can be detected. Since the exact growth time (the time that newly formed particle takes to grow from the critical cluster size (~1 1.5 nm) to the detection limit of 3 nm) is not known, the observed start and the ending of particle formation are used as nucleation start and nucleation end throughout our study (Papers I-V). 36
39 Figure 10: An example of a NPF day (class 1 event on at the SPC station, Italy). The thin blue and green vertical lines show the start and end of the particle bursts above the detection limit of 3 nm. The thin white line is the fitted curve for the constant growth rate of the nucleation mode. On this day, the estimated formation rate (J 3 ) was 12.9 cm 3 s 1 and growth rate (GR) was 8.8 nm h 1. The lower plot shows total particle concentration (N tot ) for the same day (Paper II). New particle formation typically starts after sunrise near midday at the SPC, Melpitz and Hyytiälä stations. That feature seems common with other locations as well where NPF has been observed (Papers II-IV and references therein). The direct explanation to this common feature is that sunlight induces photochemical production (due to formation of the hydroxyl radical) of condensable vapors as well as the development of the atmospheric boundary layer, both of which have been observed to have a connection with NPF. Newly formed particles grow with rates in the 1-20 nmh -1 range, depending on the availability of condensable gases. For Polar Regions the observed growth rates can be as low as 0.1 nm h -1 (Kulmala et al., 2004 and references therein). Average GR values varied between 3 nm -1 for Hyytiälä, 7 nmh -1 for SPC, and 8 nm -1 for Melpitz. The growth rates during the summer are usually several times greater than those during the winter. This gives an indication of the vital role of solar radiation on NPF. Solar radiation is more intense during summer, providing more efficient photochemistry that leads to the formation of condensable species with low volatility organics and sulfuric acid. Organic compounds having a very low saturation vapor pressure are the most likely candidates for assisting the growth of fresh nuclei; the identity of these compounds remains unknown. Based on the annual cycle of GR for Hyytiälä, it seems that the dominant condensable species in Hyytiälä are biogenic in origin (the annual cycle is 37
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms
 Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS
 STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Atmospheric Aerosols: Size Distributions and Composition
 2.1 Atmospheric Aerosols: Size Distributions and Composition The Table below (from Seinfeld and Pandis) lists typical properties of different types of atmospheric model aerosols. Note that the characteristics
2.1 Atmospheric Aerosols: Size Distributions and Composition The Table below (from Seinfeld and Pandis) lists typical properties of different types of atmospheric model aerosols. Note that the characteristics
On the roles of sulphuric acid and low-volatility organic vapours in the initial steps of atmospheric new particle formation
 Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
Charged and total particle formation and growth rates during EUCAARI 2007 campaign in Hyytiälä
 Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation
 BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
Modelling the formation of organic particles in the atmosphere
 Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods
 doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions
 The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009253, 2008 Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations C. Kuang, 1 P. H. McMurry,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009253, 2008 Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations C. Kuang, 1 P. H. McMurry,
Aerosols AP sizes AP types Sources Sinks Amount and lifetime Aerosol radiative effects. Aerosols. Trude Storelvmo Aerosols 1 / 21
 Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
New particle formation in Beijing, China: Statistical analysis of a 1-year data set
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007406, 2007 New particle formation in Beijing, China: Statistical analysis of a 1-year data set Zhijun Wu, 1 Min Hu, 1 Shang Liu, 1 Birgit
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007406, 2007 New particle formation in Beijing, China: Statistical analysis of a 1-year data set Zhijun Wu, 1 Min Hu, 1 Shang Liu, 1 Birgit
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
The contribution of boundary layer nucleation events to total particle concentrations on regional and global scales
 Author(s) 2006. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics The contribution of boundary layer nucleation events to total particle concentrations on regional
Author(s) 2006. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics The contribution of boundary layer nucleation events to total particle concentrations on regional
ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS
 UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O
 THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
Nucleation and growth of new particles in Po Valley, Italy
 Nucleation and growth of new particles in Po Valley, Italy A. Hamed, J. Joutsensaari, S. Mikkonen, L. Sogacheva, M. Dal Maso, M. Kulmala, F. Cavalli, S. Fuzzi, M. C. Facchini, S. Decesari, et al. To cite
Nucleation and growth of new particles in Po Valley, Italy A. Hamed, J. Joutsensaari, S. Mikkonen, L. Sogacheva, M. Dal Maso, M. Kulmala, F. Cavalli, S. Fuzzi, M. C. Facchini, S. Decesari, et al. To cite
Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors
 Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-393 Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors Jenni Kontkanen
Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-393 Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors Jenni Kontkanen
Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany) including the infl uence of organic compounds
 BOREAL ENVIRONMENT RESEARCH 14: 616 629 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany)
BOREAL ENVIRONMENT RESEARCH 14: 616 629 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany)
Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment.
 Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment 4th April 2017 Molteni Ugo ugo.molteni@psi.ch About me Master degree in Applied and Environmental Chemistry at the University
Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment 4th April 2017 Molteni Ugo ugo.molteni@psi.ch About me Master degree in Applied and Environmental Chemistry at the University
The contribution of sulphuric acid to atmospheric particle formation and growth: a comparison between boundary layers in Northern and Central Europe
 Atmos. Chem. Phys.,, 1 18, www.atmos-chem-phys.org/acp//1/ SRef-ID: 18-/acp/--1 European Geosciences Union Atmospheric Chemistry and Physics The contribution of sulphuric acid to atmospheric particle formation
Atmos. Chem. Phys.,, 1 18, www.atmos-chem-phys.org/acp//1/ SRef-ID: 18-/acp/--1 European Geosciences Union Atmospheric Chemistry and Physics The contribution of sulphuric acid to atmospheric particle formation
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Supplement of Total sulfate vs. sulfuric acid monomer concenterations in nucleation studies
 Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Simulating aerosol nucleation bursts in a coniferous forest
 Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
INSIGHTS INTO ATMOSPHERIC NUCLEATION
 REPORT SERIES IN AEROSOL SCIENCE N:o 111 (2010) INSIGHTS INTO ATMOSPHERIC NUCLEATION MIKKO SIPILÄ Division of Atmospheric Sciences Department of Physics Faculty of Science University of Helsinki Helsinki,
REPORT SERIES IN AEROSOL SCIENCE N:o 111 (2010) INSIGHTS INTO ATMOSPHERIC NUCLEATION MIKKO SIPILÄ Division of Atmospheric Sciences Department of Physics Faculty of Science University of Helsinki Helsinki,
Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing
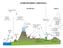 ! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Kinetic nucleation and ions in boreal forest particle formation events
 Kinetic nucleation and ions in boreal forest particle formation events L. Laakso, T. Anttila, K. E. J. Lehtinen, P. P. Aalto, M. Kulmala, U. Hõrrak, J. Paatero, M. Hanke, F. Arnold To cite this version:
Kinetic nucleation and ions in boreal forest particle formation events L. Laakso, T. Anttila, K. E. J. Lehtinen, P. P. Aalto, M. Kulmala, U. Hõrrak, J. Paatero, M. Hanke, F. Arnold To cite this version:
Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters
 Atmos. Chem. Phys.,, 6, www.atmos-chem-phys.org/acp/// Atmospheric Chemistry and Physics Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters M. Boy
Atmos. Chem. Phys.,, 6, www.atmos-chem-phys.org/acp/// Atmospheric Chemistry and Physics Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters M. Boy
Overview of the Particle Size Magnifier (PSM)
 Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Arctic Chemistry And Climate
 21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and Air Ion Spectrometer (NAIS)
 BOREAL ENVIRONMENT RESEARCH 14: 591 605 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and
BOREAL ENVIRONMENT RESEARCH 14: 591 605 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016
 ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ON MEASUREMENTS OF FORMATION, GROWTH, HYGROSCOPICITY, AND VOLATILITY OF ATMOSPHERIC ULTRAFINE PARTICLES
 REPORT SERIES IN AEROSOL SCIENCE N:o 84 (2006) ON MEASUREMENTS OF FORMATION, GROWTH, HYGROSCOPICITY, AND VOLATILITY OF ATMOSPHERIC ULTRAFINE PARTICLES TUUKKA PETÄJÄ Division of Atmospheric Sciences Department
REPORT SERIES IN AEROSOL SCIENCE N:o 84 (2006) ON MEASUREMENTS OF FORMATION, GROWTH, HYGROSCOPICITY, AND VOLATILITY OF ATMOSPHERIC ULTRAFINE PARTICLES TUUKKA PETÄJÄ Division of Atmospheric Sciences Department
Observations of new particle formation and size distributions at two different heights and surroundings in subarctic area in northern Finland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D9, 4295, doi:10.1029/2002jd002939, 2003 Observations of new particle formation and size distributions at two different heights and surroundings in subarctic
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D9, 4295, doi:10.1029/2002jd002939, 2003 Observations of new particle formation and size distributions at two different heights and surroundings in subarctic
Effect of aging on cloud nucleating properties of atmospheric aerosols
 Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN
 REPORT SERIES IN AEROSOL SCIENCE N:o 132 (2012) ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN Division of Atmospheric Sciences Department of Physics
REPORT SERIES IN AEROSOL SCIENCE N:o 132 (2012) ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN Division of Atmospheric Sciences Department of Physics
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer
 Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
Aerosols and climate. Rob Wood, Atmospheric Sciences
 Aerosols and climate Rob Wood, Atmospheric Sciences What are aerosols? Solid or liquid particles suspended in air Sizes range from a few nm to a few thousand nm Huge range of masses Where do aerosols come
Aerosols and climate Rob Wood, Atmospheric Sciences What are aerosols? Solid or liquid particles suspended in air Sizes range from a few nm to a few thousand nm Huge range of masses Where do aerosols come
Growth rates during coastal and marine new particle formation in western Ireland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014292, 2010 Growth rates during coastal and marine new particle formation in western Ireland Mikael Ehn, 1 Henri Vuollekoski, 1 Tuukka Petäjä,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014292, 2010 Growth rates during coastal and marine new particle formation in western Ireland Mikael Ehn, 1 Henri Vuollekoski, 1 Tuukka Petäjä,
Cloud Brightening and Climate Change
 Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Effects of Galactic Cosmic Rays on the Atmosphere and Climate. Jón Egill Kristjánsson, Univ. Oslo
 Effects of Galactic Cosmic Rays on the Atmosphere and Climate Jón Egill Kristjánsson, Univ. Oslo Overview of talk Hypotheses for Coupling between Galactic Cosmic Rays and Climate Observational studies
Effects of Galactic Cosmic Rays on the Atmosphere and Climate Jón Egill Kristjánsson, Univ. Oslo Overview of talk Hypotheses for Coupling between Galactic Cosmic Rays and Climate Observational studies
Nanoparticles in boreal forest and coastal environment: a comparison of observations and implications of the nucleation mechanism
 Atmos. Chem. Phys., 10, 7009 7016, 2010 doi:10.5194/acp-10-7009-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Nanoparticles in boreal forest and coastal environment:
Atmos. Chem. Phys., 10, 7009 7016, 2010 doi:10.5194/acp-10-7009-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Nanoparticles in boreal forest and coastal environment:
Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin]
![Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin] Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin]](/thumbs/86/93830655.jpg) Aerosol Challenge: Global Warming 1910s 1950s 1990s 2 1 0 +1 +2 T [Kelvin] Observed warming during 20 th century, Tapio Schneider, J. Climate, 2001 1 Aerosols are liquid or solid particles suspended in
Aerosol Challenge: Global Warming 1910s 1950s 1990s 2 1 0 +1 +2 T [Kelvin] Observed warming during 20 th century, Tapio Schneider, J. Climate, 2001 1 Aerosols are liquid or solid particles suspended in
Multicomponent aerosol dynamics model UHMA: model development and validation
 Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN
 REPORT SERIES IN AEROSOL SCIENCE N:o 122 (2011) DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN Division of Atmospheric Sciences Department
REPORT SERIES IN AEROSOL SCIENCE N:o 122 (2011) DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN Division of Atmospheric Sciences Department
Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements
 Boreal Environment Research 12: 311 321 issn 1239-6095 Helsinki 25 June 2007 2007 Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements Petri Tiitta
Boreal Environment Research 12: 311 321 issn 1239-6095 Helsinki 25 June 2007 2007 Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements Petri Tiitta
The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry
 The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry Jeff Pierce, Mat Evans, Cat Scott, Steve D'Andrea, Delphine Farmer, Erik Swietlicki and Dom Spracklen Pierce,
The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry Jeff Pierce, Mat Evans, Cat Scott, Steve D'Andrea, Delphine Farmer, Erik Swietlicki and Dom Spracklen Pierce,
Characteristics of new particle formation events and cluster ions at K-puszta, Hungary
 BOREAL ENVIRONMENT RESEARCH 14: 683 698 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Characteristics of new particle formation events and cluster ions at K-puszta, Hungary Taina
BOREAL ENVIRONMENT RESEARCH 14: 683 698 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Characteristics of new particle formation events and cluster ions at K-puszta, Hungary Taina
EAS1600 Spring 2014 Lab 06 ATMOSPHERIC AEROSOLS
 Objectives EAS1600 Spring 2014 Lab 06 ATMOSPHERIC AEROSOLS During the course of this lab we will investigate some basic connections between aerosols and climate. We will look at the aerosol scattering
Objectives EAS1600 Spring 2014 Lab 06 ATMOSPHERIC AEROSOLS During the course of this lab we will investigate some basic connections between aerosols and climate. We will look at the aerosol scattering
Arctic Oxidation Chemistry
 19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
19 July 2016 Connaught Summer Institute 1 Arctic Oxidation Chemistry Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
Differential Mobility Particle Sizer (Aerosol measurements)
 Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Anthropogenic influence on new particle formation in the marine boundary layer atmosphere. Ville Berg Malmborg
 Anthropogenic influence on new particle formation in the marine boundary layer atmosphere Ville Berg Malmborg August, 2014 Master's thesis Department of Physics, Lund University Supervisors: Adam Kristensson
Anthropogenic influence on new particle formation in the marine boundary layer atmosphere Ville Berg Malmborg August, 2014 Master's thesis Department of Physics, Lund University Supervisors: Adam Kristensson
Study of the Effects of Acidic Ions on Cloud Droplet Formation Using Laboratory Experiments
 Available online at www.sciencedirect.com ScienceDirect APCBEE Procedia 10 (2014 ) 246 250 ICESD 2014: February 19-21, Singapore Study of the Effects of Acidic Ions on Cloud Droplet Formation Using Laboratory
Available online at www.sciencedirect.com ScienceDirect APCBEE Procedia 10 (2014 ) 246 250 ICESD 2014: February 19-21, Singapore Study of the Effects of Acidic Ions on Cloud Droplet Formation Using Laboratory
Gas Particle Partitioning to OA
 Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Organic aerosol formation via sulphate cluster activation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
Cloud Condensation Nuclei Hygroscopic Parameter Kappa
 Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
The radiative effect of ion-induced inorganic nucleation in the free troposphere
 The radiative effect of ion-induced inorganic nucleation in the free troposphere Eimear M. Dunne, João Almeida, Andreas Kürten, Alexandru Rap, Kenneth S. Carslaw, and CLOUD Collaboration Citation: AIP
The radiative effect of ion-induced inorganic nucleation in the free troposphere Eimear M. Dunne, João Almeida, Andreas Kürten, Alexandru Rap, Kenneth S. Carslaw, and CLOUD Collaboration Citation: AIP
P = x i. P i. = y i. Aerosol and Aqueous Chemistry. Raoult s Law. Raoult s Law vs. Henry s Law. or C i. = HC i. = k H
 The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 4717, doi:10.1029/2003jd003664, 2003 Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 4717, doi:10.1029/2003jd003664, 2003 Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
Chapter Eight: Conclusions and Future Work
 2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
BELOW-CLOUD SCAVENGING OF AEROSOL PARTICLES BY SNOW AT AN URBAN SITE IN FINLAND. Republic of South Africa
 BELOW-CLOUD SCAVENGING OF AEROSOL PARTICLES BY SNOW AT AN URBAN SITE IN FINLAND M. PARAMONOV 1, A. VIRKKULA 1, T. GRÖNHOLM 1, S. GÖKE 1 AND L. LAAKSO 1,2 1 Department of Physics, University of Helsinki,
BELOW-CLOUD SCAVENGING OF AEROSOL PARTICLES BY SNOW AT AN URBAN SITE IN FINLAND M. PARAMONOV 1, A. VIRKKULA 1, T. GRÖNHOLM 1, S. GÖKE 1 AND L. LAAKSO 1,2 1 Department of Physics, University of Helsinki,
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Finnish Estonian air ion and aerosol workshops
 Boreal Environment Research 12: 237 245 issn 1239-6095 Helsinki 25 June 2007 2007 Finnish Estonian air ion and aerosol workshops Markku Kulmala 1) and Hannes Tammet 2) 1) Division of Atmospheric Sciences,
Boreal Environment Research 12: 237 245 issn 1239-6095 Helsinki 25 June 2007 2007 Finnish Estonian air ion and aerosol workshops Markku Kulmala 1) and Hannes Tammet 2) 1) Division of Atmospheric Sciences,
Chemistry of stabilized Criegee intermediates in the CLOUD chamber
 Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
ON THE LIFECYCLE OF AEROSOL PARTICLES: SOURCES AND DISPERSION OVER SCANDINAVIA PETER TUNVED
 ON THE LIFECYCLE OF AEROSOL PARTICLES: SOURCES AND DISPERSION OVER SCANDINAVIA PETER TUNVED Department of Meteorology Stockholm University Stockholm 2004 To my beloved family Doctoral dissertation Department
ON THE LIFECYCLE OF AEROSOL PARTICLES: SOURCES AND DISPERSION OVER SCANDINAVIA PETER TUNVED Department of Meteorology Stockholm University Stockholm 2004 To my beloved family Doctoral dissertation Department
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
PROBLEMS Sources of CO Sources of tropospheric ozone
 220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
New Particle Formation in the UT/LS:
 New Particle Formation in the UT/LS: Project Overview and Preliminary Results Li-Hao Young 1, David Benson 1, William Montanaro 1, James C. Wilson 2, and Shan-Hu Lee 1 1 Kent State University 2 University
New Particle Formation in the UT/LS: Project Overview and Preliminary Results Li-Hao Young 1, David Benson 1, William Montanaro 1, James C. Wilson 2, and Shan-Hu Lee 1 1 Kent State University 2 University
Identification of boundaries between size classes of atmospheric aerosol particles
 BOREAL ENVIRONMENT RESEARCH 21: 363 371 2016 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 23 May 2016 Identification of boundaries between size classes of atmospheric aerosol particles Hannes
BOREAL ENVIRONMENT RESEARCH 21: 363 371 2016 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 23 May 2016 Identification of boundaries between size classes of atmospheric aerosol particles Hannes
J. Schneider & Chr. Voigt - Physics and Chemistry of Aerosols and Ice Clouds
 Chapter 8 Contrails and contrail cirrus 8.1 Introduction - Terminology 8.2 Contrail formation conditions 8.3 Heterogeneous nucleation on volatile aerosol and soot 8.4 Indirect effect of soot on cirrus
Chapter 8 Contrails and contrail cirrus 8.1 Introduction - Terminology 8.2 Contrail formation conditions 8.3 Heterogeneous nucleation on volatile aerosol and soot 8.4 Indirect effect of soot on cirrus
Water adsorption on ammounium sulfate and silica nanoparticles. Keywords: nanoparticles, hygroscopic growth, water monolayers INTRODUCTION
 Water adsorption on ammounium sulfate and silica nanoparticles Keskinen Helmi 1, Jaatinen Antti 1, Miettinen Pasi 1, Romakkaniemi Sami 1, Joutsensaari Jorma 1, Smith James N. 1, and Laaksonen Ari 1,3 1
Water adsorption on ammounium sulfate and silica nanoparticles Keskinen Helmi 1, Jaatinen Antti 1, Miettinen Pasi 1, Romakkaniemi Sami 1, Joutsensaari Jorma 1, Smith James N. 1, and Laaksonen Ari 1,3 1
Chapter 9 Generation of (Nano)Particles by Growth
 Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Progress on Application of Modal Aerosol Dynamics to CAM
 Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
Annual and size dependent variation of growth rates and ion concentrations in boreal forest
 BOREAL ENVIRONMENT RESEARCH 10: 357 369 ISSN 1239-6095 Helsinki 24 October 2005 2005 Annual and size dependent variation of growth rates and ion concentrations in boreal forest Anne Hirsikko 1), Lauri
BOREAL ENVIRONMENT RESEARCH 10: 357 369 ISSN 1239-6095 Helsinki 24 October 2005 2005 Annual and size dependent variation of growth rates and ion concentrations in boreal forest Anne Hirsikko 1), Lauri
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects
 Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Atmospheric particle formation events at Värriö measurement station in Finnish Lapland
 Atmos. Chem. Phys., 4, 2015 2023, 2004 SRef-ID: 1680-7324/acp/2004-4-2015 Atmospheric Chemistry and Physics Atmospheric particle formation events at Värriö measurement station in Finnish Lapland 1998 2002
Atmos. Chem. Phys., 4, 2015 2023, 2004 SRef-ID: 1680-7324/acp/2004-4-2015 Atmospheric Chemistry and Physics Atmospheric particle formation events at Värriö measurement station in Finnish Lapland 1998 2002
A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation
 BOREAL ENVIRONMENT RESEARCH : 55 5 ISSN 9-695 Helsinki December 5 5 A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation Alf Grini )), Hannele Korhonen
BOREAL ENVIRONMENT RESEARCH : 55 5 ISSN 9-695 Helsinki December 5 5 A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation Alf Grini )), Hannele Korhonen
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) Slides from Review Sessions are posted on course website:
 Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Aerosol Effects on Water and Ice Clouds
 Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Chapter 7 Precipitation Processes
 Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Chapter One: Atmospheric Aerosols
 2004 PhD Thesis 9 Chapter One: Atmospheric Aerosols 1.1 Introduction Atmospheric aerosols have significant local, regional and global impacts. Local impacts include vehicular emissions, wood burning fires
2004 PhD Thesis 9 Chapter One: Atmospheric Aerosols 1.1 Introduction Atmospheric aerosols have significant local, regional and global impacts. Local impacts include vehicular emissions, wood burning fires
Model studies on ion-induced nucleation in the atmosphere
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D20, 4427, doi:10.1029/2002jd002140, 2002 Model studies on ion-induced nucleation in the atmosphere L. Laakso, J. M. Mäkelä, 1 L. Pirjola, and M. Kulmala
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D20, 4427, doi:10.1029/2002jd002140, 2002 Model studies on ion-induced nucleation in the atmosphere L. Laakso, J. M. Mäkelä, 1 L. Pirjola, and M. Kulmala
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility.
 Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
Empiric equations of coagulation sink of fine nanoparticles on background aerosol optimized for boreal zone
 Boreal Environment Research 19: 115 126 2014 ISSN 1239-6095 (print) ISSN 1797-2469 (online) helsinki 25 April 2014 Empiric equations of coagulation sink of fine nanoparticles on background aerosol optimized
Boreal Environment Research 19: 115 126 2014 ISSN 1239-6095 (print) ISSN 1797-2469 (online) helsinki 25 April 2014 Empiric equations of coagulation sink of fine nanoparticles on background aerosol optimized
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models
 Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
The Atmosphere. All of it. In one hour. Mikael Witte 10/27/2010
 The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
Are Cosmic Rays Changing our Climate? Jose Cardoza University of Utah Atmospheric Science Department Tuesday, February 16, 2010
 Are Cosmic Rays Changing our Climate? Jose Cardoza University of Utah Atmospheric Science Department Tuesday, February 16, 2010 OUTLINE Cosmic rays in the atmosphere The supporters The skeptics Summary
Are Cosmic Rays Changing our Climate? Jose Cardoza University of Utah Atmospheric Science Department Tuesday, February 16, 2010 OUTLINE Cosmic rays in the atmosphere The supporters The skeptics Summary
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer
 Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Aerosols and Climate
 Aerosols and Climate S K Satheesh S K Satheesh is an Assistant Professor at Centre for Atmospheric and Oceanic Sciences, Indian Institute of Science, Bangalore. His research interests include aerosols,
Aerosols and Climate S K Satheesh S K Satheesh is an Assistant Professor at Centre for Atmospheric and Oceanic Sciences, Indian Institute of Science, Bangalore. His research interests include aerosols,
