Kinetic nucleation and ions in boreal forest particle formation events
|
|
|
- Ira Heath
- 5 years ago
- Views:
Transcription
1 Kinetic nucleation and ions in boreal forest particle formation events L. Laakso, T. Anttila, K. E. J. Lehtinen, P. P. Aalto, M. Kulmala, U. Hõrrak, J. Paatero, M. Hanke, F. Arnold To cite this version: L. Laakso, T. Anttila, K. E. J. Lehtinen, P. P. Aalto, M. Kulmala, et al.. Kinetic nucleation and ions in boreal forest particle formation events. Atmospheric Chemistry and Physics, European Geosciences Union, 2004, 4 (9/10), pp <hal > HAL Id: hal Submitted on 30 Nov 2004 HAL is a multi-disciplinary open access archive for the deposit and dissemination of scientific research documents, whether they are published or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers. L archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
2 Atmos. Chem. Phys., 4, , 2004 SRef-ID: /acp/ European Geosciences Union Atmospheric Chemistry and Physics Kinetic nucleation and ions in boreal forest particle formation events L. Laakso 1, T. Anttila 1,3, K. E.J. Lehtinen 1, P. P. Aalto 1, M. Kulmala 1, U. Hõrrak 2, J. Paatero 3, M. Hanke 4, and F. Arnold 4 1 Department of Physical Sciences, P.O. Box 64, FIN University of Helsinki, Finland 2 Institute of Environmental Physics, University of Tartu, 18 Ülikooli Street, Tartu, 50090, Estonia 3 Finnish Meteorological Institute, Air Quality Research, Sahaajankatu 20E, FIN Helsinki, Finland 4 Max-Planck-Institute für Kernphysik (MPI-K), D-6900 Heidelberg, P.O. Box , Germany Received: 13 May 2004 Published in Atmos. Chem. Phys. Discuss.: 20 July 2004 Revised: 22 November 2004 Accepted: 23 November 2004 Published: 30 November 2004 Abstract. In order to gain a more comprehensive picture on different mechanisms behind atmospheric particle formation, measurement results from QUEST 2-campaign are analyzed with an aid of an aerosol dynamic model. A special emphasis is laid on air ion and charged aerosol dynamics. Model simulations indicate that kinetic nucleation of ammonia and sulphuric acid together with condensation of sulphuric acid and low-volatile organic vapours onto clusters and particles explain basic features of particle formation events as well as ion characteristics. However, an observed excess of negative ions in the diameter range nm and overcharge of 3 5 nm particles demonstrate that ions are also involved in particle formation. These observations can be explained by preferential condensation of sulphuric acid onto negatively charged clusters and particles and/or contribution of ion-induced nucleation on particle formation. According to model simulations, which assume that the nucleation rate is equal to the sulfuric acid collision rate, the relative contribution of ion-based particle formation seems to be smaller than kinetic nucleation of neutral clusters. Conducted model simulations also corroborate the recently-presented hypothesis according to which a large number of so-called thermodynamically stable clusters (TSCs) having a diameter between 1 3 nm exist in the atmosphere. TSCs were found to grow to observable sizes only under favorable conditions, e.g. when the pre-existing particle concentration was low. Correspondence to: L. Laakso (lauri.laakso@iki.fi) 1 Introduction A considerable number of field measurements indicate that formation of nanometer-size particles and their growth into climatically relevant sizes take place regularly under various atmospheric conditions (Kulmala et al., 2004c). However, the overall climatic importance of this phenomenon is unclear at present due to uncertainties in the links between these initial steps of particle formation and growth and an ultimate influence of new particles on the radiative balance of the Earth (e.g. Ramanathan et al. (2001)). In a view of these uncertainties, detailed investigations of the mechanisms behind new particle formation is warranted. Several theories have been put forward to explain nucleation and subsequent growth of stable clusters into detectable sizes. Regarding nucleation, binary nucleation of water and sulphuric acid as well as ternary nucleation involving ammonia as a third compound have been proposed as plausible candidates (Korhonen et al., 1999). The nucleation potential of organic compounds (Gaman et al., 2004; Gao et al., 2001; Bonn and Moortgat, 2003) or combination of sulphuric acid and organics (Zhang et al., 2004) is still an open question. Nucleation of iodine compounds is likely to explain massive particle bursts observed in coastal areas (ODowd et al., 2002; Burkholder et al., 2004). Furthermore, ion-induced nucleation according to which stable clusters are formed on atmospheric ions has also been investigated (Arnold, 1980; Laakso et al., 2002; Harrison and Carslaw, 2003; Wilhelm et al., 2004; Yu and Turco, 2000). Observed particle growth has been hitherto explained by condensation of various organic and inorganic vapours, heterogeneous reactions and self-coagulation (Kulmala et al., 2000a; Kerminen et al., 1997; Zhang and Wexler, 2002; 2004 Author(s). This work is licensed under a Creative Commons License.
3 2354 L. Laakso et al.: Kinetic nucleation Anttila et al., 2003). Any plausible theory, however, must predict sufficiently high growth rates since freshly-nucleated clusters are scavenged away very rapidly by pre-existing particles (Kerminen et al., 2001). In particular, it has been suggested that the presence of electric charges in freshly-formed clusters enhance the uptake of polar molecules such as sulfuric acid, thereby giving a growth advantage to charged clusters over neutral ones (Yu and Turco, 2001; Nadykto and Yu, 2003; Nadykto et al., 2004). Atmospheric ions are thus expected to be involved in both nucleation and growth, but their overall importance to particle formation is an open question at present. This is mainly due to the lack of comprehensive set of field measurement data against which different hypothesis could be tested. In order to gain a more comprehensive picture on different nucleation and growth mechanisms, this paper analyzes measurement results obtained during QUEST (Quantification of aerosol nucleation in the European boundary layer) campaign carried out in SMEAR II-station (Vesala et al., 1998; Kulmala et al., 2001) during March Results are interpreted utilizing a box model that covers the appropriate aerosol dynamical processes as well as the charge effects. The principal goals of the study are the following: (1) To present air ion and charging state measurements, (2) To assess the importance of air ions on new particle formation taking place in the measurement site, (3) To compare the measurements and model simulations for the measurement period, and (4) To investigate the possibility that kinetic nucleation explains the evolution of observed ion size distributions. The measurements and the applied numerical model are described in Sects. 2 and 3, respectively. The measurement results are discussed in Sect. 4.1 and they are compared with model simulations in Sect Conclusions follow in Sect Measurements Several different instruments were utilized during the measurement period. The mobility distribution of ions was measured with a Balanced Scanning Mobility Analyzer (BSMA) and neutral particles with a differential mobility particle sizer (DMPS). Ionizing radiation was measured by glass-fibre filters and a scintillation gamma spectrometer (Laakso et al., 2004). In addition, the natural charging state of the particles was measured by a special DMPS setup described in Sect Sulphuric acid was observed by a Chemical Ionization Mass Spectrometer (CI-MS) and ammonia by Diffusion Scrubber Flow Injection Analyzer (DS-FIA). 2.1 BSMA The Balanced Scanning Mobility Analyzer manufactured by Airel Ltd., Estonia, consists of two plain aspiration-type differential mobility analyzers, one for positive and the other for negative ions (Tammet, 2004). The two aspiration condensers are connected as a balanced bridge circuit that allows continuous variation of the driving voltage and scanning of the mobility distribution of charged clusters and nanoparticles called air ions. A large airflow rate of 44 liters per second helps to suppress the loss of air ions at the inlet of the instrument. The inlet can be closed or opened for ions using a controlled electrostatic filter and the background signal is eliminated making every second scan with a closed inlet. A mobility distribution is calculated according to the results of 9 scans performed during 3 minutes. The electric mobility range of cm 2 V 1 s 1 is divided into 16 fractions that are equally spaced on a logarithmic scale. The mobility distribution is converted to a size distribution using the algorithm developed by Tammet (1995). The size distribution is divided into 12 size classes that are equally distributed in a logarithmic space and span the diameter range nm. The sampling height was about 1.6 m above the ground. 2.2 AIS The Air Ion Spectrometer (AIS, manufactured by AIREL Ltd, Estonia) measures the mobility distribution of air ions (naturally charged clusters and aerosol particles) in the range of cm 2 V 1 s 1. The spectrometer consists of two identical cylindrical aspiration-type differential mobility analyzers, one for measuring positive ions and the other for negative ions. Each mobility analyzer has 21 collector electrodes provided with individual electrometrical amplifiers for the measuring of electrical current carried by ions of different mobilities. The simultaneous measurements enable the recording of the air ion mobility distribution, which is showing considerable variation in ion concentration and evolution in time. The air sample containing ions (flow rate is 500 cm 3 s 1 ) is sucked into the mobility analyzer through the electronically controlled electrostatic filter (switched on/off). The measurements with a closed inlet for ions are used for the verification of the offset level and noise of electrometrical amplifiers. Both mobility analyzers have a closed loop of clean sheath air. The unipolar charging of aerosol particles in the corona charger and the subsequent removal of charged particles by electrostatic filtration is used to create clean sheath airflow of 1000 cm 3 s 1. The mean mobility distributions of positive and negative air ions and distribution uncertainties are calculated averaging the results of 20 measurements recorded during a 5-minute period. The mobility distribution of ions in the range of cm 2 V 1 s 1 is presented by 28 logarithmically uniformly distributed fractions: 12 fractions in the mobility range of cm 2 V 1 s 1 and 16 fractions (two fractions per electrometrical channel) in the range of cm 2 V 1 s 1. The corresponding diameter ranges of single charged particles calculated according to the algorithm by Tammet (1995) are and nm respectively. Atmos. Chem. Phys., 4, , 2004
4 L. Laakso et al.: Kinetic nucleation DMPS Neutral particles were measured by using the differential mobility particle sizer (DMPS). The system consists of two parallel DMPS devices: the first classifies the particles between 3 and 10 nm and the second between 10 and 500 nm. Both devices uses a Hauke-type differential mobility analyzer (DMA) (Winklmayr et al., 1991) and a closed loop sheath flow arrangement (Jokinen and Mäkelä, 1997). The first device has a 10.9 cm long DMA and the second one a 28 cm long DMA. Before sizing the aerosol is neutralized with a 74 MBq 2(mCi) Krypton-85 beta source. The particle counter of the 1st device is TSI model 3025 and the 2nd is TSI model The time resolution is 10 min. The sampling height was about 2 m above the ground. 2.4 Charging state measurements A fraction of aerosol particles in the atmosphere is always charged. The particle charge distribution is determined by the ion concentration and the particle size distribution (Wiedensohler, 1990). In stationary conditions, charges are in equilibrium with its electrical environment. However, if the system changes, it takes some time until a new equilibrium is reached. In the DMPS-setup, aerosol particles are led through a charger which, due to its high ion concentration, balances the charge distribution. If, for example, particles have less charges than equilibrium requires, some more particles are charged. On the other hand, if there are too many charges, ions of opposite polarity discharge a fraction of the particles. In our studies the natural charging state of the particles having a diameter between 3 5 nm was measured with a special DMPS setup. There were two similar inlets, one with a neutralizer (see Sect. 2.3), the other without a neutralizer (a dummy-one). The DMPS-system switched between these two inlets every 75 seconds. When the concentration measured without the charger was divided by the corresponding charged particle concentration, the natural charging state of the aerosol was obtained. If the value is equal to one, aerosol particles are in a charge equilibrium, whereas values less than one represent undercharged particles and values larger than one indicate overcharge of the particles. The system was calibrated with 4 nm ammonium sulphate particles which were measured through both inlets. There were 33% more losses in the neutralizer compared to the dummy inlet and therefore the concentrations measured through charger were corrected by a factor of Gaseous sulphuric acid measurements Gaseous sulphuric acid (GSA) was measured by a chemical ionization mass spectrometer apparatus (CI-MS) built by MPI-K Heidelberg. The instrument is essentially the same as the one used for measurements of atmospheric OH, HO 2 and RO 2 radicals except for the chemical conversion parts (Hanke et al., 2002). In brief, the CI-MS used in QUEST 2 consists of four major elements including an ion trap mass spectrometer, a flow reactor, an ion source and a GSA-source used for calibration. Atmospheric air at ambient atmospheric pressure is passed through the flow reactor. Simultaneously, reagent ions of the type NO 3 -(HNO 3 ) n (with n being mostly 1) are produced in the ion source and lead to the flow reactor. There these ions undergo ion-molecule reactions of the type NO 3 -(HNO 3 ) n +H 2 SO 4 HSO 4 -(HNO 3 ) n +HNO 3 the rate coefficients of which are close to the ion-molecule collision rate coefficients. The abundance ratio of product and reagent ions is measured using the mass spectrometer. This ion abundance ratio is the base for the determination of the sulphuric acid concentration in the flow reactor. The latter is typically only about 50% of the ambient atmospheric GSAconcentration due to sulphuric acid losses to the walls of the sampling line and the flow reactor. In order to quantify these sulphuric acid losses a sulphuric acid source is used for careful calibrations. Also carefully determined is the sulphuric acid background signal of the CI-MS-instrument which dictates the GSA-detection limit. During QUEST 2 the GSAdetection limit was as low as about cm 3 corresponding to an atmospheric mole fraction of or (4 ppq). The time-resolution of the GSA-measurements was below 1 s. The sulphuric acid concentrations were, however, integrated over 60 s to reduce the statistical error. The absolute uncertainty of the measured GSA-concentration is plus or minus 30%. A paper addressing the CI-MS used and the GSA-measurements made during the QUEST 2-campaign in detail is in preparation (Scholz et al., ). 3 Model description A modified version of the model AEROION (Laakso et al., 2002) is applied here in simulating the time evolution of neutral as well as charged clusters and particles. The processes covered by the model are nucleation, coagulation, condensation of sulphuric acid and a low-volatile organic vapour. The effects of charges on these processes are taken into account comprehensively. The model is expressed schematically in Fig. 1. The model distributes neutral particles into 52 size sections which span the radius range from around 0.3 nm (radius of one sulphuric acid molecule) to 1000 nm. Each size bin is also divided to 3 charge bins, 1, 0 and +1 elementary charges. Because the chemical composition of the cluster ions may differ from the composition of the neutral clusters and particles, two additional sections are reserved for negative and positive cluster ions. All particles are assumed to 1 Scholz, S., Hanke, M., Ücker, J., and Arnold, F.: Gaseous Sulfuric Acid Measurements made in the boreal Atmosphere during the QUEST 2 campaign, paper in preparation, Atmos. Chem. Phys., 4, , 2004
5 2356 L. Laakso et al.: Kinetic nucleation Kinetic, ion-induced or homogeneous nucleation Ionizing radiation (high energy) + UVradiation SO Organic vapour condensation Coagulation - H2SO4 - Ion attach, recombination NH3 Molecules Cluster ions and neutral clusters Observable particles Particle size increases, time increases Fig. 1. Schematic picture of the aerosol-ion model. consist of water, sulphate, ammonium and an organic compound. For particles larger than 40 nm in diameter the model utilizes measured size distributions. These particles are assumed to be in a charge balance which is determined from the properties of the small ions. In practice, this leads to slightly more negative (52.5%) than positive particles (47.5%) in the diameter range above 40 nm. However, this assumption does not have a significant effect on the results. The ion production rate and temperature were taken from the measurements and the particle water uptake was calculated similarly as in the original AEROION model using average measured RH. Nucleation. A previous analysis of the measurement data indicates that nucleation taking place in the measurement site is limited by the gas-phase kinetics rather than the thermodynamics of ammonia-water-sulphuric acid system (Kulmala et al., 2000b; Kulmala, 2003). Ammonia is assumed to stabilize the clusters (Korhonen et al., 1999; Lee et al., 2003) and therefore the nucleation rate is determined by the coagulation rate of ammonium bisulphate clusters. Since the gasphase concentration of ammonia is clearly higher than that of sulphuric acid (Finlayson-Pitts and Pitts, 2000), it is reasonable to assume that the formation rate of ammonium bisulphate clusters is limited by that of sulphuric acid molecules (Vehkama ki et al., 2004). Accordingly, we assume that the collisions of clusters containing one sulfuric acid molecule result in stable particles, thus the nucleation rate is calculated using the following equation: J = 0.5KC 2 Atmos. Chem. Phys., 4, , 2004 (1) Here J is the nucleation rate, K is the coagulation coefficient between ammonium bisulphate clusters and C is the gas-phase concentration of sulphuric acid. Coagulation kernel K is obtained from Fuchs coagulation theory. We have assumed in this study that ammonia is a stabilizing compound in kinetic nucleation. However, it is possible that in stead of ammonia, sulphuric acid is stabilized by some other compound like some organic vapours having sufficiently high concentration levels in the atmosphere (Zhang et al., 2004). If the stabilizing agent is different from ammonia, the results may slightly change in numbers, but the qualitative conclusions remain the same. In any case, the used expression for nucleation is an upper limit of possible nucleation rates. If the true rate is smaller, the relative importance of ion induced nucleation would increase resulting in higher concentrations of nm ions than observed. Coagulation. Coagulation is treated similarly as in the model AEROION (Laakso et al., 2002), except that the general transition regime coagulation coefficients of Fuchs is applied in determining the coagulation rates (Marlow, 1980). The calculated coagulation coefficients are shown in Fig. 2. Because a general theory for the calculation of charged cluster coagulation in the kinetic regime is not available, we have repeated our calculations with different approximations (Fig. 2). The first set of calculations is based on Fuchs general equation in which the coagulation coefficients are corrected with free molecular enhancement factors (blue curves). However, since in the small particle limit the theory is not able to reproduce ion-ion recombination coefficients
6 L. Laakso et al.: Kinetic nucleation Scaled d p2 =0.68 nm Coagulation coefficient [m 3 s 1 ] Recombination Negative condensation enhanced Mirror charging Non scaled d p1 =d p Neutral neutral Same polarities Diameter [m] Fig. 2. Coagulation coefficients. Non-scaled coagulation coefficients result from Fuchs generalized theory whereas scaled coagulation coefficients are forced to reach ion-ion recombination and ion-neutral reaction coefficients. or ion-neutral reaction rates, we also scaled the enhancement factor so that coefficients reach these measured values at molecular size (red curves). In addition, the value for enhanced sulphuric acid condensation rate is shown. The two sets of curves represent coagulation coefficients between equally sized particles and between a single molecule (d p =0.68 nm) and a particle of certain size (d p ). Ion-aerosol attachment coefficients are calculated based on Fuchs theory (Fuchs, 1964) as in the earlier version of the model. In some calculations the two ion classes were replaced with charged clusters consisting of a single pair of sulphuric acid and ammonia molecules. The aim was to investigate the effect of chemical composition of the ions to the system. We assumed the average measured properties both for positive and negative cluster ions, cm 2 V 1 s 1 and cm 2 V 1 s 1, respectively. These properties were taken from the BSMA-measurements (vide infra). Condensation. The condensation of ammonia and sulphuric acid is treated as coagulation between molecules and particles. Ammonia is assumed to condense simultaneously with sulphuric acid so that inorganic salt present in clusters and particles is effectively ammonium bisulphate. The gasphase concentration of sulphuric acid was taken from the measurements. Preliminary model calculations indicated that the observed particle formation and growth rates can not be produced unless low-volatile organic vapours are assumed to condense onto clusters and particles. Therefore condensation of a lowvolatile organic vapour was also included into the model. The mass transfer of the organic vapour between the gas phase and particles is simulated using a version of the condensation equation of Fuchs and Sutugin (1971) which takes into account mirror Coulomb interactions (Lushnikov and Kulmala, 2004). The mass accommodation coefficient of the organic vapour is assumed unity. The condensational flux is driven by the difference (C os C os,eq ), where C os is the gas-phase concentration of the organic vapour and C os,eq its saturation vapour concentration over the cluster or particle surface. Here the model developed by Kulmala et al. (2004a) is applied in calculating C os,eq. According to the applied model, condensation of low-volatile organic vapours onto nanometer-size clusters is analogous to cloud droplet formation described by the traditional Köhler theory: the clusters activate with respect to a low-volatile organic vapour after they have reached a certain threshold size d crit. In order to save computing time, the activation process is treated here assuming that the organic vapour does not condense onto clusters having a diameter below d crit, whereas it is able to condense without any thermodynamic barrier onto clusters and particles above this size, i.e. C os,eq is equal to the zero above the surfaces of activated clusters and particles. Based on detailed thermodynamic calculations, the following equation Atmos. Chem. Phys., 4, , 2004
7 2358 L. Laakso et al.: Kinetic nucleation x Temperature [ C] RH [%] Condensation sink [s 1 ] DOY Fig. 3. Temperature and relative humidity measured during the QUEST 2-campaign at SMEAR II-station. 1 DOY Fig. 5. Condensation sink the organic vapour as a pure compound was assumed to be equal to (Anttila et al., 2003; Kulmala et al., 2004a). Golabl radiation [W m 2 ] Results 4.1 Measurements Meteorology DOY Fig. 4. Global radiation measured during the QUEST 2-campaign at SMEAR II-station. for d crit was derived (Kerminen et al., 2004): d crit = a + b T + c ln(s os) 1 + d T + e ln(s os ) Here d crit is given in nanometers, a=6.49, b= , c=0.039, d= 0.02, e=0.174, S os is the ambient saturation ratio of the organic vapour and T is the temperature. This parameterization was shown to mimic closely activation behaviour predicted by the detailed thermodynamic model (Kerminen et al., 2004). The activation diameter, d crit has values of approximately 2 nm under considered conditions. The gas-phase concentration of the organic vapour was assumed to be constant during each day and during the whole period it varied between cm 3 (Kulmala et al., 2004b). Furthermore, the saturation vapour concentration of (2) Figures 3 and 4 represent the temperature (T), relative humidity (RH) and global radiation during the days ( ). All these quantities exhibit quite similar diurnal variation throughout the whole measurement period. Only during the night there were some changes due to the below zero temperatures, which made the air drier than during the other nights. We also looked at satellite pictures for this period ( all days were clear except day 88 when there were some clouds. This can also be seen from the global radiation measurements DMPS measurements The effect of pre-existing particles on new particle formation can be conveniently quantified in terms of condensation sink (CS), i.e. the loss rate of condensing vapours onto preexisting particles. This concept is especially useful since it provides also a measure for the loss rate of freshly-formed clusters/particles due to coagulation (Dal Maso et al., 2002). Similar quantity is the so-called Fuchs surface or Fuchs area. Figure 5 shows CS during the considered days. It was relatively low during the measurement period except for day 86 and the evening of day 88. The particle size distributions measured by DMPS are shown for the considered period in Fig. 6. During days 83, Atmos. Chem. Phys., 4, , 2004
8 L. Laakso et al.: Kinetic nucleation DMPS Hyytiälä 2003 Particle diameter [m] /3 M 25/3 T 26/3 W 27/3 T 28/3 F 29/3 S Particle concentration [1/cm 3 ] dn/dlogd p [1/cm 3 ] Number of day Fig. 6. Particle concentration measured by DMPS during the days , 85, 87, and 88 apparent particle formation took place whereas during day 86 it was much weaker and lasted for a shorter period. By comparing DMPS size distributions with the time evolution of CS, it is seen that the value of CS decreases always before pronounced particle formation takes place. In contrast, a large value of CS prevented particle formation in the morning of the day 86 and only a small particle burst took place later during the day. During the afternoon of day 88, CS starts to increase suppressing growth of the nucleation mode particles. Overall, these observations illustrate the extreme sensitivity of new particle formation to the value of CS (Mönkkönen et al., 2003) Ions In Figs. 7 and 8, the evolution of the size distribution of negative and positive cluster ions and naturally charged particles (aerosol ions) based on BSMA and AIS measurements are shown, for various days during the period March There are several things that can be seen from the measurements. First, the growing modes consisting of freshlyformed clusters and particles are seen from ion measurements. These modes start growing from cluster ions sizes below 2 nm in diameter. The growing mode in the size distribution of negative ions is more pronounced (enhanced concentrations) than is the case with positive ions in the size range below 5 nm. This feature (a clear preference of negative ions) has also been found in laboratory experiments (Wilhelm et al., 2004). The sign preference can be due to an ion-induced particle formation mechanism or sign preference of condensation rate. The latter property could be connected with the geometry of sulphuric acid molecules (Nadykto et al., 2004). The same also applies for condensing organic vapours, but the effect might be smaller because these compounds do not likely condense onto clusters having a diameter below 2 nm (see the previous section). The stability of the nanoclusters might also be influenced by a different composition of negative and positive cluster ions (Eisele and Tanner, 1990; Luts and Salm, 1994; Beig and Brasseur, 2000). For example, Lovejoy et al. (2004) observed that negative sulphuric acid ions may grow without any energetic barrier in favourable conditions. According to their study, barrierless negative ion-induced nucleation is predicted if the concentration of sulphuric acid exceeds 10 7 cm 3 and RH 80% at a temperature of about 270 K. Taking into account the adiabatic cooling in uplifting air in the boundary layer, these values are also met during the studied period. A purely neutral nucleation tends to create a gap between cluster ions and larger charged particles since the charge probability increases with particle size. This is also due to the finite time needed for the particle charging. Atmos. Chem. Phys., 4, , 2004
9 2360 L. Laakso et al.: Kinetic nucleation 10 8 Negative ions BSMA Diameter [m] Julian date 10 8 Positive ions Diameter [m] Julian date dn/dlogd [1/cm 3 ] Fig. 7. Ion concentration measured with Balanced Scanning Mobility Analyzer (BSMA) during the days Fig. 8. Ion concentration measured with Air Ion Spectrometer (AIS) during the days However, this trend is balanced by the decreasing number concentrations with the increasing particle size. The measurements show that the number concentration of ion clusters in the smallest size class nm decreases slightly during the particle formation events. This feature is insensitive to the value of CS and is therefore probably connected to particle formation. The size (or mobility) distribution of cluster ions depends on the chemical composition of air (on amount of trace gases) and the concentration of aerosol particles. The latter affects the lifetime of cluster ions in the air, which depends also on the ionization rate, and therefore on the concentration of radioactive substances in the ground and in the air (e.g. radon). Variation of radon concentration due to the mixing causes approximately 10% difference for the ion production rate between day and night (Laakso et al., 2004). One explanation for the observed decrease is possibly higher concentrations of condensable vapours which grow ions to larger sizes in a shorter time. Another interesting observation is that during days 85 and 88 there is a gap in the concentrations of ions having sizes nm (Figs. 7 and 8). During these days CS is relatively high and and the gap is thus probably linked to the larger scavenging rates of charged clusters. It is also possible that during the days with high CS the nucleation mechanism differs from that of clean days Concentrations of ions and particles and the charging state of 3 5 nm particles In Fig. 9 the concentration of ions in certain intervals (from BSMA) and particles are shown. The days with clear particle formation bursts are indicated with red lines at the top of upper panel. In the same panel, the concentration of sulphuric acid is shown, together with the total number concentration measured with DMPS (3 500 nm) and the concentrations of positive and negative cluster ions. In the lower panel, the concentrations of positive (red) and negative (blue) ions in size-intervals nm and 3 5 nm Atmos. Chem. Phys., 4, , 2004
10 L. Laakso et al.: Kinetic nucleation 2361 Concentration [cm 3 ] Events N tot <1.5nm (+) H 2 SO <1.5nm ( ) Concentration [cm 3 ] nm ( ) 3 5 nm ( ) 1.5 3nm (+) 3 5 nm (+) 3 5 nm ( 1,0,+1) Charging state Overcharging Day of year Fig. 9. Particle and ion measurements during the period days In the top panel above shown are the total particle concentration, the number concentrations of ions having a diameter below <1.5 nm and the gas-phase concentration of sulphuric acid (curve has gaps). In the bottom panel, blue curves represent negative charges, red curves positive and magenta curves sum over all charges ( 1,0,+1). The stars indicate overcharge measurements. are shown. In addition, the number concentration of particles between 3 nm and 5 nm measured with DMPS is given. The charging state of the particles is shown on right axes. As it can be seen, the concentration of cluster ions was relatively constant during the aerosol formation events. This indicates that ion-induced nucleation can not be the only or the main particle formation mechanism. If we assume that clusters are produced via classical ion-induced nucleation, the nucleation rate depends on the sulphuric acid concentration and the ion production rate. Because the dependence on the sulphuric acid concentration is exponential, the nucleation rate should sometimes reach the ion production rate. This would decrease the cluster ion concentrations notably and increase significantly the concentration of charged particles having sizes nm, which is in contrast to the observations. On the other hand, mixing of the air parcels under turbulent conditions may prevent such drastic decrease in the concentrations and change this conclusion (Nilsson et al., 2001). An alternative explanation for the nearly constant concentration of cluster ions is that the nucleation rate is well below the ionization rate. It was noted that the observed formation rate of 3 nm size particles in the boreal forest was in the range of cm 3 s 1 (Mäkelä et al., 2000), which never exceeded the ion-pair production rate typical for continental areas. The key question is how rapidly cluster ions or critical clusters (e.g. H 2 SO 4 clusters) grow from initial size up to 3 nm, and how rapid is the coagulational scavenging. Observations (during BIOFOR-campaign, not published) indicated that the ion sink has a minimum before the burst of 3 nm particles or at the time when the nanoparticle concentration reaches its peak value. So the changes in cluster ion concentration due to ion-induced nucleation can be masked by the changes in the ion sink. Another interesting feature is that particles were slightly negatively overcharged (indication for ion-induced nucleation) during particle formation events that took place when the value of CS was low. Otherwise particles remained undercharged. Overall, these observations indicate that atmospheric ions are able to influence particle formation taking place at the measurement site. The third feature worth noting is that the relative concentrations of negative and positive ions in the size range of 3 5 nm depend on the ion size: negative ions were dominant in the smallest size fractions whereas particles having a diameter above 5 nm were always equally charged. There are three possible explanations for the first observation: 1) ioninduced nucleation has a negative sign preference (Kusaka et al., 1995; Wilhelm et al., 2004), 2) smaller (negative) ions charge particles more easily due to their higher mobility, and 3) the condensation rate onto positive and negative particles is different. Because large differences in the concentration Atmos. Chem. Phys., 4, , 2004
11 2362 L. Laakso et al.: Kinetic nucleation and mobility of the negative and positive cluster ions were not observed, the first explanation can not be the dominant one. In the size range 3 5 nm, the difference in the concentration of negative ions and positive ions was observed to depend on the pre-existing particle concentration. When CS had a high value these concentrations were equal whereas negative ions were otherwise dominant. 4.2 Model simulations The measurement results were analyzed using the developed numerical model. The main goal was to find whether kinetic nucleation of ammonium bisulphate clusters (or sulphuric acid + some unknown compound) together with condensation of sulphuric acid and organic vapours can explain the basic features of the observed particle formation. Regarding ion dynamics, the principal goals were to explain the following two findings: 1. particles were overcharged during particle formation events, and 2. a net unipolar charge existed in the particles with sizes and 3 5 nm in diameter. The conducted model runs are divided into the following four cases: 1. coagulation coefficients obey enhanced Fuchs coagulation coefficients 2. coagulation coefficients are scaled to reach ion-ion recombination coefficients in the limit of molecular sizes 3. coagulation coefficients obey enhanced Fuchs coagulation coefficients. In addition, the condensation rate of sulphuric acid onto negative particles is 50% larger than onto the positive particles 4. coagulation coefficients are scaled to reach ion-ion recombination coefficients and the condensation rate of sulphuric acid onto negative particles is 50% larger than onto the positive particles Each of these four cases is divided further into three subcases: a) cluster ions consist of a compound X of which mass and mobility are varied b) positive ions consist of the compound X and negative ones ammonium bisulphate c) both negative and positive ions consist of ammonium bisulphate General features New particles were formed in the model runs in equal or higher amounts than observed, the maximum difference being within a factor of three. The timing of particle formation events was also predicted well in all cases. Furthermore, the high CS inhibited particle formation during the day 86, and particle formation characteristics for polluted days 85 and 88 were different than for the days 83, 84 and 87. However, the charge characteristics and concentrations of nanometer-sized ions were different in different cases. The particles were always undercharged in cases 1c and 2c. Assuming different compositions for negative and positive cluster ions, slightly more negative than positive nm particles were formed. In addition, the modelled concentrations of the and 3 5 nm charged particles were of the same order of the magnitude as measured, varying from 100 cm 3 to 800 cm 3. These sets of model runs reproduced also the overcharged/undercharged events. Of all combinations, the measurements were best explained when different condensation rates of sulphuric acid onto negative and positive particles were assumed, i.e. by simulation sets 3) and 4) listed above. The results from these model runs are shown in Figs. 10 and 11 and they can be compared with the corresponding observations shown in Figs. 6 8 and Existence of neutral clusters The model calculations also suggest that a large number of thermodynamically stable clusters (TSCs) exist below the DMPS detection limit (Fig. 10). The number concentration of clusters having diameter between 1 and 3 nm varied between 10 5 cm 3 during the particle formation events and approximately 10 cm 3 in the night. The corresponding variation in charged cluster concentration was 500 cm 3 and 10 cm 3. A small fraction of these charged clusters belong to large cluster ions. Freshly-formed TSCs are scavenged away rapidly by larger particles and their existence requires a continuous source of new clusters. The model results indicate that a majority of TSCs are neutral. Furthermore, most of TSCs can not be distinguished from the cluster ions because they are not able to reach larger sizes due to the rapid coagulation scavenging. These clusters stabilize the cluster ion distribution and cause the observed fact that the cluster ion concentration is not influenced by particle formation. Initial ions may charge these clusters and, on the other hand, TSCs may form by recombination of ion clusters (Arnold, 1980). The extremely small size of TSCs makes their atmospheric observation through ion mobility measurements complicated. The best approach for this is perhaps an ion mass spectrometer which would measure ion masses in the size range amu (c.f. Eichkorn et al. (2002); Wilhelm et al. (2004)). If one would detect combinations of sulphuric acid and ammonia, the existence of stable ammonium bisulphate Atmos. Chem. Phys., 4, , 2004
12 L. Laakso et al.: Kinetic nucleation 2363 Fig. 10. Charged and neutral particles predicted by model calculations. Ions are assumed to consist 50% of ammonium bisulphate and 50% of the unknown compound. Coagulation coefficients are scaled to reach ion-ion recombination coefficient in the small cluster limit. The sulphuric acid condensation rate is enhanced for negative clusters/particles. The measured sulphuric acid concentration used as an input value is also shown for comparison. Fig. 11. Fractional concentrations of negative, positive and neutral particles (see Fig. 10 for more details). Atmos. Chem. Phys., 4, , 2004
13 2364 L. Laakso et al.: Kinetic nucleation clusters could be inferred. However, e.g. the mass of initial core ions could make the interpretation of the measurements complicated. There are also other possibilities for these measurements based on the properties of traditional condensation particle counters Possibility of binary ion-induced nucleation The possibility of binary water-sulphuric acid nucleation was also investigated. The theory for ion-induced binary nucleation predicts that the critical cluster consists of approximately 3 6 sulphuric acid molecules. According to the performed model calculations, however, this leads to significantly larger ion concentrations in the diameter range nm than what was observed. In addition, the concentration of cluster ions is significantly decreased which is in contrast with observations. Furthermore, assuming that 1) clusters are produced via ternary ion-induced nucleation and 2) ammonium bisulphate clusters are stable, the number concentration of charged clusters remained many orders of magnitude lower than that of neutral clusters. Thus, even if charged clusters grow faster, the main contribution to over 3 nm particle concentrations results from neutral clusters Role of condensing organic vapours in particle formation Two sets of model simulations were performed to investigate the sensitivity of the results to the properties of the organic vapour. First the organic vapour was removed entirely from the system, i.e. its gas-phase concentration was set equal to zero. This lead to such small growth rates that no particles appeared to the diameter range >3nm. It can thus be concluded that the observed gas-phase concentrations of sulphuric acid alone cannot explain the particle formation taking place at the measurement site. In the other case the organic vapour was assumed to condense on all clusters and particles without any thermodynamic barrier, i.e. C os,eq was set equal to zero. Because the organic vapour condensed onto all clusters regardless of their size, this resulted in particle concentrations that were more than one order of magnitude higher than the observed ones. This result gives a support to the existence of a certain threshold size below which low-volatile organic vapours do not contribute significantly to the cluster growth. 5 Conclusions In this study, particle formation events observed in a boreal forest site were investigated utilizing measurements and model calculations. Basic features of the measurement results were successfully explained using an aerosol dynamic model that incorporates recent insights into nucleation and condensational growth of freshly-formed clusters. The model runs led to the following conclusions. Nucleation taking place in the measurement site is probably limited by gas-phase kinetics rather than thermodynamics, i.e. by the coagulation rate of ammonium bisulphate (or some other stabile compound) clusters. Kinetic nucleation produces a large number of mainly neutral stable clusters. Moreover, a clear majority of freshly-formed clusters are predicted to have sizes below 3 nm in diameter. This finding corroborates the recently presented hypothesis according to which a large number of so-called thermodynamically stable clusters (TSCs) exist below the detection limit of current instrumentation (Kulmala et al., 2000b). The obtained results are also in agreement with the following picture on cluster and particle growth in boreal forests. First, freshly-formed TSCs are grown by self-coagulation and condensation of sulphuric acid into a threshold size which is around 2 nm in diameter. After reaching this size, clusters activate with respect to low-volatile organic vapour(s) that grow the activated clusters rapidly into detectable sizes via condensation. A distinguishable particle formation ensues only under favourable conditions since TSCs are scavenged away very rapidly by background particles. This highlights the role of a pre-existing particle population in modulating atmospheric particle formation. The observed excess of negative ions in the diameter range nm and the overcharge of 3 5 nm particles demonstrate that ions are also involved in particle formation. These observations can be explained if sulphuric acid condenses preferably onto negatively charged clusters and particles. Another possibility is the contribution of ion-induced nucleation on particle formation. According to model simulations, which assume that the nucleation rate is determined by sulfuric acid collision rate, the relative importance of ion-based particle formation is probably smaller than kinetic nucleation of neutral clusters and subsequent growth driven by condensation of sulphuric acid and organic vapours. Edited by: A. Laaksonen References Anttila, T., Kerminen, V.-M., Kulmala, M., Laaksonen, A., and O Dowd, C.: Modelling the formation of organic particles in the atmosphere, Atmos. Chem. Phys., 4, , 2003, SRef-ID: /acp/ Arnold, F.: Multi-Ion complexes in the Stratosphere-Implications for trace gases and aerosol, Nature, 284, , Beig, G. and Brasseur, G.: Model of tropospheric ion composition: A first attempt, J. Geophys. Res., 105, , Bonn, B. and Moortgat, G.: Sesquiterpene ozonolysis: Origin of atmospheric new particle formation from biogenic hydrocarbons, Geophys. Res. Lett., 30, , Burkholder, J. B., Lovejoy, E. R., Ravishankara, A. R., and Curtius, J.: Laboratory studies of the homogeneous nucleation of iodine Atmos. Chem. Phys., 4, , 2004
14 L. Laakso et al.: Kinetic nucleation 2365 oxides, Atmos. Chem. Phys., 4, 19 34, 2004, SRef-ID: /acp/ Dal Maso, M., Kulmala, M., Lehtinen, K., Mäkelä, J., Aalto, P., and O Dowd, C.: Condensation and coagulation sinks and formation of nucleation mode particles in coastal and boreal forest boundary layers, J. Geophys. Res., 107, doi: /2001jd , Eichkorn, S., Wilhelm, S., Aufmhoff, H., Wohlfrom, K., and Arnold, F.: Cosmic ray-induced aerosol formation: First observational evidence from aircraft-based ion mass spectrometer measurements in the upper troposphere, Geophys. Res. Lett., 29 (14), doi: /2002gl015044, Eisele, F. and Tanner, D.: Identification of ions in continental air, J. Geophys. Res., 95, , Finlayson-Pitts, B. J. and Pitts, J. N.: Chemistry of the Upper and Lower Atmosphere, Academic Press., Fuchs, N. A.: The mechanics of Aerosol, Pergamon, New York, Fuchs, N. A. and Sutugin, A. G.: High-dispersed aerosols, in: Topics in Current Aerosol Research, edited by: Hidy, G. M. and Brock, J. R., vol. 2, pp. 1 60, Pergamon, Oxford, Gaman, A. I., Kulmala, M., Vehkamäki, H., Napari, I., Mircea, M., and Facchini, M. C.: Binary homogeneous nucleation in watersuccinic acid and water-glutaric acid systems, J. Chem. Phys., 120, , Gao, S., Hegg, A., Frick, G., Caffrey, P., Pasternack, L., Cantrell, C., Sullivan, W., Ambrusko, J., Albrechcinski, T., and Kirchstetter, T.: Experimental and modelling studies of secondary organic aerosol formation and some applications to the marine boundary layer, J. Geophys. Res, 106, , Hanke, M., Uecker, J., Reiner, T., and Arnold, F.: Atmospheric peroxy radicals: ROXMAS, a new mass-spectrometric methodology for speciated measurements of HO 2 and Sigma RO 2 and first results, Int. J. Mass Spectr., 213, 91 99, Harrison, R. and Carslaw, K.: Ion-aerosol cloud processes in the lower atmosphere, Rev. Geophys., 41, doi: /2002rg , Jokinen, V. and Mäkelä, J. M.: Closed loop arrangement with critical orifice for DMA sheath/excess flow system, J. Aerosol Sci., 28, , Kerminen, V.-M., Wexler, A. S., and Potukuchi, S.: Growth of freshly nucleated particles in the troposphere: roles of NH 3, H 2 SO 4, HNO 3, and HCl, J. Geophys. Res., 102, , Kerminen, V. M., Pirjola, L., and Kulmala, M.: How signifigantly does coagulation scavening limit atmospheric particle production?, J. Geophys. Res., 106, , Kerminen, V.-M., Anttila, T., Lehtinen, K. E. J., and Kulmala, M.: Parameterization for atmospheric new-particle formation: application to a system involving sulfuric acid and condensable watersoluble organic vapors, Aerosol Sci. Tech., accepted, Korhonen, P., Kulmala, M., Laaksonen, A., Viisanen, Y., McGraw, R., and Seinfeld, J.: Ternary nucleation of H 2 SO 4, NH 3 and H 2 O in the atmosphere, J. Geophys. Res., 104, , Kulmala, M.: How particles nucleate and grow, Science, 302, , Kulmala, M., Korhonen, P., Laakso, L., and Pirjola, L.: Nucleation in boreal forest boundary layer, Environ. Chem. Physics, 22, 46 53, 2000a. Kulmala, M., Pirjola, L., and Mäkelä, J. M.: Stable sulphate clusters as a source of new atmospheric particles, Nature, 404, 66 69, 2000b. Kulmala, M., Hämeri, K., Aalto, P., Mäkelä, J., Pirjola, L., Nilsson, E. D., Buzorius, G., Rannik, Ü., Dal Maso, M., Seidl, W., Hoffmann, T., Jansson, R., Hansson, H.-C., O Dowd, C., and Viisanen, Y.: Overview of the international project on biogenic aerosol formation in the boreal forest (BIOFOR), Tellus B, 53, , Kulmala, M., Kerminen, V.-M., Anttila, T., Laaksonen, A., and O Dowd, C. D.: Organic aerosol formation via sulphate cluster activation, J. Geophys. Res., 109, doi: /2003jd , 2004a. Kulmala, M., Laakso, L., Lehtinen, K., Riipinen, I., Dal Maso, M., Anttila, T., Hõrrak, U., Vana, M., and Tammet, H.: Initial steps of aerosol growth, Atmos. Chem. Phys., 4, , 2004b. Kulmala, M., Vehkamäki, H., Petäjä, T., Dal Maso, M., Lauri, A., Kerminen, V.-M., Birmili, W., and McMurry, P.: Formation and growth rates of ultrafine atmospheric particles: a review of observations, J. Aerosol Sci., 35, , 2004c. Kusaka, I., Wang, Z.-G., and Seinfeld, J. H.: Ion-induced nucleation, II, Polarizable multipolar molecules, J. Chem. Phys., 103, , Laakso, L., Mäkelä, J., Pirjola, L., and Kulmala, M.: Model studies on ion-induced nucleation in the atmosphere, J. Geophys. Res., 107, 4427, doi: /2002jd, Laakso, L., Hõrrak, U., Paatero, J., Petäjä, T., Tammet, H., Joutsensaari, J., Lehtinen, K., and Kulmala, M.: Ion production rate in a boreal forest based on ion, particle and radiation measurements, Atmos. Chem. Phys. Discuss., 4, , Lee, S.-H., Reeves, J., Wilson, J., Hunton, D., Viggiano, A., Miller, T., Ballenthin, J., and Lait, L.: Particle Formation by Ion Nucleation in the Upper Troposphere and Lower Stratosphere, Science, 301, , Lovejoy, E., Curtius, J., and Froyd, K.: Atmospheric ion-induced nucleation of sulfuric acid and water, J. Geophys. Res., 109, doi: /2003jd , Lushnikov, A. and Kulmala, M.: Charging of aerosol particles in the near free-molecule regime, Europhys. J. D, 29, , Luts, A. and Salm, J.: Chemical composition of small atmospheric ions near the ground, J. Geophys. Res., 99, , Mäkelä, J. M., Dal Maso, M., Pirjola, L., Keronen, P., Laakso, L., Kulmala, M., and Laaksonen, A.: Charasteristics of the atmospheric particle formation events observed at a boreal forest site in southern Finland, Boreal Environ. Res., 5, , Marlow, W.: Derivation of aerosol collision rates for singular attractive contact potentials, J. Chem. Phys., 73, , Mönkkönen, P., Koponen, I. K., Lehtinen, K. E. J., Uma, R., Srinivasan, D., Hämeri, K., and Kulmala, M.: Death of nucleation and Aitken mode particles: observations at extreme atmospheric conditions and their theoretical explanation, J. Aero. Sci., 35, 6, , Nadykto, A. and Yu, F.: Uptake of neutral polar vapour molecules by charged particles: Enhancement due to dipole-charge interaction, J. Geophys. Res., 108 (D23), doi: /2003jd , Nadykto, A. B., Al Natsheh, A., Yu, F., Mikkelsen, K., and Ruuskanen, J.: Effect of molecular structure and hydration on the uptake of gas-phase sulfuric acid by charged clusters/ultrafine particles, Atmos. Chem. Phys., 4, , 2004
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS
 STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms
 Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
Identification and classification of the formation of intermediate ions measured in boreal forest
 Atmos. Chem. Phys., 7, 201 210, 2007 Author(s) 2007. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Identification and classification of the formation of intermediate
Atmos. Chem. Phys., 7, 201 210, 2007 Author(s) 2007. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Identification and classification of the formation of intermediate
Initial steps of aerosol growth
 Atmos. Chem. Phys., 4, 2553 2560, 2004 SRef-ID: 1680-7324/acp/2004-4-2553 European Geosciences Union Atmospheric Chemistry and Physics Initial steps of aerosol growth M. Kulmala 1, L. Laakso 1, K. E. J.
Atmos. Chem. Phys., 4, 2553 2560, 2004 SRef-ID: 1680-7324/acp/2004-4-2553 European Geosciences Union Atmospheric Chemistry and Physics Initial steps of aerosol growth M. Kulmala 1, L. Laakso 1, K. E. J.
Modelling the formation of organic particles in the atmosphere
 Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation
 BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
Annual and size dependent variation of growth rates and ion concentrations in boreal forest
 BOREAL ENVIRONMENT RESEARCH 10: 357 369 ISSN 1239-6095 Helsinki 24 October 2005 2005 Annual and size dependent variation of growth rates and ion concentrations in boreal forest Anne Hirsikko 1), Lauri
BOREAL ENVIRONMENT RESEARCH 10: 357 369 ISSN 1239-6095 Helsinki 24 October 2005 2005 Annual and size dependent variation of growth rates and ion concentrations in boreal forest Anne Hirsikko 1), Lauri
Charged and total particle formation and growth rates during EUCAARI 2007 campaign in Hyytiälä
 Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Simulating aerosol nucleation bursts in a coniferous forest
 Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
The contribution of sulphuric acid to atmospheric particle formation and growth: a comparison between boundary layers in Northern and Central Europe
 Atmos. Chem. Phys.,, 1 18, www.atmos-chem-phys.org/acp//1/ SRef-ID: 18-/acp/--1 European Geosciences Union Atmospheric Chemistry and Physics The contribution of sulphuric acid to atmospheric particle formation
Atmos. Chem. Phys.,, 1 18, www.atmos-chem-phys.org/acp//1/ SRef-ID: 18-/acp/--1 European Geosciences Union Atmospheric Chemistry and Physics The contribution of sulphuric acid to atmospheric particle formation
Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements
 Boreal Environment Research 12: 311 321 issn 1239-6095 Helsinki 25 June 2007 2007 Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements Petri Tiitta
Boreal Environment Research 12: 311 321 issn 1239-6095 Helsinki 25 June 2007 2007 Road-side measurements of aerosol and ion number size distributions: a comparison with remote site measurements Petri Tiitta
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions
 The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
Observations of new particle formation and size distributions at two different heights and surroundings in subarctic area in northern Finland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D9, 4295, doi:10.1029/2002jd002939, 2003 Observations of new particle formation and size distributions at two different heights and surroundings in subarctic
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D9, 4295, doi:10.1029/2002jd002939, 2003 Observations of new particle formation and size distributions at two different heights and surroundings in subarctic
Finnish Estonian air ion and aerosol workshops
 Boreal Environment Research 12: 237 245 issn 1239-6095 Helsinki 25 June 2007 2007 Finnish Estonian air ion and aerosol workshops Markku Kulmala 1) and Hannes Tammet 2) 1) Division of Atmospheric Sciences,
Boreal Environment Research 12: 237 245 issn 1239-6095 Helsinki 25 June 2007 2007 Finnish Estonian air ion and aerosol workshops Markku Kulmala 1) and Hannes Tammet 2) 1) Division of Atmospheric Sciences,
Supplement of Total sulfate vs. sulfuric acid monomer concenterations in nucleation studies
 Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Organic aerosol formation via sulphate cluster activation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 4717, doi:10.1029/2003jd003664, 2003 Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 4717, doi:10.1029/2003jd003664, 2003 Uptake of neutral polar vapor molecules by charged clusters/particles: Enhancement due to dipole-charge interaction
Commentary on cloud modelling and the mass accommodation coefficient of water
 Commentary on cloud modelling and the mass accommodation coefficient of water A. Laaksonen, T. Vesala, M. Kulmala, P. M. Winkler, P. E. Wagner To cite this version: A. Laaksonen, T. Vesala, M. Kulmala,
Commentary on cloud modelling and the mass accommodation coefficient of water A. Laaksonen, T. Vesala, M. Kulmala, P. M. Winkler, P. E. Wagner To cite this version: A. Laaksonen, T. Vesala, M. Kulmala,
Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and Air Ion Spectrometer (NAIS)
 BOREAL ENVIRONMENT RESEARCH 14: 591 605 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and
BOREAL ENVIRONMENT RESEARCH 14: 591 605 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Long-term fi eld measurements of charged and neutral clusters using Neutral cluster and
Nanoparticles in boreal forest and coastal environment: a comparison of observations and implications of the nucleation mechanism
 Atmos. Chem. Phys., 10, 7009 7016, 2010 doi:10.5194/acp-10-7009-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Nanoparticles in boreal forest and coastal environment:
Atmos. Chem. Phys., 10, 7009 7016, 2010 doi:10.5194/acp-10-7009-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Nanoparticles in boreal forest and coastal environment:
The role of ammonia in sulfuric acid ion induced nucleation
 The role of ammonia in sulfuric acid ion induced nucleation I. K. Ortega, T. Kurtén, H. Vehkamäki, M. Kulmala To cite this version: I. K. Ortega, T. Kurtén, H. Vehkamäki, M. Kulmala. The role of ammonia
The role of ammonia in sulfuric acid ion induced nucleation I. K. Ortega, T. Kurtén, H. Vehkamäki, M. Kulmala To cite this version: I. K. Ortega, T. Kurtén, H. Vehkamäki, M. Kulmala. The role of ammonia
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Model studies on ion-induced nucleation in the atmosphere
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D20, 4427, doi:10.1029/2002jd002140, 2002 Model studies on ion-induced nucleation in the atmosphere L. Laakso, J. M. Mäkelä, 1 L. Pirjola, and M. Kulmala
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D20, 4427, doi:10.1029/2002jd002140, 2002 Model studies on ion-induced nucleation in the atmosphere L. Laakso, J. M. Mäkelä, 1 L. Pirjola, and M. Kulmala
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters
 Atmos. Chem. Phys.,, 6, www.atmos-chem-phys.org/acp/// Atmospheric Chemistry and Physics Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters M. Boy
Atmos. Chem. Phys.,, 6, www.atmos-chem-phys.org/acp/// Atmospheric Chemistry and Physics Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters M. Boy
New particle formation in Beijing, China: Statistical analysis of a 1-year data set
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007406, 2007 New particle formation in Beijing, China: Statistical analysis of a 1-year data set Zhijun Wu, 1 Min Hu, 1 Shang Liu, 1 Birgit
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007406, 2007 New particle formation in Beijing, China: Statistical analysis of a 1-year data set Zhijun Wu, 1 Min Hu, 1 Shang Liu, 1 Birgit
Modal structure of the atmospheric aerosol particle size spectrum for nucleation burst days in Estonia
 Boreal Environment Research 12: 361 373 issn 1239-6095 Helsinki 25 June 2007 2007 Modal structure of the atmospheric aerosol particle size spectrum for nucleation burst days in Estonia Anna Pugatšova,
Boreal Environment Research 12: 361 373 issn 1239-6095 Helsinki 25 June 2007 2007 Modal structure of the atmospheric aerosol particle size spectrum for nucleation burst days in Estonia Anna Pugatšova,
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Is eucalyptol the cause of nocturnal events observed in Australia?
 BOREAL ENVIRONMENT RESEARCH 14: 66 615 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Is eucalyptol the cause of nocturnal events observed in Australia? Ismael K. Ortega 1), Tanja
BOREAL ENVIRONMENT RESEARCH 14: 66 615 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Is eucalyptol the cause of nocturnal events observed in Australia? Ismael K. Ortega 1), Tanja
Formation of binary ion clusters from polar vapours: effect of the dipole-charge interaction
 Atmos. Chem. Phys., 4, 385 389, 24 SRef-ID: 68-7324/acp/24-4-385 Atmospheric Chemistry and Physics Formation of inary ion clusters from polar vapours: effect of the dipole-charge interaction A. B. Nadykto
Atmos. Chem. Phys., 4, 385 389, 24 SRef-ID: 68-7324/acp/24-4-385 Atmospheric Chemistry and Physics Formation of inary ion clusters from polar vapours: effect of the dipole-charge interaction A. B. Nadykto
Multicomponent aerosol dynamics model UHMA: model development and validation
 Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
Water Vapour Effects in Mass Measurement
 Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors
 Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-393 Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors Jenni Kontkanen
Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-393 Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors Jenni Kontkanen
On the roles of sulphuric acid and low-volatility organic vapours in the initial steps of atmospheric new particle formation
 Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
STUDIES OF NEW PARTICLE FORMATION IN THE EUROPEAN BOUNDARY LAYER
 REPORT SERIES IN AEROSOL SCIENCE No 112 (2010) STUDIES OF NEW PARTICLE FORMATION IN THE EUROPEAN BOUNDARY LAYER AMAR HAMED Department of Physics and Mathematics Faculty of Science and Forestry University
REPORT SERIES IN AEROSOL SCIENCE No 112 (2010) STUDIES OF NEW PARTICLE FORMATION IN THE EUROPEAN BOUNDARY LAYER AMAR HAMED Department of Physics and Mathematics Faculty of Science and Forestry University
Case report on the article Water nanoelectrolysis: A simple model, Journal of Applied Physics (2017) 122,
 Case report on the article Water nanoelectrolysis: A simple model, Journal of Applied Physics (2017) 122, 244902 Juan Olives, Zoubida Hammadi, Roger Morin, Laurent Lapena To cite this version: Juan Olives,
Case report on the article Water nanoelectrolysis: A simple model, Journal of Applied Physics (2017) 122, 244902 Juan Olives, Zoubida Hammadi, Roger Morin, Laurent Lapena To cite this version: Juan Olives,
Atmospheric particle formation events at Värriö measurement station in Finnish Lapland
 Atmos. Chem. Phys., 4, 2015 2023, 2004 SRef-ID: 1680-7324/acp/2004-4-2015 Atmospheric Chemistry and Physics Atmospheric particle formation events at Värriö measurement station in Finnish Lapland 1998 2002
Atmos. Chem. Phys., 4, 2015 2023, 2004 SRef-ID: 1680-7324/acp/2004-4-2015 Atmospheric Chemistry and Physics Atmospheric particle formation events at Värriö measurement station in Finnish Lapland 1998 2002
New Particle Formation in the UT/LS:
 New Particle Formation in the UT/LS: Project Overview and Preliminary Results Li-Hao Young 1, David Benson 1, William Montanaro 1, James C. Wilson 2, and Shan-Hu Lee 1 1 Kent State University 2 University
New Particle Formation in the UT/LS: Project Overview and Preliminary Results Li-Hao Young 1, David Benson 1, William Montanaro 1, James C. Wilson 2, and Shan-Hu Lee 1 1 Kent State University 2 University
Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009253, 2008 Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations C. Kuang, 1 P. H. McMurry,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009253, 2008 Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations C. Kuang, 1 P. H. McMurry,
ATMOSPHERIC AEROSOLS: FORMATION AND GROWTH
 ATMOSPHERIC AEROSOLS: FORMATION AND GROWTH Markku Kulmala University of Helsinki, Department of Physical Sciences, Division of Atmospheric Sciences, P.O. Pox 64, FIN-00014, University of Helsinki, Finland
ATMOSPHERIC AEROSOLS: FORMATION AND GROWTH Markku Kulmala University of Helsinki, Department of Physical Sciences, Division of Atmospheric Sciences, P.O. Pox 64, FIN-00014, University of Helsinki, Finland
Diurnal variation of tropospheric temperature at a tropical station
 Diurnal variation of tropospheric temperature at a tropical station K. Revathy, S. R. Prabhakaran Nayar, B. V. Krishna Murthy To cite this version: K. Revathy, S. R. Prabhakaran Nayar, B. V. Krishna Murthy.
Diurnal variation of tropospheric temperature at a tropical station K. Revathy, S. R. Prabhakaran Nayar, B. V. Krishna Murthy To cite this version: K. Revathy, S. R. Prabhakaran Nayar, B. V. Krishna Murthy.
Characteristics of new particle formation events and cluster ions at K-puszta, Hungary
 BOREAL ENVIRONMENT RESEARCH 14: 683 698 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Characteristics of new particle formation events and cluster ions at K-puszta, Hungary Taina
BOREAL ENVIRONMENT RESEARCH 14: 683 698 29 ISSN 1239-695 (print) ISSN 1797-2469 (online) Helsinki 31 August 29 Characteristics of new particle formation events and cluster ions at K-puszta, Hungary Taina
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation
 Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
Seasonal variations of trace gases, meteorological parameters, and formation of aerosols in boreal forests
 BOREAL ENVIRONMENT RESEARCH 10: 493 510 ISSN 1239-6095 Helsinki 14 December 2005 2005 Seasonal variations of trace gases, meteorological parameters, and formation of aerosols in boreal forests Yulia S.
BOREAL ENVIRONMENT RESEARCH 10: 493 510 ISSN 1239-6095 Helsinki 14 December 2005 2005 Seasonal variations of trace gases, meteorological parameters, and formation of aerosols in boreal forests Yulia S.
REPORT SERIES IN AEROSOL SCIENCE
 REPORT SERIES IN AEROSOL SCIENCE N:o 59 (2003) RESEARCH UNIT ON PHYSICS, CHEMISTRY AND BIOLOGY OF ATMOSPHERIC COMPOSITION AND CLIMATE CHANGE: PROGRESS REPORT AND PROCEEDINGS HYYTIALA 12.-14.3.2003 Helsinki
REPORT SERIES IN AEROSOL SCIENCE N:o 59 (2003) RESEARCH UNIT ON PHYSICS, CHEMISTRY AND BIOLOGY OF ATMOSPHERIC COMPOSITION AND CLIMATE CHANGE: PROGRESS REPORT AND PROCEEDINGS HYYTIALA 12.-14.3.2003 Helsinki
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS
 HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O
 THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
A Simple Model for Cavitation with Non-condensable Gases
 A Simple Model for Cavitation with Non-condensable Gases Mathieu Bachmann, Siegfried Müller, Philippe Helluy, Hélène Mathis To cite this version: Mathieu Bachmann, Siegfried Müller, Philippe Helluy, Hélène
A Simple Model for Cavitation with Non-condensable Gases Mathieu Bachmann, Siegfried Müller, Philippe Helluy, Hélène Mathis To cite this version: Mathieu Bachmann, Siegfried Müller, Philippe Helluy, Hélène
Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods
 doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany) including the infl uence of organic compounds
 BOREAL ENVIRONMENT RESEARCH 14: 616 629 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany)
BOREAL ENVIRONMENT RESEARCH 14: 616 629 2009 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 31 August 2009 Connection between new particle formation and sulphuric acid at Hohenpeissenberg (Germany)
Growth rates during coastal and marine new particle formation in western Ireland
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014292, 2010 Growth rates during coastal and marine new particle formation in western Ireland Mikael Ehn, 1 Henri Vuollekoski, 1 Tuukka Petäjä,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014292, 2010 Growth rates during coastal and marine new particle formation in western Ireland Mikael Ehn, 1 Henri Vuollekoski, 1 Tuukka Petäjä,
Estimation and Modeling of the Full Well Capacity in Pinned Photodiode CMOS Image Sensors
 Estimation and Modeling of the Full Well Capacity in Pinned Photodiode CMOS Image Sensors Alice Pelamatti, Vincent Goiffon, Magali Estribeau, Paola Cervantes, Pierre Magnan To cite this version: Alice
Estimation and Modeling of the Full Well Capacity in Pinned Photodiode CMOS Image Sensors Alice Pelamatti, Vincent Goiffon, Magali Estribeau, Paola Cervantes, Pierre Magnan To cite this version: Alice
ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS
 UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
Overview of the Particle Size Magnifier (PSM)
 Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Identification of boundaries between size classes of atmospheric aerosol particles
 BOREAL ENVIRONMENT RESEARCH 21: 363 371 2016 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 23 May 2016 Identification of boundaries between size classes of atmospheric aerosol particles Hannes
BOREAL ENVIRONMENT RESEARCH 21: 363 371 2016 ISSN 1239-6095 (print) ISSN 1797-2469 (online) Helsinki 23 May 2016 Identification of boundaries between size classes of atmospheric aerosol particles Hannes
Easter bracelets for years
 Easter bracelets for 5700000 years Denis Roegel To cite this version: Denis Roegel. Easter bracelets for 5700000 years. [Research Report] 2014. HAL Id: hal-01009457 https://hal.inria.fr/hal-01009457
Easter bracelets for 5700000 years Denis Roegel To cite this version: Denis Roegel. Easter bracelets for 5700000 years. [Research Report] 2014. HAL Id: hal-01009457 https://hal.inria.fr/hal-01009457
A wide-range multi-channel Air Ion Spectrometer
 Boreal nvironment Research 12: 247 264 issn 1239-6095 Helsinki 25 June 2007 2007 A wide-range multi-channel Air Ion Spectrometer Aadu Mirme 1), duard Tamm 1), Genrik Mordas 2), Marko Vana 1)2), Janek Uin
Boreal nvironment Research 12: 247 264 issn 1239-6095 Helsinki 25 June 2007 2007 A wide-range multi-channel Air Ion Spectrometer Aadu Mirme 1), duard Tamm 1), Genrik Mordas 2), Marko Vana 1)2), Janek Uin
Thermally-Stimulated Current Investigation of Dopant-Related D- and A+ Trap Centers in Germanium for Cryogenic Detector Applications
 Thermally-Stimulated Current Investigation of Dopant-Related D- and A+ Trap Centers in Germanium for Cryogenic Detector Applications J. Domange, E. Olivieri, N. Fourches, A. Broniatowski To cite this version:
Thermally-Stimulated Current Investigation of Dopant-Related D- and A+ Trap Centers in Germanium for Cryogenic Detector Applications J. Domange, E. Olivieri, N. Fourches, A. Broniatowski To cite this version:
Lee Mauldin Airmodus University of Helsinki University of Colorado
 Nitrate Ion Chemical Ionization Lee Mauldin Airmodus University of Helsinki University of Colorado Co axial Nitrate Ion Chemical Ionization Used extensively for OH, H 2 SO 4, and MSA measurements Ground
Nitrate Ion Chemical Ionization Lee Mauldin Airmodus University of Helsinki University of Colorado Co axial Nitrate Ion Chemical Ionization Used extensively for OH, H 2 SO 4, and MSA measurements Ground
DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN
 REPORT SERIES IN AEROSOL SCIENCE N:o 122 (2011) DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN Division of Atmospheric Sciences Department
REPORT SERIES IN AEROSOL SCIENCE N:o 122 (2011) DIRECT DETECTION OF ATMOSPHERIC PARTICLE FORMATION USING THE NEUTRAL CLUSTER AND AIR ION SPECTROMETER HANNA MANNINEN Division of Atmospheric Sciences Department
Chemistry of stabilized Criegee intermediates in the CLOUD chamber
 Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
Trench IGBT failure mechanisms evolution with temperature and gate resistance under various short-circuit conditions
 Trench IGBT failure mechanisms evolution with temperature and gate resistance under various short-circuit conditions Adel Benmansour, Stephane Azzopardi, Jean-Christophe Martin, Eric Woirgard To cite this
Trench IGBT failure mechanisms evolution with temperature and gate resistance under various short-circuit conditions Adel Benmansour, Stephane Azzopardi, Jean-Christophe Martin, Eric Woirgard To cite this
NEGATIVE ION IMAGING IN FIELD ION MICROSCOPY
 NEGATIVE ION IMAGING IN FIELD ION MICROSCOPY R. Schmitz, L. Bütfering, F. Röllgen To cite this version: R. Schmitz, L. Bütfering, F. Röllgen. NEGATIVE ION IMAGING IN FIELD ION MICROSCOPY. Journal de Physique
NEGATIVE ION IMAGING IN FIELD ION MICROSCOPY R. Schmitz, L. Bütfering, F. Röllgen To cite this version: R. Schmitz, L. Bütfering, F. Röllgen. NEGATIVE ION IMAGING IN FIELD ION MICROSCOPY. Journal de Physique
Measurements of the ion concentrations and conductivity over the Arabian. Sea during the ARMEX
 Measurements of the ion concentrations and conductivity over the Arabian Sea during the ARMEX Devendraa Siingh, S.D. Pawar, V. Gopalakrishnan and A. K. Kamra Indian Institute of Tropical Meteorology, Pune,
Measurements of the ion concentrations and conductivity over the Arabian Sea during the ARMEX Devendraa Siingh, S.D. Pawar, V. Gopalakrishnan and A. K. Kamra Indian Institute of Tropical Meteorology, Pune,
Lorentz force velocimetry using small-size permanent magnet systems and a multi-degree-of-freedom force/torque sensor
 Lorentz force velocimetry using small-size permanent magnet systems and a multi-degree-of-freedom force/torque sensor D Hernández, C Karcher To cite this version: D Hernández, C Karcher. Lorentz force
Lorentz force velocimetry using small-size permanent magnet systems and a multi-degree-of-freedom force/torque sensor D Hernández, C Karcher To cite this version: D Hernández, C Karcher. Lorentz force
A new simple recursive algorithm for finding prime numbers using Rosser s theorem
 A new simple recursive algorithm for finding prime numbers using Rosser s theorem Rédoane Daoudi To cite this version: Rédoane Daoudi. A new simple recursive algorithm for finding prime numbers using Rosser
A new simple recursive algorithm for finding prime numbers using Rosser s theorem Rédoane Daoudi To cite this version: Rédoane Daoudi. A new simple recursive algorithm for finding prime numbers using Rosser
The condensation particle counter battery (CPCB): A new tool to investigate the activation properties of nanoparticles
 Aerosol Science 38 (2007) 289 304 www.elsevier.com/locate/jaerosci The condensation particle counter battery (CPCB): A new tool to investigate the activation properties of nanoparticles Markku Kulmala
Aerosol Science 38 (2007) 289 304 www.elsevier.com/locate/jaerosci The condensation particle counter battery (CPCB): A new tool to investigate the activation properties of nanoparticles Markku Kulmala
Intercomparison of air ion spectrometers: an evaluation of results in varying conditions
 doi:10.5194/amt-4-805-2011 Author(s) 2011. CC Attribution 3.0 License. Atmospheric Measurement Techniques Intercomparison of air ion spectrometers: an evaluation of results in varying conditions S. Gagné
doi:10.5194/amt-4-805-2011 Author(s) 2011. CC Attribution 3.0 License. Atmospheric Measurement Techniques Intercomparison of air ion spectrometers: an evaluation of results in varying conditions S. Gagné
Methylation-associated PHOX2B gene silencing is a rare event in human neuroblastoma.
 Methylation-associated PHOX2B gene silencing is a rare event in human neuroblastoma. Loïc De Pontual, Delphine Trochet, Franck Bourdeaut, Sophie Thomas, Heather Etchevers, Agnes Chompret, Véronique Minard,
Methylation-associated PHOX2B gene silencing is a rare event in human neuroblastoma. Loïc De Pontual, Delphine Trochet, Franck Bourdeaut, Sophie Thomas, Heather Etchevers, Agnes Chompret, Véronique Minard,
Dynamic Thermal Analysis of a Power Amplifier
 Dynamic Thermal Analysis of a Power Amplifier J. Banaszczyk, G. De Mey, M. Janicki, A. Napieralski, B. Vermeersch, P. Kawka To cite this version: J. Banaszczyk, G. De Mey, M. Janicki, A. Napieralski, B.
Dynamic Thermal Analysis of a Power Amplifier J. Banaszczyk, G. De Mey, M. Janicki, A. Napieralski, B. Vermeersch, P. Kawka To cite this version: J. Banaszczyk, G. De Mey, M. Janicki, A. Napieralski, B.
IMPROVEMENTS OF THE VARIABLE THERMAL RESISTANCE
 IMPROVEMENTS OF THE VARIABLE THERMAL RESISTANCE V. Szekely, S. Torok, E. Kollar To cite this version: V. Szekely, S. Torok, E. Kollar. IMPROVEMENTS OF THE VARIABLE THERMAL RESIS- TANCE. THERMINIC 2007,
IMPROVEMENTS OF THE VARIABLE THERMAL RESISTANCE V. Szekely, S. Torok, E. Kollar To cite this version: V. Szekely, S. Torok, E. Kollar. IMPROVEMENTS OF THE VARIABLE THERMAL RESIS- TANCE. THERMINIC 2007,
The influence of the global atmospheric properties on the detection of UHECR by EUSO on board of the ISS
 The influence of the global atmospheric properties on the detection of UHECR by EUSO on board of the ISS C. Berat, D. Lebrun, A. Stutz, E. Plagnol To cite this version: C. Berat, D. Lebrun, A. Stutz, E.
The influence of the global atmospheric properties on the detection of UHECR by EUSO on board of the ISS C. Berat, D. Lebrun, A. Stutz, E. Plagnol To cite this version: C. Berat, D. Lebrun, A. Stutz, E.
Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS)
 Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS) Jason Olfert, Brookhaven National Laboratory Jian Wang, Brookhaven National Laboratory Measurement
Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS) Jason Olfert, Brookhaven National Laboratory Jian Wang, Brookhaven National Laboratory Measurement
Smart Bolometer: Toward Monolithic Bolometer with Smart Functions
 Smart Bolometer: Toward Monolithic Bolometer with Smart Functions Matthieu Denoual, Gilles Allègre, Patrick Attia, Olivier De Sagazan To cite this version: Matthieu Denoual, Gilles Allègre, Patrick Attia,
Smart Bolometer: Toward Monolithic Bolometer with Smart Functions Matthieu Denoual, Gilles Allègre, Patrick Attia, Olivier De Sagazan To cite this version: Matthieu Denoual, Gilles Allègre, Patrick Attia,
A new feedback mechanism linking forests, aerosols, and climate
 SRef-ID: 1680-7324/acp/2004-4-557 Atmospheric Chemistry and Physics A new feedback mechanism linking forests, aerosols, and climate M. Kulmala 1, T. Suni 1, K. E. J. Lehtinen 1, M. Dal Maso 1, M. Boy 1,
SRef-ID: 1680-7324/acp/2004-4-557 Atmospheric Chemistry and Physics A new feedback mechanism linking forests, aerosols, and climate M. Kulmala 1, T. Suni 1, K. E. J. Lehtinen 1, M. Dal Maso 1, M. Boy 1,
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Airborne measurements of nucleation mode particles I: coastal nucleation and growth rates
 Atmos. Chem. Phys., 7, 1491 151, 27 www.atmos-chem-phys.net/7/1491/27/ Author(s) 27. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Airborne measurements of nucleation
Atmos. Chem. Phys., 7, 1491 151, 27 www.atmos-chem-phys.net/7/1491/27/ Author(s) 27. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Airborne measurements of nucleation
LAWS OF CRYSTAL-FIELD DISORDERNESS OF Ln3+ IONS IN INSULATING LASER CRYSTALS
 LAWS OF CRYSTAL-FIELD DISORDERNESS OF Ln3+ IONS IN INSULATING LASER CRYSTALS A. Kaminskii To cite this version: A. Kaminskii. LAWS OF CRYSTAL-FIELD DISORDERNESS OF Ln3+ IONS IN INSULAT- ING LASER CRYSTALS.
LAWS OF CRYSTAL-FIELD DISORDERNESS OF Ln3+ IONS IN INSULATING LASER CRYSTALS A. Kaminskii To cite this version: A. Kaminskii. LAWS OF CRYSTAL-FIELD DISORDERNESS OF Ln3+ IONS IN INSULAT- ING LASER CRYSTALS.
SOLAR RADIATION ESTIMATION AND PREDICTION USING MEASURED AND PREDICTED AEROSOL OPTICAL DEPTH
 SOLAR RADIATION ESTIMATION AND PREDICTION USING MEASURED AND PREDICTED AEROSOL OPTICAL DEPTH Carlos M. Fernández-Peruchena, Martín Gastón, Maria V Guisado, Ana Bernardos, Íñigo Pagola, Lourdes Ramírez
SOLAR RADIATION ESTIMATION AND PREDICTION USING MEASURED AND PREDICTED AEROSOL OPTICAL DEPTH Carlos M. Fernández-Peruchena, Martín Gastón, Maria V Guisado, Ana Bernardos, Íñigo Pagola, Lourdes Ramírez
A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation
 BOREAL ENVIRONMENT RESEARCH : 55 5 ISSN 9-695 Helsinki December 5 5 A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation Alf Grini )), Hannele Korhonen
BOREAL ENVIRONMENT RESEARCH : 55 5 ISSN 9-695 Helsinki December 5 5 A combined photochemistry/aerosol dynamics model: model development and a study of new particle formation Alf Grini )), Hannele Korhonen
Ideal Coulomb plasma approximation in line shape models: problematic issues
 Ideal Coulomb plasma approximation in line shape models: problematic issues Joël Rosato, Hubert Capes, Roland Stamm To cite this version: Joël Rosato, Hubert Capes, Roland Stamm. Ideal Coulomb plasma approximation
Ideal Coulomb plasma approximation in line shape models: problematic issues Joël Rosato, Hubert Capes, Roland Stamm To cite this version: Joël Rosato, Hubert Capes, Roland Stamm. Ideal Coulomb plasma approximation
Charging State of Aerosols during Particle Formation Events in an Urban Environment and Its Implications for Ion-Induced Nucleation
 Aerosol and Air Quality Research, 16: 348 36, 216 Copyright Taiwan Association for Aerosol Research ISSN: 168-8584 print / 271-149 online doi: 1.429/aaqr.215.3.148 Charging State of Aerosols during Particle
Aerosol and Air Quality Research, 16: 348 36, 216 Copyright Taiwan Association for Aerosol Research ISSN: 168-8584 print / 271-149 online doi: 1.429/aaqr.215.3.148 Charging State of Aerosols during Particle
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113, D06202, doi: /2007jd009302, 2008
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009302, 2008 Nucleation mode particles in upslope valley winds at Mount Norikura, Japan: Implications for the vertical extent of new particle
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009302, 2008 Nucleation mode particles in upslope valley winds at Mount Norikura, Japan: Implications for the vertical extent of new particle
Jianfei Peng et al. Correspondence to: Jianfei Peng Min Hu and Renyi Zhang
 Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Quantum efficiency and metastable lifetime measurements in ruby ( Cr 3+ : Al2O3) via lock-in rate-window photothermal radiometry
 Quantum efficiency and metastable lifetime measurements in ruby ( Cr 3+ : Al2O3) via lock-in rate-window photothermal radiometry A. Mandelis, Z. Chen, R. Bleiss To cite this version: A. Mandelis, Z. Chen,
Quantum efficiency and metastable lifetime measurements in ruby ( Cr 3+ : Al2O3) via lock-in rate-window photothermal radiometry A. Mandelis, Z. Chen, R. Bleiss To cite this version: A. Mandelis, Z. Chen,
Gas Particle Partitioning to OA
 Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing
 ! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Laboratory Studies of H2So4/H2O Binary Homogeneous Nucleation from the So2+Oh Reaction: Evaluation of the Experimental Setup and Preliminary Results
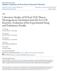 Kent State University Digital Commons @ Kent State University Libraries Biostatistics, Environmental Health, and Epidemiology Publications Biostatistics, Environmental Health and Epidemiology 8 Laboratory
Kent State University Digital Commons @ Kent State University Libraries Biostatistics, Environmental Health, and Epidemiology Publications Biostatistics, Environmental Health and Epidemiology 8 Laboratory
Differential Mobility Particle Sizer (Aerosol measurements)
 Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Diurnal variation in the concentration of air ions of different mobility classes in a rural area
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4653, doi:10.1029/2002jd003240, 2003 Diurnal variation in the concentration of air ions of different mobility classes in a rural area Urmas Hõrrak, Jaan
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4653, doi:10.1029/2002jd003240, 2003 Diurnal variation in the concentration of air ions of different mobility classes in a rural area Urmas Hõrrak, Jaan
Determination of absorption characteristic of materials on basis of sound intensity measurement
 Determination of absorption characteristic of materials on basis of sound intensity measurement R. Prascevic, A. Milosevic, S. Cvetkovic To cite this version: R. Prascevic, A. Milosevic, S. Cvetkovic.
Determination of absorption characteristic of materials on basis of sound intensity measurement R. Prascevic, A. Milosevic, S. Cvetkovic To cite this version: R. Prascevic, A. Milosevic, S. Cvetkovic.
Sound intensity as a function of sound insulation partition
 Sound intensity as a function of sound insulation partition S. Cvetkovic, R. Prascevic To cite this version: S. Cvetkovic, R. Prascevic. Sound intensity as a function of sound insulation partition. Journal
Sound intensity as a function of sound insulation partition S. Cvetkovic, R. Prascevic To cite this version: S. Cvetkovic, R. Prascevic. Sound intensity as a function of sound insulation partition. Journal
On Symmetric Norm Inequalities And Hermitian Block-Matrices
 On Symmetric Norm Inequalities And Hermitian lock-matrices Antoine Mhanna To cite this version: Antoine Mhanna On Symmetric Norm Inequalities And Hermitian lock-matrices 015 HAL Id: hal-0131860
On Symmetric Norm Inequalities And Hermitian lock-matrices Antoine Mhanna To cite this version: Antoine Mhanna On Symmetric Norm Inequalities And Hermitian lock-matrices 015 HAL Id: hal-0131860
A criterion for new particle formation in the sulfur-rich Atlanta atmosphere
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2005jd005901, 2005 A criterion for new particle formation in the sulfur-rich Atlanta atmosphere P. H. McMurry, 1 M. Fink, 1 H. Sakurai, 1 M. R. Stolzenburg,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2005jd005901, 2005 A criterion for new particle formation in the sulfur-rich Atlanta atmosphere P. H. McMurry, 1 M. Fink, 1 H. Sakurai, 1 M. R. Stolzenburg,
